Comparative Study of Four TiO2-Based Photocatalysts to Degrade 2,4-D in a Semi-Passive System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design and Setup
2.2.2. Anodization of Titanium Mesh
2.2.3. Sample Analysis
3. Results and Discussion
3.1. Photocatalyst Characterization
3.2. TiO2 Photospheres
3.3. Anodized Mesh
3.4. Anodized Plate
3.5. Electro-Photo Catalysis Using Anodized Titanium Mesh
3.6. Comparison of Three Forms of the Photocatalyst and Electro-Photocatalysis
4. Conclusions
- The feasibility of using four types of TiO2 photocatalysts under UV-LED irradiation (i.e., λ = 365 nm) in a semi-passive system was investigated.
- Energy consumption (J/cm3) was used to rank the degradation efficiency of the photocatalysts. Photospheres (80.3 J/cm3) and anodized titanium mesh (80.3 J/cm3) showed similar efficiencies followed by electro-photocatalysis (112.2 J/cm3) and the anodized plate (114.5 J/cm3).
- Although the electro-photocatalysis rate of reaction was 64% higher than the photocatalysis with anodized plates, and 46% higher than the photocatalysis using the anodized mesh, it required additional energy and control during the photocatalytic degradation process.
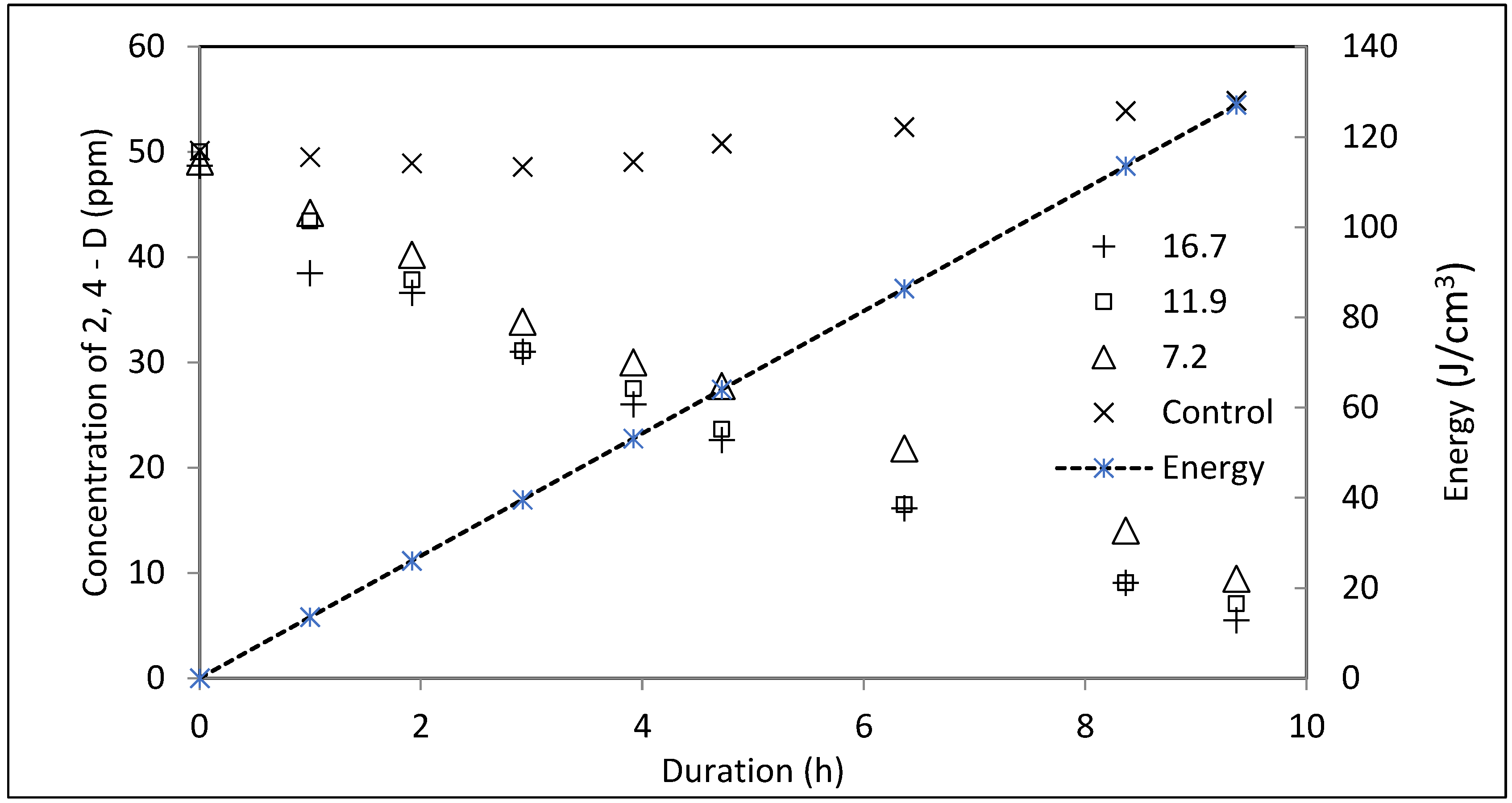
- Increasing the loadings of the photospheres enhanced the kinetics of the degradation reaction from 4.12 mg L−1 h−1 to 4.55 mg L−1 h−1, but further increases in the loadings reduced the rate of reaction to 4.36 mg L−1 h−1. The variation in the rate of reaction, validated the deactivation of the originally activated species of TiO2 due to the collision mechanism and the UV screening effect.
- Though the degradation rate and efficiency was high with photospheres, they still would require a separation step after treatment. This minimizes their attractiveness for application under ambient environments.
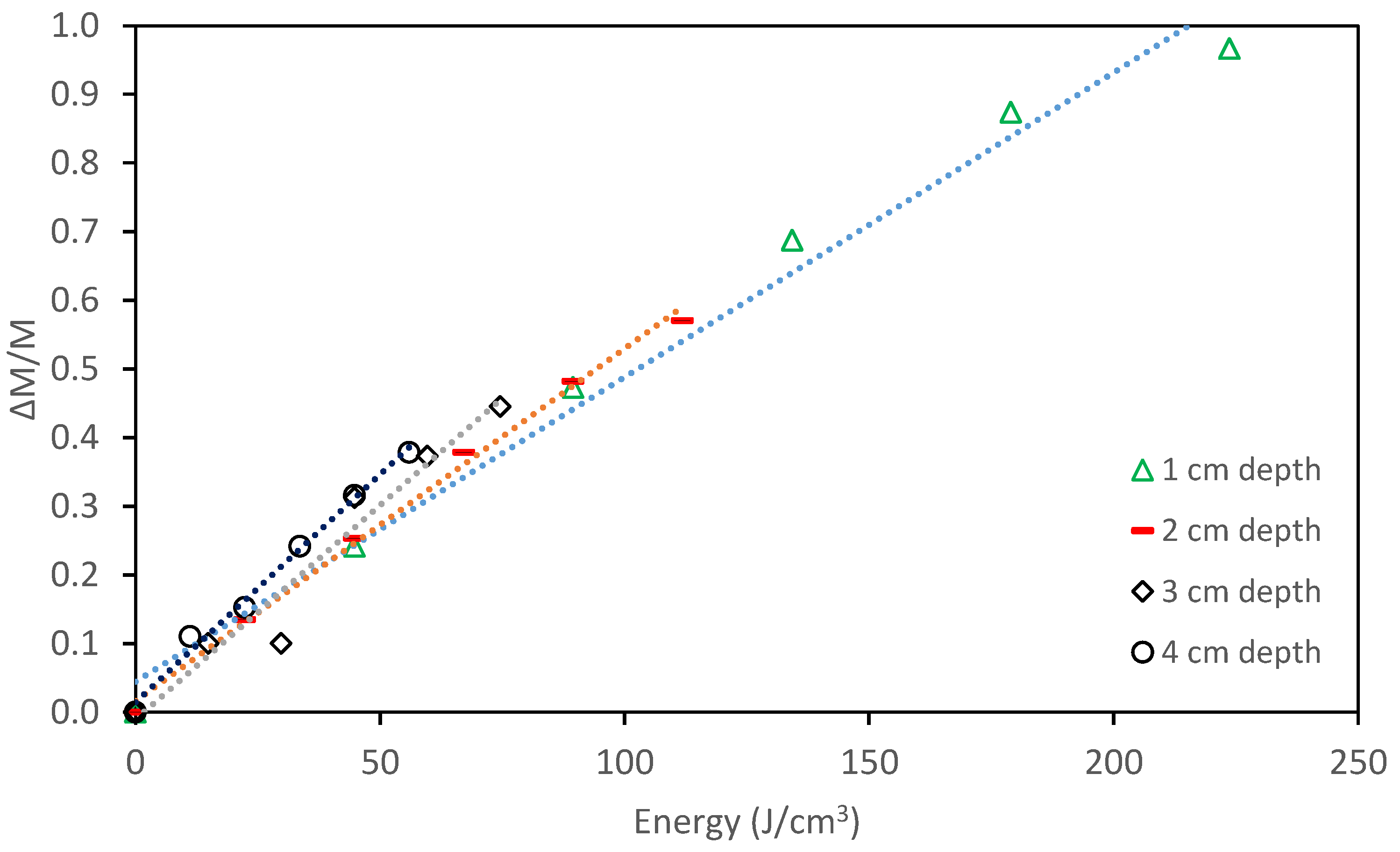
- Studying the depth of the photocatalyst from the surface showed at the range of 1 cm to 4 cm, the effect of the mass of the contaminant in the solution supersedes the effect of the light penetration.
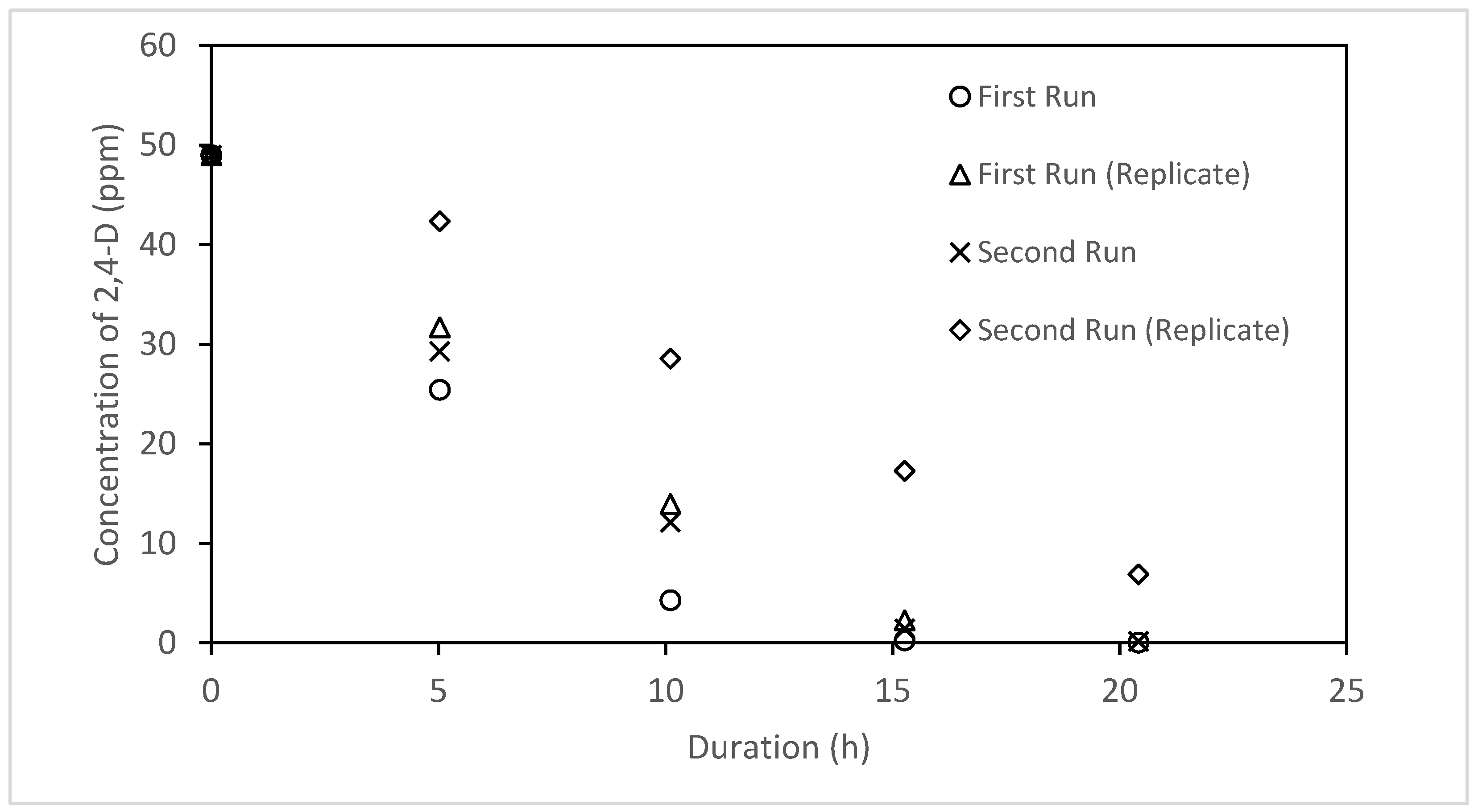
- Anodized mesh showed 29% higher efficiency with 56% surface area of the anodized plate, but its performance varied during repetitive usage. The resulted variation is associated with the ability of the anodization process to generate a uniform mesh with a stable photocatalyst on its surface. This emphasizes the importance of improving the anodization process to produce a robust and uniform mesh which will be considered in future studies.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical evidence for the mechanism of the primary state of photosynthesis. Bull. Chem. Soc. Jpn. 1971, 44, 1148–1150. [Google Scholar] [CrossRef]
- Mondal, K. Recent advances in the synthesis of metal oxide nanofibers and their environmental remediation applications. Inventions 2017, 2, 9. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Qu, Y. TiO2-based photocatalytic process for purification of polluted water: Bridging fundamentals to applications. J. Nanomater. 2013, 2013, 319637. [Google Scholar] [CrossRef]
- Toor, A.P.; Verma, A.; Jotshi, C.K.; Bajpai, P.K.; Singh, V. Photocatalytic degradation of 3, 4-dichlorophenol using TiO2 in a shallow pond slurry reactor. Indian J. Chem. Technol. 2005, 12, 75–81. [Google Scholar]
- Yu, L.; Achari, G.; Langford, C.H. LED-based photocatalytic treatment of pesticides and chlorophenols. J. Environ. Eng. 2013, 139, 1146–1151. [Google Scholar] [CrossRef]
- Ikehata, K.; El-Din, M.G.; Snyder, S.A. Ozonation and advanced oxidation treatment of emerging organic pollutants in water and wastewater. Ozone Sci. Eng. 2008, 30, 21–26. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 224–245. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Blake, D.M. Bibliography of work on the heterogeneous photocatalytic removal of hazardous compounds from water and air. Natl. Renew. Energy Lab. 2001, 4, 1–265. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, S.; Du, M.; Ye, X.; Wang, Y.; Ye, J. Titanium Dioxide (TiO2) Mesocrystals: Synthesis, Growth Mechanisms and Photocatalytic Properties. Catalysis 2019, 9, 91. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2015, 42, 2–14. [Google Scholar] [CrossRef]
- Lavand, A.B.; Malghe, Y.S. Visible light photocatalytic degradation of 4-chlorophenol using C/ZnO/CdS nanocomposite. J. Saudi Chem. Soc. 2015, 19, 471–478. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.J. Tungsten oxides for photocatalysis, electrochemistry, and phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured tungsten oxide–properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Smith, Y.R.; Sarma, B.; Mohanty, S.K.; Misra, M. Light-assisted anodized TiO2 nanotube arrays. ACS Appl. Mater. Interfaces 2012, 4, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Brock, J. Materials and Methods for Photocatalyzing Oxidation of Organic Compounds on Water. U.S. Patent 4,997,576, 5 March 1991. Available online: http://www.freepatentsonline.com/4997576.html (accessed on 26 March 2019).
- Gjipalaj, J.; Alessandri, I. Easy recovery, mechanical stability, enhanced adsorption capacity and recyclability of alginate-based TiO2 macrobead photocatalysts for water treatment. J. Environ. Chem. Eng. 2017, 5, 1763–1770. [Google Scholar] [CrossRef]
- Robert, D.; Keller, V.; Keller, N. Immobilization of a semi-conductor photocatalyst on solid supports, methods, materials and applications. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications; Lu, M., Pichat, P., Eds.; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2013; pp. 145–172. ISBN 9783527645411. [Google Scholar]
- Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 solar photocatalytic reactor systems: Selection of reactor design for scale-up and commercialization—Analytical review. Catalysts 2016, 6, 138. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Murugesan, V. Photocatalytic decomposition of leather dye comparative study of TiO2 supported on alumina and glass beads. J. Photochem. Photobiol. A Chem. 2002, 148, 153–159. [Google Scholar] [CrossRef]
- Sirisuk, A.; Hill, C.G.; Anderson, M.A. Photocatalytic degradation of ethylene over thin films of titania supported on glass rings. Catal. Today 1999, 54, 159–164. [Google Scholar] [CrossRef]
- Portjanskaja, E.; Krichevskaya, M.; Preis, S.; Kallas, J. Photocatalytic oxidation of humic substances with TiO2-coated glass micro-spheres. Environ. Chem. Lett. 2004, 2, 123–127. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Zeng, Q.; Xi, M.; Xu, W.; Li, X. Preparation of titanium dioxide nanotube arrays on titanium mesh by anodization in (NH4)2SO4/NH4F electrolyte. Mater. Corros. 2013, 64, 1001–1006. [Google Scholar] [CrossRef]
- Liao, J.; Lin, S.; Zhang, L.; Pan, N.; Cao, X.; Li, J. Photocatalytic degradation of methyl orange using a TiO2/Ti mesh electrode with 3D nanotube arrays. ACS Appl. Mater. Interfaces 2012, 4, 171–177. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem.–Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Jun, Y.; Park, J.H.; Kang, M.G. The preparation of highly ordered TiO2 nanotube arrays by an anodization method and their applications. Chem. Commun. 2012, 48, 6456–6471. [Google Scholar] [CrossRef]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Hahn, R.; Schmuki, P. Self-organized, free-standing TiO2 nanotube membrane for flow-through photocatalytic applications. Nano Lett. 2007, 7, 1286–1289. [Google Scholar] [CrossRef]
- Motola, M.; Satrapinskyy, L.; Roch, T.; Šubrt, J.; Kupčík, J.; Klementová, M.; Jakubičková, M.; Peterka, F.; Plesch, G. Anatase TiO2 nanotube arrays and titania films on titanium mesh for photocatalytic NOXremoval and water cleaning. Catal. Today 2017, 287, 59–64. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, G.; Yang, X. Preparation of Ti mesh supported WO3/TiO2 nanotubes composite and its application for photocatalytic degradation under visible light. Mater. Lett. 2015, 145, 216–218. [Google Scholar] [CrossRef]
- Cunha, D.L.; Kuznetsov, A.; Achete, C.A.; da Hora Machado, A.E.; Marques, M. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. PeerJ. 2018, 4464, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hartley, A.C.; Moss, J.B.; Keesling, K.J.; Moore, N.J.; Glover, J.D.; Boyd, J.E. PMMA-titania floating macrospheres for the photocatalytic remediation of agro-pharmaceutical wastewater. Water Sci. Technol. 2017, 75, 1362–1369. [Google Scholar] [CrossRef]
- Xing, Z.; Li, J.; Wang, Q.; Zhou, W.; Tian, G.; Pan, K.; Tian, C.; Zou, J.; Fu, H. A floating porous crystalline TiO2 ceramic with enhanced photocatalytic performance for wastewater decontamination. Eur. J. Inorg. Chem. 2013, 2411–2417. [Google Scholar] [CrossRef]
- Magalhães, F.; Moura, F.C.C.; Lago, R.M. TiO2/LDPE composites: A new floating photocatalyst for solar degradation of organic contaminants. Desalination 2011, 276, 266–271. [Google Scholar] [CrossRef]
- Magalhães, F.; Lago, R.M. Floating photocatalysts based on TiO2 grafted on expanded polystyrene beads for the solar degradation of dyes. Sol. Energy 2009, 83, 1521–1526. [Google Scholar] [CrossRef]
- Bahreini, Z.; Heydari, V.; Hekmat, A.N.; Taheri, M.; Vahid, B.; Moradkhannejhad, L. A comparative study of photocatalytic degradation and mineralisation of an azo dye using supported and suspended nano-TiO2 under UV and sunlight irradiations. Pigment Resin Technol. 2016, 45, 119–125. [Google Scholar] [CrossRef]
- Salinaro, A.; Emeline, A.V.; Zhao, J.; Hidaka, H.; Ryabchuk, V.K.; Serpone, N. Terminology, relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. Part I: Suggested protocol. Pure Appl. Chem. 1999, 71, 303–320. [Google Scholar] [CrossRef]
- Turolla, A. Heterogeneous Photocatalysis and Electro-Photocatalysis on Nanostructured Titanium Dioxide for Water and Wastewater Treatment: Process Assessment, Modelling and Optimization. Ph.D. Thesis, Polytechnic University of Milan, Milan, Italy, 2014. [Google Scholar]
- Zlamal, M.; Macak, J.M.; Schumulu, P.; Josef, K. Electrochemically assisted photocatalysis on self-organized TiO2 nanotubes. Electrochem. Commun. 2007, 9, 2822–2826. [Google Scholar] [CrossRef]
- Turolla, A.; Fumagalli, M.; Bestetti, M.; Antonelli, M. Electro-photocatalytic decolorization of an azo dye on TiO2 self-organized nanotubes in a laboratory scale reactor. Desalination 2012, 285, 377–382. [Google Scholar] [CrossRef]
- Wu, T.N.; Pan, T.C.; Chen, L.C. Electro-photocatalysis of aqueous methyl tert-butyl ether on a titanium dioxide coated electrode. Electrochim. Acta 2012, 86, 170–176. [Google Scholar] [CrossRef]
- Shen, Y.; Li, F.; Li, S.; Liu, D.; Fan, L.; Zhang, Y. Electrochemically enhanced photocatalytic degradation of organic pollutant on β-PbO2-TNT/Ti/TNT bifunctional electrode. Int. J. Electrochem. Sci. 2012, 7, 8702–8712. [Google Scholar]
- Krieger, R. (Ed.) Hayes’ Handbook of Pesticide Toxicology; Elsevier Science & Technology: Saint Louis, MO, USA, 2010; ISBN 9780080922010. [Google Scholar]
- Health Canada Special Review of 2,4-D: Proposed Decision for Consultation. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/consultations/re-evaluation-note/2016/special-review-2-4-d/document.html#s2 (accessed on 8 February 2017).
- Guillard, C.; Amalric, L.; D’Oliveira, J.C.; Delpart, H.; Hoang-Van, C.; Pichat, P. Heterogeneous photocatalysis: Use in water treatment and involvement in atmopheric chemistry. In Aquatic and Surface Photochemistry; Helz, G.R., Zepp, R.G., Crosby, D.G., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 369–386. ISBN 0873718712. [Google Scholar]
- Meunier, L.; Pilichowski, J.F.; Boule, P. Photochemical behaviour of 1,4-dichlorobenzene in aqueous solution. Can. J. Chem. Can. Chim. 2001, 79, 1179–1186. [Google Scholar] [CrossRef]
- Heydari, G. Passive or Semi-Passive Photocatalytic Treatment of Organic Pollutants in Water. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2018. [Google Scholar]
- Radwan, E.K.; Yu, L.; Achari, G.; Langford, C.H. Photocatalytic ozonation of pesticides in a fixed bed flow through UVA-LED photoreactor. Environ. Sci. Pollut. Res. 2016, 23, 21313–21318. [Google Scholar] [CrossRef] [PubMed]
- Eskandarian, M.R.; Choi, H.; Fazli, M.; Rasoulifard, M.H. Effect of UV-LED wavelengths on direct photolytic and TiO2 photocatalytic degradation of emerging contaminants in water. Chem. Eng. J. 2016, 300, 414–422. [Google Scholar] [CrossRef]
- Yu, L. Light Emitting Diode Based Photochemical Treatment of Contaminants in Aqueous Phase; University of Calgary: Calgary, AB, Canada, 2014. [Google Scholar]
- Casterjon-Sanchez, V.H.; Lopez, R.; Ramon-Gonzalez, M.; Enriquez-Perez, A.; Camasho-Lopez, M.; Villa-Sanchez, G. Annealing Control on the Anatase/Rutile Ratio of Nanostructured Titanium Dioxide Obtained by Sol-Gel. Crystals 2018, 9, 22. [Google Scholar] [CrossRef]
- Topalov, A.S.; Sojic, D.V.; Molnar-Gabor, D.A.; Abramovic, B.F.; Comor, M.I. Photocatalytic activity of synthesized nanosized TiO2 towards the degradation of herbicide mecoprop. Appl. Catal. B Environ. 2004, 54, 125–133. [Google Scholar] [CrossRef]
- Aguer, J.; Blachère, F.; Boule, P.; Garaudee, S.; Guillard, C. Photolysis of dicamba (3,6-dichloro-2-methoxybenzoic acid) in aqueous solution and dispersed on solid supports. Int. J. Photoenergy 2000, 2, 81–86. [Google Scholar] [CrossRef]
- Fogarty, A.M.; Traina, S.J.; Tuovinen, O.H. Determination of Dicamba by Reverse-Phase HPLC. J. Liq. Chromatogr. 1994, 17, 2667–2674. [Google Scholar] [CrossRef]
- Castellote, M.; Bengtsson, N. Principles of TiO2 photocatalysis. In Applications of Titanium Dioxide Photocatalysis to Construction Materials: State-of-the-Art Report of the RILEM Technical Committee 194-TDP; Ohama, Y., Van Gemert, D., Eds.; Springer: Dordrecht, The Netherland, 2011; pp. 5–9. ISBN 9789400712966. [Google Scholar]
- Li, L.; Wang, M. Advanced nanomaterials for solar photocatalysis. In Advanced Catalytic Materials–Photocatalysis and Other Current Trends; INTECH: London, UK, 2016; pp. 169–230. ISBN 9789535122449. [Google Scholar]
- Calza, P.; Sakkas, V.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, R.; Choi, H.; Bhattacharjee, C. Involvement of process parameters and various modes of application of TiO2 nanoparticles in heterogeneous photocatalysis of pharmaceutical wastes—A short review. RSC Adv. 2014, 4, 57250–57266. [Google Scholar] [CrossRef]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef] [PubMed]
- Neppolian, B.; Choi, H.C.; Sakthivel, S.; Arabindoo, B.; Murugesan, V. Solar/UV-induced photocatalytic degradation of three commercial textile dyes. J. Hazard. Mater. 2002, 89, 303–317. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Tsydenova, O.; Batoev, V.; Batoeva, A. Solar-enhanced advanced oxidation processes for water treatment: Simultaneous removal of pathogens and chemical pollutants. Int. J. Environ. Res. Public Health 2015, 12, 9542–9561. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Lee, K.; Kim, D.; Roy, P.; Paramasivam, I.; Birajdar, B.I.; Spiecker, E.; Schmuki, P. Anodic formation of thick anatase TiO2 mesosponge layers for high efficiency photocatalysis. Mater. Sci. 2010, 7, 1–10. [Google Scholar] [CrossRef]
- Schmidt-Stein, F.; Thiemann, S.; Berger, S.; Hahn, R.; Schmuki, P. Mechanical properties of anatase and semi-metallic TiO2 nanotubes. Acta Mater. 2010, 58, 6317–6323. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Z. Anodic formation of ordered TiO2 nanotube arrays: Effects of electrolyte temperature and anodization potential. J. Phys. Chem. C 2009, 113, 4026–4030. [Google Scholar] [CrossRef]
- Yu, J.; Wang, B. Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl. Catal. B Environ. 2010, 94, 295–302. [Google Scholar] [CrossRef]
- Jarosz, M.; Kapusta-Kołodziej, J.; Jaskuła, M.; Sulka, G.D. Effect of different polishing methods on anodic titanium dioxide formation. J. Nanomater. 2015, 2015, 295126. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Chekir, N.; Boukendakdji, H.; Igoud, S.; Taane, W. Solar energy for the benefit of water treatment: Solar photoreactor. Procedia Eng. 2012, 33, 174–180. [Google Scholar] [CrossRef]
- Mackak, J.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Scumula, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Hashempour, M.; Vicenzo, A.; Franz, S.; Badiei, A.; Behnajady, M.A.; Bestetti, M. High-temperature stable anatase-type TiO2 nanotube arrays: A study of the structure-activity relationship. Appl. Catal. B Environ. 2016, 185, 119–132. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B Environ. 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Li, X.Z.; Liu, H.L.; Li, F.B.; Mak, C.L. Photoelectrocatalytic oxidation of Rhodamine B in aqueous soultion using Ti/TiO2 mesh photoelectrodes. J. Environ. Sci. Heal. Part A 2007, 37, 55–69. [Google Scholar] [CrossRef]
- Leshuk, T.; Krishnakumar, H.; de Oliveira Livera, D.; Gu, F. Floating photocatalysts for passive solar degradation of naphthenic acids in oil sands process-affected water. Water 2018, 10, 202. [Google Scholar] [CrossRef]
- Wang, J.; He, B.; Kong, X.Z. A study on the preparation of floating photocatalyst supported by hollow TiO2 and its performance. Appl. Surf. Sci. 2015, 327, 406–412. [Google Scholar] [CrossRef]
- Yuan, J.; An, Z.-G.; Zhang, J.-J.; Li, B. Synthesis and properties of hollow glass spheres/TiO2 composite. Imaging Sci. Photochem. 2012, 30, 447–455. [Google Scholar]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
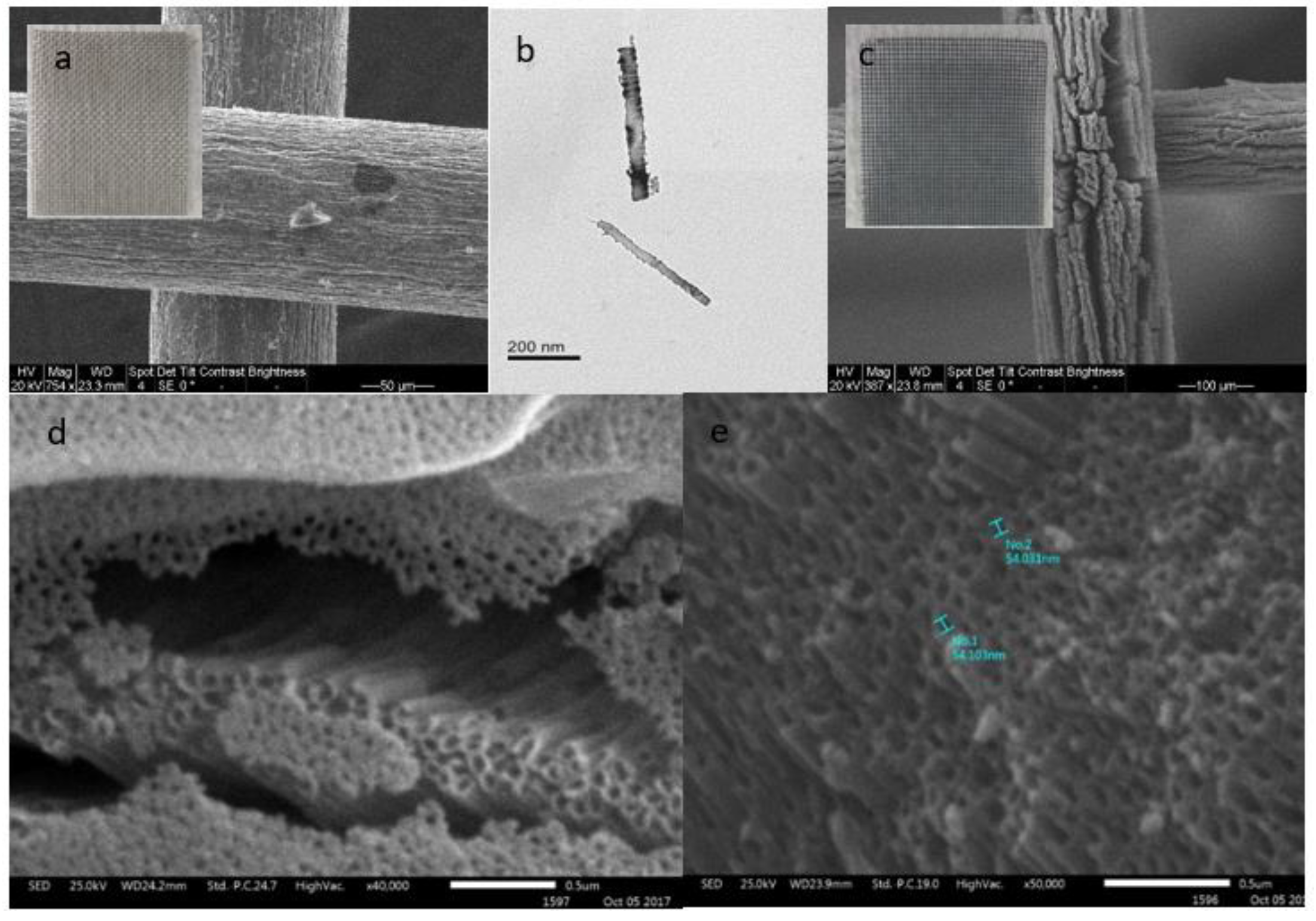
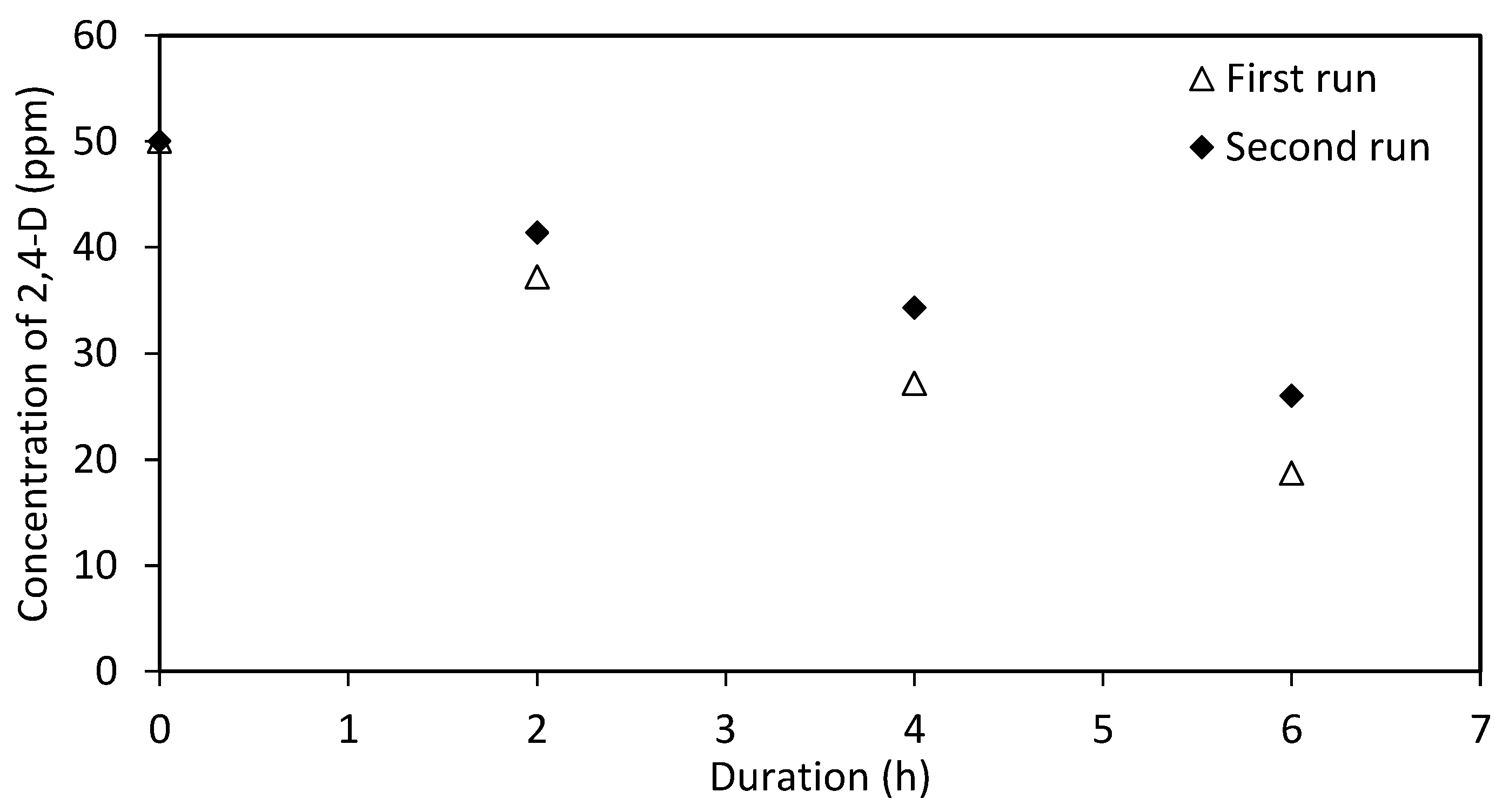

| Crystal Phase (weight %) | |||
|---|---|---|---|
| Photocatalyst | Anatase | Rutile | Titanium |
| Mesh | 91.8 | 3.7 | 2.8 |
| Plate | 81.9 | 0.1 | 17.3 |
| Photospheres | 72.7 | - | - |
| Photocatalyst Loading (mg/cm2) | K (mg L−1 h−1) | t½ (h) | Degradation (%) |
|---|---|---|---|
| 7.2 | 4.12 | 6.05 | 80 |
| 11.9 | 4.55 | 5.48 | 86 |
| 16.7 | 4.36 | 5.72 | 89 |
| Photocatalyst | Surface Area of the Photocatalyst (cm2) | Energy (J/cm3) | T1/2 (h) | K Value (mg L−1 h−1) | R2 |
|---|---|---|---|---|---|
| Anodized plate | 4.97 | 114.5 | 7.97 | 3.07 | 0.99 |
| Anodized mesh | 2.76 | 80.3 | 7.09 | 3.45 | 0.99 |
| Electro-photocatalysis | 2.76 | 112.2 | 4.86 | 5.04 | 0.99 |
| Photospheres | 12.56 | 80.3 | 5.48 | 4.55 | 0.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydari, G.; Hollman, J.; Achari, G.; Langford, C.H. Comparative Study of Four TiO2-Based Photocatalysts to Degrade 2,4-D in a Semi-Passive System. Water 2019, 11, 621. https://doi.org/10.3390/w11030621
Heydari G, Hollman J, Achari G, Langford CH. Comparative Study of Four TiO2-Based Photocatalysts to Degrade 2,4-D in a Semi-Passive System. Water. 2019; 11(3):621. https://doi.org/10.3390/w11030621
Chicago/Turabian StyleHeydari, Gisoo, Jordan Hollman, Gopal Achari, and Cooper H. Langford. 2019. "Comparative Study of Four TiO2-Based Photocatalysts to Degrade 2,4-D in a Semi-Passive System" Water 11, no. 3: 621. https://doi.org/10.3390/w11030621
APA StyleHeydari, G., Hollman, J., Achari, G., & Langford, C. H. (2019). Comparative Study of Four TiO2-Based Photocatalysts to Degrade 2,4-D in a Semi-Passive System. Water, 11(3), 621. https://doi.org/10.3390/w11030621





