Removal of Pb(II) by Pellicle-Like Biofilm-Producing Methylobacterium hispanicum EM2 Strain from Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Identification of Methylobacterium hispanicum EM2 Strain
2.2. Effect of Pb(II) on Bacterial Growth
2.3. Biosorption Profile
2.4. Study of Biosorption and Kinetic Isotherms
2.5. Scanning Electron Microscope Energy Dispersive X-ray Spectroscopy Analysis
2.6. Fourier-Transform Infrared Spectroscopy Analysis
2.7. Pb(II) Removal from Sewage Water
3. Results and Discussion
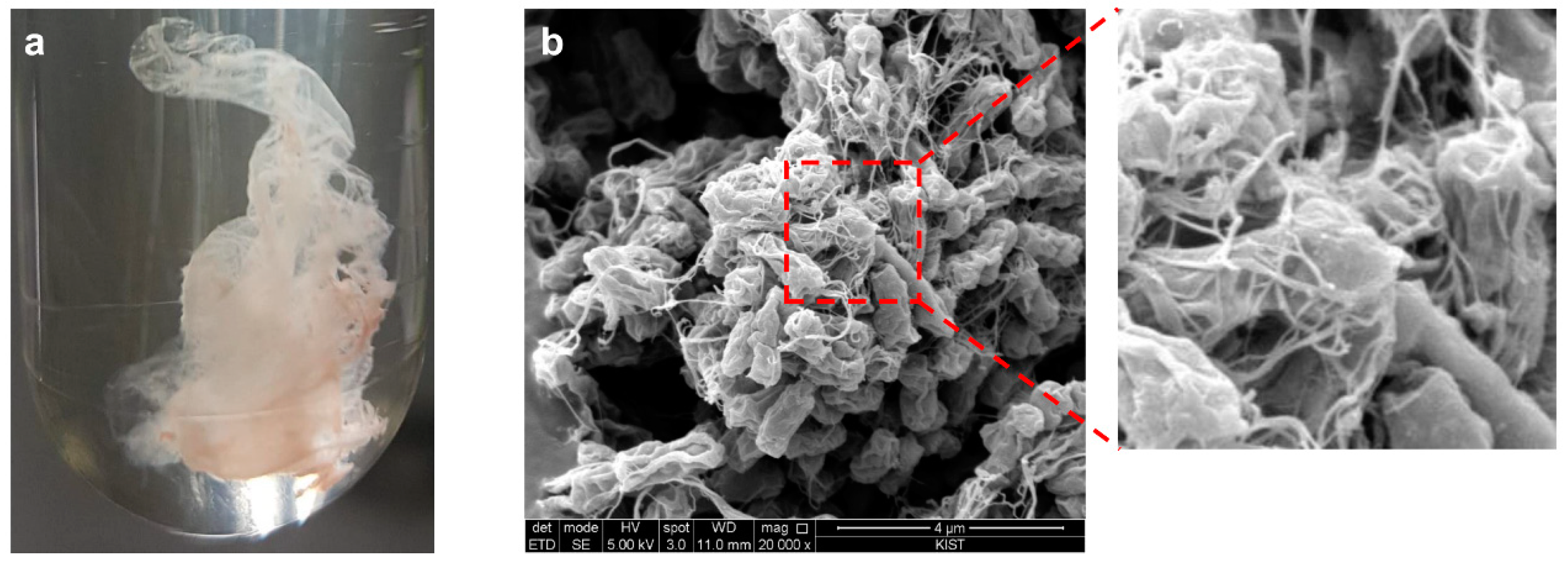
3.1. Identification of the Pellicle-Like Biofilm-Producing M. hispanicum EM2 Strain
3.2. Resistance to Pb(II)
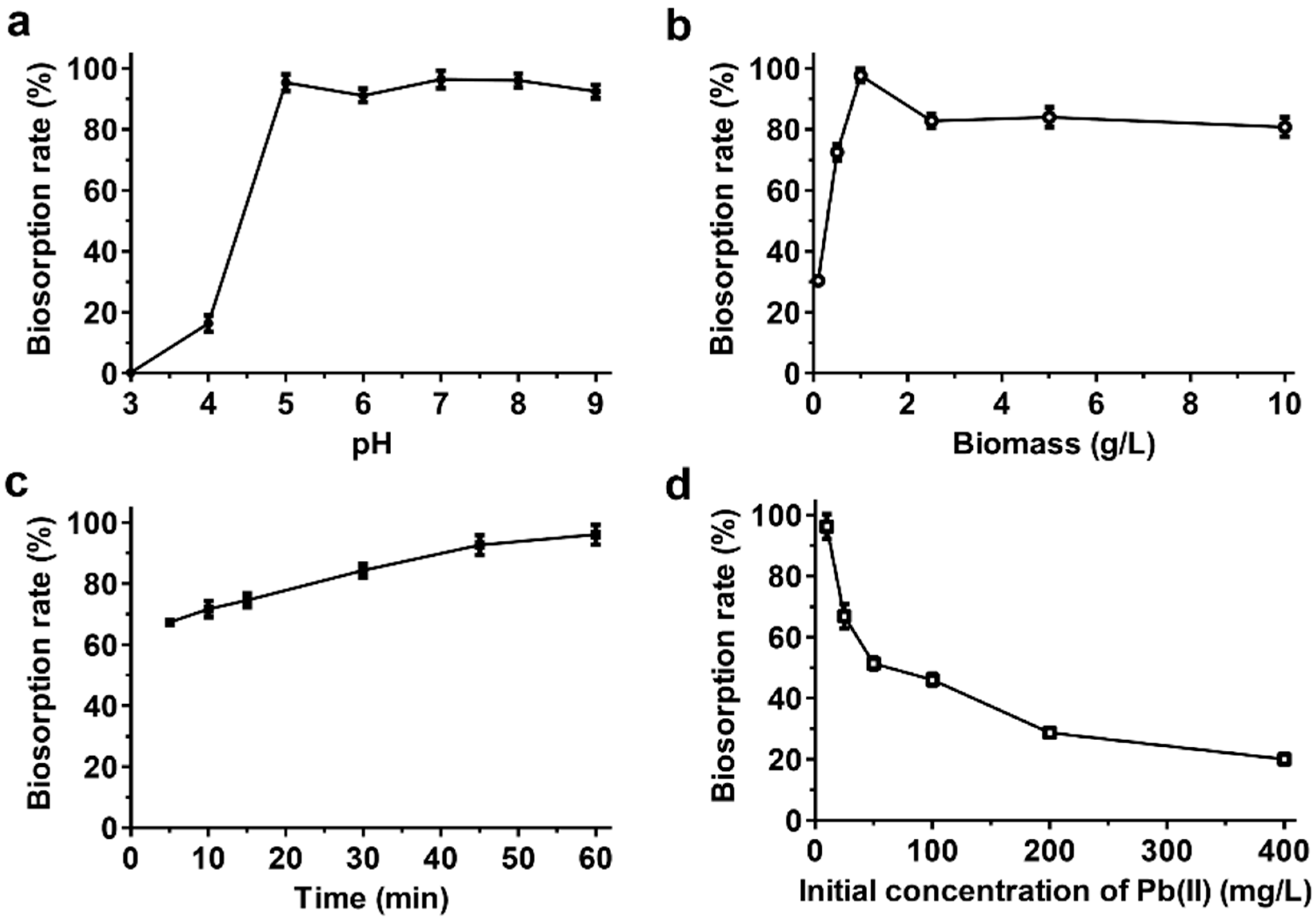
3.3. Optimization and Isotherm and Kinetic Modeling of the Biosorption of Pb(II)
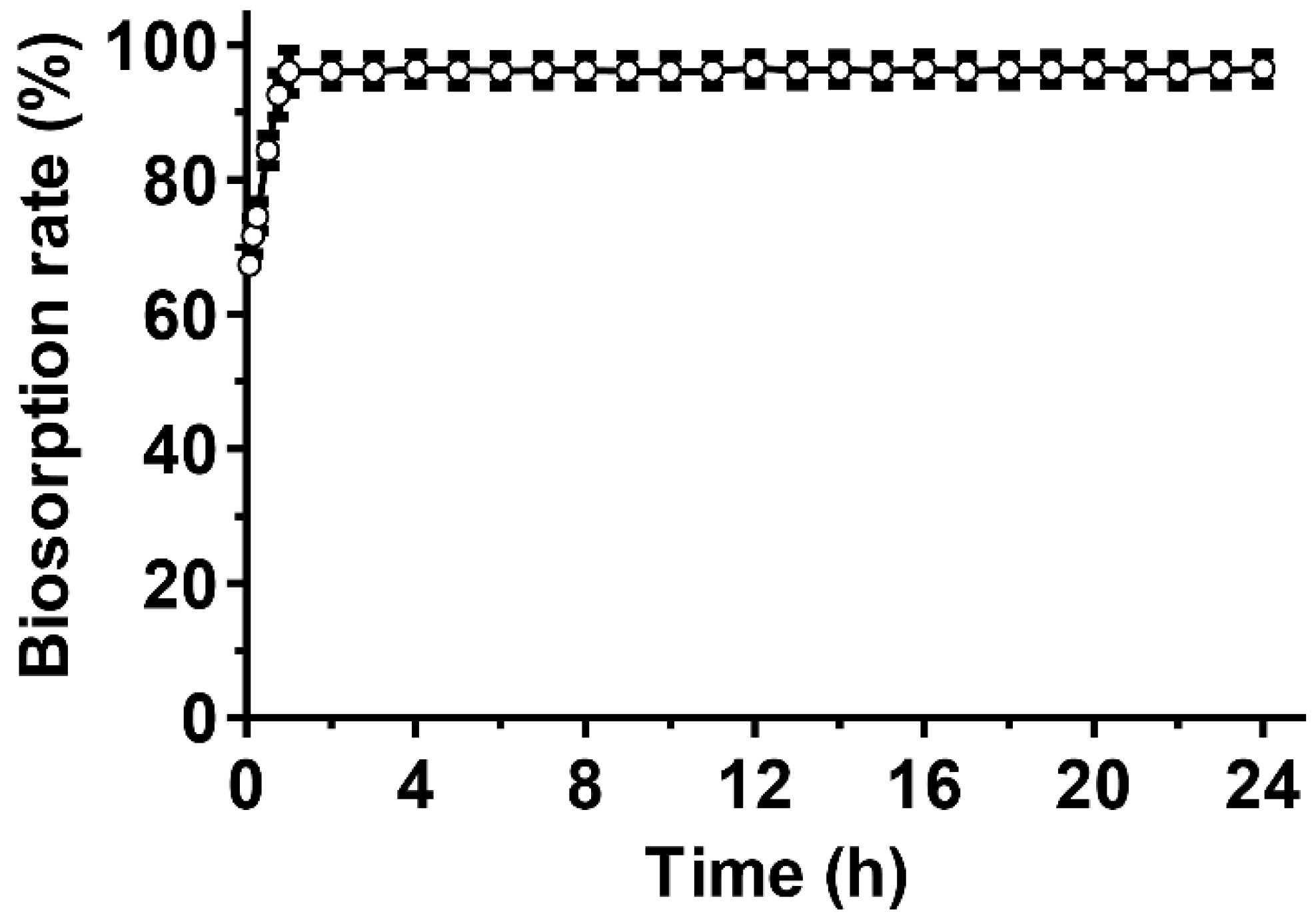
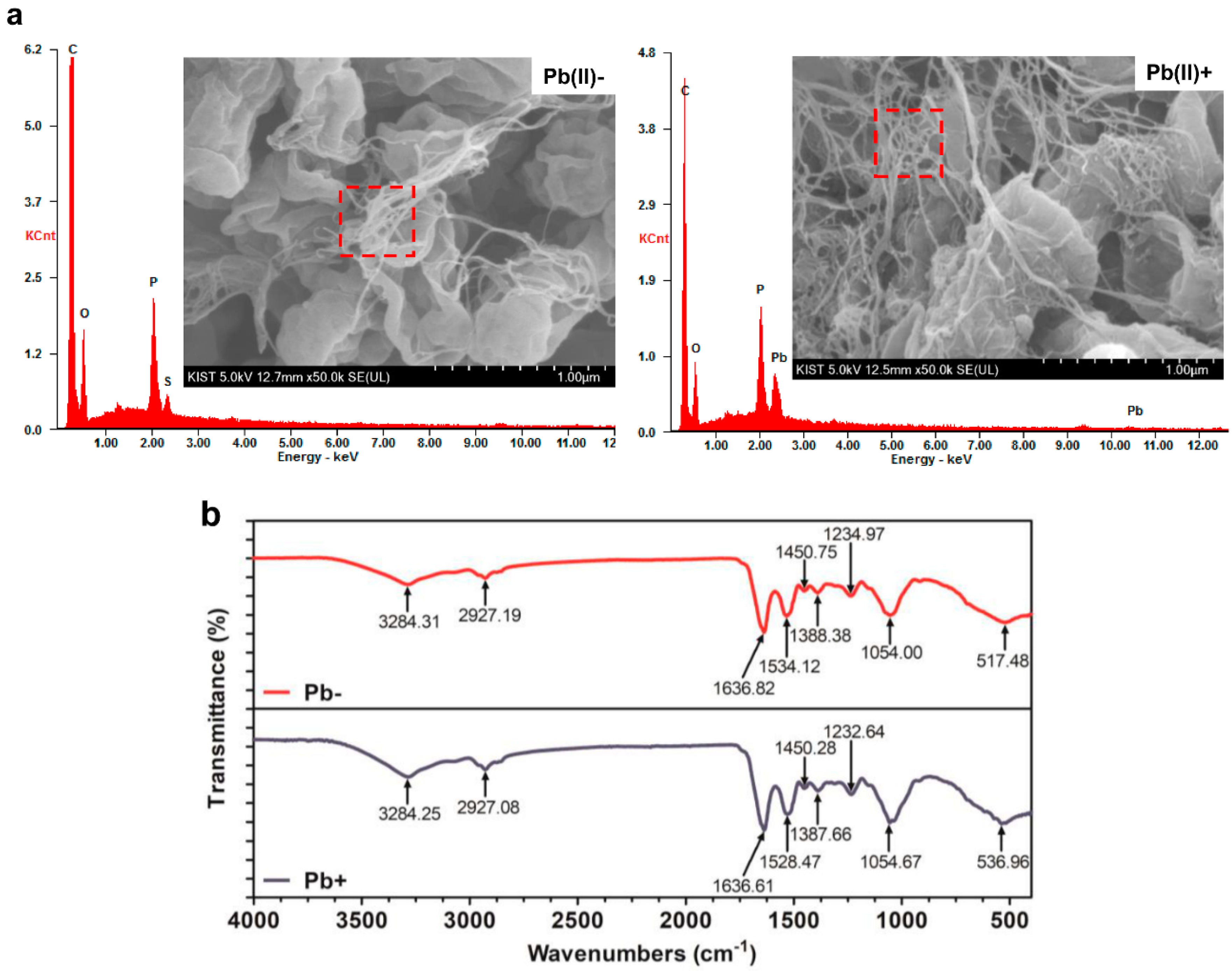
3.4. Removal of Pb(II) Using the EM2 Strain
3.5. Removal of Pb(II) from Heavy-Metal-Contaminated Sewage Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neeti, K.; Prakash, T. Effects of heavy metal poisoning during pregnancy. Int. Res. J. Environ. Sci. 2013, 2, 88–92. [Google Scholar]
- Naik, M.M.; Dubey, S.K. Lead resistant bacteria: Lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotoxicol. Environ. Saf. 2013, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Merganpour, A.M.; Nekuonam, G.; Tomaj, O.A.; Kor, Y.; Safari, H.; Karimi, K.; Kheirabadi, V. Efficiency of lead removal from drinking water using cationic resin purolite. Environ. Health Eng. Manag. 2015, 2, 41–45. [Google Scholar]
- Zhao, D.; Yu, Y.; Chen, J.P. Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA. Water Res. 2016, 101, 564–573. [Google Scholar] [CrossRef]
- Esalah, J.O.; Weber, M.E.; Vera, J.H. Removal of lead from aqueous solutions by precipitation with sodium di-(n-octyl) phosphinate. Sep. Purif. Technol. 1999, 18, 25–36. [Google Scholar] [CrossRef]
- Matlock, M.M.; Howerton, B.S.; Atwood, D.A. Chemical precipitation of lead from lead battery recycling plant wastewater. Ind. Eng. Chem. Res. 2002, 41, 1579–1582. [Google Scholar] [CrossRef]
- Kavak, D. Removal of lead from aqueous solutions by precipitation: Statistical analysis and modeling. Desalin. Water Treat. 2013, 51, 1720–1726. [Google Scholar] [CrossRef]
- Wong, P.K.; Lam, K.C.; So, C.M. Removal and recovery of Cu(II) from industrial effluent by immobilized cells of Pseudomonas putida II-11. Appl. Microbiol. Biotechnol. 1993, 39, 127–131. [Google Scholar] [CrossRef]
- Mosharaf, M.K.; Tanvir, M.Z.H.; Haque, M.M.; Haque, M.A.; Khan, M.A.A.; Molla, A.H.; Alam, M.Z.; Islam, M.S.; Talukder, M.R. Metal-adapted bacteria isolated from wastewaters produce biofilms by expressing proteinaceous curli fimbriae and cellulose nanofibers. Front. Microbiol. 2018, 9, 1334. [Google Scholar] [CrossRef]
- Soni, K.A.; Balasubramanian, A.K.; Beskok, A.; Pillai, S.D. Zeta-potential of selected bacteria in drinking water when added, starved, or exposed to minimal and rich media. Curr. Microbiol. 2007, 56, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.H.; Kim, C.J.; Oh, D.K. Metal adsorption of the polysaccharide produced from Methylobacterium organophilum. Biotechnol. Lett. 1996, 18, 1161–1164. [Google Scholar] [CrossRef]
- Kalita, D.; Joshi, S.R. Study on bioremediation of Lead by exopolysaccharide producing metallophilic bacterium isolated from extreme habitat. Biotechnol. Rep. (Amst.) 2017, 16, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Diwan, B. Bacterial exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnol. Rep. (Amst.) 2017, 13, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Völkel, S.; Fröls, S.; Pfeifer, F. Heavy metal ion stress on Halobacterium salinarum R1 planktonic cells and biofilms. Front. Microbiol. 2018, 9, 3157. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fang, H.H.P. Characterization of electrostactic binding sites of extracellular polymers by linear programmed analysis of titration data. Biotechnol. Bioeng. 2002, 80, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Fragkopoulos, A.A.; Marquez, S.M.; Kim, H.D.; Angelini, T.E.; Fernández-Nieves, A. Biofilm formation in geometries with different surface curvature and oxygen availability. New J. Phys. 2015, 17, 033017. [Google Scholar] [CrossRef]
- Lee, J.J.; Lee, Y.H.; Park, S.J.; Lim, S.Y.; Jeong, S.W.; Lee, S.Y.; Park, S.K.; Choi, H.W.; Kim, M.K.; Jung, H.Y. Deinococcus sedimenti sp nov isolated from river sediment. J. Microbiol. 2016, 54, 802–808. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-joining method- a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence-limits on phylogenies—An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, P.; Murray, R.G.E.; Wood, W.A.; Kreig, N.R. Methods for General and Molecular Bacteriology; American Society for Microbiology: Washington, DC, USA, 1994. [Google Scholar]
- Li, H.; Lin, Y.; Guan, W.; Chang, J.; Xu, L.; Guo, J.; Wei, G. Biosorption of Zn(II) by live and dead cells of Streptomyces ciscaucasicus strain CCNWHX 72-14. J. Hazard. Mater. 2010, 179, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Prithviraj, D.; Deboleena, K.; Neelu, N.; Noor, N.; Aminur, R.; Balasahe, K.; Abul, M. Biosorption of nickel by Lysinibacillus sp. BA2 native to bauxitemine. Ecotoxicol. Environ. Saf. 2014, 107, 260–268. [Google Scholar] [CrossRef]
- Gallgo, V.; García, M.T.; Ventasa, A. Methylobacterium hispanicum sp. nov. and Methylobacterium aquaticum sp. nov., isolated from drinking water. Int. J. Syst. Evol. Microbiol. 2005, 55, 281–287. [Google Scholar] [CrossRef]
- Li, K.; Ramakrishna, W. Effect of multiple metal resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J. Hazard. Mater. 2011, 189, 531–539. [Google Scholar] [CrossRef]
- Gupta, S.; Goyal, R.; Prakash, N.T. Biosequestration of lead using Bacillus strains isolated from seleniferous soils and sediments of Punjab. Environ. Sci. Pollut. Res. 2014, 21, 10186–10193. [Google Scholar] [CrossRef]
- Jebara, S.H.; Abdelkerim, S.; Fatnassi, I.C.; Chiboub, M.; Saadani, O.; Jebara, M. Identification of effective Pb resistant bacteria isolated from Lens culinaris growing in lead contaminated soils. J. Basic. Microb. 2015, 55, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Çolak, F.; Atar, N.; Yazıcıoğlu, D.; Olgun, A. Biosorption of lead from aqueous solutions by Bacillus strains possessing heavy-metal resistance. Chem. Eng. J. 2011, 173, 422–428. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process—A review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef] [PubMed]
- Wimpenny, J.; Manz, W.; Szewzyk, U. Heterogeneity in biofilms. FEMS Microbiol. Rev. 2000, 24, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Kim, S.M.; Lee, S.G.; Kim, I.S. Effect of heavy metals (Cu, Pb, and Ni) on the compositions of EPS in biofilms. Water Sci. Technol. 2001, 43, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosorption of Zn(II) from industrial effluents using sugar beet pulp and F. vesiculosus: From laboratory tests to a pilot approach. Sci. Total Environ. 2017, 598, 856–866. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of solute substances. K. Vet. akad. Handl. 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy from waters by means of natural zeolites. Wat. Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Zafar, M.N.; Nadeemb, R.; Hanif, M.A. Biosorption of nickel from protonated rice bran. J. Hazard. Mater. 2007, 143, 478–485. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, S.; Teng, C.; Song, T.; Dong, L.; Liang, J.; Bai, X.; Qu, J. Biosorption characteristic of Alcaligenes sp. BAPb.1 for removal of lead(II) from aqueous solution. 3 biotech. 2017, 7, 123. [Google Scholar] [CrossRef]
- Wang, T.; Yao, J.; Yuan, Z.; Zhao, Y.; Wang, F.; Chen, H. Isolation of lead-resistant Arthrobacter strain GQ-9 and its biosorption mechanism. Environ. Sci. Pollut. Res. 2018, 25, 3527–3538. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Jin, Y.; Zhang, C.; Gu, H.; Qu, J. Characteristics of Bacillus sp. PZ-1 and its biosorption to Pb(II). Ecotoxicol. Environ. Saf. 2015, 117, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, X.; Liu, D.; Xu, D.; Ai, Y.; Sun, X.; Zhang, M.; Gao, Y.; Zhang, Y.C.; Yang, T. A novel Pb-resistant Bacillus subtilis bacterium isolate for Co-biosorption of hazardous Sb(III) and Pb(II): Thermodynamics and application strategy. Int. J. Environ. Res. Public Health 2018, 15, 702. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tirado, V.; Green-Ruiz, C.; Gómez-Gil, B. Cu and Pb biosorption on Bacillus thioparans strain U3 in aqueous solution: Kinetic and equilibrium studies. Chem. Eng. J. 2012, 181, 352–359. [Google Scholar] [CrossRef]
- Chatterjee, S.K.; Bhattacharjee, I.; Chandra, G. Biosorption of heavy metals from industrial waste water by Geothermalis thermodenitrificans. J. Hazard. Mater. 2010, 175, 117–125. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Z.M. Biosorption of Cd(II)/Pb(II) from aqueous solution by biosurfactant-producing bacteria: Isotherm kinetic characteristic and mechanism studies. Colloid Surf. B. 2013, 105, 113–119. [Google Scholar] [CrossRef]
- Oh, S.E.; Hassan, S.; Joo, J.H. Biosorption of heavy metals by lyophilized cells of Pseudomonas stutzeri. World J. Microbiol. Biotechnol. 2009, 25, 1771–1778. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Biosorption of lead from aqueous solutions by green algae Spirogyra species: Kinetics and equilibrium strudies. J. Hazard. Mater. 2008, 152, 407–414. [Google Scholar] [CrossRef]
- Chen, S.H.; Cheow, Y.L.; Ng, S.L.; Ting, A.S.Y. Mechanisms for metal removal established via electron microscopy and spectroscopy: A case study on metal tolerant fungi Penicillium simplicissium. J. Hazard. Mater. 2019, 363, 394–402. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, V.; Guzmán-Moreno, J.; Rodríguez-González, V.; Flores-de la Torre, J.A.; Ramírez-Santoyo, R.M.; Vidales-Rodríguez, L.E. Biosorption of lead phosphates by lead-tolerant bacteria as a mechanism for lead immobilization. World J. Microbiol. Biotechnol. 2017, 33, 150. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, X.; Zang, T.; Hu, Y.; Hu, X.; Ren, G.; Xu, X.; Qu, J. Biosorption of Lead(II) by Arthrobacter sp. 25: Process optimization and mechanism. J. Microbiol. Biotechnol. 2016, 26, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
| Experimental value (isotherms) | Langmuir Isotherm | Freundlich Isotherm | ||||
| qexp (mg/g) | Qmax (mg/g) | KL (L/mg) | R2 | KF (L/g) | n | R2 |
| 79.84 | 85.47 | 0.025 | 0.96 | 6.876 | 2.31 | 0.98 |
| Experimental Value (Kinetics) | Pseudo-First-Order | Pseudo-Second-Order | ||||
| qexp (mg/g) | qcal (mg/g) | K1 (L/min) | R2 | qcal (mg/g) | K2 (g/mg/min) | R2 |
| 9.32 | 2.61 | −0.01 | 0.97 | 9.07 | 0.04 | 0.98 |
| Biosorbents | Removal Efficiency | Maximum Removal Capacity | Biomass Dosage | Optimum Conditions | Reference |
|---|---|---|---|---|---|
| Alcaligenes sp. | 85.2% | 56.8 mg/g | 1.5 g/L | pH 5, 35 °C, 0.5 h, 100 mg/L | [41] |
| Arthrobacter sp. GQ-9 | 21.62% | 17.56 mg/g | 1.2 g/L | pH 5.5, 35 °C, 4 h, 100 mg/L | [42] |
| Bacillus sp. PZ-1 | 93.01% | 15.38 mg/g | 40 g/L | pH 5, 15 °C, 0.3 h, 400 mg/L | [43] |
| Bacillus subtilis | 90.81% | 17.34 mg/g | 6 mg/L | pH 5, 35 °C, 0.6 h, 25 mg/L | [44] |
| Bacillus thioparans U3 | 94.2% | 210.1 mg/g | 0.5 g/L | pH 4, 30 °C, 0.3 h, 40 mg/L | [45] |
| Geobacillus thermodenitrificans | 36.86% | 32.26 mg/g | 0.3 g/L | pH 4.5, 65 °C, 12 h, 175 mg/L | [46] |
| Pseudomonas sp. LKS06 | 90% | 77.8 mg/g | 1 g/L | pH 6, 30 °C, 2 h, 50 mg/L | [47] |
| Pseudomonas stutzeri KCCM 34719 | 88% | 142 mg/g | 1 g/L | pH 6, 30 °C, 0.5 h, 300 mg/L | [48] |
| M. hispanicum EM2 | 96% | 79.84 mg/g | 1 g/L | pH 7, 30 °C, 1 h, 10 mg/L | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-W.; Kim, H.K.; Yang, J.E.; Choi, Y.J. Removal of Pb(II) by Pellicle-Like Biofilm-Producing Methylobacterium hispanicum EM2 Strain from Aqueous Media. Water 2019, 11, 2081. https://doi.org/10.3390/w11102081
Jeong S-W, Kim HK, Yang JE, Choi YJ. Removal of Pb(II) by Pellicle-Like Biofilm-Producing Methylobacterium hispanicum EM2 Strain from Aqueous Media. Water. 2019; 11(10):2081. https://doi.org/10.3390/w11102081
Chicago/Turabian StyleJeong, Sun-Wook, Hyo Kyeong Kim, Jung Eun Yang, and Yong Jun Choi. 2019. "Removal of Pb(II) by Pellicle-Like Biofilm-Producing Methylobacterium hispanicum EM2 Strain from Aqueous Media" Water 11, no. 10: 2081. https://doi.org/10.3390/w11102081
APA StyleJeong, S.-W., Kim, H. K., Yang, J. E., & Choi, Y. J. (2019). Removal of Pb(II) by Pellicle-Like Biofilm-Producing Methylobacterium hispanicum EM2 Strain from Aqueous Media. Water, 11(10), 2081. https://doi.org/10.3390/w11102081





