Urban Watercourses in Peril: Implications of Phthalic Acid Esters on Aquatic Ecosystems Caused by Urban Sprawl
Abstract
1. Introduction
2. Materials and Methods
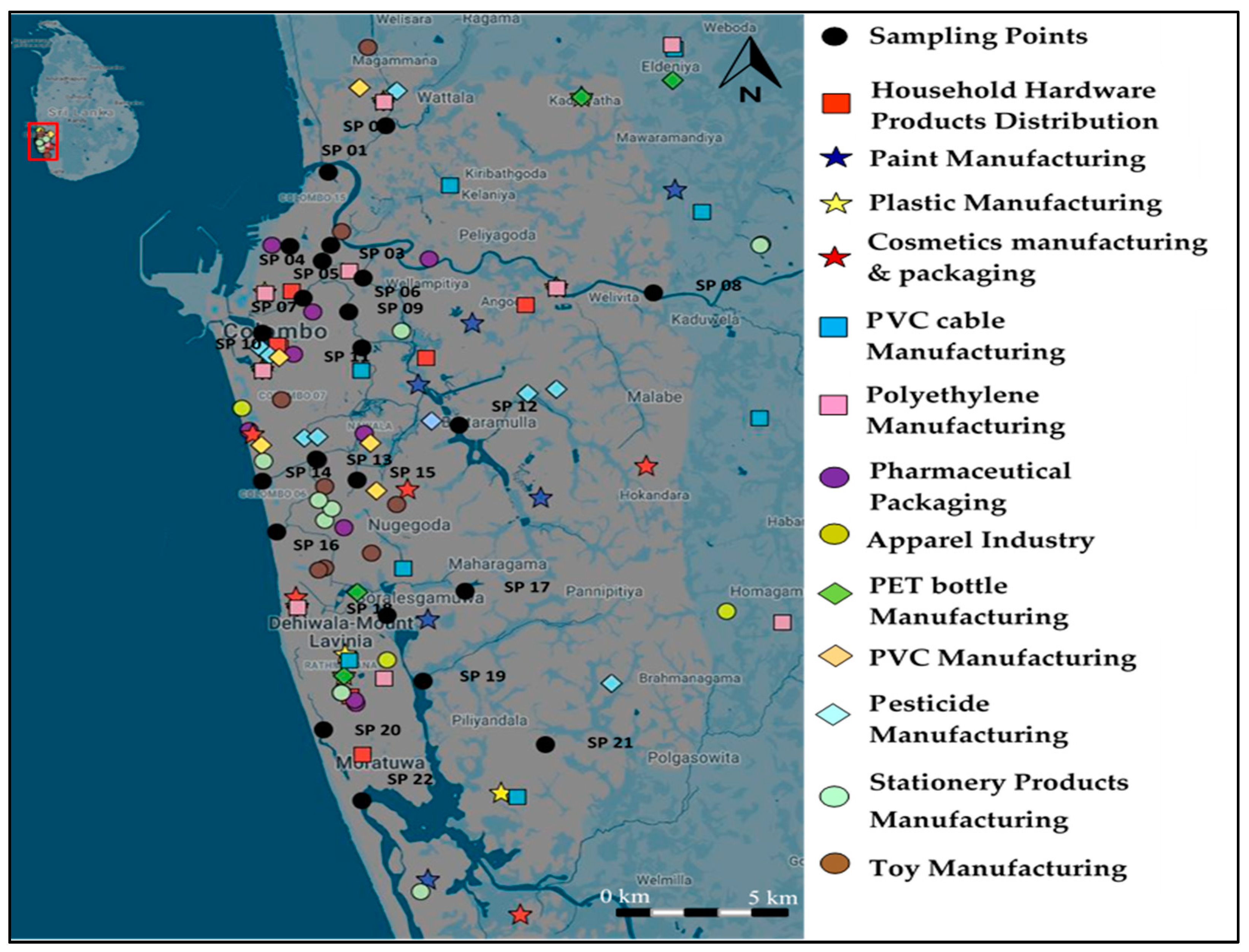
2.1. Study Area and Sampling Locations
2.2. Sample Collection
2.3. In situ Water Quality Measurements
2.4. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Occurrence of PAEs in Watercourses
3.2. Correlation of PAE Concentrations with Receiving Water Quality
3.3. Behavior of PAEs in the Aquatic Environment
3.4. Possible Sources and Implications of PAEs in Watercourses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paluselli, A.; Fauvelle, V.; Schmidt, N.; Galgani, F.; Net, S.; Sempéré, R. Distribution of phthalates in Marseille Bay (NW Mediterranean Sea). Sci. Total Environ. 2018, 621, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, P.; Lu, Y.; Wang, X.; Xhu, N.; Dang, Z. Enhancement of photocatalytic degradation of dimethyl phthalate with nano-TiO2 immobilized onto hydrophobic layered double hydroxides: A mechanism study. J. Hazard. Mater. 2013, 246, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, J.J.; Knight, T.L. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: A systematic review and meta-analysis. Environ. Health Perspect. 2008, 116, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, M.; Ma, X.; Wang, W.; Wang, C.; Zang, X.; Wang, Z. Extraction of phthalate esters from water and beverages using a graphene-based magnetic nanocomposite prior to their determination by HPLC. Microchim. Acta 2012, 177, 23–30. [Google Scholar] [CrossRef]
- Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K. Titanium dioxide mediated photocatalytic degradation of dibutyl phthalate in aqueous solution-kinetics, mineralization and reaction mechanism. Chem. Eng. J. 2006, 125, 59–66. [Google Scholar] [CrossRef]
- Wallner, P.; Kundi, M.; Hohenblum, P.; Scharf, S.; Hutter, H. Phthalates Metabolites, Consumer Habits and Health Effects. Int. J. Environ. Res. Public Health 2016, 13, 717. [Google Scholar] [CrossRef]
- Ito, Y.; Yamanoshita, O.; Asaeda, N.; Tagawa, Y.; Lee, C.; Aoyama, T.; Ichihara, G.; Furuhashi, K.; Kamijima, M.; Gonzalez, F.J.; et al. Di (2-ethylhexyl) phthalate Induces Hepatic Tumorigenesis through a Peroxisome Proliferator-activated Receptor α-independent Pathway. J. Occup. Health 2007, 49, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Huang, X.; Gao, D.; Liu, G.; Fang, C.; Shang, Y.; Du, J.; Zhao, Y.; Lv, L.; Song, K. Phthalate esters in surface water of Songhua River watershed associated with land use types, Northeast China. Environ. Sci. Pollut. Res. Int. 2018, 25, 7688–7698. [Google Scholar] [CrossRef] [PubMed]
- Roslev, P.; Vorkamp, K.; Aarup, J.; Frederiksen, K.; Nielsen, P.H. Degradation of phthalate esters in an activated sludge wastewater treatment plant. Water Res. 2007, 41, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Peijnenburg, W.J.G.M.; Struijs, J. Occurrence of phthalate esters in the environment of the Netherlands. Ecotoxicol. Environ. Saf. 2006, 63, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council of Ministers of the Environment. Canadian Water Quality Guidelines for the Protection of Aquatic Life; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 1999; p. 146. [Google Scholar]
- Mittermeier, A.; Volkel, W.; Fromme, H. Kinetics of the phthalate metabolites mono-2-ethylhexyl phthalate (MEHP) and mono-n-butyl phthalate (MnBP) in male subjects after a single oral dose. Toxicol. Lett. 2016, 252, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Karlovsky, P. Removal of the endocrine disrupter butyl benzyl phthalate from the environment. Appl. Microbiol. Biotechnol. 2010, 87, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Liu, K. Developmental toxicity and cardiac effects of butyl benzyl phthalate in zebrafish embryos. Aquat. Toxicol. 2017, 192, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, J.; Gong, Z.; Zhang, N.; Duan, H. Occurance, special distribution, historical trend and ecological risk of phthalate esters in the Jiulong River, Southeast China. Sci. Total Environ. 2016, 580, 388–397. [Google Scholar] [CrossRef] [PubMed]
- EPA Method 8061A, Phthalate Esters by Gas Chromatography with Electron Capture Detection (GC/ECD). Available online: https://www.epa.gov/sites/production/files/2015-12/documents/8061a.pdf (accessed on 6 February 2019).
- Xu, X.; Li, X. Sorption behavior of benzyl butyl phthalate on marine sediments: Equilibrium assessments, effects of organic carbon content, temperature and salinity. Mar. Chem. 2009, 115, 66–71. [Google Scholar] [CrossRef]
- Xu, X.; Li, X. Adsorption behavior of dibutyl phthalate on marine sediments. Mar. Pollut. Bull. 2008, 57, 403–408. [Google Scholar] [CrossRef]
- Staples, C.A.; Peterson, D.R.; Parkerton, T.F.; Adams, W.J. The environment fate of phthalate esters: A literature review. Chemosphere 1997, 35, 667–749. [Google Scholar] [CrossRef]
- Howard, P.H.; Banerjee, S.; Robillard, K.H. Measurement of water solubilities, octanol/water partition coefficients and vapor pressures of commercial phthalate esters. Environ. Toxicol. Chem. 1985, 4, 653–661. [Google Scholar] [CrossRef]
- DeFoe, L.; Holcombe, G.W.; Hammermeister, D.E.; Beisinger, K.E. Solubility and toxicity of eight phthalate esters to four aquatic organisms. Environ. Toxicol. Chem. 1990, 9, 623–636. [Google Scholar] [CrossRef]
- Osipoff, R.J.; Hutzler, N.J.; Crittenden, J.C. Interaction of Specific Organic Chemicals Percolating through a Soil. In Proceedings of the 35th Industrial Waste Conference, Lafayette, IN, USA, 13–15 May 1980; Volume 35, pp. 17–23. [Google Scholar]
- Russell, D.J.; McDuffie, B. Chemo dynamic Properties of Phthalates: Partitioning and Soil Mimation. Chemosphere 1986, 15, 1003–1021. [Google Scholar] [CrossRef]
- USEPA’s Estimation Program Interference Suits 4.1; United States Environment Protection Agency, 2012. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 6 February 2019).
- Wolfe, N.L.; Bums, L.A.; Steen, W.C. Use of Linear Free Energy Relationships and an Evaluative Model to Assess the Fate and Transport of Phthalate Esters in the Aquatic Environment. Chemosphere 1980, 9, 393–402. [Google Scholar] [CrossRef]
- Ritsema, R.; Cofino, W.P.; Frintrop, P.C.M.; Brinkman, U.A. Trace-Level Analysis of Phthalate Esters in surface water and suspended particulate matter by means of capillary gas chromatography with electron capture and mass selective detection. Chemosphere 1989, 18, 2161–2175. [Google Scholar]
- Furtmarm, K. Phthalate in der Aquatischen Umvelt; Landesant fur Wasser, und Abfall Nordhein-Westfalen: Dusseldorf, Germany, 1993; 197p and Appendices. [Google Scholar]
- Wei, X.; Shi, Y.; Fei, Y.; Chen, J.; Lv, B.; Chen, Y.; Zheng, H.; Shen, J.; Zhu, L. Removal of trace phthalate esters from water by thin-film composite nanofiltration hollow fiber membranes. Chem. Eng. J. 2017, 292, 382–388. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, W.; Shi, X.; Yu, X.; Li, M.; Xiao, L.; Cui, Y. Health risk of semi-volatile organic pollutants in Wujin river inflow into Taihu Lake. Ecotoxicology 2011, 20, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Hu, G.; Sun, C.; Chen, S.; Yang, M.; Li, J.; Zhao, Y.; Wang, H. Trace analysis of persistent toxic substances in the main stream of Jiangsu section of the Yangtze River, China. Environ. Sci. Pollut. Res. Int. 2010, 18, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xia, X.; Sha, Y. Distribution of phthalic acid esters in Wuhan section of the Yangtze River, China. J. Hazard. Mater. 2007, 154, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Dargnat, C.; Blanchard, M.; Chevreuil, M.; Teil, M.J. Occurrence of phthalate esters in the Seine River estuary (France). Hydrol. Process. 2009, 23, 1192–1201. [Google Scholar] [CrossRef]
- Fromme, H.; Kuchler, T.; Otto, T.; Pilz, K.; Muller, J.; Wenzel, A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002, 36, 1429–1438. [Google Scholar] [CrossRef]
- Hashizume, K.; Nanya, J.; Toda, C.; Yasui, T.; Nagano, H.; Kojima, N. Phthalate esters detected in various water samples and biodegradation of the phthalates by microbes isolated from river water. Biol. Pharm. Bull. 2002, 25, 209–214. [Google Scholar] [CrossRef]
- Bastos, P.M.; Haglund, P. The use of comprehensive two-dimensional gas chromatography and structure-activity modeling for screening and preliminary risk assessment of organic contaminants in soil, sediment, and surface water. J. Soils Sediments 2012, 12, 1079–1088. [Google Scholar] [CrossRef]
- Olujimi, O.O.; Fatoki, O.S.; Odendaal, J.P.; Daso, A.P. Chemical monitoring and temporal variation in levels of endocrine disrupting chemicals (priority phenols and phthalate esters) from selected wastewater treatment plant and freshwater systems in Republic of South Africa. Microchem. J. 2012, 101, 11–23. [Google Scholar] [CrossRef]
- Sánchez-Avila, J.; Fernandez-Sanjuan, M.; Vicente, J.; Lacorte, S. Development of a multi residue method for the determination of organic micro pollutants in water, sediment and mussels using gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 6799–6811. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Liompart, M.; Garcia-Jares, C.; Garcia-Monteagudo, J.C.; Cela, R. Ultrasound-assisted emulsification–microextraction of emergent contaminants and pesticides in environmental waters. J. Chromatogr. A 2008, 1190, 27–38. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Liu, C.; Liao, C.S.; Chang, B.V. Occurrence and microbial degradation of PAEs esters in Taiwan river sediments. Chemosphere 2002, 49, 1295–1299. [Google Scholar] [CrossRef]
- Thomsen, M.; Carlsen, L.; Hvidt, S. Solubilities and surface activities of phthalates investigated by surface tension measurements. Environ. Toxicol. Chem. 2001, 20, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Earls, A.O.; Axford, I.P.; Braybrook, J.H. Gas chromatography-mass spectrometry determination of the migration of phthalate plasticizers from polyvinyl chloride toys and childcare articles. J. Chromatogr. A 2003, 983, 237–246. [Google Scholar] [CrossRef]
- Stringer, R.; Labunska, I.; Santillo, D.; Johnston, P.; Siddorn, J.; Stephenson, A. Concentration of phthalate esters and identification of other additives in PVC children’s toys. Environ. Sci. Pollut. Res. Int. 2000, 7, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bouma, K.; Schakel, D.J. Migration of phthalates from PVC toys into saliva simulant by dynamic extraction. Food Addit. Contam. 2002, 19, 602–610. [Google Scholar] [CrossRef]
- Rodgers, K.M.; Rudel, R.A.; Just, A.C. Phthalates in food packaging, consumer products, and indoor environments. In Toxicants in Food Packaging and Household Plastics, Molecular and Integrative Toxicology; Snedeker, S.M., Ed.; Springer: London, UK, 2014; pp. 31–59. [Google Scholar]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.J.; Herrmann, R. Estimation of the environmental contamination by phthalic acid esters leaching from household wastes. Sci. Total Environ. 1997, 208, 49–57. [Google Scholar] [CrossRef]
- Bosnir, J.; Puntaric, D.; Galic, A.; Skes, I.; Dijanic, T.; Klaric, M.; Grgic, M.; Curkovic, M.; Smit, Z. Migration of phthalates from plastic containers into soft drinks and mineral water. Food Technol. Biotechnol. 2006, 45, 91–95. [Google Scholar]
- Calafat, A.M.; Needham, L.L.; Silva, M.J.; Lambert, G. Exposure to Di-(2-Ethylhexyl) Phthalate Among Premature Neonates in a Neonatal Intensive Care Unit. Pediatrics 2004, 113, 429–434. [Google Scholar] [CrossRef]
- Edgren, D.E.; Carpenter, H.A.; Bhatti, G.K.; Ayer, A.D. Encinal Pharmaceutical investments LLC. U.S. Patent US6,217,905, 7 May 1990. [Google Scholar]
- Houlihan, J.; Brody, C.; Schwan, B. Not Too Pretty: Phthalates, Beauty Products and the FDA; Environmental Working Group: Washington, DC, USA, 2002. [Google Scholar]
- Latini, G.; Knipp, G.; Mantovani, A.; Macrovecchio, M.L.; Chiarelli, F.; Soder, O. Endocrine disruptors and human health. Mini-Rev. Med. Chem. 2010, 10, 1–10. [Google Scholar] [CrossRef]
- Koniecki, D.; Wang, R.; Moody, R.P.; Zhu, J. Phthalates in cosmetic and personal care products: Concentrations and possible dermal exposure. Environ. Res. 2011, 111, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.C.; Peterson, K.E.; Meeker, J.D.; Sanchez, B.N.; Zhang, Z.; Cantoral, A.; Solano, M.; Tellez-Rojo, M.M. Bisphenol A and phthalates in utero and in childhood: Association with child BMI z-score and adiposity. Environ. Res. 2017, 156, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.A.; Fla, O.; Bushland, R.C. Pyrethrin and Dibenzyl Phthalate Insecticide. U.S. Patent 2,428,494, 7 October 1947. [Google Scholar]
- Schripp, T.; Salthammer, T.; Fauck, C.; Beko, G.; Weschler, C.J. Latex paint as a delivery vehicle for diethylphthalate and di-n- butylphthalate: Predictable boundary layer concentrations and emission rates. Sci. Total Environ. 2014, 494–495, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.A. Aqueous Composition for Plasticizing Paint Prior to Strip. U.S. Patent US6,468,952B1, 28 December 1995. [Google Scholar]
- Kitchloo, P.V.; Sallavanti, R.A. Infusion of Dye Using Plasticizer. U.S. Patent US6,719,812B1, 11 April 2000. [Google Scholar]
- Lippmann, D.C.; Conn, G. Wearing Apparel. U.S. Patent 2,360,953, 24 October 1942. [Google Scholar]
| Sample ID | Sampling Site | Description of the Sampling Site | pH | Temp. 1 (°C) | Salinity 2 (%) |
|---|---|---|---|---|---|
| SP 1 | Sri Wickrama canal | About 5 m wide, velocity <1.0 m/min, brackish, fecally polluted | 7.45 ± 0.12 | 29.3 ± 0.1 | 0.7 ± 0.1 |
| SP 2 | Peliyagoda canal | About 4 m wide, velocity <1.0 m/min, brackish, fecally polluted | 7.50 ± 0.10 | 30.7 ± 0.2 | 0.3 ± 0.0 |
| SP 3 | Main Drain | About 6 m wide, velocity <1.0 m/min, brackish, fecally polluted | 7.65 ± 0.14 | 30.0 ± 0.1 | 0.9 ± 0.2 |
| SP 4 | Beira lake-North lock gate | About 6 m wide, velocity <2.0 m/min, brackish, fecally polluted | 7.55 ± 0.12 | 28.2 ± 0.2 | 0.9 ± 0.3 |
| SP 5 | St. Sebastian canal (North) | About 6 m wide, velocity <1.0 m/min, brackish, fecally polluted | 7.30 ± 0.15 | 29.7 ± 0.2 | 0.4 ± 0.1 |
| SP 6 | Kittampahuwa canal | About 4 m wide, velocity <1.0 m/min, brackish, fecally polluted | 6.90 ± 0.20 | 28.6 ± 0.1 | 0.4 ± 0.1 |
| SP 7 | St. Sebastian canal (South) | About 6 m wide, velocity <1.0 m/min, brackish, fecally polluted | 7.40 ± 0.31 | 30.6 ± 0.2 | 0.1 ± 0.0 |
| SP 8 | Raggahawatta canal | About 4 m wide, velocity <5.0 m/min, fresh, fecally polluted | 6.45 ± 0.20 | 30.5 ± 0.1 | 0.0 ± 0.0 |
| SP 9 | Dematagoda canal | About 6 m wide, velocity <2.0 m/min, brackish, fecally polluted | 8.60 ± 0.22 | 31.1 ± 0.20 | 0.2 ± 0.0 |
| SP 10 | Beira lake-East | Lake, very slow water movements, brackish, fecally polluted | 8.90 ± 0.12 | 30.7 ± 0.2 | 0.7 ± 0.2 |
| SP 11 | Kimbula canal | About 2 m wide, velocity <2.0 m/min, brackish, fecally polluted | 8.10 ± 0.21 | 29.8 ± 0.2 | 0.1 ± 0.0 |
| SP 12 | Diyawanna lake | Lake, very slow water movements, fresh, fecally polluted | 7.45 ± 0.31 | 30.7 ± 0.3 | 0.0 ± 0.0 |
| SP 13 | Kirulapona canal | About 5 m wide, velocity <2.0 m/min, fresh, fecally polluted | 7.70 ± 0.28 | 28.5 ± 0.2 | 0.1 ± 0.0 |
| SP 14 | Wellawatta canal | About 6 m wide, velocity <2.0 m/min, brackish, fecally polluted | 7.65 ± 0.26 | 30.5 ± 0.1 | 0.9 ± 0.3 |
| SP 15 | Poorwarama canal | About 6 m wide, velocity <2.0 m/min, brackish, fecally polluted | 7.30 ± 0.25 | 31.5 ± 0.2 | 0.1 ± 0.0 |
| SP 16 | Dehiwela canal | About 6 m wide, velocity <3.0 m/min, brackish, fecally polluted | 8.25 ± 0.25 | 28.4 ± 0.3 | 2.4 ± 0.6 |
| SP 17 | Maharagama Ela | About 2 m wide, velocity <1.0 m/min, fresh, fecally polluted | 5.80 ± 0.32 | 27.9 ± 0.3 | 0.0 ± 0.0 |
| SP 18 | Nadimala canal | About 2 m wide, velocity <1.0 m/min, fresh, fecally polluted | 6.60 ± 0.42 | 28.7 ± 0.2 | 0.0 ± 0.0 |
| SP 19 | Weras Ganga | About 10 m wide, velocity <2.0 m/min, fresh, fecally polluted | 7.45 ± 0.34 | 29.5 ± 0.3 | 0.0 ± 0.0 |
| SP 20 | Ratmalana canal | About 2 m wide, velocity <1.0 m/min, fresh, fecally polluted | 5.45 ± 0.21 | 30.8 ± 0.4 | 0.0 ± 0.0 |
| SP 21 | Kesbawa lake | Lake, very slow water movements, fresh, fecally polluted | 7.20 ± 0.30 | 27.5 ± 0.2 | 0.0 ± 0.0 |
| SP 22 | Bolgoda lake | Lake, very slow water movements, fresh, fecally polluted | 7.20 ± 0.30 | 30.6 ± 0.3 | 0.1 ± 0.0 |
| PAE | LOD (µg/L) | LOQ (µg/L) | Recovery Efficiency for External Standards (RSD %); N = 6 | Recovery Efficiency for DPP (RSD %); N = 6 | ||
|---|---|---|---|---|---|---|
| 1 µg/L | 5 µg/L | Field Blank | Procedural Blank | |||
| DMP | 0.3 | 0.5 | 98–102 (8%) | 95–120 (5%) | NA | NA |
| DEP | 0.3 | 0.5 | 98–103 (5%) | 95–120 (3%) | NA | NA |
| DBP | 0.3 | 0.5 | 98–104 (6%) | 95–120 (3%) | NA | NA |
| BBP | 0.5 | 1 | 95–110 (8%) | 95–120 (5%) | NA | NA |
| DEHP | 0.5 | 1 | 95–110 (8%) | 95–120 (4%) | NA | NA |
| DnOP | 0.5 | 1 | 95–110 (8%) | 95–120 (5%) | NA | NA |
| DPP | 0.3 | 0.5 | NA | NA | 95–105 (4%) | 95–110 (6%) |
| Sample | PAE Concentration (µg/L) | |||||
|---|---|---|---|---|---|---|
| DMP | DEP | DBP | BBP | DEHP | DnOP | |
| SP 1 | <0.5 | 22.0 ± 3 | 17.0 ± 1 | <1 | 58 ± 2 | <1 |
| SP 2 | <0.5 | 37.0 ± 15 | 16.0 ± 5 | <1 | 165 ± 136 | <1 |
| SP 3 | <0.5 | 20.5 ± 8 | 26.0 ± 11 | <1 | 105 ± 54 | <1 |
| SP 4 | <0.5 | 61.0 ± 39 | 27.5 ± 2 | 61 ± 4 | 82 ± 16 | <1 |
| SP 5 | <0.5 | 26.0 ± 7 | 15.5 ± 3 | <1 | 70 ± 6 | <1 |
| SP 6 | <0.5 | <0.5 | <0.5 | <1 | 73 ± 1 | <1 |
| SP 7 | <0.5 | 31.0 ± 17 | 20.0 ± 15 | <1 | 110 ± 56 | <1 |
| SP 8 | <0.5 | < 0.5 | 32.0 ± 11 | <1 | 44 ± 2 | <1 |
| SP 9 | <0.5 | 110.5 ± 51 | 20.5 ± 3 | 72 ± 3 | 114 ± 23 | <1 |
| SP 10 | <0.5 | 265.0 ± 25 | 27.5 ± 4 | 75 ± 11 | 137 ± 6 | <1 |
| SP 11 | <0.5 | <0.5 | <0.5 | <1 | 102 ± 2 | <1 |
| SP 12 | <0.5 | <0.5 | <0.5 | <1 | 21 ± 3 | <1 |
| SP 13 | <0.5 | 27.5 ± 1 | 27.0 ± 17 | <1 | 53 ± 3 | <1 |
| SP 14 | <0.5 | 42.0 ± 7 | 24.5 ± 5 | <1 | 12 ± 1 | <1 |
| SP 15 | <0.5 | 25.0 ± 1 | 19.0 ± 3 | <1 | 93 ± 12 | <1 |
| SP 16 | <0.5 | 48.0 ± 17 | 24.0 ± 3 | <1 | 90 ± 22 | <1 |
| SP 17 | <0.5 | 25.0 ± 8 | 21.0 ± 3 | <1 | 108 ± 50 | <1 |
| SP 18 | <0.5 | <0.5 | <0.5 | <1 | 62 ± 2 | <1 |
| SP 19 | <0.5 | 246.0 ± 26 | 21.5 ± 6 | 69 ± 6 | 101 ± 45 | <1 |
| SP 20 | <0.5 | 26.0 ± 13 | 27.0 ± 7 | 108 ± 7 | 134 ± 1 | <1 |
| SP 21 | <0.5 | 2.5 ± 1 | 2.5 ± 1 | <1 | <1 | <1 |
| SP 22 | <0.5 | 61.0 ± 15 | 20.5 ± 4 | <1 | 98 ± 20 | <1 |
| Country | PAE Concentration (µg/L) | ||||||
|---|---|---|---|---|---|---|---|
| DMP | DEP | DBP | BBP | DEHP | DnOP | References | |
| Sri Lanka 1 | <0.5 | 2.5–265.0 | 1.0–32.0 | 61–108 | 12–165 | <1 | This Study |
| China 2 | 0.065–0.208 | 0.140–0.334 | 0.190–4.762 | <0.001 | 0.364–2.682 | 0.001–0.621 | [28] |
| China 3 | <0.001 | <0.001 | 6.825 | 0.21 | 5.196 | NM | [29] |
| China 4 | <0.010 | 0.098–0.197 | 0.146–0.225 | <0.010 | 0.582–2.05 | 0.010–0.059 | [30] |
| China 5 | <0.001 | <0.001 | 35.65 | NM | 54.73 | 0.84 | [31] |
| France 6 | <0.01 | <0.015 | 0.086 | <0.005 | 0.090 | <0.01 | [32] |
| Germany 7 | NM | NM | 0.12–8.80 | <0.02 | 0.33–97.80 | NM | [33] |
| Japan 8 | <0.03 | <0.03 | 3.34 | NM | 0.97 | NM | [34] |
| Sweden 9 | 0.40 | 0.63 | 6.8 | 0.17 | NM | NM | [35] |
| South Africa 10 | ND | 53–813 | 1343–5600 | 1–140 | 107–361 | 1–5 | [36] |
| Spain 11 | 0.003–0.008 | 0.300–1.742 | NM | 0.005–0.122 | 0.014–0.180 | NM | [37] |
| Spain 12 | 0.158 | 0.261 | <0.125 | 0.029 | 0.133 | NM | [38] |
| Taiwan 13 | NM | 0.6–2.5 | 1–13.5 | <0.6 | 1–18.5 | NM | [39] |
| pH | Temperature | Salinity | ||
|---|---|---|---|---|
| DEP | Correlation coefficient | 0.23 | 0.39 | −0.09 |
| p-value | 0.30 | 0.07 | 0.67 | |
| DBP | Correlation coefficient | 0.26 | 0.03 | −0.06 |
| p-value | 0.24 | 0.88 | 0.76 | |
| BBP | Correlation coefficient | 0.13 | 0.14 | −0.15 |
| p-value | 0.58 | 0.54 | 0.50 | |
| DEHP | Correlation coefficient | 0.29 | 0.25 | −012 |
| p-value | 0.19 | 0.26 | 0.60 |
| Sample | Type of Industry | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| SP 1 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SP 2 | 1 | - | - | - | 3 | 1 | - | - | 1 | 3 | 1 | 1 | - |
| SP 3 | - | 1 | 1 | 1 | - | 1 | 1 | 1 | 2 | 2 | 5 | 3 | - |
| SP 4 | 2 | 2 | 1 | 1 | 1 | 3 | 3 | 2 | 3 | 3 | 5 | 4 | 1 |
| SP 5 | 2 | 2 | 1 | 1 | 1 | 3 | 3 | 2 | 3 | 3 | 5 | 4 | 1 |
| SP 6 | 1 | 1 | - | - | - | 1 | - | 2 | 2 | - | 5 | 3 | - |
| SP 7 | - | - | - | - | - | - | - | 1 | - | 1 | - | - | - |
| SP 8 | - | 1 | - | 1 | 2 | 2 | - | 2 | - | 1 | 1 | 2 | - |
| SP 9 | 1 | 1 | - | - | - | 1 | 2 | 1 | 1 | 1 | 1 | 2 | - |
| SP 10 | - | - | 3 | - | 1 | - | 2 | - | 1 | 1 | 2 | - | - |
| SP 11 | 2 | 4 | - | - | - | 1 | 2 | 2 | 2 | 1 | 5 | 4 | - |
| SP 12 | - | - | - | 2 | - | 2 | 2 | 2 | 1 | 2 | 3 | 4 | 1 |
| SP 13 | 2 | 2 | - | - | - | 1 | 2 | 1 | 2 | - | 3 | 2 | - |
| SP 14 | 1 | 1 | - | - | - | - | 2 | 1 | 1 | - | 4 | 1 | - |
| SP 15 | 2 | - | - | - | - | - | 1 | 1 | 2 | - | 1 | - | - |
| SP 16 | 4 | 3 | - | 1 | 2 | 2 | 1 | - | - | 1 | - | 1 | - |
| SP 17 | 1 | - | - | - | - | - | - | - | - | - | - | 1 | - |
| SP 18 | 3 | - | - | - | 2 | 2 | - | - | - | - | - | 1 | 1 |
| SP 19 | 1 | 1 | - | 1 | 2 | 2 | - | - | - | 1 | 2 | 1 | 1 |
| SP 20 | 1 | 1 | - | 1 | 1 | 1 | 2 | - | - | 1 | - | - | 1 |
| SP 21 | - | - | 1 | - | 1 | - | 1 | - | - | - | 1 | - | |
| SP 22 | - | 2 | - | 1 | - | 1 | - | 2 | - | - | - | 2 | - |
| Type | Industry | Percentages by Weight in Final Products | References | |||||
|---|---|---|---|---|---|---|---|---|
| DMP | DEP | DBP | BBP | DEHP | DnOP | |||
| 1 | Stationery products distributors | 10 1 | 38 1 | 4 1 | 20 1 | 1 [41] | ||
| 2 | Toy manufacturing | 0.1 2 | 30–40 3 | 1 2 | 2 [42], 3 [43] | |||
| 3 | Household-hardware product manufacturing | 14 4 | 14–40 5 | 4 [44], 5 [45] | ||||
| 4 | Plastic manufacturing | 67 6 | 6 [46] | |||||
| 5 | PET bottle manufacturing | 56 7 | 43 7 | 7 [47] | ||||
| 6 | PVC Cable manufacturing | 40 8 | 8 [48] | |||||
| 7 | Pharmaceutical packaging | 2.7 9 | 2.7 9 | 9 [49] | ||||
| 8 | Cosmetic and personal care product packaging | 1 10 | 72 10 | 8 10 | 6 10 | 4 10 | 10 [50] | |
| 9 | PVC manufacturing | 40–50 11, 67 12 | 11 [51], 12 [52] | |||||
| 10 | Polyethylene manufacturing | 20 13 | 13 [53] | |||||
| 11 | Pesticide packaging | 2 14 | 2 14 | 1 14 | 14 [54] | |||
| 12 | Paint manufacturing | 4–10 15 | 1 15 | 10 15 | 4–10 16 | 15 [55], 16 [56] | ||
| 13 | Apparel | 1–4 17 | 10–60 18 | 10–60 18 | 17 [57], 18 [58] | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaweera, M.; Danushika, G.; Bandara, N.; Dissanayake, J.; Gunawardana, B.; Manatunge, J.; Zoysa, K. Urban Watercourses in Peril: Implications of Phthalic Acid Esters on Aquatic Ecosystems Caused by Urban Sprawl. Water 2019, 11, 519. https://doi.org/10.3390/w11030519
Jayaweera M, Danushika G, Bandara N, Dissanayake J, Gunawardana B, Manatunge J, Zoysa K. Urban Watercourses in Peril: Implications of Phthalic Acid Esters on Aquatic Ecosystems Caused by Urban Sprawl. Water. 2019; 11(3):519. https://doi.org/10.3390/w11030519
Chicago/Turabian StyleJayaweera, Mahesh, Gimhani Danushika, Nilanthi Bandara, Janith Dissanayake, Buddhika Gunawardana, Jagath Manatunge, and Kasun Zoysa. 2019. "Urban Watercourses in Peril: Implications of Phthalic Acid Esters on Aquatic Ecosystems Caused by Urban Sprawl" Water 11, no. 3: 519. https://doi.org/10.3390/w11030519
APA StyleJayaweera, M., Danushika, G., Bandara, N., Dissanayake, J., Gunawardana, B., Manatunge, J., & Zoysa, K. (2019). Urban Watercourses in Peril: Implications of Phthalic Acid Esters on Aquatic Ecosystems Caused by Urban Sprawl. Water, 11(3), 519. https://doi.org/10.3390/w11030519