Skeletal Deformity of Scoliosis in Gilthead Seabreams (Sparus aurata): Association with Changes to Calcium-Phosphor Hydroxyapatite Salts and Collagen Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Sampling
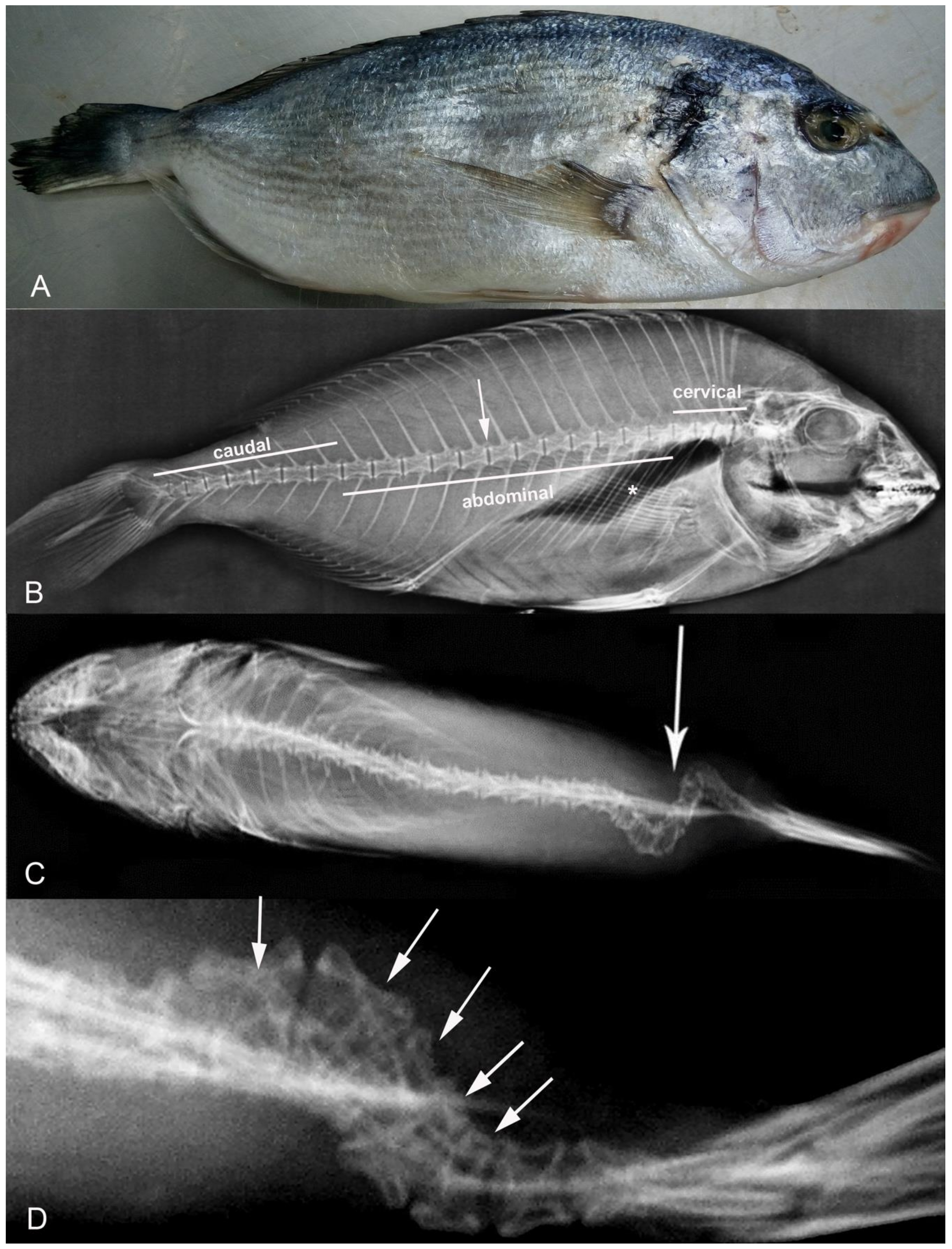
2.2. X-ray
2.3. Ca and P Level Measurement
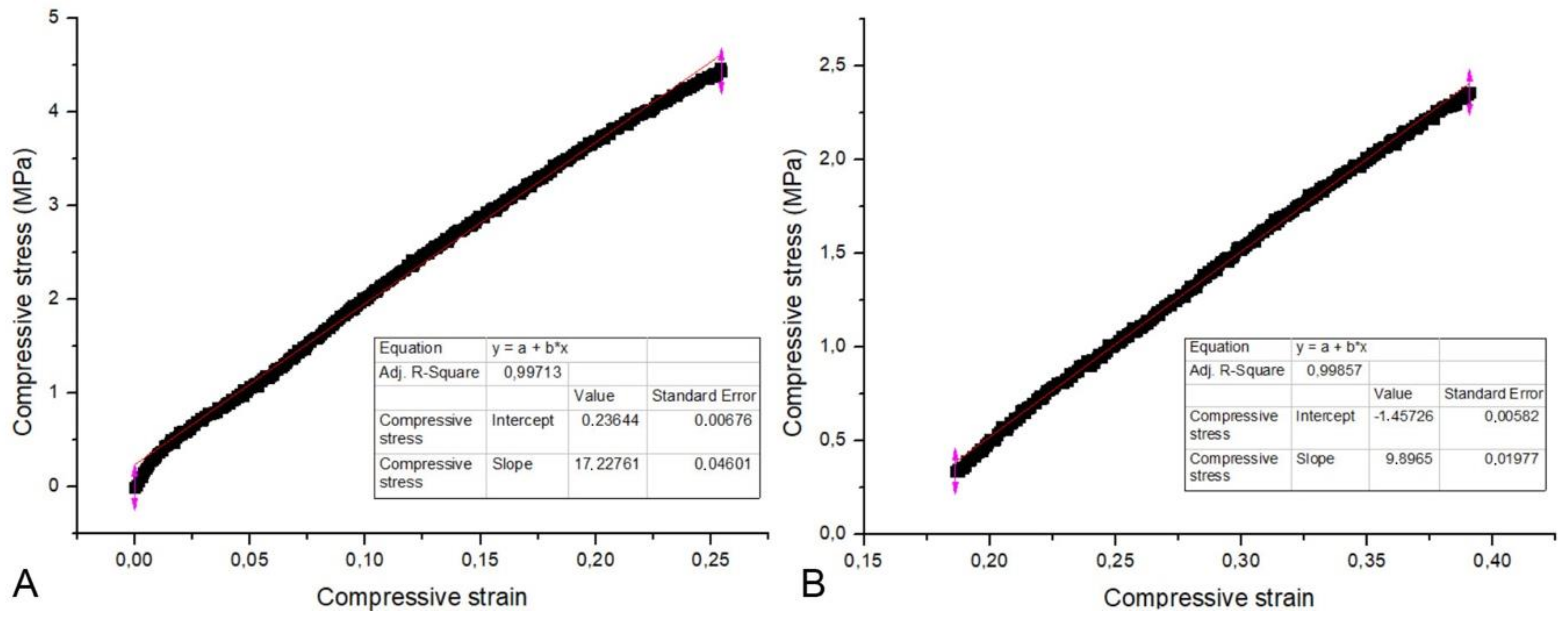
2.4. Compressive Strength and Modulus of Elasticity
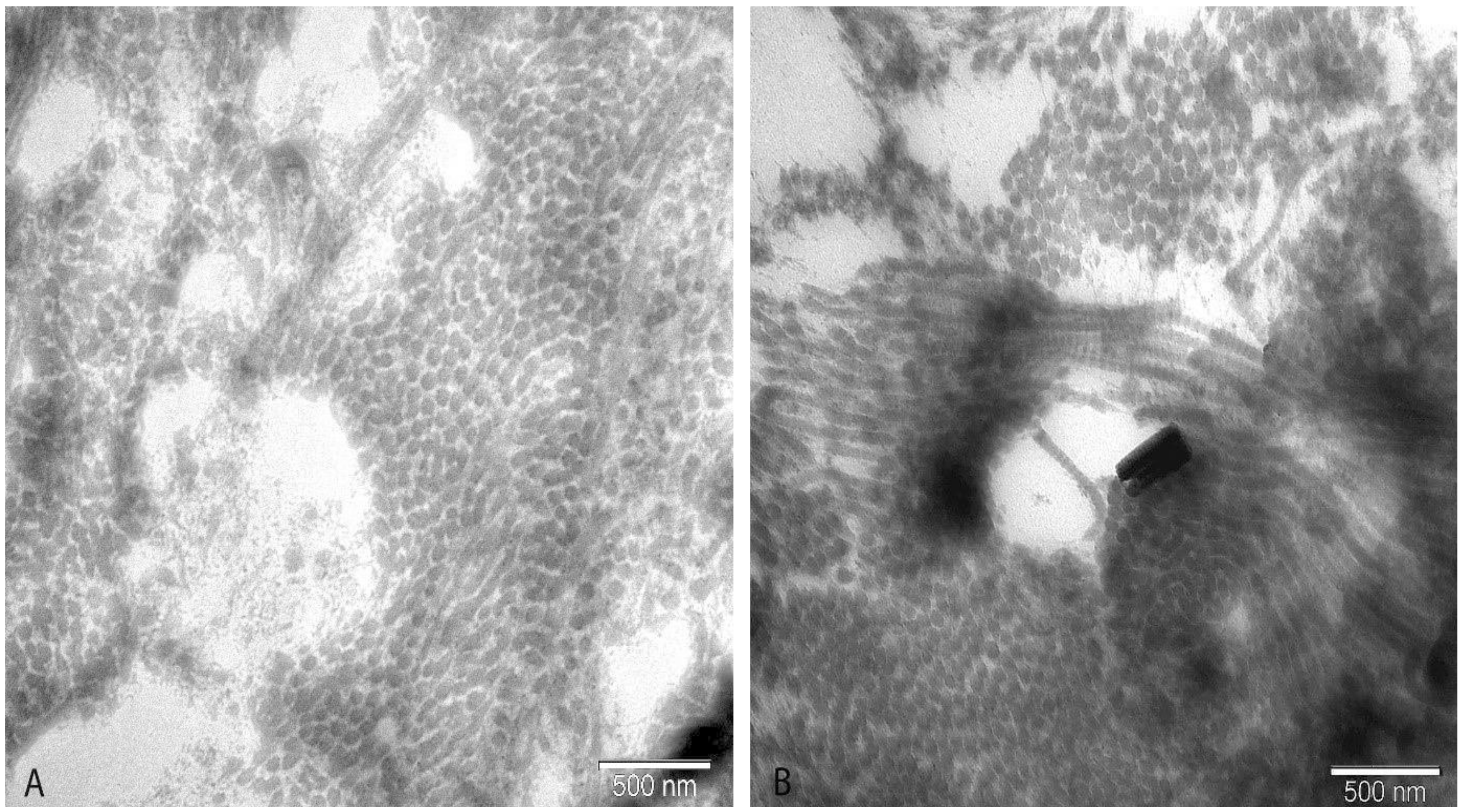
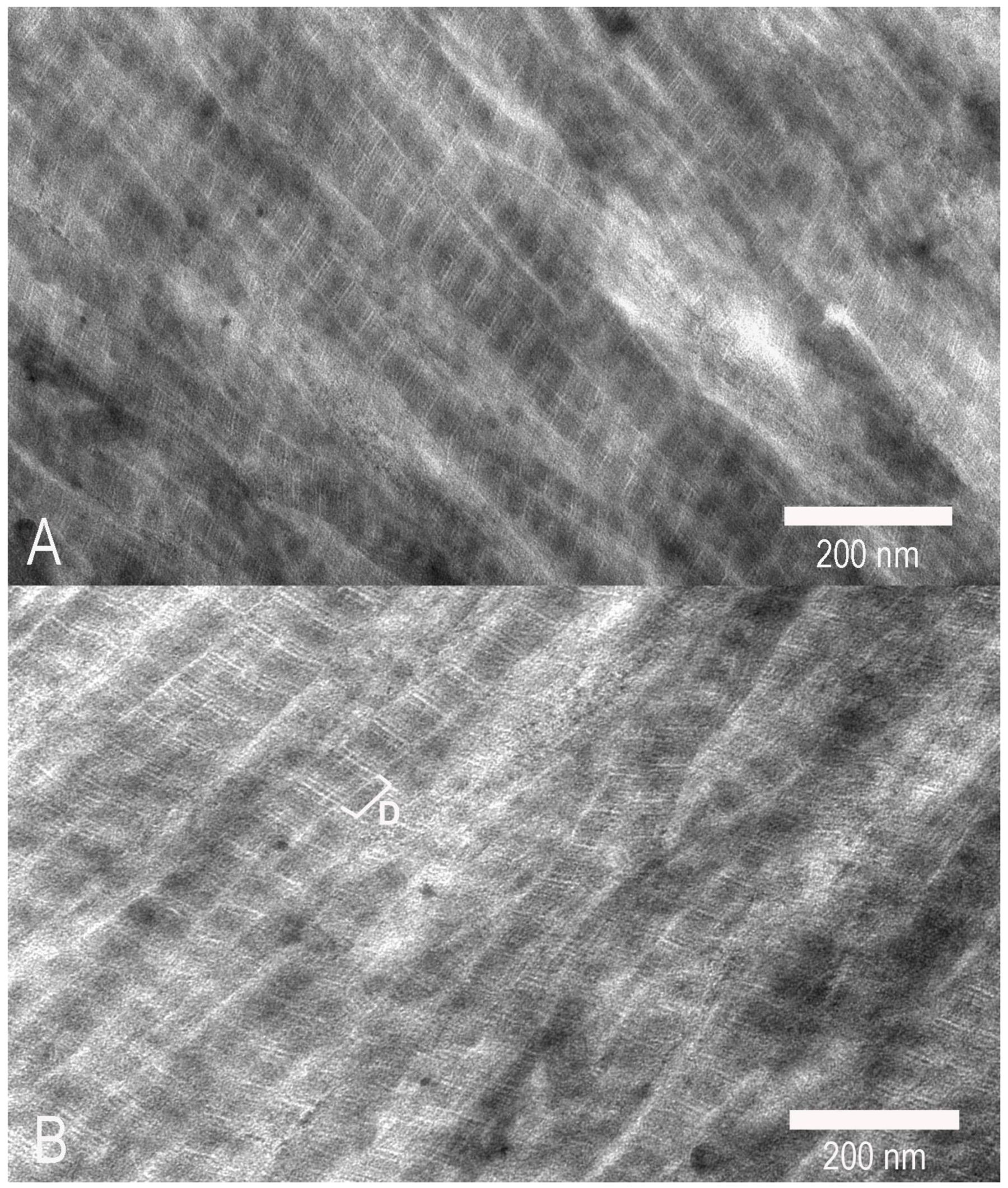
2.5. Electron Microscopy
2.6. Statistical Analysis
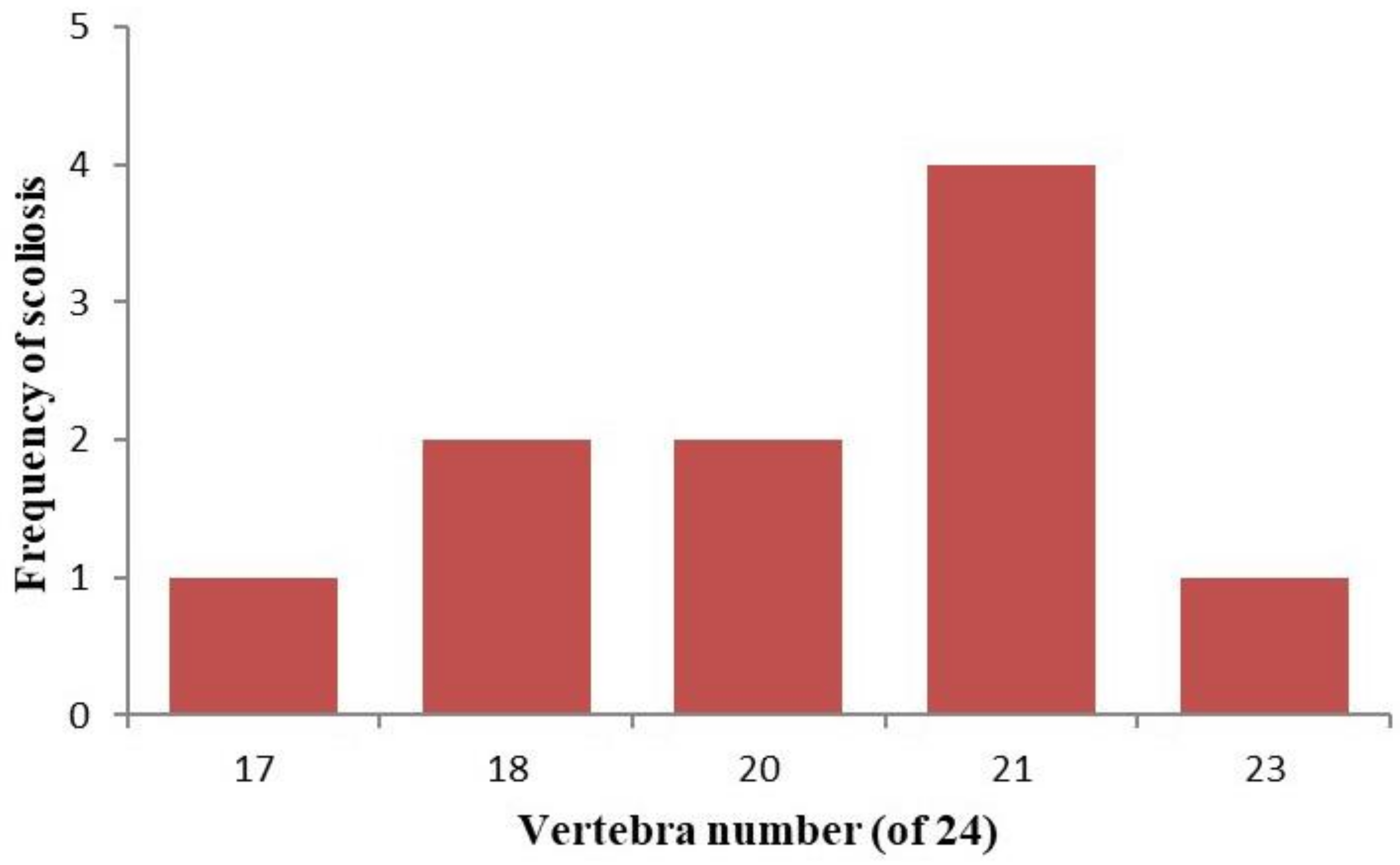
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations, Cultured Aquatic Species Information Program, Sparus aurata (Linnaeus, 1758). Available online: http:// www.fao.org/fishery/culturedspecies/Sparus aurata/en (accessed on 16 November 2018).
- European Commission. Annual Economic Report of EU Aquaculture Sector 2016. Available online: https://stecf.jrc.ec.europa.eu/documents/43805/1491449/_2016-10_STECF+16-19+-+EU+Aquaculture_JRCxxx.pdf (accessed on 10 December 2017).
- Boglione, C.; Gavaia, P.; Koumoundouros, G.; Gisbert, E.; Moren, M.; Fontagné, S.; Witten, P.E. Skeletal anomalies in reared European fish larvae and juveniles. Part 1: Normal and anomalous skeletogenic processes. Rev. Aquac. 2013, 5, 99–120. [Google Scholar] [CrossRef]
- Fernández, I.; Hontoria, F.; Ortiz-Delgado, J.B.; Kotzamanis, Y.; Estévez, A.; Zambonino-Infante, J.L.; Gisbert, E. Larval performance and skeletal deformities in farmed gilthead sea bream (Sparus aurata) fed with graded levels of Vitamin A enriched rotifers (Brachionus plicatilis). Aquaculture 2008, 283, 102–115. [Google Scholar] [CrossRef]
- Georgakopoulou, E.; Katharios, P.; Divanach, P.; Koumoundouros, G. Effect of temperature on the development of skeletal deformities in gilthead seabream (Sparus aurata Linnaeus, 1758). Aquaculture 2010, 308, 13–19. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Gagliardi, F.; Divanach, P.; Boglione, C.; Cataudella, S.; Kentouri, M. Normal and abnormal osteologic development of caudal fin in Sparus aurata L. fry. Aquaculture 1997, 14, 215–226. [Google Scholar] [CrossRef]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef]
- Moss, M.L. Studies of the acellular bone of teleost fish. I. Morphological and systematic variation. Acta Anatom. 1961, 46, 343–362. [Google Scholar] [CrossRef]
- Benjamin, M.; Ralphs, J.R. Extracellular matrix of connective tissues in the heads of teleosts. J. Anat. 1991, 179, 137–148. [Google Scholar]
- Parry, D.A.; Barnes, G.R.; Craig, A.S. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc. R. Soc. Lond. B Biol. Sci. 1978, 203, 305–321. [Google Scholar]
- Baek, G.H.; Carlin, G.J.; Vogrin, T.M.; Woo, S.L.; Harner, C.D. Quantitative analysis of collagen fibrils of human cruciate and meniscofemoral ligaments. Clin. Orthop. 1998, 357, 205–211. [Google Scholar] [CrossRef]
- Ottani, V.; Franchi, M.; De Pasquale, V.; Leonardi, L.; Morocutti, M.; Ruggeri, A. Collagen fibril arrangement and size distribution in monkey oral mucosa. J. Anat. 1998, 192, 321–328. [Google Scholar] [CrossRef]
- Gallop, P.M.; Paz, M.A. Posttranslational protein modifications, with special attention to collagen and elastin. Physiol. Rev. 1975, 55, 418–487. [Google Scholar] [CrossRef]
- Adams, E. Invertebrate collagens. Marked differences from vertebrate collagens appear in only a few invertebrate groups. Science 1978, 202, 591–598. [Google Scholar] [CrossRef]
- Bailey, A. The nature of collagen. Compr. Biochem. 1968, 26, 297–424. [Google Scholar]
- Miller, A. Molecular packing in collagen fibrils. In Biochemistry of Collagen; Ramachandran, G.N., Reddi, A.H., Eds.; Plenum Press: New York, NY, USA, 1976; pp. 85–136. [Google Scholar]
- Lee, D.D.; Glimcher, M.J. Three dimensional spacial relationship between collagen fibrils and the inorganic calcium phosphate crystals of pickeral and herring bone. J. Mol. Biol. 1991, 217, 487–501. [Google Scholar] [CrossRef]
- Sato, K.; Yoshinaka, R.; Sato, M.; Tomit, J. Biochemical characterization of collagen in myocommata and endomysium fractions of carp and spotted mackerel muscle. J. Food Sci. 1989, 54, 1511–1514. [Google Scholar] [CrossRef]
- Yoshinaka, R.; Sato, K.; Anbe, H.; Sato, M.; Shimizu, Y. Distribution of collagen in body muscle of fishes with different swimming modes. Comp. Biochem. Physiol. 1988, 89, 147–151. [Google Scholar] [CrossRef]
- Tzaphlidou, M.; Berillis, P. Structural alterations caused by lithium in skin andliver collagen using an image processing method. J. Trace Microprobe Tech. 2002, 20, 493–504. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: London, UK, 1996. [Google Scholar]
- Halver, J.E.; Ashley, L.M.; Smith, R.R. Ascorbic acid requirements of coho salmon and rainbow trout. Trans Am Fish Soc 1969, 98, 762–771. [Google Scholar] [CrossRef]
- Weisbrode, S.E.; Doige, C.E. Bones and joints. In Thompson’s Specialized Veterinary Pathology; McGavin, M.D., Carlton, W.W., Zachary, J.F., Eds.; Mosby: St. Louis, MI, USA, 2001; pp. 499–536. [Google Scholar]
- Moro, L.; Romanello, M.; Favia, A.; Lamanna, M.P.; Lozupone, E. Posttranslational modifications of bone collagen type I are related to the function of rat femoral regions. Calcif. Tissue Int. 2000, 66, 151–156. [Google Scholar] [CrossRef]
- Lim, C.; Lowell, R.T. Pathology of the vitamin C syndrome in channel catfish (Ictalurus punctatus). J. Nutr. 1978, 108, 1137–1146. [Google Scholar] [CrossRef]
- Sanatamaría, J.A.; Andrades, J.A.; Herráez, P.; Fernández-Llebrez, P.; Becerra, J. Perinotochordal connective sheet of gilthead sea bream larvae (Sparus aurata, L.) affected by axial malformations: An histochemical and immunocytochemical study. Anat. Rec. 1994, 240, 248–254. [Google Scholar] [CrossRef]
- Berillis, P.; Panagiotopoulos, N.; Boursiaki, V.; Karapanagiotidis, I.T.; Mente, E. Vertebrae length and ultra-structure measurements of collagen fibrils and mineral content in the vertebrae of lordotic gilthead seabreams (Sparus aurata). Micron 2015, 75, 27–33. [Google Scholar] [CrossRef]
- Prockop, D.J.; Berg, R.A.; Kivirikko, K.I.; Vitto, J. Intracellular steps in the biosynthesis of collagen. In Biochemistry of Collagen; Ramachandran, G.N., Reddi, A.H., Eds.; Plenum: New York, NY, USA, 1976; pp. 163–273. [Google Scholar]
- Chapman, J.A. Molecular organisation in the collagen fibril. In Connective Tissue Matrix; Hukins, D.W.L., Ed.; Macmillan: London, UK, 1984; pp. 89–132. [Google Scholar]
- Pedrini, V.A.; Ponseti, I.V.; Dohrman, S.C. Glycosaminoglycans of intervertebral disc in AIS. J. Lab. Clin. Med. 1973, 82, 938–950. [Google Scholar]
- Roberts, S.; Menage, J.; Eisenstein, S.M. The cartilage endplate and intervertebral disc in scoliosis: Calcification and other sequelae. J. Orthop. Res. 1993, 11, 747–757. [Google Scholar] [CrossRef]
- Kobielarz, M.; Szotek, S.; Głowacki, M.; Dawidowicz, J.; Pezowicz, C. Qualitative and quantitative assessment of collagen and elastin in annulus fibrosus of the physiologic and scoliotic intervertebral discs. J. Mech. Behav. Biomed. Mater. 2016, 62, 45–56. [Google Scholar] [CrossRef]
- Tzaphlidou, M. The role of collagen in bone structure: An image processing approach. Micron 2005, 36, 593–601. [Google Scholar] [CrossRef]
- Lall, S.P. The minerals. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press Inc.: San Diego, CA, USA, 2002; pp. 259–308. [Google Scholar]
- Lall, S.P.; Lewis-McCrea, L.M. Role of nutrients in skeletal metabolism and pathology in fish—An overview. Aquaculture 2007, 267, 3–19. [Google Scholar] [CrossRef]
- Ozawa, M.; Suzuki, S. Microstructural development of natural hydroxyapatite originated from fish-bone waste through heat treatment. J. Am. Ceram. Soc. 2002, 85, 1315–1317. [Google Scholar] [CrossRef]
- Kranenbarg, S.; Van Cleynenbreugel, T.; Schipper, H.; Van Leeuwen, J. Adaptive bone formation in acellular vertebrae of sea bass (Dicentrarchus labrax L.). J. Exp. Biol. 2005, 208, 3493–3502. [Google Scholar] [CrossRef]
- Ortiz-Delgado, J.B.; Fernández, I.; Sarasquete, C.; Gisbert, E. Normal and histopathological organization of the opercular bone and vertebrae in gilthead sea bream Sparus aurata. Aquat. Biol. 2014, 21, 67–84. [Google Scholar] [CrossRef]
- Burger, E.H.; Klein-Nulend, J. Mechanotransduction in bone–role of the lacuno-canalicular network. FASEB J. 1999, 13, 101–112. [Google Scholar] [CrossRef]
- Kihara, M.; Ogata, S.; Kawano, N.; Kubota, I.; Yamaguchi, R. Lordosis induction in juvenile red sea bream, Pagrus major, by high swimming activity. Aquaculture 2002, 212, 149–158. [Google Scholar] [CrossRef]
- Pang, P.K. Calcitonin and ultimobranchial glands in fishes. J. Exp. Zool. A Ecol. Genet. Physiol. 1971, 178, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.J.; Mehrle, P.M.; Mayer, F.L.; Jones, J.R. Method to evaluate mechanical properties of bone in fish. Trans. Am. Fish. Soc. 1981, 110, 708–717. [Google Scholar] [CrossRef]
- Nishimoto, S.K.; Araki, N.; Robinson, F.D.; Waite, J.H. Discovery of bone gamma-carboxyglutamic acid protein in mineralized scales. The abundance and structure of Lepomis macrochirus bone gamma-carboxyglutamic acid protein. J. Biol. Chem. 1992, 267, 11600–11605. [Google Scholar] [PubMed]
- Porter, M.E.; Long, J.H. Vertebrae in compression: Mechanical behavior of arches and centra in the gray smooth-hound shark (Mustelus californicus). J. Morphol. 2010, 271, 366–375. [Google Scholar] [CrossRef]
- Porter, M.E.; Beltrán, J.L.; Koob, T.J.; Summers, A.P. Material properties and biochemical composition of mineralized vertebral cartilage in seven elasmobranch species (Chondrichthyes). J. Exp. Biol. 2006, 209, 2920–2928. [Google Scholar] [CrossRef]
- McHenry, M.J.; Pell, C.A.; Long, J.H.J. Mechanical control of swimming speed: Stiffness and axial wave form in undulating fish models. J. Exp. Biol. 1995, 198, 2293–2305. [Google Scholar]
- Berillis, P. Factors that can lead to the development of skeletal deformities in fishes: A review. J. FisheriesSciences.com 2015, 9, 17–23. [Google Scholar]

| Diameter (nm) | D-Period (nm) | |
|---|---|---|
| Scoliotic seabreams | 43.18 a ± 0.28 (810) | 55.69 a ± 0.60 (80) |
| Non-deformed seabreams | 46.68 b ± 0.44 (539) | 55.95 a ± 1.12 (53) |
| Cervical (mm) | Abdominal (mm) | Caudal (mm) | |
|---|---|---|---|
| Non-deformed seabreams | 4.73 a ± 0.19 (n = 40) | 7.47 b ± 0.10 (n = 120) | 6.43 d ± 0.20 (n = 80) |
| Scoliotic seabreams | 4.92 a ± 0.16 (n = 40) | 6.95 c ± 0.08 (n = 120) | 5.36 e ± 0.18 (n = 80) |
| Cervical (mm) | Abdominal (mm) | Caudal (mm) | |
|---|---|---|---|
| Non-deformed seabreams | 0.65 a ± 0.30 (n = 40) | 0.70 b ± 0.20 (n = 120) | 0.70 c ± 0.30 (n = 70) |
| Seabreams with scoliosis | 0.60 a ± 0.20 (n = 40) | 0.60 b ± 0.35 (n = 120) | 0.50 d ± 0.20 (n = 70) |
| Ca (at %) | P (at %) | Ca/P (Level) | M.E. (MPa) | |
|---|---|---|---|---|
| Non-deformed seabreams | 58.18 a ± 0.92 (n = 30) | 32.80 b ± 0.64 (n = 30) | 1.79 c ± 0.07 (n = 30) | 20.79 d ± 2.32 (n = 10) |
| Seabreams with scoliosis | 57.76 a ± 0.70 (n = 30) | 32.45 b ± 0.39 (n = 30) | 1.78 c ± 0.04 (n = 30) | 6.81 e ± 1.52 (n = 10) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boursiaki, V.; Theochari, C.; Zaoutsos, S.P.; Mente, E.; Vafidis, D.; Apostologamvrou, C.; Berillis, P. Skeletal Deformity of Scoliosis in Gilthead Seabreams (Sparus aurata): Association with Changes to Calcium-Phosphor Hydroxyapatite Salts and Collagen Fibers. Water 2019, 11, 257. https://doi.org/10.3390/w11020257
Boursiaki V, Theochari C, Zaoutsos SP, Mente E, Vafidis D, Apostologamvrou C, Berillis P. Skeletal Deformity of Scoliosis in Gilthead Seabreams (Sparus aurata): Association with Changes to Calcium-Phosphor Hydroxyapatite Salts and Collagen Fibers. Water. 2019; 11(2):257. https://doi.org/10.3390/w11020257
Chicago/Turabian StyleBoursiaki, Vaia, Charitini Theochari, Stefanos P. Zaoutsos, Eleni Mente, Dimitris Vafidis, Chrisoula Apostologamvrou, and Panagiotis Berillis. 2019. "Skeletal Deformity of Scoliosis in Gilthead Seabreams (Sparus aurata): Association with Changes to Calcium-Phosphor Hydroxyapatite Salts and Collagen Fibers" Water 11, no. 2: 257. https://doi.org/10.3390/w11020257
APA StyleBoursiaki, V., Theochari, C., Zaoutsos, S. P., Mente, E., Vafidis, D., Apostologamvrou, C., & Berillis, P. (2019). Skeletal Deformity of Scoliosis in Gilthead Seabreams (Sparus aurata): Association with Changes to Calcium-Phosphor Hydroxyapatite Salts and Collagen Fibers. Water, 11(2), 257. https://doi.org/10.3390/w11020257








