Avoiding Conflicts between Future Freshwater Algae Production and Water Scarcity in the United States at the Energy-Water Nexus
Abstract
1. Introduction
1.1. Greenhouse-Gas (GHG) Emissions
1.2. Net Water Availability (Water Surplus)
1.3. Implications for Stream Habitat
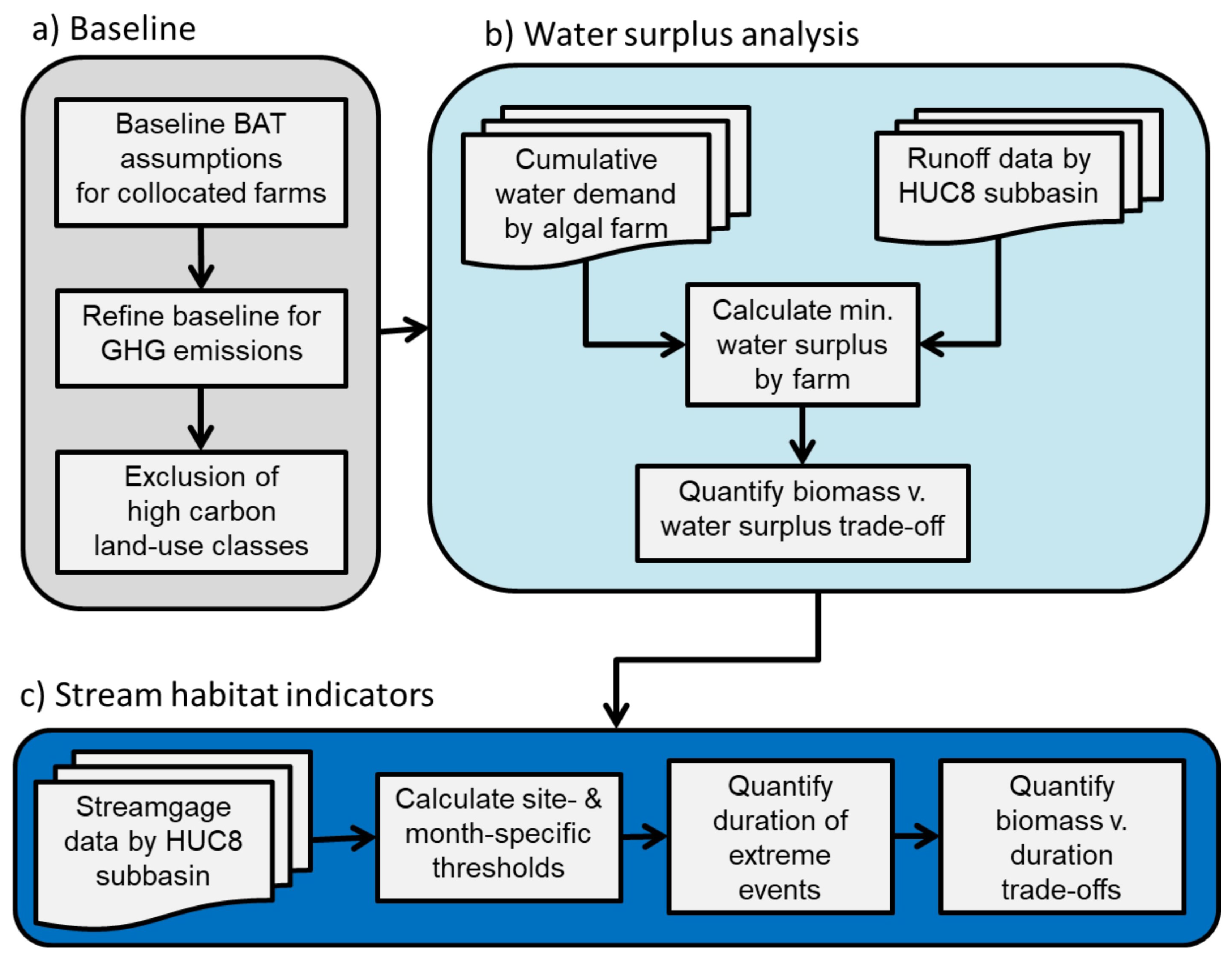
2. Materials and Methods
2.1. Setting a Low-Carbon Baseline
2.2. Biomass Modeling Using the Biomass Assessment Tool (BAT)
2.3. Net Water Availability (Water Surplus)
2.4. Implications for Stream Habitat
2.4.1. Selection of Representative Stream Gages
2.4.2. Duration of Extreme Stream Habitat Conditions
3. Results
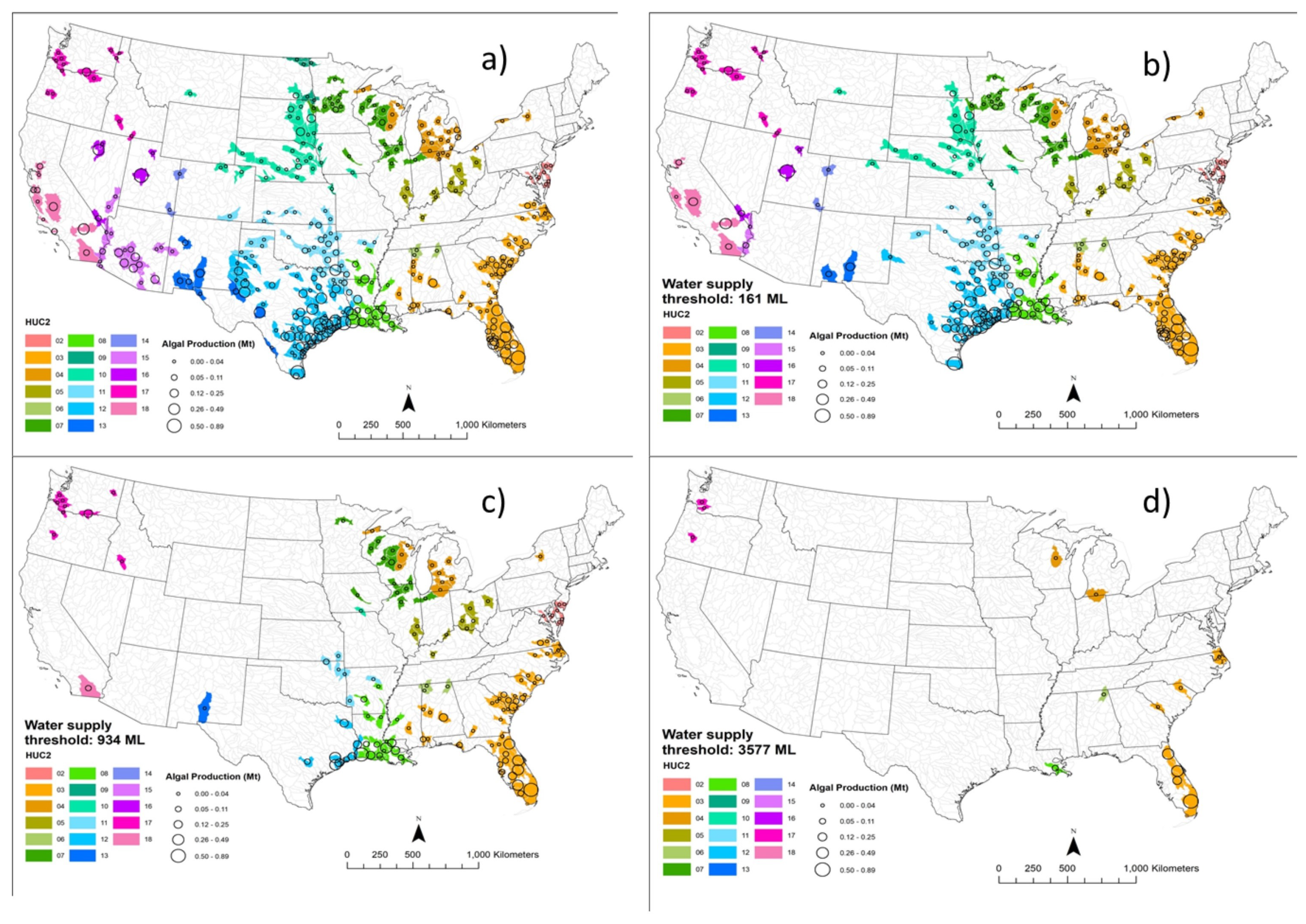
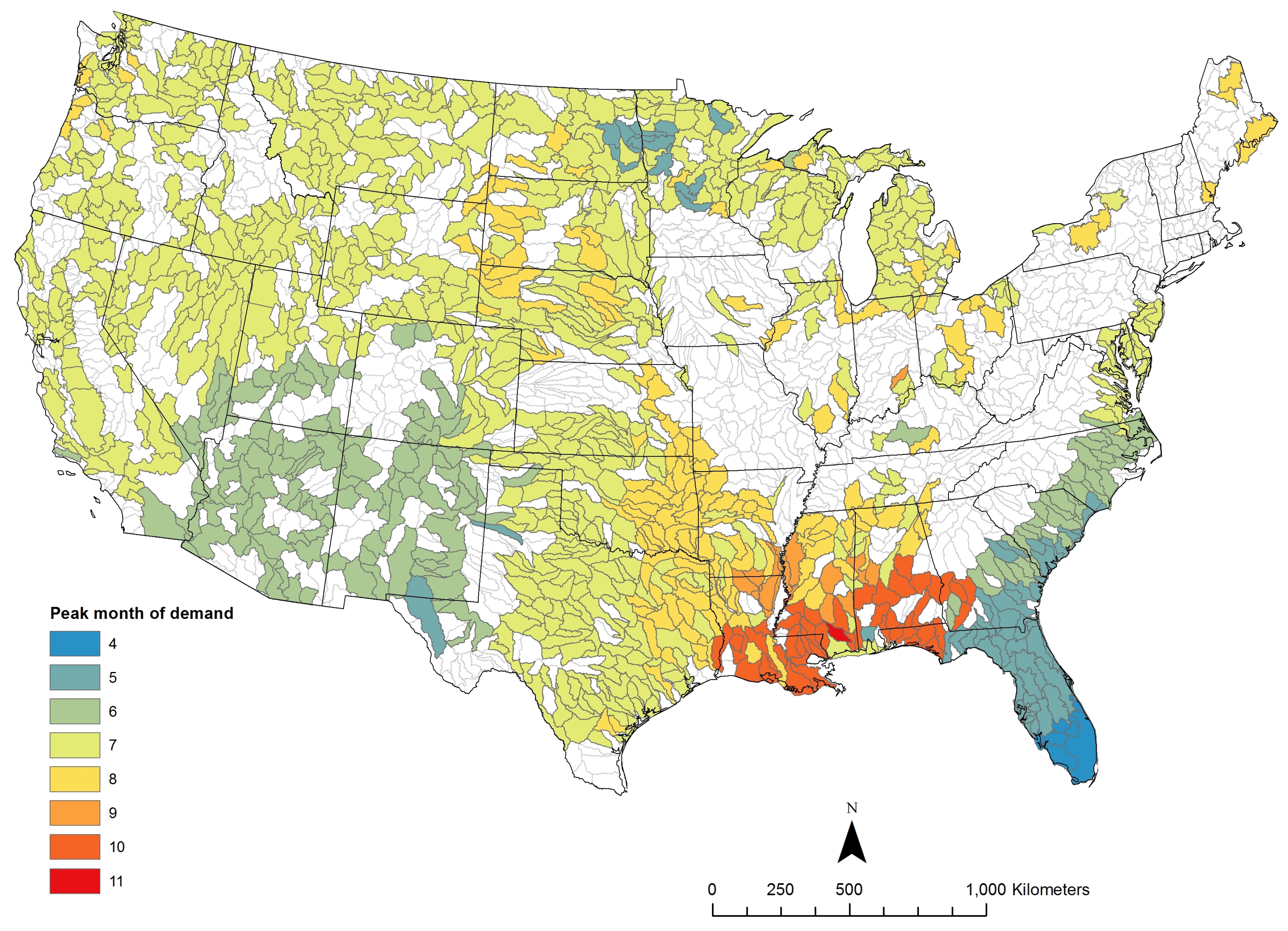
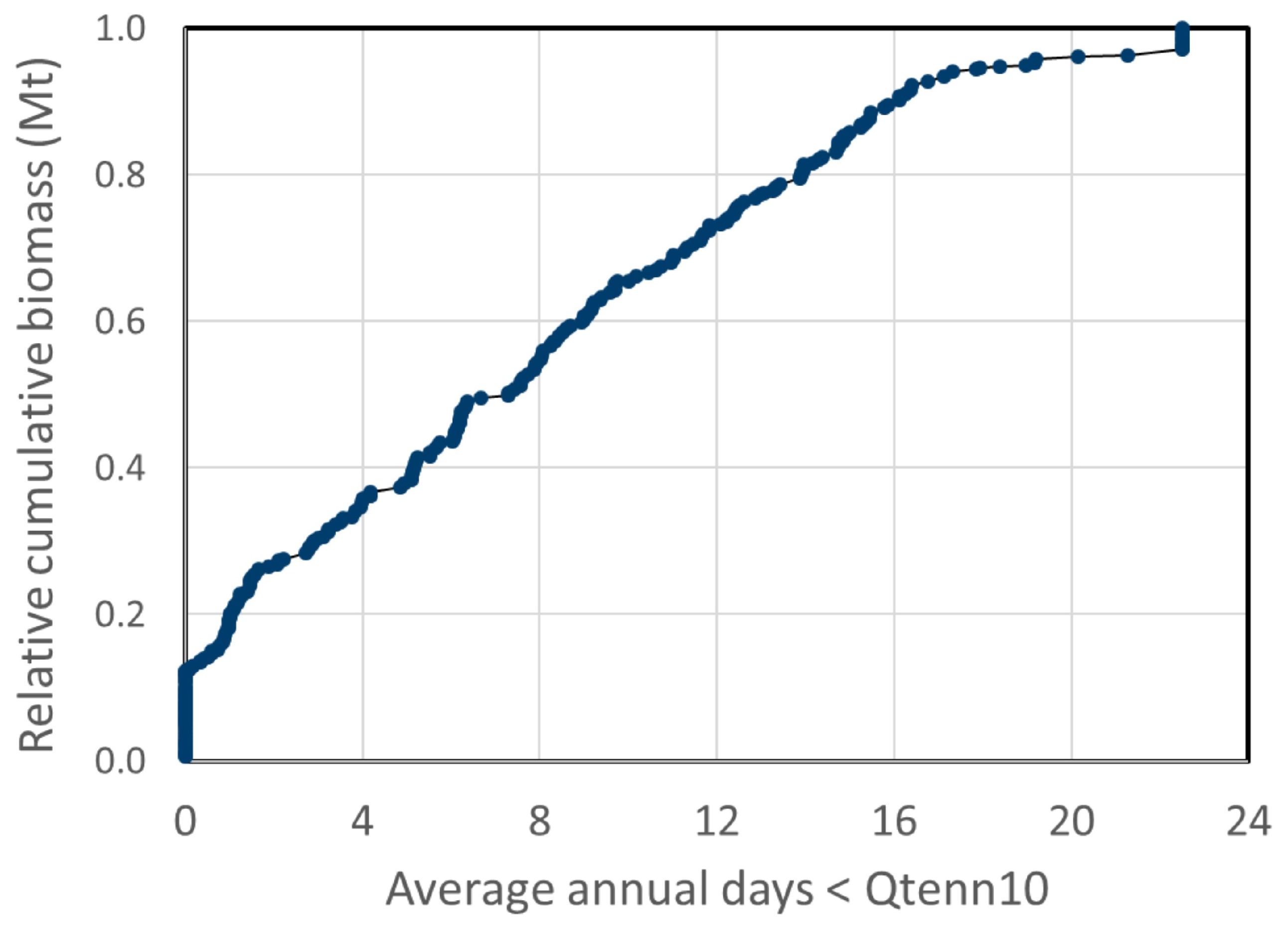
3.1. Potential Algae Production
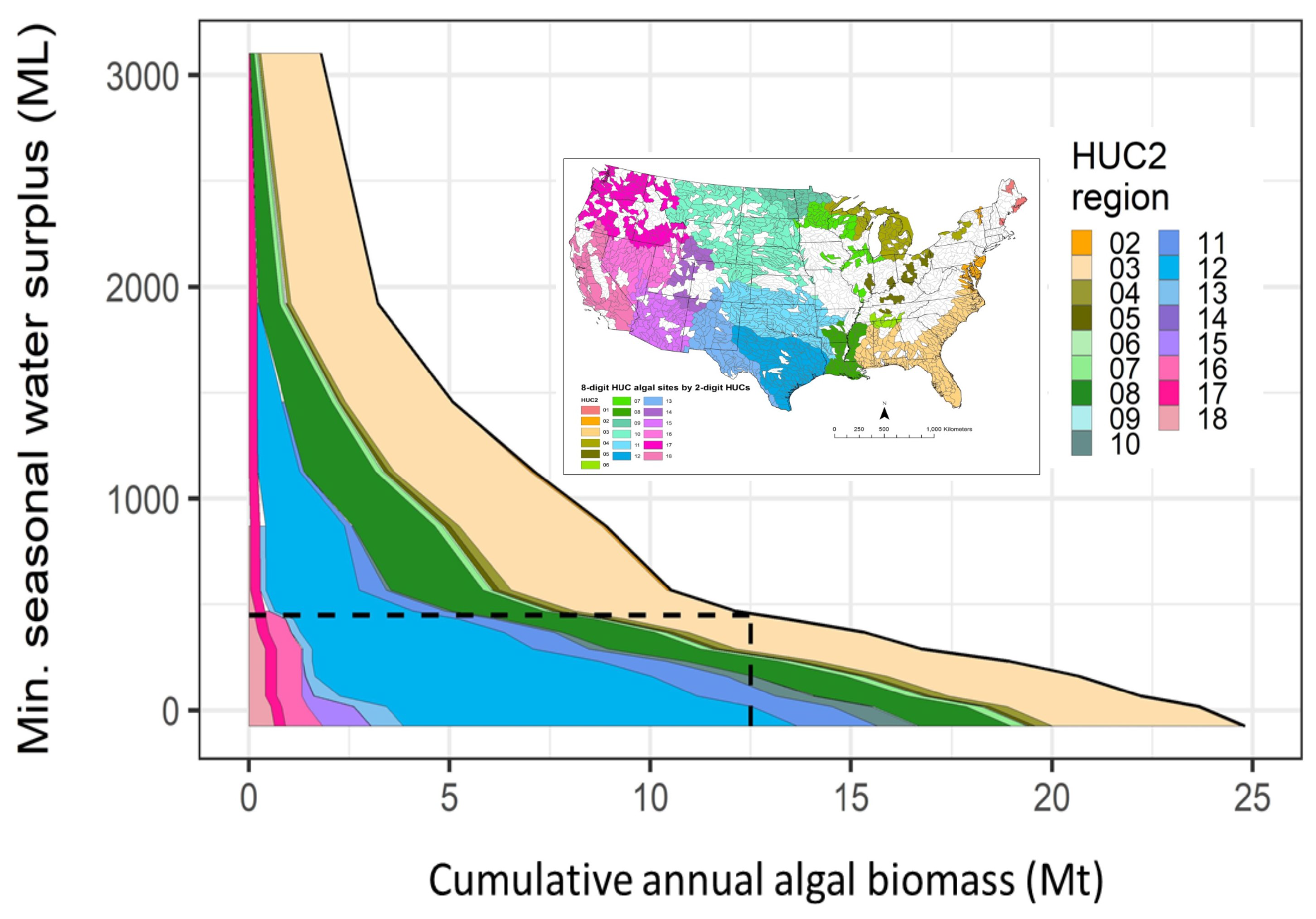
3.2. Net Water Availability (Water Surplus)
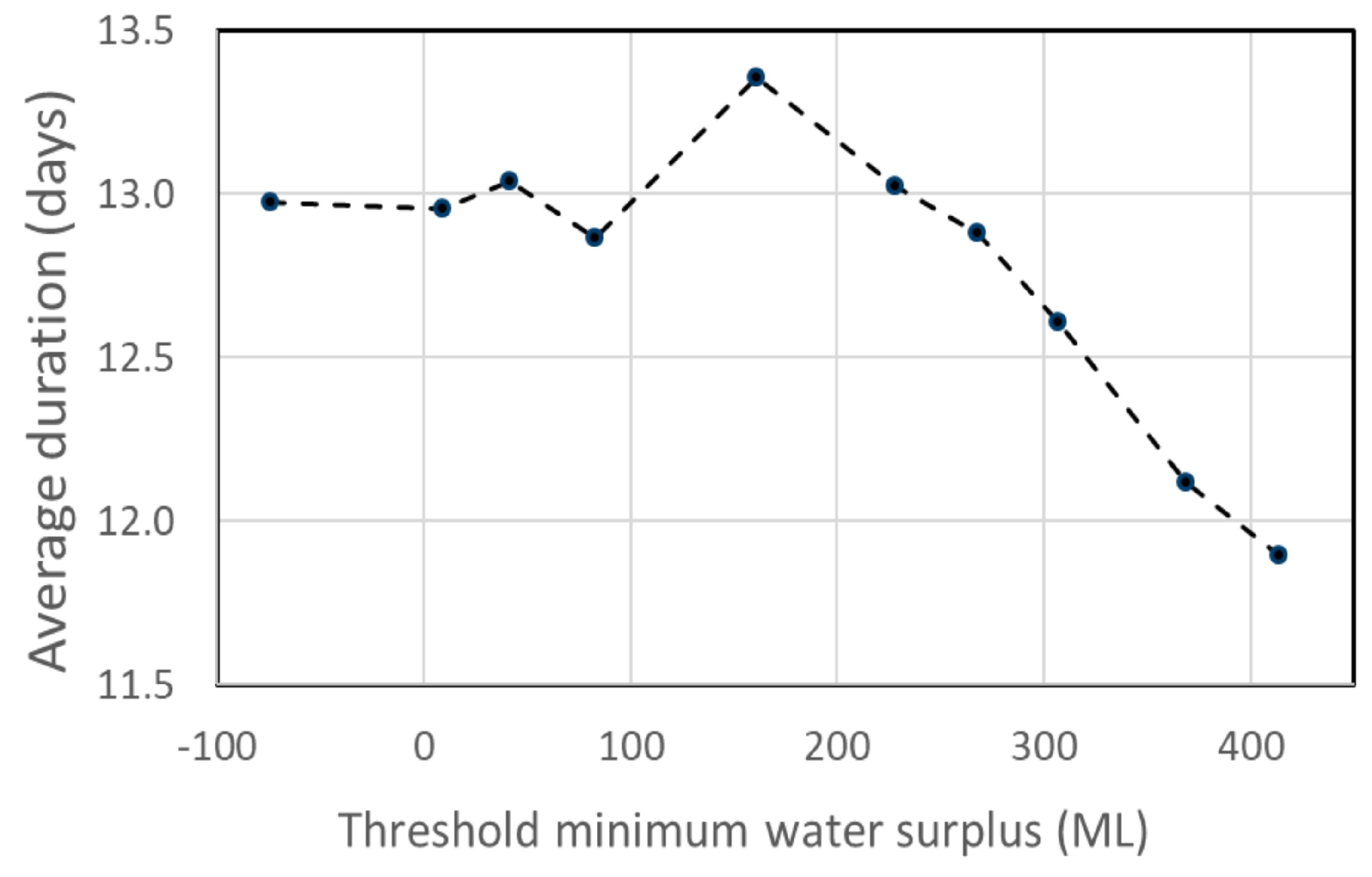
3.3. Implications for Stream Habitat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohtar, R.H.; Shafiezadeh, H.; Blake, J.; Daher, B. Economic, social, and environmental evaluation of energy development in the Eagle Ford shale play. Sci. Total Environ. 2019, 646, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Daher, B.; Lee, S.H.; Kaushik, V.; Blake, J.; Askariyeh, M.H.; Shafiezadeh, H.; Zamaripa, S.; Mohtar, R.H. Towards bridging the water gap in Texas: A water-energy-food nexus approach. Sci. Total Environ. 2019, 647, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Tan, R.R.; Aviso, K.B.; Promentilla, M.A.B.; Sulaiman, N.M.N. Study of microalgae cultivation systems based on integrated analytic hierarchy process-life cycle optimization. Clean Technol. Environ. Policy 2017, 19, 2075–2088. [Google Scholar] [CrossRef]
- Venteris, E.R.; Skaggs, R.L.; Wigmosta, M.S.; Coleman, A.M. A national-scale comparison of resource and nutrient demands for algae-based biofuel production by lipid extraction and hydrothermal liquefaction. Biomass Bioenergy 2014, 64, 276–290. [Google Scholar] [CrossRef]
- Venteris, E.R.; McBride, R.C.; Coleman, A.M.; Skaggs, R.L.; Wigmosta, M.S. Siting Algae Cultivation Facilities for Biofuel Production in the United States: Trade-Offs between Growth Rate, Site Constructability, Water Availability, and Infrastructure. Environ. Sci. Technol. 2014, 48, 3559–3566. [Google Scholar] [CrossRef] [PubMed]
- Venteris, E.R.; Skaggs, R.L.; Coleman, A.M.; Wigmosta, M.S. A GIS cost model to assess the availability of freshwater, seawater, and saline groundwater for algal biofuel production in the United States. Environ. Sci. Technol. 2013, 47, 4840–4849. [Google Scholar] [CrossRef]
- Wigmosta, M.S.; Coleman, A.M.; Skaggs, R.J.; Huesemann, M.H.; Lane, L.J. National microalgae biofuel production potential and resource demand. Water Resour. Res. 2011, 47. [Google Scholar] [CrossRef]
- Coleman, A.M.; Abodeely, J.M.; Skaggs, R.L.; Moeglein, W.A.; Newby, D.T.; Venteris, E.R.; Wigmosta, M.S. An integrated assessment of location-dependent scaling for microalgae biofuel production facilities. Algal Res. 2014, 5, 79–94. [Google Scholar] [CrossRef]
- Baumber, A. Enhancing ecosystem services through targeted bioenergy support policies. Ecosyst. Serv. 2017, 26, 98–110. [Google Scholar] [CrossRef]
- Jager, H.I.; Efroymson, R.A. Can upstream biofuel production increase the flow of downstream ecosystem goods and services? Biomass Bioenergy 2018, 114, 125–131. [Google Scholar] [CrossRef]
- Selosse, S.; Ricci, O. Carbon capture and storage: Lessons from a storage potential and localization analysis. Appl. Energy 2017, 188, 32–44. [Google Scholar] [CrossRef]
- Edmundson, S.J.; Wilkie, A.C. Landfill leachate—A water and nutrient resource for algae-based biofuels. Environ. Technol. 2013, 34, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Norvill, Z.N.; Shilton, A.; Guieysse, B. Emerging contaminant degradation and removal in algal wastewater treatment ponds: Identifying the research gaps. J. Hazard. Mater. 2016, 313, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. The possible roles of algae in restricting the increase in atmospheric CO2 and global temperature. Eur. J. Phycol. 2017, 52, 506–522. [Google Scholar] [CrossRef]
- Vasseur, C.; Bougaran, G.; Garnier, M.; Hamelin, J.; Leboulanger, C.; Le Chevanton, M.; Mostajir, B.; Sialve, B.; Steyer, J.P.; Fouilland, E. Carbon conversion efficiency and population dynamics of a marine algae-bacteria consortium growing on simplified synthetic digestate: First step in a bioprocess coupling algal production and anaerobic digestion. Bioresour. Technol. 2012, 119, 79–87. [Google Scholar] [CrossRef]
- US Department of Energy Efroymson (Lead). Billion Ton 2016 Report: Volume II. Environmental Effects; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016.
- Gerbens-Leenes, P.W.; Xu, L.; de Vries, G.J.; Hoekstra, A.Y. The blue water footprint and land use of biofuels from algae. Water Resour. Res. 2014, 50, 8549–8563. [Google Scholar] [CrossRef]
- Kasischke, E.S.; Amiro, B.D.; Barger, N.N.; French, N.H.F.; Goetz, S.J.; Grosse, G.; Harmon, M.E.; Hicke, J.A.; Liu, S.G.; Masek, J.G. Impacts of disturbance on the terrestrial carbon budget of North America. J. Geophys. Res. Biogeosci. 2013, 118, 303–316. [Google Scholar] [CrossRef]
- Medeiros, D.L.; Sales, E.A.; Kiperstok, A. Energy production from microalgae biomass: Carbon footprint and energy balance. J. Clean. Prod. 2015, 96, 493–500. [Google Scholar] [CrossRef]
- Yeh, S.; Berndes, G.; Mishra, G.S.; Wani, S.P.; Neto, A.E.; Suh, S.; Karlberg, L.; Heinke, J.; Garg, K.K. Evaluation of water use for bioenergy at different scales. Biofuels Bioprod. Biorefin. 2011, 5, 361–374. [Google Scholar] [CrossRef]
- Gerbens-Leenes, W.; Hoekstra, A.Y.; van der Meer, T.H. The water footprint of bioenergy. Proc. Natl. Acad. Sci. USA 2009, 106, 10219–10223. [Google Scholar] [CrossRef]
- Berndes, G. Future biomass energy supply: The consumptive water use perspective. Int. J. Water Resour. Dev. 2008, 24, 235–245. [Google Scholar] [CrossRef]
- Frank, E.D.; Elgowainy, A.; Han, J.; Wang, Z.C. Life cycle comparison of hydrothermal liquefaction and lipid extraction pathways to renewable diesel from algae. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 137–158. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Zhang, X.Z.; Hu, Q.A.; Sommerfeld, M.; Chen, Y.S. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Canter, C.E.; Davis, R.; Urgun-Demirtas, M.; Frank, E.D. Infrastructure associated emissions for renewable diesel production from microalgae. Algal Res. 2014, 5, 195–203. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Rogers, J.N.; Rosenberg, J.N.; Guzman, B.J.; Oh, V.H.; Mimbela, L.E.; Ghassemi, A.; Betenbaugh, M.J.; Oyler, G.A.; Donohue, M.D. A critical analysis of paddlewheel-driven raceway ponds for algal biofuel production at commercial scales. Algal Res. 2014, 4, 76–88. [Google Scholar] [CrossRef]
- Efroymson, R.; Coleman, A.; Wigmosta, M.; Pattullo, M.; Mayes, M.; Langholtz, M. Qualitative Analysis of Environmental Effects of Algae Production; Report ORNL/TM-2016/727; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2017. [Google Scholar] [CrossRef]
- Shurin, J.B.; Mandal, S.; Abbott, R.L. Trait diversity enhances yield in algal biofuel assemblages. J. Appl. Ecol. 2014, 51, 603–611. [Google Scholar] [CrossRef]
- Arita, C.Q.; Yilmaz, O.; Barlak, S.; Catton, K.B.; Quinn, J.C.; Bradley, T.H. A geographical assessment of vegetation carbon stocks and greenhouse gas emissions on potential microalgae-based biofuel facilities in the United States. Bioresour. Technol. 2016, 221, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Wu, W.H.; Tran-Gyamfi, M.; Lane, T.; Pate, R.; Wu, B. Comprehensive bioconversion of algae to liquid fuels and intermediate value products. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2016; Volume 251. [Google Scholar]
- De Maria, P.D. On the Use of Seawater as Reaction Media for Large-Scale Applications in Biorefineries. Chemcatchem 2013, 5, 1643–1648. [Google Scholar] [CrossRef]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef]
- Brauman, K.A.; Richter, B.D.; Postel, S.; Malsy, M.; Flörke, M. Water depletion: An improved metric for incorporating seasonal and dry-year water scarcity into water risk assessments. Elem. Sci. Anthr. 2016, 4, 000083. [Google Scholar] [CrossRef]
- Helmbrecht, J.; Pastor, J.; Moya, C. Smart solution to improve water-energy nexus for water supply systems. Procedia Eng. 2017, 186, 101–109. [Google Scholar] [CrossRef]
- Garbe, J.; Beevers, L.; Pender, G. Valuing the Effect of Freshwater Over-abstraction on Fish Species. In Proceedings of the 35th IAHR World Congress, Vols I and II; International Association for Hydro-Environment Engineering and Research (IAHR): Madrid, Spain; 2013; pp. 2769–2778. [Google Scholar]
- Poff, N.L.; Allan, J.D.; Bain, M.B.; Karr, J.R.; Prestegaard, K.L.; Richter, B.D.; Sparks, R.E.; Stromberg, J.C. The natural flow regime. BioScience 1997, 47, 769–784. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L. Redundancy and the choice of hydrologic indices for characterizing streamflow regimes. River Res. Appl. 2003, 19, 101–121. [Google Scholar] [CrossRef]
- McManamay, R.A.; Brewer, S.; Jager, H.I.; Troia, M.J. Organizing environmental flow frameworks to meet hydropower mitigation needs. Environ. Manag. 2016, 58, 365–385. [Google Scholar] [CrossRef]
- Poff, N.L.; Richter, B.D.; Arthington, A.H.; Bunn, S.E.; Naiman, R.J.; Kendy, E.; Acreman, M.; Apse, C.; Bledsoe, B.P.; Freeman, M.C.; et al. The ecological limits of hydrologic alteration (ELOHA): A new framework for developing regional environmental flow standards. Freshw. Biol. 2010, 55, 147–170. [Google Scholar] [CrossRef]
- McCullough, D.A. Are coldwater fish populations of the United States actually being protected by temperature standards? Freshw. Rev. 2010, 3, 147–197. [Google Scholar] [CrossRef]
- Richter, A.; Kolmes, S.A. Maximum temperature limits for chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Rev. Fish. Sci. 2005, 13, 23–49. [Google Scholar] [CrossRef]
- Nadeau, T.L.; Leibowitz, S.G.; Wigington, P.J., Jr.; Ebersole, J.L.; Fritz, K.M.; Coulombe, R.A.; Comeleo, R.L.; Blocksom, K.A. Validation of rapid assessment methods to determine streamflow duration classes in the Pacific Northwest, USA. Environ. Manag. 2015, 56, 34–53. [Google Scholar] [CrossRef]
- Jager, H. Thinking outside the channel: Timing pulse flows to benefit salmon via indirect pathways. Ecol. Model. 2014, 217, 117–127. [Google Scholar] [CrossRef]
- McCullough, D.A.; Bartholow, J.M.; Jager, H.I.; Beschta, R.L.; Cheslak, E.F.; Deas, M.L.; Ebersole, J.L.; Foott, J.S.; Johnson, S.L.; Marine, K.R.; et al. Research in thermal biology: Burning questions for coldwater stream fishes. Rev. Fish. Sci. 2009, 17, 90–115. [Google Scholar] [CrossRef]
- Eaton, J.G.; Scheller, R.M. Effects of climate warming on fish thermal habitat in streams of the United States. Limnol. Oceanogr. 1996, 41, 1109–1115. [Google Scholar] [CrossRef]
- Efroymson, R.; Coleman, A.; Wigmosta, M.; Schoenung, S.; Sokhansanj, S.; Langholtz, M.; Davis, M. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; Langholtz, M., Stokes, B., Eaton, L., Eds.; ORNL/TM-2016/160; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016; Book Section 7; p. 448. [Google Scholar]
- Perkin, J.S.; Gido, K.B.; Costigan, K.H.; Daniels, M.D.; Johnson, E.R. Fragmentation and drying ratchet down Great Plains stream fish diversity. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 25, 639–655. [Google Scholar] [CrossRef]
- Mistak, J.L.; Hayes, D.B.; Bremigan, M.T. Food habits of coexisting salmonines above and below Stronach Dam in the Pine River, Michigan. Environ. Biol. Fishes 2003, 67, 179–190. [Google Scholar] [CrossRef]
- Jager, H.I.; King, A.W.; Gangrade, S.; Haines, A.; DeRolph, C.; Naz, B.S.; Ashfaq, M. Will future climate change increase the risk of violating minimum flow and maximum temperature thresholds below dams in the Pacific Northwest? Clim. Risk Manag. 2018, 21, 69–84. [Google Scholar] [CrossRef]
- Olden, J.D.; Naiman, R.J. Incorporating thermal regimes into environmental flows assessments: modifying dam operations to restore freshwater ecosystem integrity. Freshw. Biol. 2010, 55, 86–107. [Google Scholar] [CrossRef]
- Sesma-Martin, D. The river’s light: Water needs for thermoelectric power generation in the Ebro River basin, 1969–2015. Water 2019, 11, 441. [Google Scholar] [CrossRef]
- Cook, M.A.; King, C.W.; Webber, M.E. Implications of Thermal Discharge Limits On Future Power Generation in Texas. In Proceedings of the Asme International Mechanical Engineering Congress and Exposition, San Diego, CA, USA, 15–21 November 2013; ASME International Mechanical Engineering Congress and Exposition: San Diego, CA, USA, 2014; Volume 6b. [Google Scholar]
- Stewart, R.J.; Wollheim, W.M.; Miara, A.; Vorosmarty, C.J.; Fekete, B.; Lammers, R.B.; Rosenzweig, B. Horizontal cooling towers: Riverine ecosystem services and the fate of thermoelectric heat in the contemporary Northeast US. Environ. Res. Lett. 2013, 8, 025010. [Google Scholar] [CrossRef]
- Cook, M.A.; King, C.W.; Davidson, F.T.; Webber, M.E. Assessing the impacts of droughts and heat waves at thermoelectric power plants in the United States using integrated regression, thermodynamic, and climate models. Energy Rep. 2015, 1, 193–203. [Google Scholar] [CrossRef]
- Efroymson, R.; Dale, V. Environmental indicators for sustainable production of algal biofuels. Ecol. Indic. 2015, 49, 1–13. [Google Scholar] [CrossRef]
- Lopez-Diaz, D.C.; Lira-Barragan, L.F.; Rubio-Castro, E.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Optimal location of biorefineries considering sustainable integration with the environment. Renew. Energy 2017, 100, 65–77. [Google Scholar] [CrossRef]
- Huesemann, M.; Crowe, B.; Waller, P.; Chavis, A.; Hobbs, S.; Edmundson, S.; Wigmosta, M. A validated model to predict microalgae growth in outdoor pond cultures subjected to fluctuating light intensities and water temperatures. Algal Res. 2016, 13, 195–206. [Google Scholar] [CrossRef]
- Smakhtin, V.U. Low flow hydrology: A review. J. Hydrol. 2001, 240, 147–186. [Google Scholar] [CrossRef]
- Reilly, C.F.; Kroll, C.N. Estimation of 7-day, 10-year low-streamflow statistics using baseflow correlation. Water Resour. Res. 2003, 39. [Google Scholar] [CrossRef]
- Essig, D.; Mebane, C.; Hillman, T. Update of Bull Trout Temperature Requirements; Idaho Department of Environmental Quality: Boise, Idaho, 2003. [Google Scholar]
- Falcone, J. GAGES II (Geospatial Attributes of Gages for Evaluating Streamflow) Summary Report; USGS: Reston, VA, USA, 2011.
- Xu, H.; Lee, U.; Coleman, A.M.; Wigmosta, M.S.; Wang, M. Assessment of algal biofuel resource potential in the United States with consideration of regional water stress. Algal Res. 2019, 37, 30–39. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Global Gray Water Footprint and Water Pollution Levels Related to Anthropogenic Nitrogen Loads to Fresh Water. Environ. Sci. Technol. 2015, 49, 12860–12868. [Google Scholar] [CrossRef] [PubMed]
- Loehman, E.T.; Charney, S. Further down the road to sustainable environmental flows: Funding, management activities and governance for six western US states. Water Int. 2011, 36, 873–893. [Google Scholar] [CrossRef]
- Null, S.E.; Prudencio, L. Climate change effects on water allocations with season dependent water rights. Sci. Total Environ. 2016, 571, 943–954. [Google Scholar] [CrossRef]
- Podolak, C.J.P.; Doyle, M. Conditional water rights in the western United States: Introducting uncertainty to prior appropriation? J. Am. Water Resour. Assoc. 2015, 51, 14–32. [Google Scholar] [CrossRef]
- Hadian, S.; Madani, K. A system of systems approach to energy sustainability assessment: Are all renewables really green? Ecol. Indic. 2015, 52, 194–206. [Google Scholar] [CrossRef]
- Dieter, C.; Maupin, M.; Caldwell, R.; Harris, M.; Ivahnenko, T.; Lovelace, J.; Barber, N.; Linsey, K. Estimated Use of Water in the United States in 2015; Report U.S. Geological Survey Circular 1441; US Geological Survey: Cantonsville, MD, USA, 2018. [CrossRef]
- Schoenung, S.; Efroymson, R.; Langholtz, M. Considerations for the Design of a Gas Transport System for Co-Location of Microalgae Cultivation with CO2 Sources; Technical Report; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2019. [Google Scholar]
- Sharma, B.; Brandes, E.; Khanchi, A.; Birrell, S.; Heaton, E.; Miguez, F.E. Evaluation of Microalgae Biofuel Production Potential and Cultivation Sites Using Geographic Information Systems: A Review. Bioenergy Res. 2015, 8, 1714–1734. [Google Scholar] [CrossRef]
- Laamanen, C.A.; Shang, H.L.; Ross, G.M.; Scott, J.A. A model for utilizing industrial off-gas to support microalgae cultivation for biodiesel in cold climates. Energy Convers. Manag. 2014, 88, 476–483. [Google Scholar] [CrossRef]
- Zhao, X.B.; Yan, W.; Wang, Y.Q.; Lu, W.; Ma, S. Simulation study on the design of key technical parameters in marine environment sounding with fully polarimetric synthetic aperture radar based on ocean surface scattering model. Acta Phys. Sin. 2014, 63, 21. [Google Scholar] [CrossRef]
- Bennett, M.C.; Turn, S.Q.; Chan, W.Y. A methodology to assess open pond, phototrophic, algae production potential: A Hawaii case study. Biomass Bioenergy 2014, 66, 168–175. [Google Scholar] [CrossRef]
- Resurreccion, E.P.; Colosi, L.M.; White, M.A.; Clarens, A.F. Comparison of algae cultivation methods for bioenergy production using a combined life cycle assessment and life cycle costing approach. Bioresour. Technol. 2012, 126, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.E.; Fishman, D.B.; Frank, E.D.; Johnson, M.C.; Jones, S.B.; Kinchin, C.M.; Skaggs, R.L.; Venteris, E.R.; Wigmosta, M.S. Integrated Evaluation of Cost, Emissions, and Resource Potential for Algal Biofuels at the National Scale. Environ. Sci. Technol. 2014, 48, 6035–6042. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jager, H.I.; Efroymson, R.A.; Baskaran, L.M. Avoiding Conflicts between Future Freshwater Algae Production and Water Scarcity in the United States at the Energy-Water Nexus. Water 2019, 11, 836. https://doi.org/10.3390/w11040836
Jager HI, Efroymson RA, Baskaran LM. Avoiding Conflicts between Future Freshwater Algae Production and Water Scarcity in the United States at the Energy-Water Nexus. Water. 2019; 11(4):836. https://doi.org/10.3390/w11040836
Chicago/Turabian StyleJager, Henriette I., Rebecca A. Efroymson, and Latha M. Baskaran. 2019. "Avoiding Conflicts between Future Freshwater Algae Production and Water Scarcity in the United States at the Energy-Water Nexus" Water 11, no. 4: 836. https://doi.org/10.3390/w11040836
APA StyleJager, H. I., Efroymson, R. A., & Baskaran, L. M. (2019). Avoiding Conflicts between Future Freshwater Algae Production and Water Scarcity in the United States at the Energy-Water Nexus. Water, 11(4), 836. https://doi.org/10.3390/w11040836




