Abstract
Understanding the factors that regulate phytoplankton and zooplankton is an important goal of aquatic ecologists; however, much remains unknown because of complex interactions between phytoplankton, zooplankton, and fish. Zooplankton, in particular cladocerans, can be regulated by bottom–up factors either via food quantity or food quality in terms of polyunsaturated fatty acids (PUFA) or phosphorus (P) contents in phytoplankton. Fish can recycle nutrients and in turn change the PUFA and P contents of algal resources, thus modifying bottom–up regulation. Furthermore, fish can change phytoplankton structure through consumption of cladocerans which selectively graze phytoplankton. We conducted a mesocosm (300 L) experiment to determine how trophic state and fish affected crustacean dynamics. The mesocosms were filled with water containing natural plankton from the eutrophic Lake Jorzec and mesotrophic Lake Majcz (Northeastern Poland), and we manipulated fish presence/absence. We also conducted a complementary life-table experiment to determine how trophic state and fish nonconsumptively affected demographic parameters of the dominant cladocerans in the mesocosms. Small and large cladoceran species responded differently to food quantity and quality. Small-bodied Ceriodaphnia were regulated mainly by resource concentrations (i.e., food quantity), while large species were limited by PUFAs (i.e., food quality). Fish likely increased food quality in terms of PUFA, primarily eicosapentaenoic acids (EPA), thus providing conditions for more successful development of Daphnia than in the fish-free treatments. Phosphorus in the seston was likely limiting for zooplankton. However, food quality in terms of phosphorus was likely less important than PUFA because zooplankton can accumulate nutrients in their body.
1. Introduction
Crustacean zooplankton are regulated by algal resources not only in terms of carbon (C), but also in terms of other essential compounds. In particular, the content of phosphorus (P), nitrogen (N), and polyunsaturated fatty acids (PUFA, including eicosapentaenoic acids (EPA) and docosahexaenoic acids (DHA)) is important for zooplankton development and reproduction. These substances may limit the growth of zooplankton, especially in mesotrophic and eutrophic conditions where C concentrations exceed threshold food concentrations. Shortages of key dietary elements like C, N, and P for consumer metabolic demands can alter the synthesis of major macromolecules such as lipids, proteins, and nucleic acids [1]. However, zooplankton can regulate the content of these elements in their body, retaining those that are in shortage while excreting those that are in excess through homeostasis [2,3,4].
Sundbom and Vrede [5] suggested that growth limitation in cladoceran zooplankton by PUFAs was a secondary effect of P limitation. They considered that nutrient stress caused changes in the biochemical composition of Daphnia, which in turn slowed growth. Therefore, P limitation can determine fatty acid composition, which in turn adversely affects growth. As Gulati showed, the importance of fatty acids for daphnids increased with a decrease in the C:P ratios of algal resources from 673 to 59 µmol/µmol [6].
The elemental and biochemical composition of crustaceans differs between species, resulting in species-specific food quality requirements. Lake crustacean communities are typically dominated by copepods and cladocerans, which differ in their nutritional requirements. Copepods have a high N:P (ca. 30–50) ratio in their tissues and therefore relatively high N and low P demands for their development [7]. By contrast, cladocerans have a lower tissue N:P (ca. 14) and thus relatively higher P and lower N demands [3]. Cladocerans are also more vulnerable to EPA limitation in nature [8], while copepods have higher requirements for DHA [9,10]. Thus, food quality can help to determine crustacean community structure.
Small- and large-bodied species of cladocerans respond differently to environmental factors [11], and they are likely to have different levels of threshold concentrations of C (abundance of food resources), C:P ratios, and/or EPA. Large cladocerans have lower threshold food concentrations (i.e., food concentration at which population death rate equals birth rate) than small-bodied species [12,13,14,15]. However, P requirements are higher in large-bodied species than in small-bodied species, because P is used for somatic growth [16]. Therefore, large-bodied species can be more vulnerable to P limitation. Sikora et al. [17] also showed that small-bodied Daphnia species were less vulnerable to EPA shortage [18].
Zooplankton food quality is also dependent on phytoplankton composition. For example, total fatty acids of green algae were 40% PUFA, while cyanobacteria were only 6% [6,19]. Phytoplankton quality may also depend on its P and N content [20]. Planktivorous fish have the ability to alter phytoplankton quantity and quality indirectly through grazing and nutrient cycling. Fish can change phytoplankton composition indirectly through selective consumption of cladocerans which prefer food items from 1 to 30 mm [21]. Copepods are better able to escape fish predators than cladocerans because they have higher locomotor activity [22,23,24]. For example, copepods escaped fish attacks 90% of the time, while Daphnia avoided predator attacks only 15% of the time [25]. Field studies [26] were in accordance with the above experimental data, demonstrating that cladocerans were selectively consumed by fish despite higher density of copepods. Furthermore, fish prefer larger cladocerans [14,27]. Therefore, fish selectively reduce the abundance of the most effective filter feeders, mainly large daphnids, thus leading to increases of edible particulate food items for zooplankton [28].
Fish can also exert bottom–up effects on zooplankton by indirectly altering phytoplankton communities. For example, fish excrete nutrients into the water column that can stimulate phytoplankton growth and alter its species composition [29]. Fish can also stimulate or inhibit the development of individual phytoplankton taxa passing through their guts in “viable gut passage” [30,31].
The goal of this experiment was to determine how fish and trophic state affected crustacean community dynamics, with particular emphasis on cladocerans. We conducted a mesocosm experiment where we manipulated the presence of fish in water from a mesotrophic and a eutrophic reservoir. We were interested in how fish affected cladocerans both indirectly through changes in algal food quantity and quality and directly through predation. We also conducted supplementary life-table experiments in order to better understand how the experimental treatments affect the demographic parameters and estimate population growth rates of the dominant large- and small-bodied cladoceran species in the absence of direct predation by the fish. We predicted that fish would enhance the quality of algal resources (e.g., PUFA and/or nutrient content), while at the same time structuring cladoceran communities through size-selective predation. We also hypothesized that small and large cladoceran species would respond differently to variations in algal quantity and quality based on the differences in threshold concentrations and nutritional requirements discussed above.
2. Materials and Methods
The experiment was carried out in a series of 12 plastic mesocosms (300-L total volume, 0.94 × 0.64 × 0.50 m), half of which were filled with water containing natural phytoplankton and zooplankton from the eutrophic Lake Jorzec (northeastern Poland, Mazury Lakes, lake area 41.9 ha, max depth 11.6 and mean depth 5.5 m) and the other half filled with water from the mesotrophic Lake Majcz (area 163.5 ha, max depth 16.4 m, mean depth 6 m [32]). The mesocosms were kept on the shore of Lake Mikolajki at the Research Station of the Nencki Institute of Experimental Biology, Polish Academy of Sciences. We manipulated the presence/absence of fish in the mesocosms for a total of 4 treatments: water from the eutrophic lake without fish (E); water from the mesotrophic lake without fish (M); water from the eutrophic lake with fish (EF); and water from the mesotrophic lake with fish (MF). Each treatment was replicated in triplicate mesocosms, and the experiment was conducted for 31 days. To create the fish treatments, one individual ruff Gymnocephalus cernuus (Linnaeus, 1758) between 7.5–11 cm (standard length) was added to each mesocosm. Fish were kept in 5 L boxes that were suspended in the mesocosms. The boxes had large slots that allowed zooplankton to pass freely, but kept the fish inside. The fish were let out of the cage for only one hour (between 20:00 and 21:00) each day to feed freely. Previous research has shown that fish can exhibit unrealistically high predation rates in mesocosm studies, and the cages were used to limit predation on zooplankton throughout the experiment [33]. The mesocosms were open to the atmosphere, but in the event of rain, they were covered with a polyethylene multilayer film to prevent contamination.
Crustacean zooplankton samples were collected using a 2.6 L Limnos sampler every 10 days from the center of each mesocosm after they were gently mixed and fixed with 4% formaldehyde. The most abundant cladocerans were Chydorus sphaericus (O. F. Müller, 1776), Bosmina longirostris (O. F. Müller, 1776), Ceriodaphnia pulchella Sars, 1862, and Diaphanosoma brachyurum (Liévin, 1848). Since large daphnids were absent in the water from the mesotrophic and eutrophic lakes, we added Daphnia magna Straus, 1820 (originating from Binnensee, Germany) and Daphnia pulicaria Forbes, 1893 (originating from Lake Brome, Canada) into each mesocosm at densities of 1.0 ind. L−1 for each species at the beginning of the experiment on Day 1 to study the different responses of large and small cladoceran species. Copepod communities were represented by Eudiaptomus graciloides (Lilljeborg, 1888), Mesocyclops leuckarti (Claus, 1857), and Thermocyclops oithonoides (Sars G.O., 1863). Crustaceans were identified according to species. The average animal length was used to estimate the wet weight of crustaceans by applying the equations after Błędzki and Rybak [34].
Phytoplankton samples were collected after thoroughly mixing the water in the mesocosms on the same sampling dates as the zooplankton samples. Samples were preserved with Utremel solution and 4% formaldehyde. Phytoplankton samples were concentrated by sedimentation [35] and counted under a light microscope (Nikon Optiphot 2). Cell sizes were measured under a microscope using an ocular micrometer. Algae biomass was calculated based on cell size and their approximations to simple geometric shapes [36,37]. The size structure of phytoplankton was represented by three size classes: <30 µm; 30–50 µm, and >50 µm. Crustaceans mainly consume algal cells <30 µm [21], while algae >50 µm are generally too large for consumption.
Chlorophyll a was estimated using a PHYTOPAM fluorimeter (WALZ, Germany) that individually measured the concentrations of green, cyanobacteria, and brown algae (diatoms and dinoflagellates) on the same dates as the plankton samples. All the water chemistry samples were also collected at a 10-day interval and analyzed using standard methods [38]. Concentrations of N–NO3, N–NO2, N–NH4, and P–PO4 were determined via a Dionex ICS 1100 ion chromatograph; total phosphorus (TP) and total nitrogen (TN) were measured using a Shimadzu. The total concentration of inorganic nitrogen was determined as the sum of nitrate, nitrite, and ammonium.
Temperature and dissolved oxygen concentrations were measured daily from the center of each mesocosm using a WTW multi-parameter probe 3410 with optical sensor FDO925. Concentrations of dissolved oxygen varied from 8 to 10 mg/L, indicating that there was no oxygen limitation in the mesocosms. Electrical conductivity of water which varied from 271–354 μS/cm was measured with a Hatch probe.
We collected seston (all the particles and live organisms that passed through a 100 µm mesh sieve) for elemental (C, P, N) and fatty acid (FA) analyses (EPA, DHA, and total FAs) on the first and final days of the experiment. One sample (5–10 L) was filtered onto precombusted glass–fiber GF/F filters (Whatman, USA) until intensive color developed on the filter. The fatty acid subsamples were dried for 30 minutes and then transferred into a chloroform–methanol mixture and frozen. Filters for organic carbon, phosphorus, and nitrogen were dried at ambient temperature overnight and stored dry in a desiccator until further analyses.
Samples of zooplankton for elemental (C, P, N) and fatty acid (FA) analyses (EPA, DHA, and total FAs) were also taken on the first and final days of the experiment. Preliminarily, 40–50 L of water was passed through a 100 µm mesh sieve to remove large items (filamentous algae, sticks, etc.) from collected material on the sieve. Then, the zooplankton on the sieve was dried with the filter paper and divided into subsampls for fatty acid and elemental analyses. Subsamples for fatty acids were weighed and placed into a chloroform–methanol mixture and frozen. Subsamples of zooplankton phosphorus, nitrogen, and carbon were weighed and kept at 75 °C overnight and then stored in a desiccator. We did not collect seston and zooplankton samples for nutrient and PUFA analysis during the course of the experiment because we did not want to disturb the plankton community dynamics.
Organic carbon (C) and nitrogen (N) were measured using a Flash EA 1112 NC Soil/MAS 200 elemental analyzer (ThermoQuest, Milan, Italy), as described in [39]. Calibration curves for the elemental analyzer were generated using aspartic acid and standard soil reference material. Contents of particulate P were estimated following the conventional photocolorimetric method [40]. The background P content of the filters was preliminarily measured and subtracted from the sample values. We used the modified procedure for fatty acid analyses of the seston and zooplankton as described in detail elsewhere [41,42]. Briefly, filters with the collected seston and biomass of zooplankton were homogenized with glass beads in a mixture of chloroform–methanol three times. Three portions of extracted lipids in chloroform–methanol were combined and rotoevaporated. The total lipid extract was methylated in a two-step way, by heating for 10 min at 90 °C in 0.2 M sodium methoxide methanolic solution, then a portion of 1 M H2SO4 methanolic solution up to acidic reaction was added, and the mixture were again heated for 10 min at 90 °C. The methylation was stopped with the addition of a portion of saturated NaCl solution. Fatty acid methyl esters (FAMEs) were extracted from the reaction mixture with hexane and washed two times with a portion of saturated NaCl solution. FAMEs were analyzed and identified using a gas chromatograph-mass spectrometer (6890/5975C, ‘Agilent Technologies’, Santa Clara, CA, USA) equipped with a 30 m long, 0.25 mm internal diameter capillary column HP-FFAP. The main instrument conditions have been described elsewhere [42]. Peaks of FAMEs were identified by their retention time and mass spectra, comparedd to those in the integrated data base NIST-2005 and to available authentic standards (Sigma-Aldrich, St Louis, MO, USA). FAME peaks were quantified by their area compared to a peak area of the internal standard, nonadecanoic acid methyl ester that was added to samples prior to extraction as a portion of a chloroform solution. An example of a chromatogram of FAMEs is given in Figure 1.
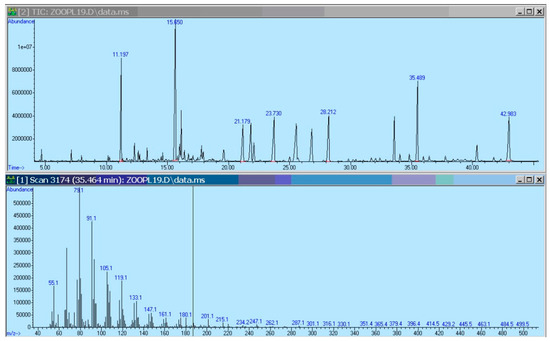
Figure 1.
An example of an experimental chromomatogram. The upper panel is the chromatogram for fatty acids from total lipids of zooplankton from mesotrophic conditions. The lower panel is a mass-spectrum of the fatty acid peak located at 35.489 min.
We conducted life-table experiments beginning on the 12th day of the mesocosm experiment. We waited to start the life table experiments until zooplankton communities adjusted to the conditions in the mesocosms. Demographic parameters can depend on the female prehistory conditions. Therefore, we used females to start our life tables that developed in mesocosms from their birth to the third clutch. Life-tables were performed in 500 mL bottles that were filled with water from each mesocosm after filtration to remove crustaceans and other large items through a 100 µm mesh sieve. Water in the bottles was exchanged at two-day intervals, and the bottles were thoroughly washed. The bottles were hung in the center of each mesocosm in the middle water layer. The openings of the bottles were covered with a sieve (50 µm) through which the crustaceans could not get out, while phytoplankton easily penetrated into the bottle. In each of the 12 mesocosms, initially, 7–10 newborn specimens of one of the three dominant species of cladocerans, namely, D. magna, D. pulicaria, and C. pulchella were placed in individual bottles. In total, each mesocosm had three bottles (one with each of the three species) such that the treatments and replicates in the life-table experiment matched the mesocosm experiment. Every other day, living individuals were removed from the bottle, counted, and returned. We recorded the duration of development until maturity and the number of eggs in each clutch. We limited our observations to the third clutch because previous studies on cladoceran life histories have shown that later clutches contribute negligibly to population growth rate (r) [43,44]. We measured the concentrations of chlorophyll in each bottle and mesocosm every other day to control for potential discrepancies in food concentrations between bottles and corresponding mesocosms.
We used one-way ANOVA with Fisher’s LSD post hoc tests to determine how nutrient concentrations, namely, sum of inorganic nitrogen compounds (N–NO3, N–NO2, and N–NH4), P–PO4 and N:P ratio, concentrations of total chlorophyll, chlorophyll of diatoms and dinoflagellates and green algae, and biomasses of copepods and cladocerans, D. magna, D. pulicaria, and C. pulchella differed between the four experimental treatments (M, MF, E, and EF). Concentrations of cyanobacteria chlorophyll were compared between E and EF using Mann–Whitney nonparametric U-test. The independent variable was treatment. The data from Day 1 of the experiments were not used in the statistical analysis because there was not yet an effect of fish on the plankton communities. The figures of the nutrient and biological parameters were made using log10-transformed data. We also used one-way ANOVA to compare concentrations (C, N, P) and ratios (C:N, C:P, N:P) of the element content in seston and zooplankton, and also concentrations of indicators of biochemical quality (EPA, DHA, Total FA) and their contents per organic carbon (EPA:C, DHA:C, Sum FA:C) in seston and zooplankton between the four treatments. Ratios C:P, C:N and EPA:C, DHA:C, and Sum FA:C were compared between zooplankton and seston using Mann–Whitney nonparametric U-test. We used one-way ANOVA to compare demographic parameters of D. magna, D. pulicaria, and C. pulchella in the life-table experiments. Effects of treatments (M, MF, E, EF) and time on phytoplankton biomasses of different size groups were compared by two-way repeated measures ANOVA (RM ANOVA). In the absence of normal distribution (Kolmogorov–Smirnov one-sample test for normality), the Kruskal–Wallis (H) test was used. Statistical analyses were performed in PAST, version 3.20.
Canonical correspondence analysis of fatty acid composition of seston was performed using STATISTICA software, version 9.0 (StatSoft, Inc., Tulsa, Oklahoma, USA). Dependence of demographic parameters of D. magna, D. pulicaria, and C. pulchella on total chlorophyll concentration was calculated using regression analysis, which was carried out using STATISTICA software, version 9.0 (StatSoft, Inc.).
3. Results
The concentrations of N and P and the N:P ratio in the water did not differ between the treatments (Figure 2). The concentration of P remained relatively stable throughout the experiment, likely due to P regeneration. However, the concentration of N decreased throughout the experiment, and as a result, the N: P ratio also decreased.
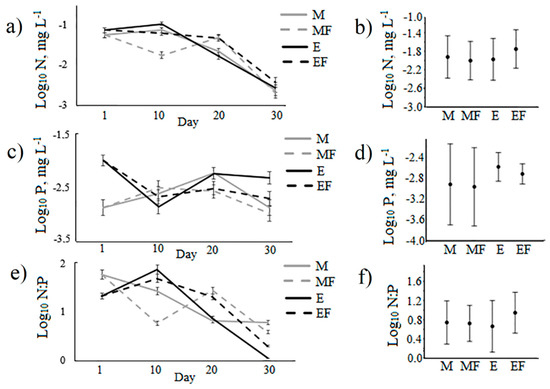
Figure 2.
Dynamics of nutrient concentrations in the experiment (a, c, e) and treatment means (b, d, f); M—mesotrophic conditions, MF—mesotrophic conditions with introduced fish, E—eutrophic conditions, EF—eutrophic conditions with introduced fish.
The concentrations of total chlorophyll and diatom and dinoflagellate chlorophyll were significantly higher in the treatments with fish than in the corresponding treatments without fish at both trophic levels (Figure 3). The highest concentrations of total chlorophyll and diatoms were in the EF treatment, and the lowest were in the M treatment. In the MF and E treatments, the concentrations of chlorophyll were not statistically different, but they were higher than in M and lower than in EF. The concentration of green algae chlorophyll was not statistically different between the treatments. Cyanobacteria were either absent or rare in mesotrophic treatments. In the eutrophic treatments, cyanobacteria chlorophyll was higher in the fish treatment than in the eutrophic treatment without fish.
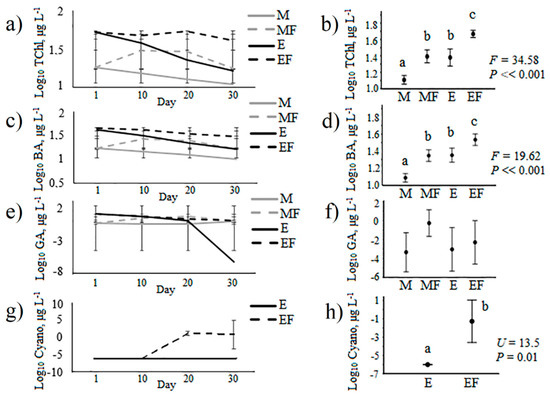
Figure 3.
Dynamics of chlorophyll concentrations in the experiment (a, c, e, g) and treatment means (b, d, f, h); different letters indicate significant differences at P < 0.05 after Fisher’s LSD post hoc test (F) and Mann–Whitney nonparametric U-test (U). TChl—concentrations of total chlorophyll, BA—chlorophyll of diatoms and dinoflagellates, GA—green algae and Cyano—cyanobacteria, M—mesotrophic conditions, MF—mesotrophic conditions with introduced fish, E—eutrophic conditions, EF—eutrophic conditions with introduced fish.
The seston C:N and C:P ratios did not differ between the treatments or from the start to the end of the experiment (Table 1). C:P mean ratios varied from 176.97 to 743 µmol/µmol (or from 68.5 to 287.6 mg/mg). Therefore, there could be a shortage of phosphorus in the seston for zooplankton, since there is evidence that 90–100 µmol/µmol can be limiting [45,46,47]. By contrast, seson N:P ratios did differ between treatments.

Table 1.
Results of one-way ANOVA comparing means (±SE) of ratios of indicators of nutritive quality of seston in experimental mesocosms: E—eutrophic, M—mesotrophic, F—fish, i—initial (June) date, f—final (July) date, C—organic carbon, N—nitrogen, P—phosphorus, F—Fisher’s test, and its significance, P—significant values are given in bold); means labeled with the same letter are not significantly different at P < 0.05 after Fisher’s LSD post hoc test (in the absence of normal distribution, the Kruskal–Wallis test was used).
Results of canonical correspondence analysis of fatty acid (FA) composition (% of total FAs) in seston are given in Figure 4. At the start of experiment, FA composition did not differ considerably between eutrophic (E) and mesotrophic (M) mesocosms (Figure 4a). However, there were differences in the M treatment due to higher levels of docosahexaenoic acid (DHA, 22:6n-3) (Figure 4). At the end of the experiment, higher levels of FAs with odd numbers of carbon atoms and iso-FAs with branched carbon chain, which are the markers of bacteria, were characteristic of seston in all the treatments except EF (Figure 4a,b). EF separated from the other treatments at the end of experiment due to high levels of 16:3n-3, 16:2n-6 and 18:3n-3 which are markers of green algae (Figure 4a). This indicated that fish dramatically changed the phytoplankton structure in the eutrophic conditions and, therefore, fatty acid composition.
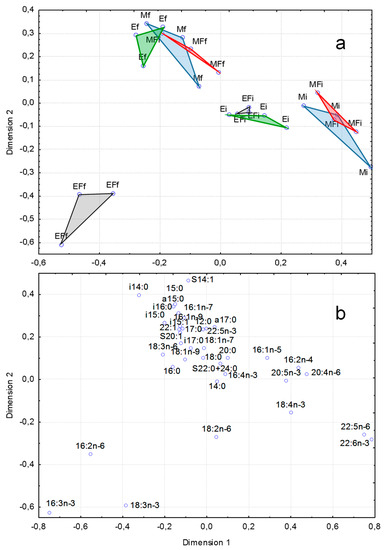
Figure 4.
Results of canonical correspondence analysis of fatty acid (FA) composition (% of total FAs) of seston: E—eutrophic, M—mesotrophic conditions, F—fish, initial (June) date, f—final (July) date. a—ordination of seston samples according to their FA composition; b- ordination of sestonic FAs.
Mean concentrations of EPA and DHA in the seston were significantly higher in all the treatments at the start of the experiment than at the end of the experiment, but there were no differences between the treatments (Table 2). At the start, total FA concentrations in eutrophic treatments, regardless of the presence or absence of fish, were higher than those in mesotrophic treatments. By contrast, at the end of the experiment, total FA concentrations in EF were significantly higher than in E. Total FA concentrations in mesotrophic treatments either with fish or without fish did not significantly differ between the start and the end of the experiment.

Table 2.
Results of one-way ANOVA comparing means (± SE) of concentrations of indicators of nutritive quality of seston in experimental mesocosms: EPA—eicosapentaenoic acid, DHA—docosahexaenoic acid, Total FA—sum of all fatty acids, E—eutrophic, M—mesotrophic, F—fish, i—initial (June) date, f—final (July) date; means labelled with the same letter are not significantly different at P < 0.05 after Tukey’s HSD post hoc test.
Contents of fatty acids per organic carbon (C) in the seston (mg/g) are given in Table 3. EPA:C ratios were similar in all the treatments except for low values observed in EF at the end of the experiment (Table 3), which probably means a significant decrease of the relative abundance of diatoms in the phytoplankton community. DHA:C tended to be higher at the start of the experiment in all the treatments than at the end of the experiment (Table 3), which probably represents a decrease in the relative abundance of chrysophytes over the course of the experiment in all the treatments. The total FA:C ratio did not differ between the treatments or between the start and the end of the experiment (Table 3).

Table 3.
Results of one-way ANOVA comparing means (± SE) of content per organic carbon (C) of indicators of nutritive quality of seston in experimental mesocosms: EPA—eicosapentaenoic acid, DHA—docosahexaenoic acid, Sum FA—sum of all fatty acids, E—eutrophic, M—mesotrophic, F—fish, i—initial (June) date, f—final (July) date; means labelled with the same letter are not significantly different at P < 0.05 after Tukey’s HSD post hoc test. If ANOVA is insignificant (P > 0.05), letter labels are absent.
At the beginning of the experiment, chrysophytes had the highest biomass in all the treatments (44%–61% of the total algal biomass) (Figure 5), with Dinobryon sp. dominating. At the end of the experiment, the green filamentous algae Oedogonium sp. and Mougeotia sp. contributed 42%–59% to the total algae biomass. Cyanobacteria Limnothrix redekeii Van Goor and Oscillatoria sp. dominated in the EF treatment at the end of the experiment (28% of the total algal biomass), while their biomass remained insignificant in the other treatments, including in the mesotrophic treatment with fish (4%). These results were in accordance with DHA:C analysis in seston, which showed that chrysophytes declined in all the treatments.
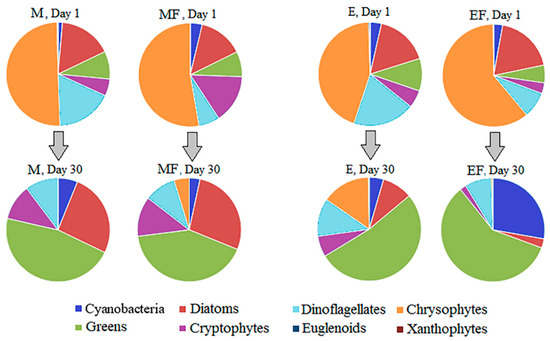
Figure 5.
Taxonomic structure of phytoplankton community on Day 1 and Day 30 in M—mesotrophic conditions, MF—mesotrophic conditions with introduced fish, E—eutrophic conditions, EF—eutrophic conditions with introduced fish.
The size structure of algae changed with time and in response to fish (Figure 6, Table 4). In all of the treatments, algae between 30 and 50 µm disappeared by the end of the experiment. In the EF treatment, the biomass of algae <30 μm decreased, while the biomass of algae >50 μm increased relative to the other treatments.
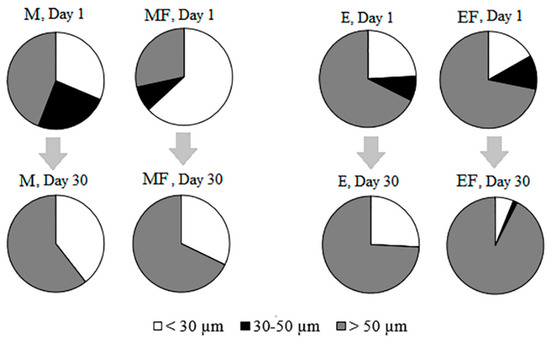
Figure 6.
Size structure of phytoplankton on Day 1 and Day 30 in M—mesotrophic conditions, MF—mesotrophic conditions with introduced fish, E—eutrophic conditions, EF—eutrophic conditions with introduced fish.

Table 4.
Results of two-way repeated measures ANOVA (RM ANOVA) with factors treatment (M, MF, E, EF) and time. M—mesotrophic conditions, MF—mesotrophic conditions with introduced fish, E—eutrophic conditions, EF—eutrophic conditions with introduced fish. Significant results (p < 0.05) are shown in bold.
The biomass of cladocerans did not differ between eutrophic and mesotrophic conditions in the fish treatments, or in the fish-free treatments (Figure 7). However, their biomass was significantly reduced in fish treatments relative to that in fish-free treatments in the mesotrophic conditions. The biomass of copepods did not differ between the treatments of the experiment.
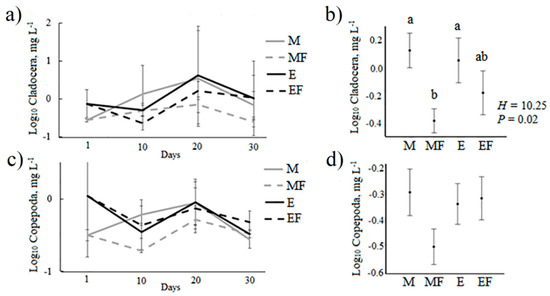
Figure 7.
Dynamics of biomass of crustaceans in the experiments (a, c) and treatment means (b, d); different letters indicate significant differences at P < 0.05 after Kruskal–Wallis test (H); M—mesotrophic conditions, MF—mesotrophic conditions with introduced fish, E—eutrophic conditions, EF—eutrophic conditions with introduced fish.
D. pulicaria and D. magna biomass did not differ between mesotrophic and eutrophic conditions in the free-fish treatments and between mesotrophic and eutrophic conditions in the fish treatments (Figure 8). However, the biomasses of these species in the treatments without fish were significantly higher than in the treatments with fish where they were very rare. The biomass of C. pulchella did not differ between the treatments with fish and the treatments without fish either in mesotrophic or eutrophic conditions (Figure 8). Initially, C. pulchella was more abundant in the M treatment and this species peaked earlier in M than in E treatments.
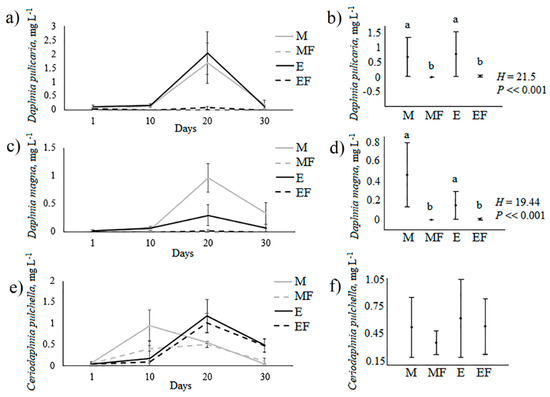
Figure 8.
Dynamics of biomasses of D. magna, D. pulicaria, and C. pulchella in the experiment (a,c,e) and treatment means (b,d,f); different letters indicate significant differences at P < 0.05 after Kruskal–Wallis test (H); M—mesotrophic conditions, MF—mesotrophic conditions with introduced fish, E—eutrophic conditions, EF—eutrophic conditions with introduced fish.
C: P and C: N in zooplankton did not differ between the treatments or between the beginning and end of the experiment (Table 5). However, C:P and C:N in zooplankton was significantly lower than in the seston in all of the treatments except one—C:N was not significantly greater in the seston in the M treatment at the end of the experiment (Table 6).

Table 5.
Results of one-way ANOVA comparing mean values (± SE) of ratios of indicators of nutritive quality of zooplankton in experimental mesocosms: E—eutrophic, LE—low-eutrophic, F—fish, i—initial (June) date, f—final (July) date, C—organic carbon, N—nitrogen, P—phosphorus, F Fisher’s test, and P significant values are given in bold; means labeled with the same letter are not significantly different at P < 0.05 after Fisher’s LSD post hoc test (in the absence of normal distribution, the Kruskal–Wallis test was used).

Table 6.
Results of Mann–Whitney comparing mean values (± SE) of ratios C:P and C:N of zooplankton and seston in experimental mesocosms: E—eutrophic, M—mesotrophic, F—fish, i—initial (June) date, f—final (July) date; means labelled with the same letter are not significantly different at P < 0.05. Significant results (P < 0.05) are shown in bold.
In zooplankton, EPA:C and total FAs did not differ between the treatments at the beginning of the experiment (Table 7). By the final day, however, both of these indicators of zooplankton quality had decreased in fish-free treatments (M and E) relative to the initial date, while in the fish treatments, these indicators remained at the initial level. Therefore, zooplankton quality in terms of EPA:C and/or FA:C in the fish treatments was higher than in the corresponding treatments without fish. The content of DHA in zooplankton was initially higher in eutrophic (E and EF) than in mesotrophic treatments (M and MF). By the end of the experiment, DHA content had decreased in the fish-free treatments (M and E) relative to initial values, while in the fish treatments (MF and EF), DHA content did not significantly change. The changes in contents of PUFAs and FAs in zooplankton did not follow corresponding changes in the seston. We suggest that assimilation and consumption of phytoplankton taxa can be different, and zooplankton content of quality indicators better characterized food conditions for zooplankton than seston quality indicators.

Table 7.
Results of one-way ANOVA comparing means (± SE) of content of indicators of nutritive quality of zooplankton in experimental mesocosms at the beginning and end of the experiment: EPA—eicosapentaenoic acid, DHA—docosahexaenoic acid, Total FA—sum of all fatty acids, E—eutrophic, M—mesotrophic, F—fish, i—initial (June) date, f—final (July) date; means labelled with the same letter are not significantly different at P < 0.05 after Tukey’s HSD post hoc test. If ANOVA is insignificant (P > 0.05), letter labels are absent.
The contents of EPA and total FAs were significantly lower in zooplankton than in phytoplankton (Table 8). DHA was lower in seston at the beginning of the experiments; however, it was reduced in seston by the end of the experiment and did not differ significantly between seston and zooplankton. In general, we can conclude that zooplankton poorly accumulated PUFA and FAs in contrast to P and N.

Table 8.
Results of Mann–Whitney (U) comparing means (± SE) of contents per organic carbon (C) of indicators of nutritive quality of zooplankton and seston in experimental mesocosms: EPA—eicosapentaenoic acid, DHA—docosahexaenoic acid, Total FA—sum of all fatty acids, E—eutrophic, M—mesotrophic, F—fish, i—initial (June) date, f—final (July) date; means labelled with the same letter are not significantly different at P < 0.05. Significant results (P < 0.05) are shown in bold.
Table 9 indicates that during the life-table experiments, the average concentrations of total chlorophyll, diatoms, and dinophytes and greens in the fish treatments were higher than in the corresponding treatments without fish. In the eutrophic conditions, cyanobacteria concentrations were higher in the fish treatments than in the treatment without fish. In the mesotrophic conditions, cyanobacteria were either absent or rare. Table 10 shows that there were no differences in resource concentrations between the mesocosm and life-table experiments except MF bottles with Daphnia species, where food concentrations were lower in life table experiments than in the corresponding mesocosms.

Table 9.
Results of one-way ANOVA comparing means ( ± SE) of chlorophyll concentrations for the period of life-table experiments in E—eutrophic, LE—low-eutrophic, F—fish treatments, F Fisher’s test; P significant values are given in bold; means labeled with the same letter are not significantly different at P < 0.05 after Fisher’s LSD post hoc test (in the absence of normal distribution, the Kruskal–Wallis test (H) was used).

Table 10.
Results of one-way ANOVA comparing means (± SE) of total chlorophyll concentrations (µg L-1) for the period of life-table experiments in mesocosms and bottles with D. pulicaria, D. magna, and C. pulchella. E—eutrophic, M—mesorophic, F—fish treatments, F Fisher’s test, and P (significant values are given in bold); means labeled with the same letter are not significantly different at P < 0.05 after Fisher’s LSD post hoc test (in the absence of normal distribution, the Kruskal–Wallis test (H) was used).
In the life-table experiments, the rate of population growth (r) differed both between the treatments and between species within individual treatments (Table 11). The population growth rate in C. pulchella was higher than that in the two species of Daphnia in all the treatments and was always positive. The minimum r in C. pulchella was observed in mesotrophic conditions, while its maximum population growth rate was recorded in eutrophic conditions with fish. Strongly negative r values were observed in both Daphnia species in the M and E treatments, i.e., these species were gradually dying out over the course of the experiment. In the two Daphnia species, r was positive in MF (although the resource concentration in this treatment was lower than in the mesocosms, see Table 10) and slightly negative in EF, but it was higher than in M and E. The survival rate of all individuals in the life-table was 100%. Therefore, r mainly depended on the clutch sizes and the duration of juvenile development until maturity. Table 11 shows that C. pulchella always had eggs after reaching maturity. Moreover, the fecundity of C. pulchella was significantly dependent on the concentration of the total chlorophyll (Figure 9). In Daphnia, there was no relationship between the fecundity and concentration of the food resource. Both Daphnia laid eggs in the treatment with fish, but eggs were extremely rare in the mesocosms without fish. In the MF treatment, in both Daphnia species, there were more than 2 eggs per female, whereas in EF, fecundity was less than one egg per female (Table 11). Therefore, fish positively affected the population growth rate of Daphnia via changing food quality and/or quantity. The juvenile developmental time until maturity (first clutch) in both Daphnia species was lower in MF and EF than in fish-free treatments, M and E. C. pulchella reached maturity for 4 days, i.e., faster than daphnids, in all the treatments except M, where the first clutch was laid in 10 days after birth. In this treatment, the average concentration of food was the lowest compared to the other treatments.

Table 11.
Demographic parameters (± SE) of D. magna, D. pulicaria, and C. pulchella in E—eutrophic, M—mesotrophic, F—fish treatments; Kruskal–Wallis (H) test and Dunn’s post hoc test (P < 0.05) were used for pairwise comparisons of population growth rate, fecundity, and time of first clutch along the columns and along the lines. P significant values are given in bold; means labeled with the same letter/asterics are not significantly different along the same columns/line. Letters are given for comparisons along the columns, while asterisks show discrepancies along the lines.
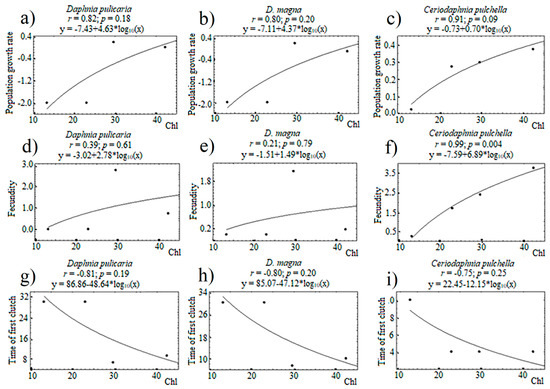
Figure 9.
Relationships between total chlorophyll (Chl) and population growth rate of D. pulicaria (a), D. magna (b), and C. pulchella (c); between total chlorophyll and fecundity of D. pulicaria (d), D. magna (e), and C. pulchella (f); between total chlorophyll and time of first clutch of D. pulicaria (g), D. magna (h), and C. pulchella (i) in the mesocosms. Above the graphs is the correlation coefficient, the significance level of the regression equation and the regression equation.
Contents of EPA+DHA in D. magna and D. pulicaria in the experiment, 0.42 ± 0.03 mg/g and 0.36 ± 0.07 mg/g, respectively, did not differ significantly: P > 0.05 after Mann–Whitney’s U-test. It means that diets of both species of Daphnia were similar.
4. Discussion
It has long been known that fish can structure zooplankton communities directly through predation. Less understood is how fish indirectly affect zooplankton through changes in algal food quantity and quality. While fish did not directly affect nutrient concentrations in the mesocosms, they did affect algal quantity and quality in terms of species composition and seston nutrient and PUFA contents. Fish recycle nutrients either via fish excretion or by changing phytoplankton composition indirectly through consumption of crustaceans. As a result, in fish mesocosms total chlorophyll, brown algae (diatoms and dinoflagellates) and cyanobacteria increased relative to fish-free treatments. Therefore, fish enhanced food concentrations for zooplankton. Moreover, fish also changed food quality since different taxa of phytoplankton have differences in their biochemical composition [6]. Fish also changed the size structure of phytoplankton. Zooplankton biomass was regulated by fish grazing on zooplankton, especially large daphnids. Small-bodied species C. pulchella and copepods did not show differences in biomass in the treatments with fish and without fish. Since fish–zooplankton interactions are very complex, we applied quantitative and qualitative indicators of seston and zooplankton to explain cladoceran species dynamics in the treatments with and without fish.
There continues to be a debate as to whether food quality or food quantity is a main driver of zooplankton dynamics. Based on the theory of the threshold food concentration (TFC), the species with higher TFC should be suppressed by species with lower TFC [14,48]. If the TFC is low, food concentration can be a limiting factor for inferior competitors because superior competitors can decrease algal resources down to its TFC. However, there is evidence indicating that food quality can also affect species dynamics. For example, phosphorus [16,45], polyunsaturated fatty acids (PUFA) or fatty acids (FAs) [6,49] can be limiting factors that constrain the development of crustaceans even if carbon concentrations are high. In our experiment, we had four treatments that were distinguished by trophic state (mesotrophic and eutrophic) and fish (presence/absence), both of which can potentially change phytoplankton structure and/or food quantity and quality. Only the small-bodied species C. pulchella showed a significant relationship between its fecundity and resource concentration. Population growth rate in C. pulchella was the highest at the greatest food concentration (EF treatment) and lowest at the smallest resource concentration (M treatment). The dynamics of C. pulchella in the mesocosms was in accordance with this result of the life-table experiment showing higher mean biomasses in the second half in the EF treatment. By contrast, both species of Daphnia had the lowest fecundity at the highest resource concentration (EF) and the highest fecundity at the intermediate resource concentration (MF). Therefore, r for daphnids was the highest in MF, and it was strongly negative in M and E. In EF, r was negative but higher than in M and E. Thus, we can conclude that small and large cladoceran species responded differently to the food concentrations. If we can assume that C. pulchella abundance was regulated by resource concentration, Daphnia abundance was rather dependent on food quality.
In general, daphnids are known as nonselective filter feeders that do not selectively consume individual food particles [50]. Their diet spectrum is restricted by the size of food items and varies from 1 to 20–30 mm [21]. Indeed, according to the contents of EPA and DHA, diets of D. magna and D. pulicaria were similar. Further, they are both large-bodied species, and they equally needed to allocate a great portion of consumed energy to their growth. For this reason, their demographic parameters changed similarly in response to the treatment effects. However, in contrast to large daphnids, C. pulchella do not need to allocate so much energy to growth and can spend more energy on reproduction. As our experiments showed, C. pulchella reached maturity earlier, and clutch sizes were always larger than in large Daphnia under the same conditions. Population growth rates of C. pulchella were always positive and higher than those of the large Daphnia. Only in MF was r of C. pulchella similar to that of both Daphnia species. It is noteworthy that despite the negative r in Daphnia in some treatments, survival was 100% everywhere; however, fecundity was close to zero in the treatments without fish. This result is in accordance with contribution theory [51]. According to this theory, daphnids prioritize energy allocation. When the concentration of resources is limiting, allocation priority of an individual is to stay alive at the expense of reproduction. When trophic conditions improve, resource partitioning is directed to provide body growth and increase clutch size. Therefore, we suggest that there was strong food limitation of Daphnia population growth in M and E treatments, while r increased in EF treatment although it was still negative and in MF where it was the highest. For C. pulchella, the best trophic conditions were in EF and the worst in M. Since its fecundity was linearly related to food concentration, we can conclude that the limiting factor for C. pulchella, was resource abundance. However, the fecundity of Daphnia species did not correlate with food abundance, a. Thus, higher population growth rates of Daphnia in the MF and EF treatments relative to corresponding treatments without fish were not related to food concentrations. We initially assumed that it could be associated with a higher share of edible size range (<30 µm) in the treatments with fish, but the data on phytoplankton size structure did not confirm this assumption, and algal taxonomic composition was also similar between these treatments. Therefore, we suggested that the Daphnia species were rather subject to limitation of resource quality.
The threshold concentration of sestonic EPA for the growth of Daphnia is equal to 13 mg L−1 [52]. In our experiments, seston EPA concentrations were much lower, and therefore, EPA may have constrained Daphnia growth rates. EPA and DHA concentrations in seston were higher at the beginning of the experiment than at the end, while FA did not differ significantly between the start and the end of the experiments in all the treatments. These data indicated that in terms of PUFAs, food quality gradually deteriorated for Daphnia. For this reason, abundance of Daphnia increased in the first half of the experiment and decreased in the second half. Content of EPA and DHA in phytoplankton, i.e., ratios EPA:C and DHA:C, less explicitly differed between the start and the end of the experiment. EPA:C was the least in EF at the end. FA:C did not differ between the treatments and between the start and the end of the experiment.
Comparing PUFA:C and/or FAs:C between zooplankton and phytoplankton, we can see that this ratio is much lower in zooplankton, indicating that zooplankton can accumulate only approximately 10% of PUFA and/or FAs relative to phytoplankton. The other part of seston EPA is likely lost, maybe because it is inaccessible for zooplankton. Despite deterioration of food quality in term of PUFA concentrations by the end of the experiment, EPA:C decreased in zooplankton only in treatments without fish. If fish were present, EPA content in zooplankton at the end was similar to that at the start of the experiment. In addition, this ratio in zooplankton at the end of the experiments was higher in the treatment with fish than in the corresponding treatments without fish. Similarly, DHA:C in zooplankton was higher in EF than in E at the end. FA:C at the end was higher in MF than in M and in EF was greater than in E. Therefore, contents of PUFA and FAs in zooplankton were higher in treatments with fish than in the corresponding treatments without fish. Based on these data, we can conclude that food conditions were worse for zooplankton in the treatments without fish. However, estimations of seston quality in terms of PUFA concentrations did not show differences between treatments with fish and without fish at the end of the experiment.
We think that since the fish significantly reduced the abundance of cladoceran species in the MF and EF treatment, the share of preferred resources by zooplankton species increased in these treatments relative to the M and E treatments, respectively. We suggest that phytoplankton species are not equally accessible for zooplankton due to their sizes or assimilation ability. Therefore, seston quality can be similar in the treatments with fish and without fish, but the edible fraction for cladoceran species can be different. The diet of cladocerans is known to be constrained by food particle size [50]. Further, they can differentially retain or assimilate particulate food items. In support, cladocerans were shown to selectively accumulate EPA from food [53,54,55,56]. Additionally, Taipale et al. [57] found that cladoceran δ 13C values did not correlate with seston δ13C values and instead correlated with the δ13C values of the different phytoplankton taxa, indicating that Daphnia selectively assimilated phytoplankton. Selective feeding of Daphnia on natural microalgal assemblages was also demonstrated experimentally by Gladyshev et al. [58]. We argue that the PUFA and FA contents of zooplankton are a better indicator of food conditions, because these indices are determined by the edible fraction of seston, which we are not able to separate when analyzing seston PUFA and FA contents.
Comparing C:P or C:N in seston or zooplankton between the treatments, there were no significant differences. C:P ratios in seston were quite high and could constrain the growth of daphnids because they were higher than the threshold for Daphnia development, which equaled to 225–375 µmol/µmol [46]. However, zooplankton C:P was significantly lower than that in seston. There is evidence in the literature that Daphnia can increase P retention if P content of resources is in shortage [21,45,59]. Feniova et al. [33] suggested that there was selective accumulation of food particles that are rich in P by Daphnia. Such mechanisms of P retention enabled crustaceans to increase P in their body. Although P content of seston in EF treatment was significantly lower than in the other treatments, P content in zooplankton did not differ significantly. With regard to C:N, there was not any mismatch between seston N content and that of zooplankton. Therefore, we do not think that there was N limitation of crustaceans in the experiment.
As mentioned above, the highest population growth rates in Daphnia species were in the MF treatment. However, contents of phosphorus and nitrogen in zooplankton in MF were not higher than in the other treatments. Therefore, we believe that PUFA and FAs, especially EPA, which is the most important component for Daphnia, can be the main limiting factors which did not allow Daphnia to reproduce in the treatments without fish. Unfortunately, we do not know explicitly why population growth rate in Daphnia was higher in MF than in EF. However, results of canonical correspondence analysis of fatty acid (FA) composition (% of total FAs) of seston showed that in EF treatment at the end of the experiment, inedible green algae were most abundant. In addition, data on taxonomic composition indicated that in eutrophic conditions, the share of attached green algae and cyanobacteria was much higher than in mesotrophic treatments. Since these algae are not accessible for zooplankton, the portion of PUFA and FAs in edible seston particles could be less in eutrophic than in mesotrophic conditions.
Small Ceriodaphnia and large Daphnia species demonstrated that they differentially responded to food conditions. While Ceriodaphnia population growth was positively related to food concentrations, Daphnia population growth was restricted by food quality, likely in terms of PUFAs and FAs. Our findings are in accordance with those of Sikora et al. [17], who showed that large-bodied species were more sensitive to low food quality than small-bodied ones in terms of PUFA and/or C:P ratio. Sikora et al. [18] showed that EPA saturation thresholds, which are defined as the minimal concentration of EPA above which the juvenile growth rate becomes saturated, increased significantly with increasing body size of the tested species. Different responses of demographic parameters of small Ceriodaphnia and large Daphnia provided different dynamics of small and large cladoceran species.
In this aspect, small Ceriodaphnia have a strategy of “patients” [60] or “stress-tolerators” [61] in the plankton, i.e., species that inhabit degraded environment, while Daphnia are typical of the strategy of “violents” [60] or ‘competitors [61] that suppress “patients” when conditions recover. The shift from “patients” to “violents” in zooplankton communities can be caused by gradual alteration in food quality. In our case, food quality in the middle of the experiments was not sufficient for Daphnia to reproduce in the treatments without fish, while in the treatment with fish, Daphnia were selectively suppressed by fish. For this reason, Daphnia were not successful in any treatment in the second half of the experiments, although during the first 10 days, when food quality was better, large daphnids reproduced and increased in biomass.
To conclude, we suggest that the small Ceriodaphnia was regulated mainly by food quantity, while large species of cladocerans were limited by PUFA and FAs. Fish likely increased food quality of the edible fraction of seston in terms of PUFA, primarily EPA, thus providing conditions for more successful development of Daphnia than in the fish-free treatments. Seston food quality in terms of PUFA and FA and nutrient concentrations appeared not to be good indicators of food conditions due to different constraints for consumption by zooplankton, including inappropriate sizes or shapes of resources and differences of assimilation or ingestion rates among algae taxa. Phosphorus in seston was likely limiting for zooplankton. However, food quality in terms of phosphorus is less important than PUFA because zooplankton can accumulate nutrients in their body. We believe that our study provides a potential mechanism (e.g., changes in food quality created by fish and different responses of small- and large-bodied species to food conditions) that can be used to help to explain patterns of cladoceran communities observed in nature. Our results showed that different life strategies of small- and large-bodied cladoceran species can provide sustainable biomass level of cladoceran communities under the variable environmental conditions due to shifts of dominance between small and large cladoceran species.
Author Contributions
I.F. participated in the experiments and wrote the manuscript; E.S. made statistical analyses; M.K. participated at all stages of the studies and processed and analyzed crustacean samples; M.I.G. and N.N.S. processed PUFA and C, N, and P data and analyzed data on fatty acids and elemental compositions in phytoplankton and zooplankton; P.D. was responsible for the methodological part of the experiments and provided crustacean and fish material for the studies; Z.G. processed and analyzed phytoplankton samples; A.G. processed and analyzed nutrient samples; Y.S. participated in the experiments; A.D. supervised and coordinated the research studies and revised and edited manuscript.
Funding
This experiment was performed with support from the Polish National Science Centre (UMO-2016/21/B/NZ8/00434). Statistical analysis and its interpretation were done with support from Russian Science Foundation (grant № 16-14-10323). Phytoplankton and zooplankton analyses were supported from the Russian Foundation for Basic Research (grant 18-54-00002 Bel_a).
Acknowledgments
We would like to thank the Research Station in Mikolajki, part of the Nencki Institute of Experimental Biology, Polish Academy of Sciences, for providing us the place and equipment for conducting our experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prater, C.; Wagner, N.D.; Frost, P.C. Seasonal effects of food quality and temperature on body stoichiometry, biochemistry, and biomass production in Daphnia populations. Limnol. Oceanogr. 2018, 63, 1727–1740. [Google Scholar] [CrossRef]
- Sterner, R.W. Daphnia growth on varying quality of Scenedesmus: Mineral limitation of zooplankton. Ecology 1993, 74, 2351–2360. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere, 1st ed.; Princeton University Press: Princeton, NJ, USA, 2002; ISSN 0-691-07491-7. [Google Scholar]
- Vanderploeg, H.A.; Nalepa, T.F.; Jude, D.J.; Mills, E.L.; Holeck, K.T.; Liebig, J.R.; Grigorovich, I.A.; Ojaveer, H. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Can. J. Fish. Aquat. Sci. 2002, 59, 1209–1228. [Google Scholar] [CrossRef]
- Sundbom, M.; Vrede, T. Effects of fatty acids and phosphorus content of food on the growth, survival and reproduction of Daphnia. Freshw. Biol. 1997, 38, 665–674. [Google Scholar] [CrossRef]
- Gulati, R.D.; DeMott, W.R. The role of food quality for zooplankton: Remarks on the state-of-the-art, perspectives and priorities. Freshw. Biol. 1997, 38, 753–768. [Google Scholar] [CrossRef]
- Elser, J.J.; Urabe, J. The stoichiometry of consumer-driven nutrient recycling: Theory, observations, and consequences. Ecology 1999, 80, 735–751. [Google Scholar] [CrossRef]
- Ravet, J.L.; Persson, J.; Brett, M.T. Threshold dietary polyunsaturated fatty acid concentrations for Daphnia pulex growth and reproduction. Inland Waters 2012, 2, 199–209. [Google Scholar] [CrossRef]
- Makhutova, O.N.; Gladyshev, M.I.; Sushchik, N.N.; Dubovskaya, O.P.; Buseva, Z.F.; Fefilova, E.B.; Semenchenko, V.P.; Kalachova, G.S.; Kononova, O.N.; Baturina, M.A. Comparison of fatty acid composition of cladocerans and copepods from lakes of different climatic zones. Contemp. Probl. Ecol. 2014, 7, 474–483. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Dubovskaya, O.P.; Buseva, Z.F.; Makhutova, O.N.; Fefilova, E.B.; Feniova, I.Y.; Semenchenko, V.P.; Kolmakova, A.A.; Kalachova, G.S.; et al. Fatty acid composition of Cladocera and Copepoda from lakes of contrasting temperature. Freshw. Biol. 2015, 60, 373–386. [Google Scholar] [CrossRef]
- Hart, R.C.; Bychek, E.A. Body size in freshwater planktonic crustaceans: An overview of extrinsic determinants and modifying influences of biotic interactions. Hydrobiologia 2011, 668, 61–108. [Google Scholar] [CrossRef]
- Achenbach, L.; Lampert, W. Effects of elevated temperatures on threshold food concentrations and possible competitive abilities of differently sized cladoceran species. Oikos 1997, 79, 469–476. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Food thresholds and body size in cladocerans. Nature 1990, 343, 638–640. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Between Hazards of Starvation and Risk of Predation: The Ecology of Off-Shore Animals Excellence in Ecology, Book 12; International Ecology Institute: Oldendorf/Luhe, Germany, 2003; p. 379. ISSN 0932-2205. [Google Scholar]
- Gliwicz, Z.M.; Lampert, W. Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 1990, 71, 691–702. [Google Scholar] [CrossRef]
- Sterner, R.W.; Schulz, K.L. Zooplankton nutrition: recent progress and a reality check. Aquat. Ecol. 1998, 32, 261–279. [Google Scholar] [CrossRef]
- Sikora, A.; Dawidowicz, P.; von Elert, E. Daphnia fed algal food grown at elevated temperature have reduced fitness. J. Limnol. 2014, 73, 421–427. [Google Scholar] [CrossRef]
- Sikora, A.; Petzoldt, T.; Dawidowicz, P.; von Elert, E. Demands of eicosapentaenoic acid (EPA) in Daphnia: are they dependent on body size? Oecologia 2016, 182, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Müller-Navarra, D.C.; Brett, M.T.; Liston, A.M.; Goldman, C.R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 2000, 403, 74–77. [Google Scholar] [CrossRef]
- Wilson, A.E. Effects of zebra mussels on phytoplankton and ciliates: A field mesocosm experiment. J. Plankton Res. 2003, 25, 905–915. [Google Scholar] [CrossRef]
- Sommer, U.; Sommer, F. Cladocerans versus copepods: the cause of contrasting top-down controls on freshwater and marine phytoplankton. Oecologia 2006, 147, 183–194. [Google Scholar] [CrossRef]
- Maia-Barbosa, P.M.; Matsumura-Tundisi, T.M. Consumption of zooplanktonic organisms by Astyanax fasciatus Cuvier, 1819 (Osteichthyes, Characidae) in Lobo (Broa) reservoir, São Carlos, SP, Brazil. Hydrobiologia 1984, 113, 171–181. [Google Scholar] [CrossRef]
- Sommer, U.; Stibor, H. Copepoda–Cladocera–Tunicata: The role of three major mesozooplankton groups in pelagic food webs. Ecol. Res. 2002, 17, 161–174. [Google Scholar] [CrossRef]
- Güntzel, A.M.; Morita Melo, I.K.; Roche, K.F.; da Silva, V.F.B.; Pompiani, P.G. Cladocerans from gut contents of fishes associated to macrophytes from Taquari River Basin, MS, Brazil. Acta Limnol. Bras. 2012, 24, 97–102. [Google Scholar] [CrossRef]
- Bohl, E. Food supply and prey selection in planktivorous Cyprinidae. Oecologia 1982, 35, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Okun, N.; Mehner, T. Distribution and feeding of juvenile fish on invertebrates in littoral reed (Phragmites) stands. Ecol. Freshw. Fish. 2005, 14, 139–149. [Google Scholar] [CrossRef]
- Brooks, J.L.; Dodson, S.I. Predation, body size and composition of plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Semenchenko, V.P.; Razlutskij, V.I.; Feniova, I.Y.; Aibulatov, D.N. Biotic relations affecting species structure in zooplankton communities. Hydrobiologia 2007, 579, 219–231. [Google Scholar] [CrossRef]
- Brabrand, A.; Bjorn, F.; Torsten, K.; Nilssen, P.J. Can iron defecation from fish influence phytoplankton production and biomass in eutrophic lakes? Limnol. Oceanogr. 1984, 29, 1330–1334. [Google Scholar] [CrossRef]
- Kolmakov, V.I.; Gladyshev, M.I. Growth and potential photosynthesis of cyanobacteria are stimulated by viable gut passage in crucian carp. Aquat. Ecol. 2003, 37, 237–242. [Google Scholar] [CrossRef]
- Kolmakov, V.I. Role of Microcystis aeruginosa passing through the digestive tracts of filter-feeding animals in eutrophic water reservoirs (review). Contemp. Probl. Ecol. 2014, 7, 455–464. [Google Scholar] [CrossRef]
- Gliwicz, Z.M.; Ghilarov, A.; Pijanowska, J. Food and predation as major factors limiting two natural populations of Daphnia cucullata Sars. Hydrobiologia 1981, 80, 205–218. [Google Scholar] [CrossRef]
- Feniova, I.; Dawidowicz, P.; Gladyshev, M.I.; Kostrzewska-Szlakowska, I.; Rzepecki, M.; Razlutskij, V.; Sushchik, N.N.; Majsak, N.; Dzialowski, A.R. Experimental effects of large-bodied Daphnia, fish and zebra mussels on cladoceran community and size structure. J. Plankton Res. 2015, 37, 611–625. [Google Scholar] [CrossRef]
- Błędzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe; Springer: Basel, Switzerland, 2016; p. 918. [Google Scholar] [CrossRef]
- Kuzmin, G.V. Phytoplankton. Methods of Ecosystem Studies in Inland Waters; Nauka: Moscow, USSR, 1975; pp. 73–87. [Google Scholar]
- Vinberg, G.G.; Lavrenteva, G.M. Guidelines for the Collection and Processing of Materials for Hydrobiological Studies in Freshwater Bodies. Phytoplankton and Its Products; GOSNIORKH: Leningrad, USSR, 1982; pp. 1–33. [Google Scholar]
- Mikheeva, T.M. Methods of quantitative estimates of nanophytoplankton (review). Hydrobiol. J. 1989, 25, 3–21. [Google Scholar]
- APHA. Standard Methods. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Gladyshev, M.I.; Sushchik, N.N.; Kolmakova, A.A.; Kalachova, G.S.; Kravchuk, E.S.; Ivanova, E.A.; Makhutova, O.N. Seasonal correlations of elemental and ω3 PUFA composition of seston and dominant phytoplankton species in a eutrophic Siberian Reservoir. Aquat. Ecol. 2007, 41, 9–23. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Sushchik, N.N.; Gladyshev, M.I.; Kalachova, G.S.; Makhutova, O.N.; Ageev, A.V. Comparison of seasonal dynamics of the essential PUFA contents in benthic invertebrates and grayling Thymallus arcticus in the Yenisei river. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2006, 145, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, M.I.; Semenchenko, V.P.; Dubovskaya, O.P.; Fefilova, E.B.; Makhutova, O.N.; Buseva, Z.F. Effect of temperature on contents of essential highly unsaturated fatty acids in freshwater zooplankton. Limnologica 2011, 41, 339–347. [Google Scholar] [CrossRef]
- Porter, K.G.; Feig, Y.S.; Vetter, E.F. Morphology, flow regimes, and filtering rates of Daphnia, Ceriodaphnia, and Bosmina fed natural bacteria. Oecologia 1983, 58, 156–163. [Google Scholar] [CrossRef]
- Pijanowska, J.; Dawidowicz, P.; Howe, A.; Weider, L.J. Predator-induced shifts in Daphnia life-histories under different food regimes. Arch. Hydrobiol. 2006, 167, 37–54. [Google Scholar] [CrossRef]
- DeMott, W.R.; Gulati, R.D.; Siewertsen, K. Effects of phosphorus-deficient diets on the carbon and phosphorus balance of Daphnia magna. Limnol. Oceanogr. 1998, 43, 1147–1161. [Google Scholar] [CrossRef]
- Brett, M.T.; Müller-Navarra, D.C.; Park, S.K. Empirical analysis of the effect of phosphorus limitation on algal food quality for freshwater zooplankton Limnol. Oceanogr. 2000, 45, 1564–1575. [Google Scholar] [CrossRef]
- Hall, S.R.; Leibold, M.A.; Lytle, D.A.; Smith, V.H. Stoichiometry and planktonic grazer composition over gradients of light, nutrients, and predation risk. Ecology 2004, 85, 2291–2301. [Google Scholar] [CrossRef][Green Version]
- Tilman, D. Niche tradeoffs, neutrality, and community structure: a stochastic theory of resource competition, invasion, and community assembly. PNAS 2004, 101, 10854–10861. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, G.; Goedkoop, W.; Markensten, H.; Sonesten, L.; Boberg, M. Seasonal variations in food quality for pelagic and benthic invertebrates in Lake Erken—The role of fatty acids. Freshw. Biol. 1997, 38, 555–570. [Google Scholar] [CrossRef]
- DeMott, W.R. The role of taste in food selection by freshwater zooplankton. Oecologia 1986, 69, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, L.V.; Vijverberg, J. Contribution analysis of body mass dynamics in Daphnia. Oecologia 2005, 144, 268–277. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Sushchik, N.N.; Dubovskaya, O.P.; Makhutova, O.N.; Kalachova, G.S. Growth rate of Daphnia feeding on seston in a Siberian reservoir: the role of essential fatty acid. Aquat. Ecol. 2008, 42, 617–627. [Google Scholar] [CrossRef]
- Taipale, S.J.; Kainz, M.J.; Brett, M.T. Diet-switching experiments show rapid accumulation and preferential retention of highly unsaturated fatty acids in Daphnia. Oikos 2011, 120, 1674–1682. [Google Scholar] [CrossRef]
- Masclaux, H.; Bec, A.; Kainz, M.J.; Perriere, F.; Desvilettes, C.; Bourdier, G. Accumulation of polyunsaturated fatty acids by cladocerans: Effects of taxonomy, temperature and food. Freshw. Biol. 2012, 57, 696–703. [Google Scholar] [CrossRef]
- Hartwich, M.; Martin-Creuzburg, D.; Wacker, A. Seasonal changes in the accumulation of polyunsaturated fatty acids in zooplankton. J. Plankton Res. 2013, 35, 121–134. [Google Scholar] [CrossRef]
- Koussoroplis, A.M.; Kainz, M.J.; Striebel, M. Fatty acid retention under temporally heterogeneous dietary intake in a cladoceran. Oikos 2013, 122, 1017–1026. [Google Scholar] [CrossRef]
- Taipale, S.J.; Vuorio, K.; Brett, M.T.; Peltomaa, E.; Hiltunen, M.; Kankaala, P. Lake zooplankton δ13C values are strongly correlated with the δ13C values of distinct phytoplankton taxa. Ecosphere 2016, 7, e01392. [Google Scholar] [CrossRef]
- Gladyshev, M.I.; Kolmakov, V.I.; Dubovskaya, O.P.; Ivanova, E.A. Studying of algae food composition of Daphnia longispina during blue-green bloom of eutrophic pond. Dokl. Akad. Nauk. 2000, 371, 556–558. [Google Scholar]
- Weidman, P.R.; Schindler, D.W.; Thompson, P.L.; Vinebrooke, R.D. Interactive effects of higher temperature and dissolved organic carbon on planktonic communities in fishless mountain lakes. Freshw. Biol. 2014, 59, 889–904. [Google Scholar] [CrossRef]
- Ramenski, L.G. Introduction to the Complex Soil-Geobotanical Investigation of Lands; Selkhozgiz: Moscow, USSR, 1938; p. 620. (In Russian) [Google Scholar]
- Grime, J.P. Plant Strategis, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons Ltd.: Chichester, UK, 2001; p. 456. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).