Potential Health Risks Linked to Emerging Contaminants in Major Rivers and Treated Waters
Abstract
1. Introduction
2. Material and Methods
3. Results and Discussion
3.1. Atrazine: Standard Herbicide, Potential Carcinogen, and Environmental Immune Disruptor
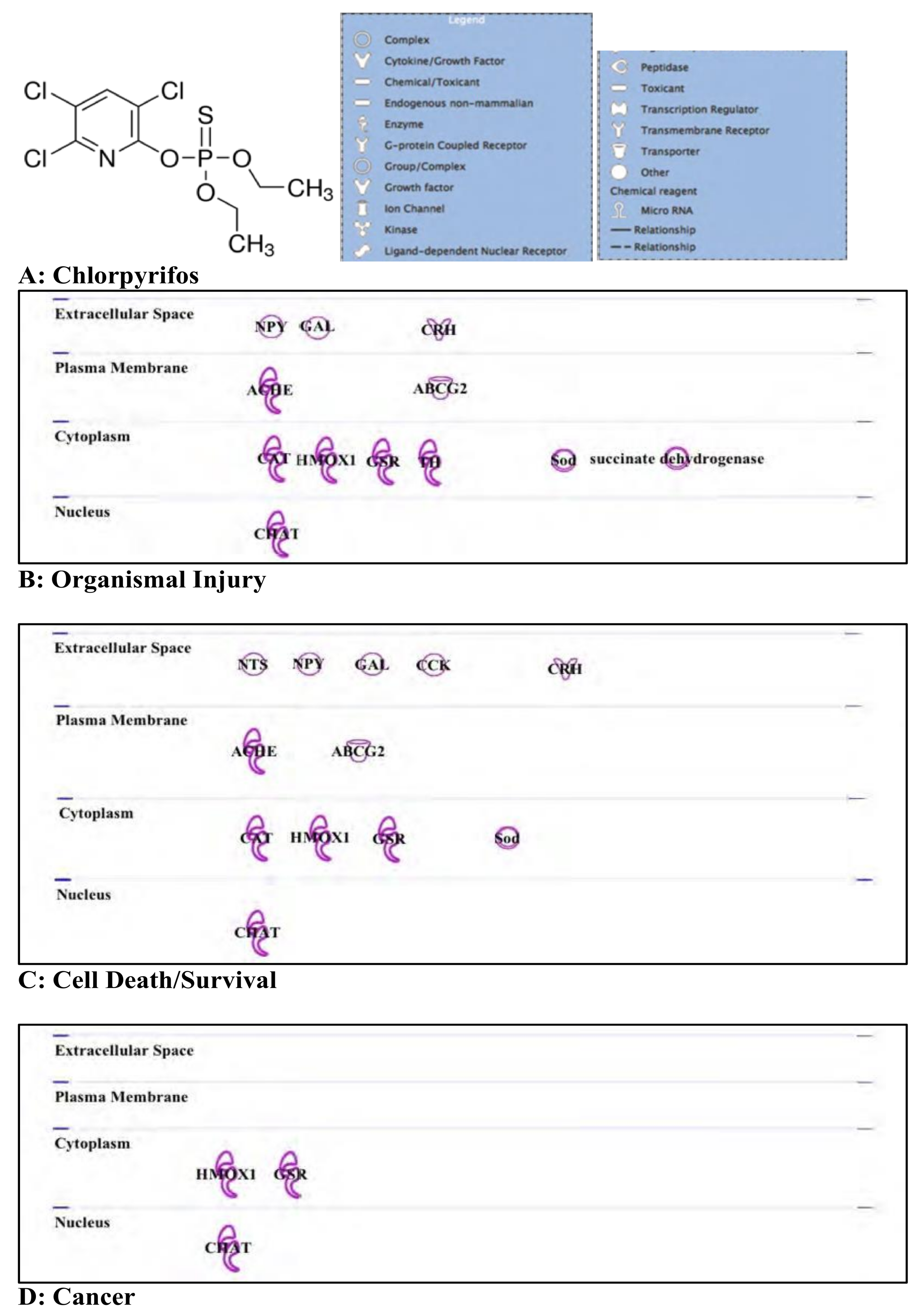
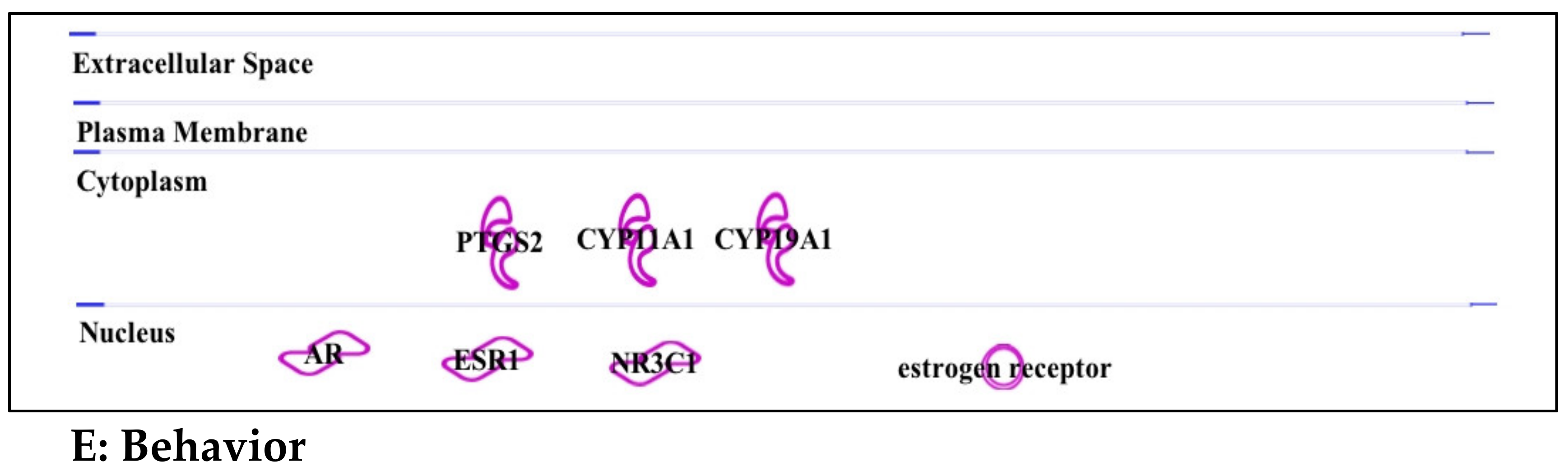
3.2. Chlorpyrifos: Organophosphates and Their Neural and Hepatic Toxicities
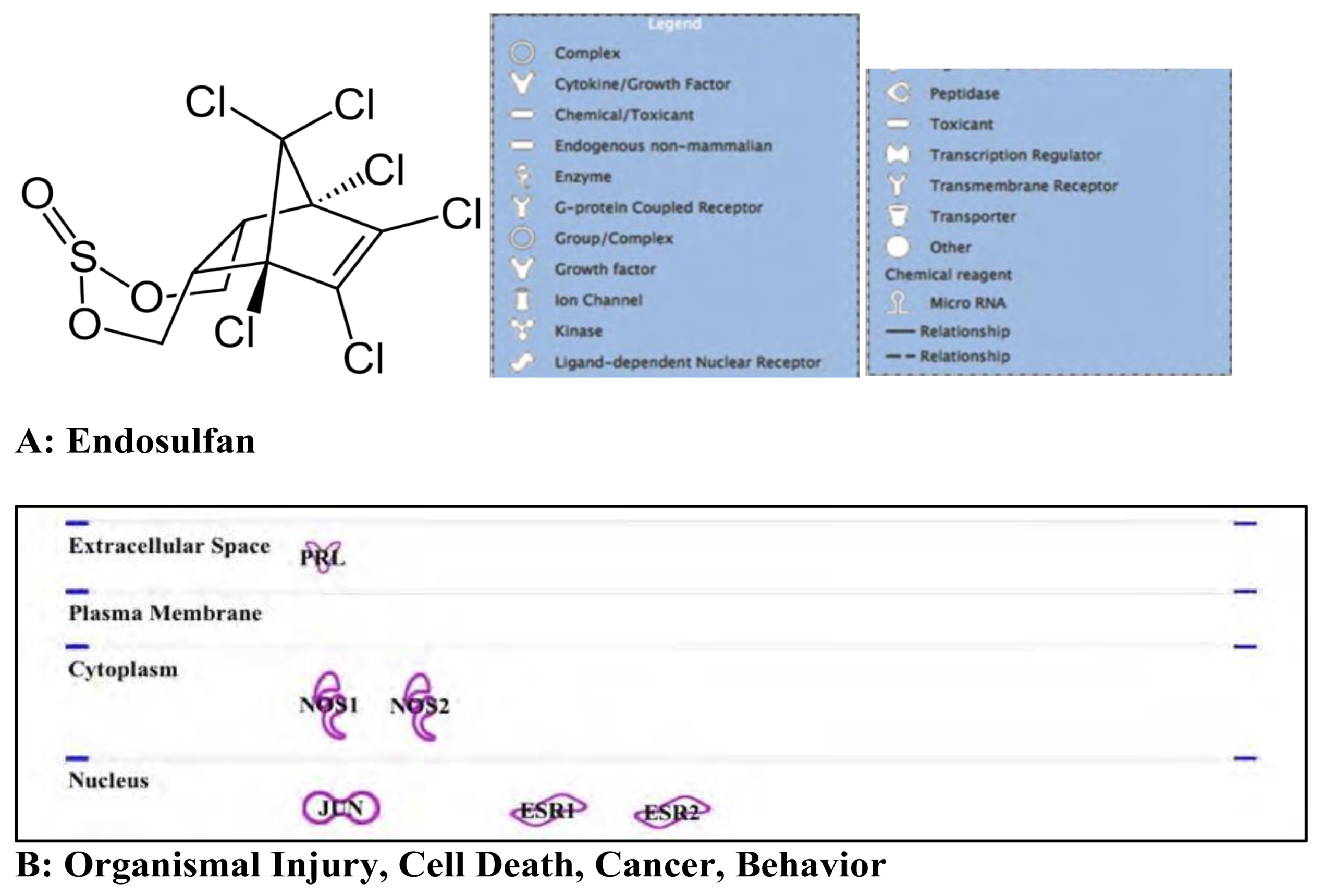
3.3. Endosulfan: Pesticide
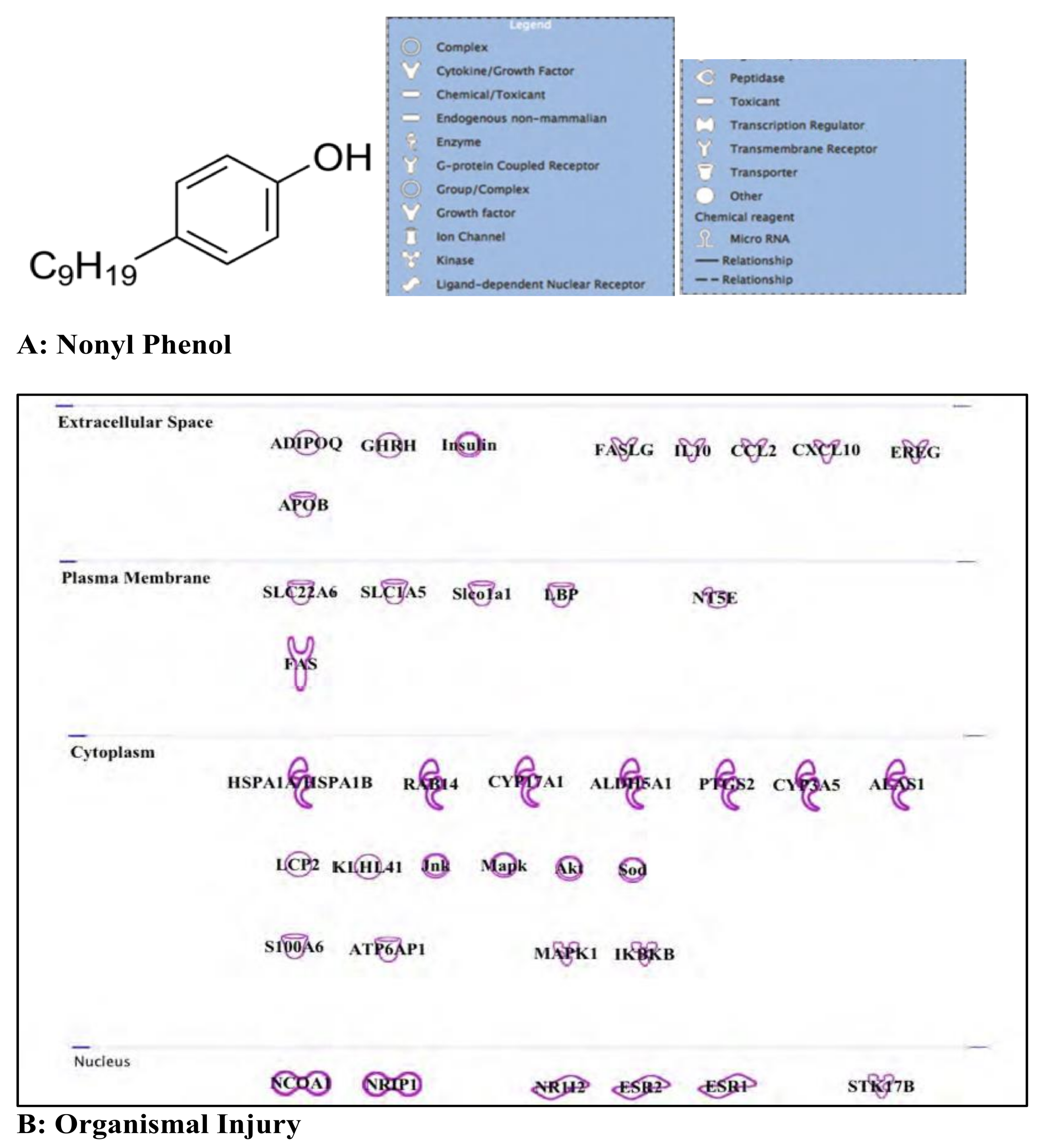
3.4. Nonyl Phenol and Nonyl Phenol Ethoxylates: Endocrine Disruptor
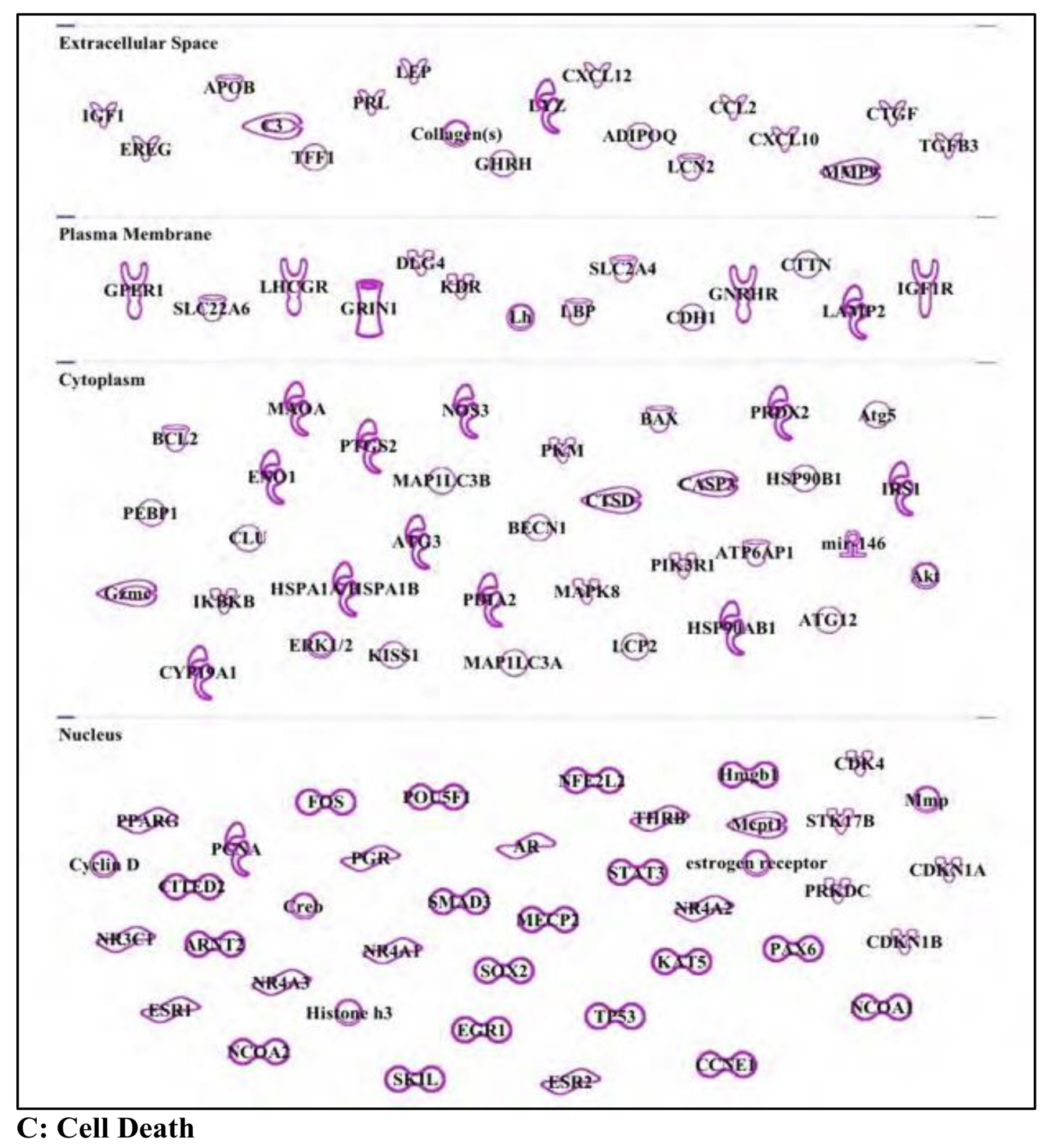
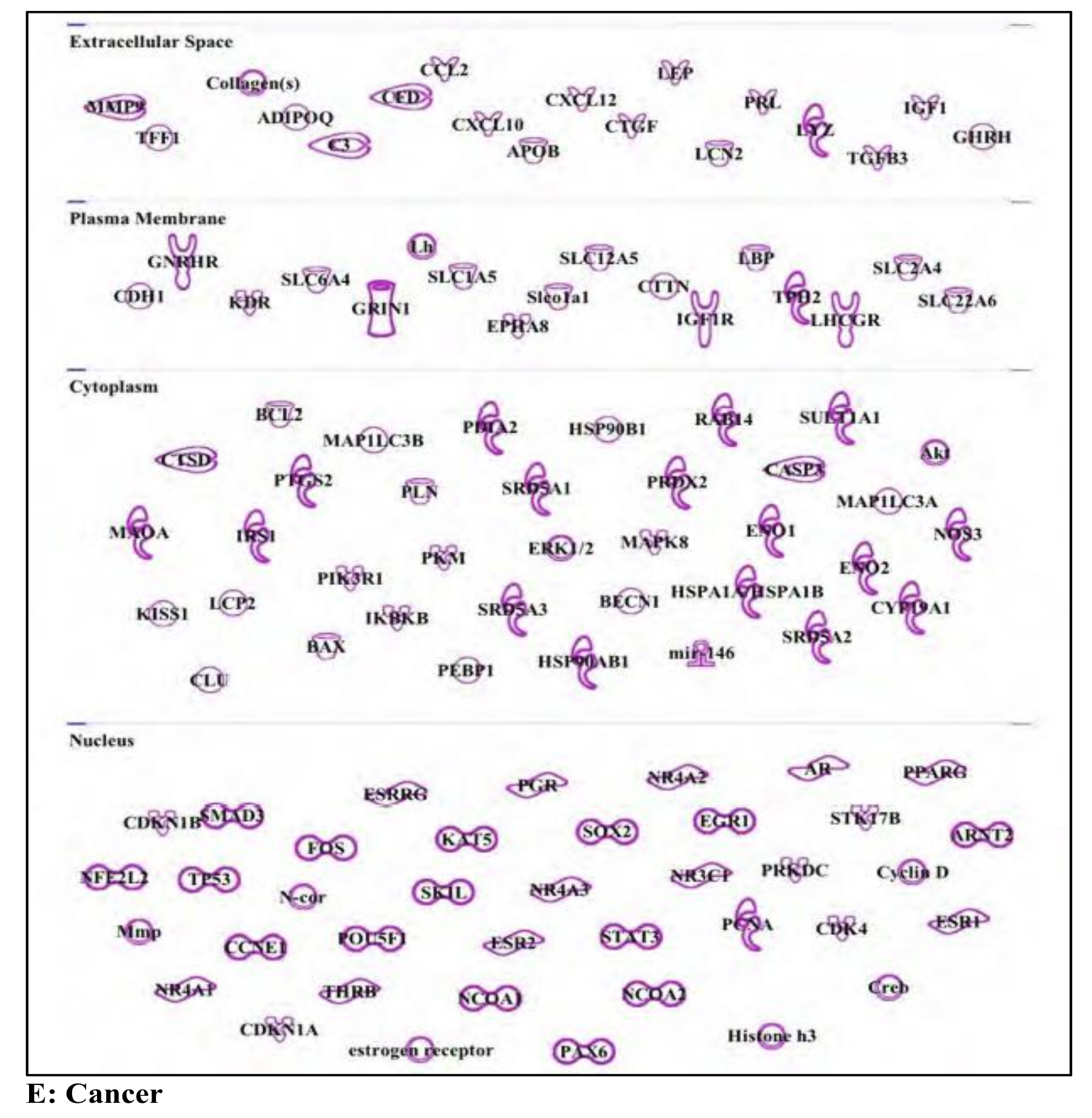
3.5. Bisphenol A: Endocrine Disruptor
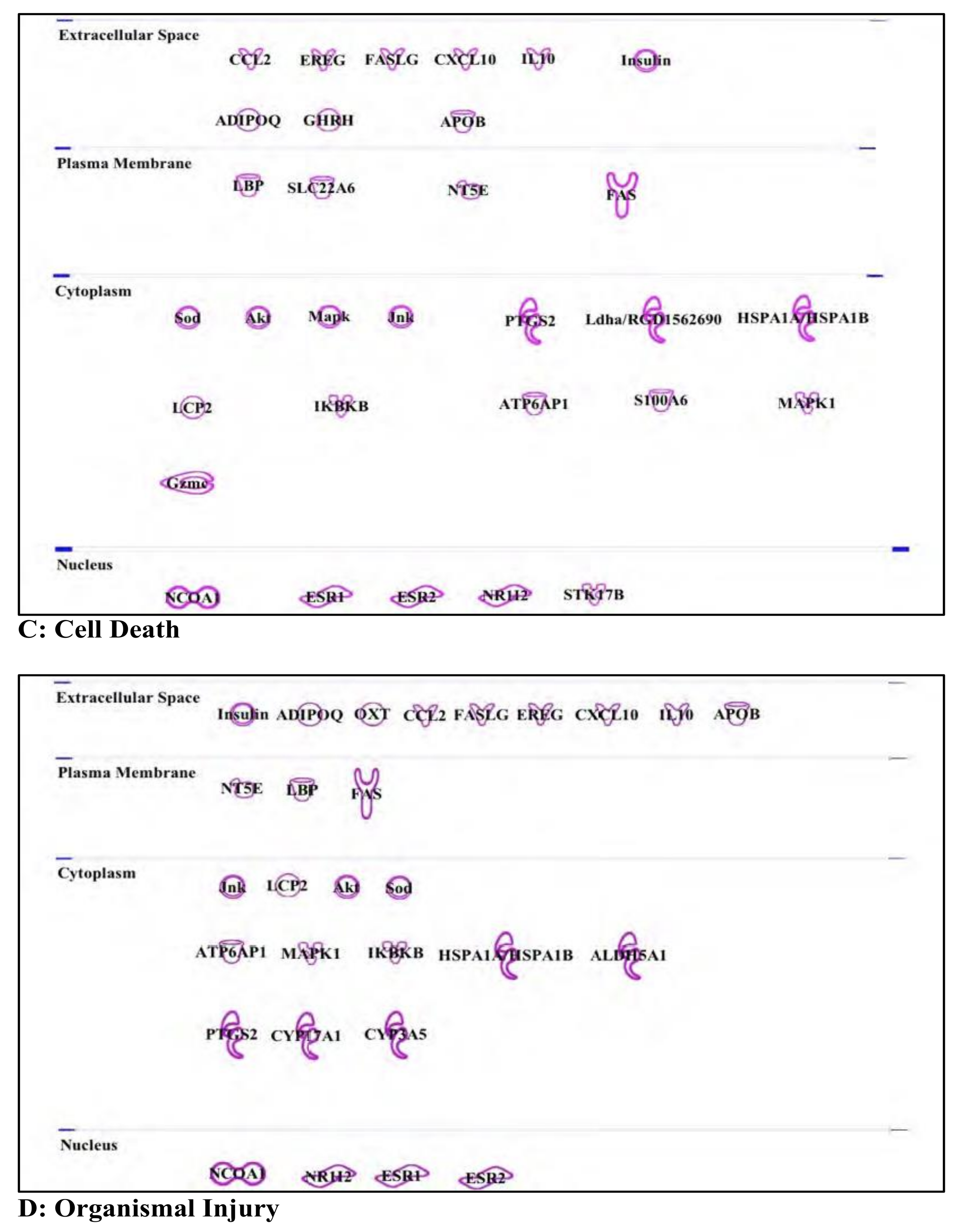
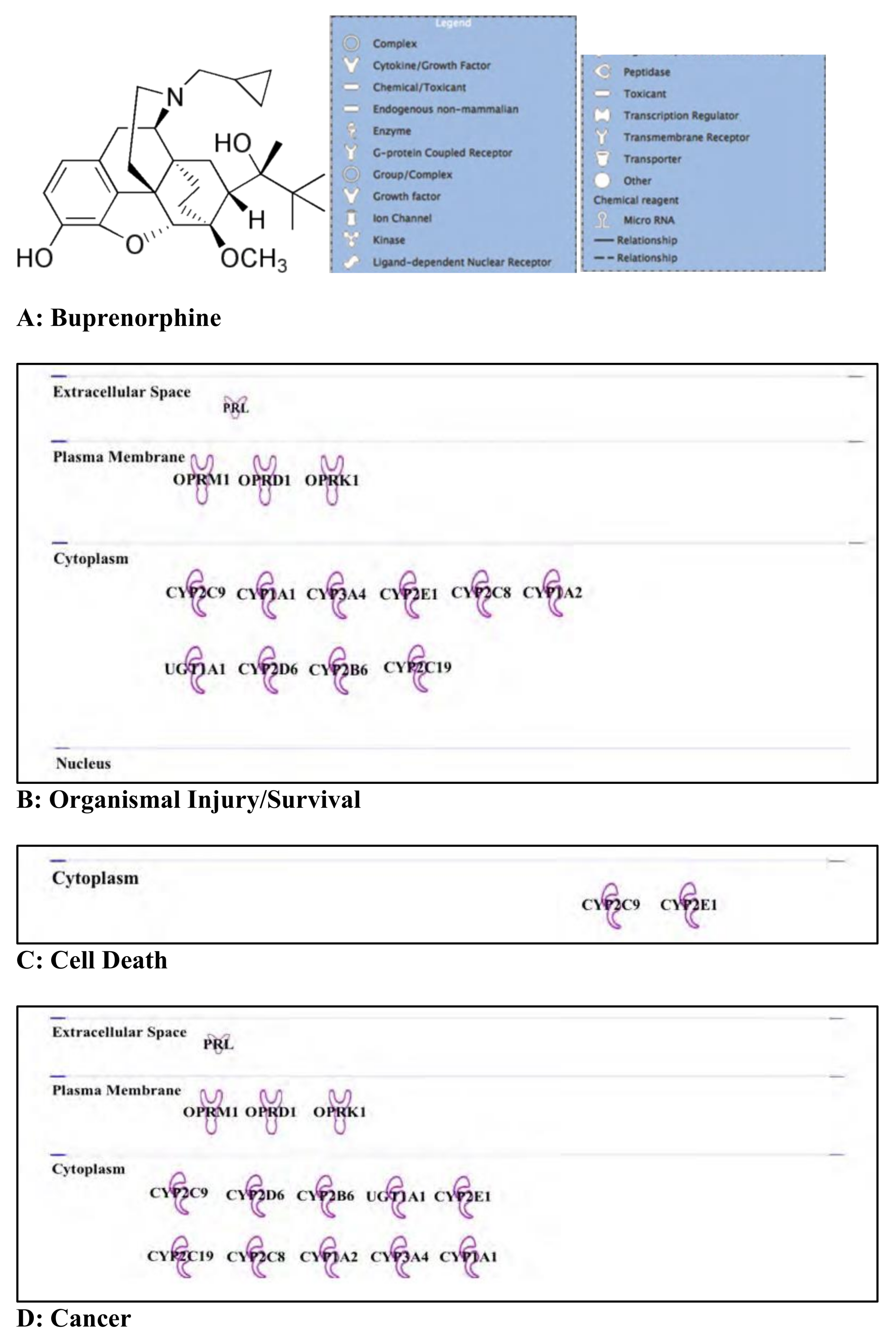
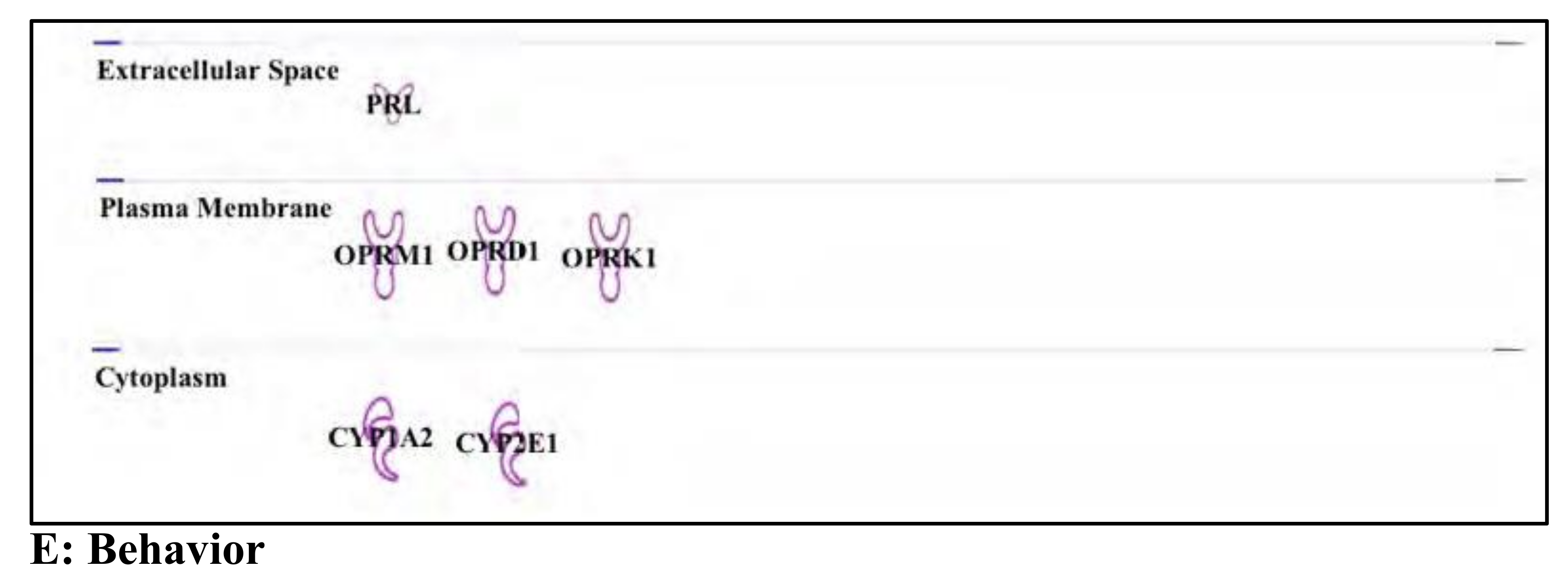
3.6. Buprenorphine: Opioid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cicmanec, J.L.S.D.; Gebbard, P.; Li, Y.; George, J.E.; Music, C.; Luna, G.; Tieman, G.; Hope, J.B. Measurement of Endocrine Disrupting Chemicals in West Virginia’s Waterways: Seasonal Comparisons for Agricultural, Industrial and Residential Areas. 2002. Available online: http://info.ngwa.org/GWOL/pdf/030777111.pdf (accessed on 11 December 2019).
- Ankley, G.E.; Francis, E.; Gray, R.; Kavlock, S.; McMaster, D.; Reese, G.; Sayles, A.; Sergeant, A.; Vallero, D. Research Plan for Endocrine Disruptors; U.S. Epa: Washington, DC, USA, 1998. [Google Scholar]
- Lin, A.Y.; Wang, X.H.; Lin, C.F. Impact of wastewaters and hospital effluents on the occurrence of controlled substances in surface waters. Chemosphere 2010, 81, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, D.; Flouriot, G.; Pakdel, F.; Saligaut, C. Effects of estrogens and endocrine-disrupting chemicals on cell differentiation-survival-proliferation in brain: contributions of neuronal cell lines. J. Toxicol. Environ. Health. Part B 2011, 14, 300–327. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Amaro, A.A.; Esposito, A.I.; Mirisola, V.; Mehilli, A.; Rosano, C.; Noonan, D.M.; Albini, A.; Pfeffer, U.; Angelini, G. Endocrine disruptor agent nonyl phenol exerts an estrogen-like transcriptional activity on estrogen receptor positive breast cancer cells. Curr. Med. Chem. 2014, 21, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Metz, L.; Yong, V.W. Review: Endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 2013, 53, 421–430. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Nagel, S.C.; Coe, B.L.; Angle, B.M.; Taylor, J.A. The estrogenic endocrine disrupting chemical bisphenol A (BPA) and obesity. Mol. Cell. Endocrinol. 2012, 354, 74–84. [Google Scholar] [CrossRef]
- Ford, A.T. Intersexuality in Crustacea: an environmental issue? Aquat. Toxicol. 2012, 108, 125–129. [Google Scholar] [CrossRef]
- Waye, A.; Trudeau, V.L. Neuroendocrine disruption: more than hormones are upset. J. Toxicol. Environ. Health. Part B 2011, 14, 270–291. [Google Scholar] [CrossRef]
- Leon-Olea, M.; Martyniuk, C.J.; Orlando, E.F.; Ottinger, M.A.; Rosenfeld, C.; Wolstenholme, J.; Trudeau, V.L. Current concepts in neuroendocrine disruption. Gen. Comp. Endocrinol. 2014, 203, 158–173. [Google Scholar] [CrossRef]
- Garcia-Closas, M.; Thompson, W.D.; Robins, J.M. Differential misclassification and the assessment of gene-environment interactions in case-control studies. Am. J. Epidemiol. 1998, 147, 426–433. [Google Scholar] [CrossRef]
- Balik-Meisner, M.; Truong, L.; Scholl, E.H.; La Du, J.K.; Tanguay, R.L.; Reif, D.M. Elucidating Gene-by-Environment Interactions Associated with Differential Susceptibility to Chemical Exposure. Environ. Health Perspect. 2018, 126, 067010. [Google Scholar] [CrossRef] [PubMed]
- Kundakovic, M.; Champagne, F.A. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav. Immun. 2011, 25, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.W.; Gilliom, R.J.; Ryberg, K.R. Pesticides in U.S. streams and rivers: Occurrence and trends during 1992–2011. Environ. Sci. Technol. 2014, 48, 11025–11030. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, K.R.; Vecchia, A.V.; Gilliom, R.J.; Martin, J.D. Pesticide Trends in Major Rivers of the United States, 1992–2010; US Geological Survey Scientific Investigations Report; USGS: Reston, VA, USA, 2014. [Google Scholar]
- Fawell, J.K.; Ohanian, E.; Giddings, M.; Toft, P.; Magara, Y.; Jackson, P. Endosulfan in Drinking Water; WHO/SDE/WSH/03.04/92; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Shivaramaiah, H.M.; Sanchez-Bayo, F.; Al-Rifai, J.; Kennedy, I.R. The fate of endosulfan in water. J. Environ. Sci. Health B 2005, 40, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Santhi, V.A.; Sakai, N.; Ahmad, E.D.; Mustafa, A.M. Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Sci. Total Environ. 2012, 427–428, 332–338. [Google Scholar] [CrossRef]
- Arnold, S.M.; Clark, K.E.; Staples, C.A.; Klecka, G.M.; Dimond, S.S.; Caspers, N.; Hentges, S.G. Relevance of drinking water as a source of human exposure to bisphenol A. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 137–144. [Google Scholar] [CrossRef]
- Rice, C.P.; Schmitz-Afonso, I.; Loyo-Rosales, J.E.; Link, E.; Thoma, R.; Fay, L.; Altfater, D.; Camp, M.J. Alkylphenol and alkylphenol-ethoxylates in carp, water, and sediment from the Cuyahoga River, Ohio. Environ. Sci. Technol. 2003, 37, 3747–3754. [Google Scholar] [CrossRef]
- Mao, Z.; Zheng, X.F.; Zhang, Y.Q.; Tao, X.X.; Li, Y.; Wang, W. Occurrence and biodegradation of nonylphenol in the environment. Int. J. Mol. Sci. 2012, 13, 491–505. [Google Scholar] [CrossRef]
- Terzic, S.; Senta, I.; Ahel, M. Illicit drugs in wastewater of the city of Zagreb (Croatia)—Estimation of drug abuse in a transition country. Environ. Pollut. 2010, 158, 2686–2693. [Google Scholar] [CrossRef]
- Tenore, P.L. Psychotherapeutic benefits of opioid agonist therapy. J. Addict. Dis. 2008, 27, 49–65. [Google Scholar] [CrossRef]
- Nefau, T.; Karolak, S.; Castillo, L.; Boireau, V.; Levi, Y. Presence of illicit drugs and metabolites in influents and effluents of 25 sewage water treatment plants and map of drug consumption in France. Sci. Total. Environ. 2013, 461–462, 712–722. [Google Scholar] [CrossRef]
- Konijnenberg, C.; Melinder, A. Prenatal exposure to methadone and buprenorphine: A review of the potential effects on cognitive development. Child Neuropsychol. 2011, 17, 495–519. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Decision of 10 March 2004 concerning the non-inclusion of atrazine in Annex I to Council Directive 91/414/EEC and the withdrawal of authorisations for plant protection products containing this active substance. In C(2004) 731. 2004/248/EC; European Commission: Brussels, Belgium, 2004. [Google Scholar]
- Boffetta, P.; Adami, H.O.; Berry, S.C.; Mandel, J.S. Atrazine and cancer: A review of the epidemiologic evidence. Eur. J. Cancer Prev. 2013, 22, 169–180. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef]
- Reichert, K.; Menzel, R. Expression profiling of five different xenobiotics using a Caenorhabditis elegans whole genome microarray. Chemosphere 2005, 61, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Andriani, G.A.; Almeida, V.P.; Faggioli, F.; Mauro, M.; Tsai, W.L.; Santambrogio, L.; Maslov, A.; Gadina, M.; Campisi, J.; Vijg, J.; et al. Whole Chromosome Instability induces senescence and promotes SASP. Sci. Rep. 2016, 6, 35218. [Google Scholar] [CrossRef]
- Liu, X.M.; Shao, J.Z.; Xiang, L.X.; Chen, X.Y. Cytotoxic effects and apoptosis induction of atrazine in a grass carp (Ctenopharyngodon idellus) cell line. Environ. Toxicol. 2006, 21, 80–89. [Google Scholar] [CrossRef]
- Jia, X.; Wang, D.; Gao, N.; Cao, H.; Zhang, H. Atrazine Triggers the Extrinsic Apoptosis Pathway in Lymphocytes of the Frog Pelophylax nigromaculata in Vivo. Chem. Res. Toxicol. 2015, 28, 2010–2018. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Gao, S.; Ren, R.; Zheng, J.; Zhang, Y. Atrazine-induced apoptosis of splenocytes in BALB/C mice. BMC Med. 2011, 9, 117. [Google Scholar] [CrossRef]
- Lee, E.J.; Jang, Y.; Kang, K.; Song, D.H.; Kim, R.; Chang, H.W.; Lee, D.E.; Song, C.K.; Choi, B.; Kang, M.J.; et al. Atrazine induces endoplasmic reticulum stress-mediated apoptosis of T lymphocytes via the caspase-8-dependent pathway. Environ. Toxicol. 2016, 31, 998–1008. [Google Scholar] [CrossRef]
- Smith, L.K.; Shah, R.R.; Cidlowski, J.A. Glucocorticoids modulate microRNA expression and processing during lymphocyte apoptosis. J. Biol. Chem. 2010, 285, 36698–36708. [Google Scholar] [CrossRef] [PubMed]
- Kay, P.; Schlossmacher, G.; Matthews, L.; Sommer, P.; Singh, D.; White, A.; Ray, D. Loss of glucocorticoid receptor expression by DNA methylation prevents glucocorticoid induced apoptosis in human small cell lung cancer cells. PLoS ONE 2011, 6, e24839. [Google Scholar] [CrossRef] [PubMed]
- Foradori, C.D.; Hinds, L.R.; Quihuis, A.M.; Lacagnina, A.F.; Breckenridge, C.B.; Handa, R.J. The differential effect of atrazine on luteinizing hormone release in adrenalectomized adult female Wistar rats. Biol. Reprod. 2011, 85, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cederbaum, A.I. Cytotoxicity and apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol. Pharm. 1998, 53, 638–648. [Google Scholar] [CrossRef]
- Alavanja, M.C.; Ross, M.K.; Bonner, M.R. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J. Clin. 2013, 63, 120–142. [Google Scholar] [CrossRef]
- Fan, W.; Yanase, T.; Morinaga, H.; Gondo, S.; Okabe, T.; Nomura, M.; Komatsu, T.; Morohashi, K.; Hayes, T.B.; Takayanagi, R.; et al. Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ. Health Perspect. 2007, 115, 720–727. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Swenberg, J.A.; Weiss, N.; Brusick, D.; Eldridge, J.C.; Stevens, J.T.; Handa, R.J.; Hovey, R.C.; Plant, T.M.; Pastoor, T.P.; et al. Atrazine and breast cancer: A framework assessment of the toxicological and epidemiological evidence. Toxicol. Sci. 2011, 123, 441–459. [Google Scholar] [CrossRef]
- Huang, P.; Yang, J.; Ning, J.; Wang, M.; Song, Q. Atrazine Triggers DNA Damage Response and Induces DNA Double-Strand Breaks in MCF-10A Cells. Int. J. Mol. Sci. 2015, 16, 14353–14368. [Google Scholar] [CrossRef]
- Midic, U.; Vincent, K.A.; VandeVoort, C.A.; Latham, K.E. Effects of long-term endocrine disrupting compound exposure on Macaca mulatta embryonic stem cells. Reprod. Toxicol. 2016, 65, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Xu, Q.; Martin, T.D.; Li, M.Z.; Demaria, M.; Aron, L.; Lu, T.; Yankner, B.A.; Campisi, J.; Elledge, S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015, 349, aaa5612. [Google Scholar] [CrossRef] [PubMed]
- Giusi, G.; Facciolo, R.M.; Canonaco, M.; Alleva, E.; Belloni, V.; Dessi’-Fulgheri, F.; Santucci, D. The endocrine disruptor atrazine accounts for a dimorphic somatostatinergic neuronal expression pattern in mice. Toxicol. Sci. 2006, 89, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Belloni, V.; Dessi-Fulgheri, F.; Zaccaroni, M.; Di Consiglio, E.; De Angelis, G.; Testai, E.; Santochirico, M.; Alleva, E.; Santucci, D. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology 2011, 279, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Jocque, H.; Sirot, L.K.; Fiumera, A.C. Effects of atrazine exposure on male reproductive performance in Drosophila melanogaster. J. Insect. Physiol. 2015, 72, 14–21. [Google Scholar] [CrossRef]
- Schmidel, A.J.; Assmann, K.L.; Werlang, C.C.; Bertoncello, K.T.; Francescon, F.; Rambo, C.L.; Beltrame, G.M.; Calegari, D.; Batista, C.B.; Blaser, R.E.; et al. Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol. Teratol. 2014, 44, 62–69. [Google Scholar] [CrossRef]
- Jowa, L.; Howd, R. Should atrazine and related chlorotriazines be considered carcinogenic for human health risk assessment? J. Environ. Sci. Health Part C 2011, 29, 91–144. [Google Scholar] [CrossRef]
- Mitra, A.K.; Faruque, F.S. Breast cancer incidence and exposure to environmental chemicals in 82 counties in Mississippi. South Med. J. 2004, 97, 259–263. [Google Scholar] [CrossRef]
- Mitra, A.K.; Faruque, F.S.; Avis, A.L. Breast cancer and environmental risks: Where is the link? J. Environ. Health 2004, 66, 24. [Google Scholar]
- Van Der Kraak, G.J.; Hosmer, A.J.; Hanson, M.L.; Kloas, W.; Solomon, K.R. Effects of atrazine in fish, amphibians, and reptiles: an analysis based on quantitative weight of evidence. Crit. Rev. Toxicol. 2014, 44 Suppl. S5, 1–66. [Google Scholar] [CrossRef]
- Goodman, M.; Mandel, J.S.; DeSesso, J.M.; Scialli, A.R. Atrazine and pregnancy outcomes: A systematic review of epidemiologic evidence. Birth Defects Res. Part B 2014, 101, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Kuckelkorn, J.; Redelstein, R.; Heide, T.; Kunze, J.; Maletz, S.; Waldmann, P.; Grummt, T.; Seiler, T.B.; Hollert, H. A hierarchical testing strategy for micropollutants in drinking water regarding their potential endocrine-disrupting effects-towards health-related indicator values. Environ. Sci. Pollut. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, L.M.; Lee, S.R.; Filipov, N.M. In vitro atrazine exposure affects the phenotypic and functional maturation of dendritic cells. Toxicol. Appl. Pharmacol. 2007, 223, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, J.; Corsini, E.; Williams, M.A.; Decker, W.; Manjili, M.H.; Otsuki, T.; Singh, N.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; et al. Chemical compounds from anthropogenic environment and immune evasion mechanisms: potential interactions. Carcinogenesis 2015, 36 Suppl. S1, S111–S127. [Google Scholar] [CrossRef] [PubMed]
- Toughan, H.; Khalil, S.R.; El-Ghoneimy, A.A.; Awad, A.; Seddek, A.S. Effect of dietary supplementation with Spirulina platensis on Atrazine-induced oxidative stress- mediated hepatic damage and inflammation in the common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2017, 149, 135–142. [Google Scholar] [CrossRef]
- Yuan, B.; Liang, S.; Jin, Y.X.; Zhang, M.J.; Zhang, J.B.; Kim, N.H. Toxic effects of atrazine on porcine oocytes and possible mechanisms of action. PLoS ONE 2017, 12, e0179861. [Google Scholar] [CrossRef]
- Ruiz-Guzman, J.A.; Gomez-Corrales, P.; Cruz-Esquivel, A.; Marrugo-Negrete, J.L. Cytogenetic damage in peripheral blood lymphocytes of children exposed to pesticides in agricultural areas of the department of Cordoba, Colombia. Mutat. Res. 2017, 824, 25–31. [Google Scholar] [CrossRef]
- Eyer, P. The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol. Rev. 2003, 22, 165–190. [Google Scholar] [CrossRef]
- Casida, J.E. Esterase inhibitors as pesticides. Science 1964, 146, 1011–1017. [Google Scholar] [CrossRef]
- Eddleston, M.; Phillips, M.R. Self poisoning with pesticides. Bmj 2004, 328, 42–44. [Google Scholar] [CrossRef]
- Namba, T. Cholinesterase inhibition by organophosphorus compounds and its clinical effects. Bull. World Health Organ. 1971, 44, 289–307. [Google Scholar] [PubMed]
- Jamal, G.A.; Hansen, S.; Julu, P.O. Low level exposures to organophosphorus esters may cause neurotoxicity. Toxicology 2002, 181–182, 23–33. [Google Scholar] [CrossRef]
- Lotti, M.; Moretto, A. Organophosphate-induced delayed polyneuropathy. Toxicol. Rev. 2005, 24, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed]
- Poet, T.S.; Timchalk, C.; Hotchkiss, J.A.; Bartels, M.J. Chlorpyrifos PBPK/PD model for multiple routes of exposure. Xenobiotica 2014, 44, 868–881. [Google Scholar] [CrossRef]
- Foxenberg, R.J.; Ellison, C.A.; Knaak, J.B.; Ma, C.; Olson, J.R. Cytochrome P450-specific human PBPK/PD models for the organophosphorus pesticides: Chlorpyrifos and parathion. Toxicology 2011, 285, 57–66. [Google Scholar] [CrossRef]
- Gao, J.; Naughton, S.X.; Beck, W.D.; Hernandez, C.M.; Wu, G.; Wei, Z.; Yang, X.; Bartlett, M.G.; Terry, A.V., Jr. Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. Neurotoxicology 2017, 62, 111–123. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Skavicus, S.; Card, J.; Levin, E.D.; Seidler, F.J. Diverse neurotoxicants target the differentiation of embryonic neural stem cells into neuronal and glial phenotypes. Toxicology 2016, 372, 42–51. [Google Scholar] [CrossRef]
- Sultana Shaik, A.; Shaik, A.P.; Jamil, K.; Alsaeed, A.H. Evaluation of cytotoxicity and genotoxicity of pesticide mixtures on lymphocytes. Toxicol. Mech. Methods 2016, 26, 588–594. [Google Scholar] [CrossRef]
- Ernst, W.R.; Jonah, P.; Doe, K.; Julien, G.; Hennigar, P. Toxicity to aquatic organisms of off-target deposition of endosulfan applied by aircraft. Environ. Toxicol. Chem. 1991, 10, 103–114. [Google Scholar] [CrossRef]
- Naqvi, S.M.; Vaishnavi, C. Bioaccumulative potential and toxicity of endosulfan insecticide to non-target animals. Comp. Biochem. Physiol. Part C Comp. Pharmacol. Toxicol. 1993, 105, 347–361. [Google Scholar] [CrossRef]
- Moses, V.; Peter, J.V. Acute intentional toxicity: endosulfan and other organochlorines. Clin. Toxicol. 2010, 48, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.L.B.; Singh, T.P. Effect of endosulfan (thiodan) on vitellogenesis and its modulation by different hormones in the vitellogenic catfish Claria batrachus. Toxicology 1992, 75, 191–198. [Google Scholar] [CrossRef]
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef]
- Rousseau, J.; Cossette, L.; Grenier, S.; Martinoli, M.G. Modulation of prolactin expression by xenoestrogens. Gen. Comp. Endocrinol. 2002, 126, 175–182. [Google Scholar] [CrossRef]
- Caride, A.; Lafuente, A.; Cabaleiro, T. Endosulfan effects on pituitary hormone and both nitrosative and oxidative stress in pubertal male rats. Toxicol. Lett. 2010, 197, 106–112. [Google Scholar] [CrossRef]
- Bradlow, H.L.; Davis, D.L.; Lin, G.; Sepkovic, D.; Tiwari, R. Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: a biologic marker of breast cancer risk. Environ. Health Perspect. 1995, 103 Suppl. S7, 147–150. [Google Scholar] [CrossRef]
- Suzuki, T.; Ide, K.; Ishida, M. Response of MCF-7 human breast cancer cells to some binary mixtures of oestrogenic compounds in-vitro. J. Pharm. Pharmacol. 2001, 53, 1549–1554. [Google Scholar] [CrossRef]
- Hu, L.; Xia, L.; Zhou, H.; Wu, B.; Mu, Y.; Wu, Y.; Yan, J. TF/FVIIa/PAR2 promotes cell proliferation and migration via PKCalpha and ERK-dependent c-Jun/AP-1 pathway in colon cancer cell line SW620. Tumour Biol. 2013, 34, 2573–25781. [Google Scholar] [CrossRef]
- Kannan, K.; Holcombe, R.F.; Jain, S.K.; Alvarez-Hernandez, X.; Chervenak, R.; Wolf, R.E.; Glass, J. Evidence for the induction of apoptosis by endosulfan in a human T-cell leukemic line. Mol. Cell. Biochem. 2000, 205, 53–66. [Google Scholar] [CrossRef]
- Ahmed, T.; Tripathi, A.K.; Ahmed, R.S.; Das, S.; Suke, S.G.; Pathak, R.; Chakraboti, A.; Banerjee, B.D. Endosulfan-induced apoptosis and glutathione depletion in human peripheral blood mononuclear cells: Attenuation by N-acetylcysteine. J. Biochem. Mol. Toxicol. 2008, 22, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Benachour, N.; Séralini, G.E. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 2008, 22, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, G.A.; Delconte, M.B.; Gomez, A.L.; Alarcon, R.; Bosquiazzo, V.L.; Luque, E.H.; Munoz-de-Toro, M.; Kass, L. Early postnatal exposure to endosulfan interferes with the normal development of the male rat mammary gland. Toxicol. Lett. 2017, 281, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gimenez, B.; Llansola, M.; Cabrera-Pastor, A.; Hernandez-Rabaza, V.; Agusti, A.; Felipo, V. Endosulfan and Cypermethrin Pesticide Mixture Induces Synergistic or Antagonistic Effects on Developmental Exposed Rats Depending on the Analyzed Behavioral or Neurochemical End Points. ACS Chem. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, R.; Raghavan, S.C. Molecular mechanism of Endosulfan action in mammals. J. Biosci. 2017, 42, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- OSPAR Convention. Recommendation on Nonylphenol-Ethoxylates; OSPAR Convention: London, UK, 2000. [Google Scholar]
- Directive, EU Cement. Amending for the 26th time the Council directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (nonylphenol, nonylphenol ethoxylate and cement). In Directive 2003/53/EC; Off. J. Eur. Commun. L; European Commission: Luxembourg, 2003. [Google Scholar]
- Li, M.H. Effects of nonylphenol on cholinesterase and carboxylesterase activities in male guppies (Poecilia reticulata). Ecotoxicol. Environ. Saf. 2008, 71, 781–786. [Google Scholar] [CrossRef]
- Gejlsbjerg, B.; Klinge, C.; Samsoe-Petersen, L.; Madsen, T. Toxicity of linear alkylbenzene sulfonates and nonylphenol in sludge-amended soil. Environ. Toxicol. Chem. 2001, 20, 2709–2716. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, J. Genotoxic effects of nonylphenol and bisphenol A exposure in aquatic biomonitoring species: Freshwater crustacean, Daphnia magna, and aquatic midge, Chironomus riparius. Bull. Environ. Contam. Toxicol. 2009, 83, 463–468. [Google Scholar] [CrossRef]
- Shen, W.Y.; Zhou, Z.L.; Li, X.J. Effects of long term exposure to bisphenol A and nonylphenol on the reproduction of zebrafish (Danio rerio). J. Fish. China 2007, 31, 59–64. [Google Scholar]
- Vazquez-Duhalt, R.; Marquez-Rocha, F.; Ponce, E.; Licea, A.F.; Viana, M.T. Nonylphenol, an integrated vision of a pollutant. Appl. Ecol. Environ. Res. 2005, 4, 1–25. [Google Scholar] [CrossRef]
- Aoki, M.; Kurasaki, M.; Saito, T.; Seki, S.; Hosokawa, T.; Takahashi, Y.; Fujita, H.; Iwakuma, T. Nonylphenol enhances apoptosis induced by serum deprivation in PC12 cells. Life Sci. 2004, 74, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.T.; Wais, J.; Crawford, D.A. Prenatal exposure to common environmental factors affects brain lipids and increases risk of developing autism spectrum disorders. Eur. J. Neurosci. 2015, 42, 2742–2760. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Justicia, H.; Wray, J.W.; Sonnenschein, C. p-Nonyl-phenol: an estrogenic xenobiotic released from "modified" polystyrene. Environ. Health Perspect. 1991, 92, 167–173. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Jobling, S.; Hoare, S.A.; Sumpter, J.P.; Parker, M.G. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology 1994, 135, 175–182. [Google Scholar] [CrossRef]
- Mendes, J.A. The endocrine disrupters: a major medical challenge. Food Chem. Toxicol. 2002, 40, 781–788. [Google Scholar] [CrossRef]
- Ferguson, S.A.; Flynn, K.M.; Delclos, K.B.; Newbold, R.R. Maternal and offspring toxicity but few sexually dimorphic behavioral alterations result from nonylphenol exposure. Neurotoxicol. Teratol. 2000, 22, 583–591. [Google Scholar] [CrossRef]
- Ponzo, O.J.; Silvia, C. Evidence of reproductive disruption associated with neuroendocrine changes induced by UV-B filters, phthalates and nonylphenol during sexual maturation in rats of both gender. Toxicology 2013, 311, 41–51. [Google Scholar] [CrossRef]
- Negishi, T.; Kawasaki, K.; Suzaki, S.; Maeda, H.; Ishii, Y.; Kyuwa, S.; Kuroda, Y.; Yoshikawa, Y. Behavioral alterations in response to fear-provoking stimuli and tranylcypromine induced by perinatal exposure to bisphenol A and nonylphenol in male rats. Environ. Health Perspect. 2004, 112, 1159–1164. [Google Scholar] [CrossRef]
- Ferguson, S.A.; Flynn, K.M.; Delclos, K.B.; Newbold, R.R.; Gough, B.J. Effects of lifelong dietary exposure to genistein or nonylphenol on amphetamine-stimulated striatal dopamine release in male and female rats. Neurotoxicol. Teratol. 2002, 24, 37–45. [Google Scholar] [CrossRef]
- Nagao, T.; Wada, K.; Marumo, H.; Yoshimura, S.; Ono, H. Reproductive effects of nonylphenol in rats after gavage administration: a two-generation study. Reprod. Toxicol. 2001, 15, 293–315. [Google Scholar] [CrossRef]
- Kim, H.; Oh, S.; Gye, M.C.; Shin, I. Comparative toxicological evaluation of nonylphenol and nonylphenol polyethoxylates using human keratinocytes. Drug Chem. Toxicol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Derakhshesh, N.; Movahedinia, A.; Salamat, N.; Hashemitabar, M.; Bayati, V. Using a liver cell culture from Epinephelus coioides as a model to evaluate the nonylphenol-induced oxidative stress. Mar. Pollut. Bull. 2017, 122, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Twaddle, N.C.; Vanlandingham, M.; Fisher, J.W. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2010, 247, 158–165. [Google Scholar] [CrossRef]
- Antherieu, S.; Ledirac, N.; Luzy, A.P.; Lenormand, P.; Caron, J.C.; Rahmani, R. Endosulfan decreases cell growth and apoptosis in human HaCaT keratinocytes: partial ROS-dependent ERK1/2 mechanism. J. Cell. Physiol. 2007, 213, 177–186. [Google Scholar] [CrossRef]
- Masoner, J.R.; Kolpin, D.W.; Furlong, E.T.; Cozzarelli, I.M.; Gray, J.L.; Schwab, E.A. Contaminants of emerging concern in fresh leachate from landfills in the conterminous United States. Environ. Sci. Process. Impacts 2014, 16, 2335–2354. [Google Scholar] [CrossRef]
- Dsikowitzky, L.; Botalova, O.; Illgut, S.; Bosowski, S.; Schwarzbauer, J. Identification of characteristic organic contaminants in wastewaters from modern paper production sites and subsequent tracing in a river. J. Hazard. Mater. 2015, 300, 254–262. [Google Scholar] [CrossRef]
- Lu, J.; Wu, J.; Stoffella, P.J.; Wilson, P.C. Uptake and distribution of bisphenol A and nonylphenol in vegetable crops irrigated with reclaimed water. J. Hazard. Mater. 2015, 283, 865–870. [Google Scholar] [CrossRef]
- Adewale, H.B.; Jefferson, W.N.; Newbold, R.R.; Patisaul, H.B. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol. Reprod. 2009, 81, 690–699. [Google Scholar] [CrossRef]
- Aldad, T.S.; Rahmani, N.; Leranth, C.; Taylor, H.S. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil. Steril. 2011, 96, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Newbold, R.R.; Bucher, J.R.; Camacho, L.; Delclos, K.B.; Lewis, S.M.; Vanlandingham, M.; Churchwell, M.I.; Twaddle, N.C.; McLellen, M.; et al. NIEHS/FDA CLARITY-BPA research program update. Reprod. Toxicol. 2015, 58, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, S.; Di Renzo, G.; Cuocolo, R.; Amantea, B.; Leo, A.; Taglialatela, M.; Annunziato, L. Evidence for a differential interaction of buprenorphine with opiate receptor subtypes controlling prolactin secretion. Eur. J. Pharmacol. 1988, 145, 257–260. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Afshari, G.; Mard, S.A.; Khodadadi, A.; Hashemitabar, M. Preventive effects of procyanidin A2 on glucose homeostasis, pancreatic and duodenal homebox 1, and glucose transporter 2 gene expression disturbance induced by bisphenol A in male mice. J. Physiol. Pharmacol. 2016, 67, 243–252. [Google Scholar]
- Baker, D.R.; Barron, L.; Kasprzyk-Hordern, B. Illicit and pharmaceutical drug consumption estimated via wastewater analysis. Part A: Chemical analysis and drug use estimates. Sci. Total Environ. 2014, 487, 629–641. [Google Scholar] [CrossRef]
- Xie, M.; Bu, P.; Li, F.; Lan, S.; Wu, H.; Yuan, L.; Wang, Y. Neonatal bisphenol A exposure induces meiotic arrest and apoptosis of spermatogenic cells. Oncotarget 2016, 7, 10606–10615. [Google Scholar] [CrossRef]
- Arslan-Alaton, I.; Olmez-Hanci, T.; Dogan, M.; Ozturk, T. Zero-valent aluminum-mediated degradation of Bisphenol A in the presence of common oxidants. Water Sci. Technol. 2017, 76, 2455–2464. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I.; Dogan, M.; Khoei, S.; Fakhri, H.; Korkmaz, G. Enhanced degradation of micropollutants by zero-valent aluminum activated persulfate: assessment of toxicity and genotoxic activity. Water Sci. Technol. 2017, 76, 3195–3204. [Google Scholar] [CrossRef]
- Ding, Z.M.; Jiao, X.F.; Wu, D.; Zhang, J.Y.; Chen, F.; Wang, Y.S.; Huang, C.J.; Zhang, S.X.; Li, X.; Huo, L.J. Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem. Biol. Interact 2017, 278, 222–229. [Google Scholar] [CrossRef]
- Jones, H.E.; Johnson, R.E.; Jasinski, D.R.; O’Grady, K.E.; Chisholm, C.A.; Choo, R.E.; Crocetti, M.; Dudas, R.; Harrow, C.; Huestis, M.A.; et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Allouche, S.; Le Marec, T.; Coquerel, A.; Noble, F.; Marie, N. Striatal dopamine D1 and D2 receptors are differentially regulated following buprenorphine or methadone treatment. Psychopharmacology 2015, 232, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Namasivayam, V. Endocrine disrupting chemicals in the atmosphere: Their effects on humans and wildlife. Environ. Int. 2015, 76, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Pedraz-Cuesta, E.; Fredsted, J.; Jensen, H.H.; Bornebusch, A.; Nejsum, L.N.; Kragelund, B.B.; Pedersen, S.F. Prolactin Signaling Stimulates Invasion via Na(+)/H(+) Exchanger NHE1 in T47D Human Breast Cancer Cells. Mol. Endocrinol. 2016, 30, 693–708. [Google Scholar] [CrossRef][Green Version]
- Fitzgerald, P.; Dinan, T.G. Prolactin and dopamine: what is the connection? A review article. J. Psychopharmacol. 2008, 22, 12–19. [Google Scholar] [CrossRef]
- Ausio, J. MeCP2 and the enigmatic organization of brain chromatin. Implications for depression and cocaine addiction. Clin. Epigenet. 2016, 8, 58. [Google Scholar] [CrossRef]
- Kugawa, F.; Arae, K.; Ueno, A.; Aoki, M. Buprenorphine hydrochloride induces apoptosis in NG108-15 nerve cells. Eur. J. Pharmacol. 1998, 347, 105–112. [Google Scholar] [CrossRef]
- Ejaredar, M.; Lee, Y.; Roberts, D.J.; Sauve, R.; Dewey, D. Bisphenol A exposure and children’s behavior: A systematic review. J. Expo. Sci. Environ. Epidemiol. 2016. [Google Scholar] [CrossRef]
- Corella, D.; Ordovas, J.M. Basic Concepts in Molecular Biology Related to Genetics and Epigenetics. Rev. Esp. Cardiol. 2017, 70, 744–753. [Google Scholar] [CrossRef]
- Gely-Pernot, A.; Hao, C.; Becker, E.; Stuparevic, I.; Kervarrec, C.; Chalmel, F.; Primig, M.; Jegou, B.; Smagulova, F. The epigenetic processes of meiosis in male mice are broadly affected by the widely used herbicide atrazine. BMC Genom. 2015, 16, 885. [Google Scholar] [CrossRef]
- Wirbisky-Hershberger, S.E.; Sanchez, O.F.; Horzmann, K.A.; Thanki, D.; Yuan, C.; Freeman, J.L. Atrazine exposure decreases the activity of DNMTs, global DNA methylation levels, and dnmt expression. Food Chem. Toxicol. 2017, 109, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Gely-Pernot, A.; Kervarrec, C.; Boudjema, M.; Becker, E.; Khil, P.; Tevosian, S.; Jegou, B.; Smagulova, F. Exposure to the widely used herbicide atrazine results in deregulation of global tissue-specific RNA transcription in the third generation and is associated with a global decrease of histone trimethylation in mice. Nucl. Acids Res. 2016, 44, 9784–9802. [Google Scholar] [CrossRef] [PubMed]
- McBirney, M.; King, S.E.; Pappalardo, M.; Houser, E.; Unkefer, M.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Winchester, P.; Skinner, M.K. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS ONE 2017, 12, e0184306. [Google Scholar] [CrossRef] [PubMed]
- Milesi, M.M.; Varayoud, J.; Ramos, J.G.; Luque, E.H. Uterine ERalpha epigenetic modifications are induced by the endocrine disruptor endosulfan in female rats with impaired fertility. Mol. Cell. Endocrinol. 2017, 454, 1–11. [Google Scholar] [CrossRef]
- Ghosh, K.; Chatterjee, B.; Jayaprasad, A.G.; Kanade, S.R. The persistent organochlorine pesticide endosulfan modulates multiple epigenetic regulators with oncogenic potential in MCF-7 cells. Sci. Total Environ. 2017. [Google Scholar] [CrossRef]
- Hung, C.H.; Yang, S.N.; Kuo, P.L.; Chu, Y.T.; Chang, H.W.; Wei, W.J.; Huang, S.K.; Jong, Y.J. Modulation of cytokine expression in human myeloid dendritic cells by environmental endocrine-disrupting chemicals involves epigenetic regulation. Environ. Health Perspect. 2010, 118, 67–72. [Google Scholar] [CrossRef][Green Version]
- Hung, C.H.; Yang, S.N.; Wang, Y.F.; Liao, W.T.; Kuo, P.L.; Tsai, E.M.; Lee, C.L.; Chao, Y.S.; Yu, H.S.; Huang, S.K.; et al. Environmental alkylphenols modulate cytokine expression in plasmacytoid dendritic cells. PLoS ONE 2013, 8, e73534. [Google Scholar] [CrossRef][Green Version]
- Ajj, H.; Chesnel, A.; Pinel, S.; Plenat, F.; Flament, S.; Dumond, H. An alkylphenol mix promotes seminoma derived cell proliferation through an ERalpha36-mediated mechanism. PLoS ONE 2013, 8, e61758. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, X.; Li, D.K.; Yang, F.; Pan, H.; Li, T.; Miao, M.; Li, R.; Yuan, W. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PLoS ONE 2017, 12, e0178535. [Google Scholar] [CrossRef]
- Bansal, A.; Rashid, C.; Xin, F.; Li, C.; Polyak, E.; Duemler, A.; van der Meer, T.; Stefaniak, M.; Wajid, S.; Doliba, N.; et al. Sex- and Dose-Specific Effects of Maternal Bisphenol A Exposure on Pancreatic Islets of First- and Second-Generation Adult Mice Offspring. Environ. Health Perspect. 2017, 125, 097022. [Google Scholar] [CrossRef]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in reproduction: Epigenetic effects. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- McLemore, G.L.; Lewis, T.; Jones, C.H.; Gauda, E.B. Novel pharmacotherapeutic strategies for treatment of opioid-induced neonatal abstinence syndrome. Semin. Fetal Neonatal Med. 2013, 18, 35–41. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.J.; Leamon, M.H.; Finnegan, L.P.; Fassbender, C. Opioid dependence and pregnancy: Minimizing stress on the fetal brain. Am. J. Obs. Gynecol. 2017, 216, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Ausio, J.; Georgel, P.T. MeCP2 and CTCF: Enhancing the cross-talk of silencers. Biochem. Cell Biol. 2017, 95, 593–608. [Google Scholar] [CrossRef] [PubMed]






| EDC | Concentration (ppb) | µg L−1 | µg.K−1 (in Sediments) | Presence in Tap/Drinking Water |
|---|---|---|---|---|
| Atrazine (ppb1) | 0.19 to 1.88 (Ohio River)1 | Detected in 28 U.S. states2 | ||
| Bis-Phenol A | 0.016–0.5 [26,27,28,29] | Detected in Asia, Europe, North America [26] | ||
| Chlorpyrifos | 0.24 (ground water, MN)3 | 0–2.828 [30] | Detected in drinking water3 [30] | |
| Endosulfan | <1 (WHO), 0.020–0.11 [31] | Detected in drinking water [31] | ||
| Nonyl Phenol | 0.1–0.5 (Ohio Tributary) [32] 0000.1-0.0027 (Tap water, Chongqing China [28]) | 75–340 (Ohio Tributary) [32] | Detected in drinking water [28,32] | |
| Buprenorphine | 0.042–0.195 (sewage water, Paris) France [7] | Detected in waste water [7,33,34] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessler, J.; Dawley, D.; Crow, D.; Garmany, R.; Georgel, P.T. Potential Health Risks Linked to Emerging Contaminants in Major Rivers and Treated Waters. Water 2019, 11, 2615. https://doi.org/10.3390/w11122615
Kessler J, Dawley D, Crow D, Garmany R, Georgel PT. Potential Health Risks Linked to Emerging Contaminants in Major Rivers and Treated Waters. Water. 2019; 11(12):2615. https://doi.org/10.3390/w11122615
Chicago/Turabian StyleKessler, James, Diane Dawley, Daniel Crow, Ramin Garmany, and Philippe T. Georgel. 2019. "Potential Health Risks Linked to Emerging Contaminants in Major Rivers and Treated Waters" Water 11, no. 12: 2615. https://doi.org/10.3390/w11122615
APA StyleKessler, J., Dawley, D., Crow, D., Garmany, R., & Georgel, P. T. (2019). Potential Health Risks Linked to Emerging Contaminants in Major Rivers and Treated Waters. Water, 11(12), 2615. https://doi.org/10.3390/w11122615




