Abstract
Inorganic mercury (Hg2+) pollution of water reserves, especially drinking water, is an important issue in the environmental and public health field. Mercury is reported to be one of the most dangerous elements in nature since its accumulation and ingestion can lead to a series of permanent human diseases, affecting the kidneys and central nervous system. All the conventional approaches for assaying Hg2+ have some limitations in terms of bulky instruments and the cost and time required for the analysis. Here, we describe a miniaturizable and high-throughput bioluminescence sensor for Hg2+ detection in water, which combines the specificity of a living bacterial Hg2+ reporter cell, used as sensing element, with the performance of a silicon photomultiplier, used as optical detector. The proposed system paves the basis for portable analysis and low reactants consumption. The aim of the work is to propose a sensing strategy for total inorganic mercury evaluation in water. The proposed system can lay the basis for further studies and validations in order to develop rapid and portable technology that can be used in situ providing remote monitoring.
1. Introduction
The term ‘chemical contamination’ is used when chemicals are present where they should not be, or when their concentration is higher than national or international regulatory standards. Examples of chemical contaminants are pesticides, salts, toxins, drugs, and metals. They infiltrate in water intended for drinking and contact recreation, defined as “recreational activities involving a significant risk of ingestion of water, including wading by children, swimming, water skiing, diving, and surfing.” [1]. The presence of a contaminant may lead to an exposure that translates into effects on public health. However, the risks from chemical contamination may be smaller than that from microbiological hazards [2,3]. Nonetheless, for water-users’ safety, it is important to be able to measure a component and to ensure that it is within its regulatory threshold [4]. In assessing the risk from a particular chemical contaminant, the likelihood of exposure is as important as the type of recreational activity and the routes of exposure. Eventually, taking into consideration contamination by metals, their chemical form may significantly affect their solubility and absorption, and this should be taken into account in assessing any potential risks from exposure [5].
Exposure to mercury (Hg) is a cause of concern for the general population and recreational anglers because mercury compounds can accumulate in water plants and fishes [6,7]. Mercury exists in three forms that have different properties and toxicity: elemental or metallic mercury (Hg0), inorganic mercury compounds (mercuric, Hg2+ and mercurous ion, Hg1+), and organic mercury compounds. Elemental mercury is frequently used in dental amalgam and is released into the air when fossil fuels are burned. It is also used in some industrial processes. Thus, people may be exposed to elemental mercury when they breathe air containing elemental mercury vapors. Very high mercury vapor concentrations can quickly cause severe lung damage. At low vapor concentrations over a long time, neurological disturbances, memory problems, skin rash, and kidney abnormalities may occur. Moreover, in the body, elemental mercury can be converted to inorganic mercury [8,9]. Inorganic mercury compounds are formed when it combines with other elements, such as sulfur or oxygen. Mercury salts occur naturally but are also created in some industrial processes and in the production of e.g., cosmetic skin creams. If accumulated by ingestion, inorganic mercury can be very irritating and corrosive to the digestive system. If repeatedly eaten or applied to the skin for a long period of time, inorganic mercury can cause neurological disturbances, skin rash, and kidney failure [10]. Organic mercury compounds form when mercury combines with carbon. In particular, methylation of inorganic mercury is an important process in water and occurs in both fresh water and seawater. Some microorganisms (e.g., Pseudomonas spp. or anaerobic bacteria) in water and soil can convert elemental and inorganic mercury into organic mercury compounds (e.g., methylmercury, MeHg), which accumulates in the food chain as consequence of rapid diffusion in aquatic biota. People may be exposed to MeHg when they eat contaminated fish or shellfish. Large amounts of methylmercury eaten over time can cause damage to the nervous system. Moreover, it can pass through the placenta, causing developmental abnormalities and cerebral palsy to infants born to women who were poisoned during pregnancy [11,12].
Mercury toxicity is heavily dependent on its chemical form and its concentration in water is expected to be very low. More precisely, for both drink usage and contact recreation the tolerance value is 1 µg/L of total Hg [4]. Lower values, 0.02–0.01 µg/L are, instead, related to water for non-contact recreation (i.e., aquatic recreational pursuits, including fishing, commercial and recreational boating, not involving a significant risk of ingestion [1]). Different analytical techniques have been used for mercury determination at low concentrations. Spectrometric techniques such as cold vapor-atomic absorption spectrophotometry (CV-AAS), atomic emission flame spectrometry (AEF), or inductively coupled plasma techniques such as inductively coupled plasma optical emission spectroscopy (ICP-OES), inductively coupled plasma atomic emission spectroscopy (ICP-AES), inductively coupled plasma mass spectrometry (ICP-MS), and cold vapor atomic fluorescence spectroscopy (CV-AFS) are widely used [13,14]. ICP-MS, in particular, has an advantage of low detection limits and wide range of linearity in the determination of mercury [15]. However, for all these techniques the sample preparation step is very critical and may be time-consuming. In fact, because of mercury volatile character, care must be taken in sample preparation to ensure that it is not lost prior to analysis. Finally, a sample preparation step (either preconcentration or separation) seems to be essential to improve the quantification of a very low concentration of mercury content in biological and environmental samples [16,17].
For speciation and analysis of mercury in traces, the usage of non-chromatographic methods has several advantages in terms of speed of analysis and inexpensiveness. Anyway, for the complete speciation of mercury in both biological and environmental samples chromatographic methods are more suitable [18]. For this reason, combined techniques such as gas chromatograph-spectrometric methods (gas chromatography coupled with atomic fluorescence spectrometry, GC-AFS) [19,20] and high-performance liquid chromatography-spectrometric methods (high-performance liquid chromatography–atomic absorption spectrometry, HPLC-AAS, high performance liquid chromatography using inductively coupled plasma-optical emission spectrometry HPLC-ICP-OES) [21,22,23] have been developed. However, most of these techniques have some limitations in terms of bulky instruments, reactant volumes used, cost and time required for the analysis. Moreover, they are generally incompatible with routine on-site measurements.
During the last few years, there has been a growing interest in developing alternative detection strategies capable of overcoming the intrinsic limitations of the analytical methods mentioned above. Many attempts have been made on analytical instrumentation miniaturization, as to achieve on-site inorganic mercury analysis on real water samples. In this regard, sensors based on electrochemical techniques, optical methods employing colorimetry, fluorimetry and surface-enhanced Raman scattering (SERS) are promising due to their high sensitivity and selectivity, rapid analysis time, cost effectiveness and miniaturization [24,25,26,27,28,29,30]. However, these techniques still exhibit some features limiting their practical use, such as the inability to discriminate between different mercury species and cross-sensitivity towards other metal ions. For these reasons, more simple and fast procedures, which are enough sensitive to replace all the aforementioned methods, are needed.
More recently, determination of mercury by biosensors has received widespread attention for its inexpensive and easy-to use properties. A biosensor associates a bioactive compound (enzyme, antibody, microorganism, plant cells, algae, fungi, protozoa, etc.) which can specifically recognize the species of interest with a transducing element [31]. However, also these techniques may show some drawbacks. The main limitation of enzyme-based biosensors is that the inhibition of enzymatic reaction by mercury is not specific, which leads to poor selectivity. Hence, different heavy metal ions may cause cross sensitivity. Moreover, antibody-based biosensors have some limitations because of reaction conditions, such as temperature, pH, and ionic strength, that can affect the activity of the antibodies [32].
Whole cell-based biosensors are based on biosensing cells, such as microorganisms, which can be natural or recombinant [33]. The use of whole cells has many advantages. The whole cells can be more easily cultivated with respect to enzymes. Hence, whole cell-based biosensors are usually cheaper than enzyme-based ones. Moreover, due to specific metabolic pathways used in microorganisms, microbial sensors have the potential for more selective analysis with respect to enzyme reactions [34]. If compared to antibody-based biosensors, whole cell-based biosensors show a higher resistance to environmental conditions (e.g., significant changes in pH, temperature, or ionic strength) [35]. Finally, sample preparation or preconcentration, usually, is not necessary [36]. On the contrary, depending on the stuff involved, the technology may be not suitable for on-site contaminant determination, as in ref [36] for mercury.
We describe a new variant sensor for potential high-throughput Hg2+ detection in water, which is based on a bioluminescent Escherichia coli Hg2+ reporter embedded within a single-photon sensitive small-dimension silicon photomultiplier (SiPM) optical detector. Whole-cell biosensors are the result of a powerful combination between synthetic biology approaches and microengineering, which is an appropriate step towards the future improvement of the environmental monitoring [37]. The cells sensing the Hg2+ in the aqueous sample produce luciferase in a highly specific manner [38,39,40]. We present an innovative strategy for the surveillance of chemical risks in water. Biosensor sensitivity is described through an optical characterization, compared to the state-of-the-art, for the chemiluminescence detection with a commercial luminometer.
2. Materials and Methods
2.1. Materials
Filtered sterile 10X phosphate-buffered saline solution, or PBS, (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 and 1.47 mM KH2PO4, pH = 7, Fisher Scientific, Hampton, NH, USA) was diluted 10 fold for usage. Sterile Luria Bertani (LB) broth was purchased from Sigma-Aldrich (St. Louis, MO, USA). Mercury(II) Chloride, HgCl2, powder (Sigma) was dissolved in filtered sterile PBS to get a 25 mM stock solution, and used for the analysis. The 4 mL glass vials (14.7 mm × 45 mm) were from Lab Logistics Group International GmbH. Cuvettes are 12.5 mm × 12.5 mm × 45 mm disposable ultraviolet (UV)-cuvettes from Brand. The SiPM used as detector is produced by Fondazione Bruno Kessler (Trento, Italy).
The luminometer used for comparison was a TriStar² S LB 942 Modular Monochromator Multimode Reader luminometer.
2.2. Hg2+ Reporter Cell Preparation
The sensing element for Hg2+ detection in water is an Escherichia coli DH5-α strain containing the plasmid pSB403-merR_luxCDABE in which the merR regulatory gene and its cognate Pmer promoter, from the mer resistance operon, is fused to the genes for bacterial luciferase (luxCDABE). To prepare the cell suspension for the assay, the E. coli strain was plated on nutrient agar medium containing 25 μg/mL of tetracycline at 37 °C for 24 h. A single colony was picked and regrown in 10 mL liquid Luria Broth (LB) medium plus tetracycline at 37 °C and 150 rpm until the culture turbidity (optical density at 600 nm) was equal to 0.6. This corresponds to cell concentration of ≈6 × 108 cells/mL. Then, the cell culture was diluted 10-fold in peptone water minimal medium and used for the assay.
2.3. Hg Reporter Assay in Water
The mechanism of E. coli reporter induction by mercury is schematically represented in Figure 1. Basically, the merR gene of pSB403-merR_luxCDABE plasmid in E. coli expresses the MerR transcriptional repressor that blocks transcription from Pmer and thus prevents luciferase to be made by the cell. In presence of Hg2+, MerR allosterically changes its conformation and releases repression on Pmer, which triggers transcription from the lux genes, codifying for both the flavoenzyme bacterial luciferase and its long-chain aldehyde substrate n-decanal [41], giving the following reaction:
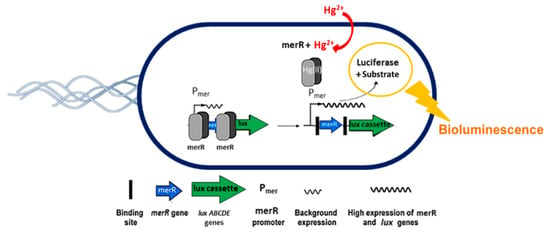
Figure 1.
Mechanism of E. coli reporter induction by Hg2+ (freely inspired figure [43]).
Once expressed, the bacterial luciferase catalyzes the oxidation of a reduced flavin mononucleotide (FMNH2) and the aldehyde substrate (RCHO) by molecular oxygen to yield oxidized FMN, a carboxylic acid (RCOOH) and water with concomitant blue-green light (hv) at 490 nm [42].
Depending on the amount of mercury in water the cells produce more luciferase and, in aerobic conditions, the intensity of the light increases.
Hg2+ standards for the optical assay were prepared suspending the HgCl2 powder in PBS 1X (0.1mM) to get a 25 mM solution and diluting it in sterile water, down to concentrations of 0.25; 1; 2.5; 10; 25; 50 and 100 µg/L.
For the bioreporter assay, 180 µL of cell culture prepared as described above, were mixed with 20 µL of Hg2+ standards, or with tap water as blank. The 8 samples were transferred to 500 µL tubes and incubated at 32 °C and 350 rpm for 1 h. After 1 h incubation, assay mixtures were transferred into the measurement cuvette inside the SiPM based optical system for the luminescence analysis.
The first luminescence test was performed preparing samples in a final volume of 2 mL and acquiring the luminescence by a commercial Charge-Coupled Device (CCD camera), with an exposure time of 20 s.
As a comparison, similar assay mixtures were prepared and transferred into a 96 well plate, for measurement of bioluminescence in a TriStar luminometer. The same experiments were performed three times.
2.4. Optical Sensor for the Hg Sensing in Water
The optical system and detector used for the Hg reporter luminescence characterization are shown in Figure 2. The sensing system, Figure 2a, is packaged inside a dark box and consists of a cuvette, containing the E. coli reporter cells, placed on the top of a silicon photomultiplier, SiPM, sensor that collects the bioluminescence signal coming from the induced cells. The SiPM sensor, Figure 2b, is a small size (250 µm × 250 µm), low cost, easily managed, pixelated photodetector [44,45] composed by an array of 100 microcells, i.e., passively quenched single-photon avalanche diodes (SPAD), with 25µm pitch (fill factor > 72%), based on the RGB-HD technology (from Fondazione Bruno Kessler (FBK)) [46], having peak sensitivity in the green spectral range. SiPMs are used in medical imaging, scientific experiments and biology, being able to achieve a single photon sensitivity but also having a high dynamic range. The SiPM signal is typically amplified and the resulting output is composed by a series of pulses, each one indicating the detection of a photon (or noise event). In this case instead, the readout is more simple: the system measure directly the current flowing through the SiPM.
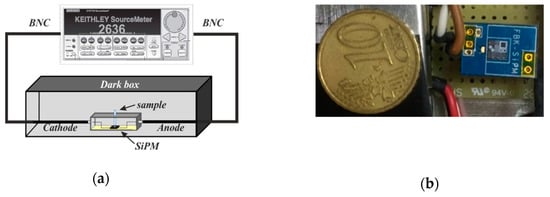
Figure 2.
(a) Scheme of the optical sensing system for bioluminescence analysis of the integrated Hg reporter cells. (b) Detail of the silicon photomultiplier (SiPM) sensor (the 250 µm × 250 µm SiPM structure is included in a bigger test chip, mounted on Fondazione Bruno Kessler (FBK) custom PCB/package).
The SiPM is biased at −29 V through a 2636 Keithley Source Meter, which directly measured the time-dependent SiPM current, referred as the “signal”. The biasing condition was chosen in order to maximize the signal-to-noise-ratio (SNR). Current–voltage curves from −34V to −28V were acquired in dark and during the experiment (bioluminescence acquisition) and the best SNR voltage determined. A custom-developed Labview software controls the bias voltage, the measurement conditions and the acquisition of the SiPM output current. The signal was acquired for 1 min and elaborated offline by a routine developed in MATLAB.
3. Results
3.1. First Optical Check of Hg2+ Reporter-Induced Bioluminescence
Assays of Hg2+ reporter activity started with a first macroscopic check, performed by a CCD camera, of the bioluminescence trend as function of Hg2+ concentration in water samples. E. coli reporter bioluminescence increases in comparison to a water-only control, from 0.25; 1; 2.5; 10; 25; 50 and 100 µg/L Hg2+ treatment in 2 mL of water as shown in Figure 3.
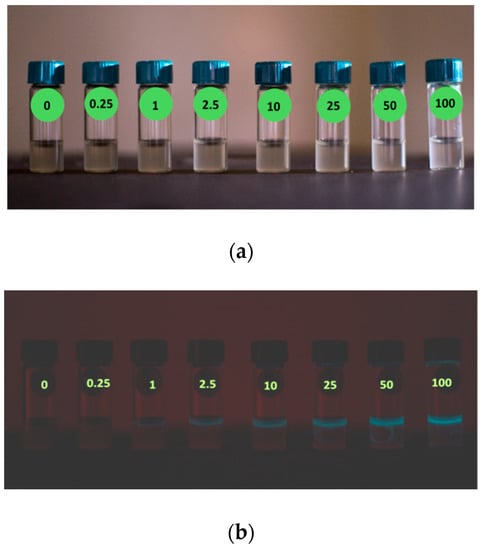
Figure 3.
Bioluminescence of the E. coli reporter to 0 (tap water), 0.25; 1; 2.5; 10; 25; 50 or 100 µg/L Hg2+ treatment in water: (a) light conditions; (b) dark conditions (20 s exposure time).
A weak halo of the light blue luciferase luminescence was visible starting on top of solution of the reporter sample treated with 1 µg/L Hg2+, and its intensity gradually increased with the concentration of pollutant. The reason of this partial distribution of the luminescence signal inside the glass vial was that solutions remained static during the image acquisition (and during the 5 min before). The agitation is a key element for the bioluminescence reaction (see Section 2) since it enhances the oxygen diffusion and its uptake by those bacteria that are far away from the air interface of suspension, allowing the luciferase reaction to proceed.
We repeated the experiment using the 0 and 100 µg/L Hg2+ treated samples under 300 rpm magnetic agitation during the images acquisition and the results are reported in Figure 4.
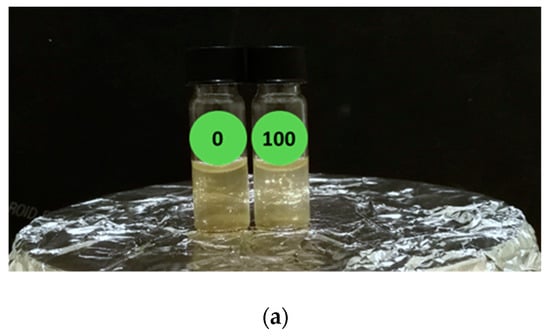
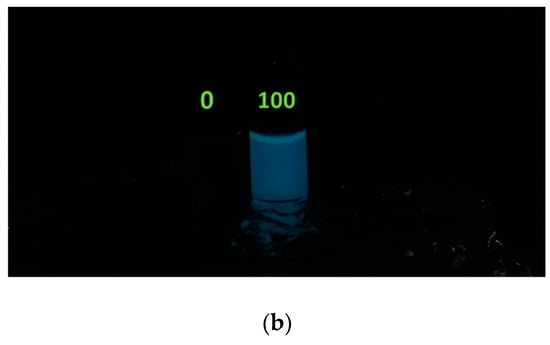
Figure 4.
Luminescent responsivity of 0 and 100 µg/L Hg2+ treated E. coli reporter samples under agitation: (a) light conditions; (b) dark conditions.
In this case, a diffused and enhanced luminescent signal is visible in the 100 µg/L Hg2+ compared to the zero. The enhanced luminescence on the liquid sample surface has two causes: the presence of a higher O concentration at the interface with the solution. Cells close to the air/water interface have a higher oxygen influx than deeper cells; hence, the bioluminescence mechanism can occur. Moreover, there is an optical effect, a sort of “lens-effect”, at the air/liquid interface when the solution is illuminated (see Figure 4a).
3.2. Hg2+ Reporter Activity Validation by Silicon Photomultiplier (SiPM)-Based Sensing System
Once verified the bacteria are working as sensing element by visual inspection, the E. coli reporter activity was verified by measuring Hg2+ induced bioluminescence in the miniaturized SiPM-based sensing system described in Section 2.
For the analysis, we spotted the 200 µL samples of E. coli reporter, treated with 0, 0.25; 1; 2.5; 10; 25; 50 and 100 µg/L Hg2+, directly into the cuvette of the miniaturized system. The luminescent signal was acquired for 1 min, after 1 h induction and the results are reported in Figure 5 (biasing conditions are reported in the experimental section).
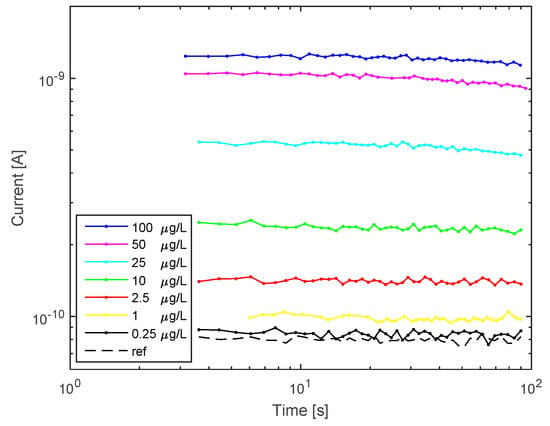
Figure 5.
Mercury-induced bioluminescence detected by the SiPM sensor: E. coli reporter samples treated with 0 (reference, black line); 0.25 (yellow line); 1 (green line); 2.5 (purple line); 10 (blue line); 25 (red line); 50 (brown line) and 100 (light blue line) µg/L Hg2+, for 1 h; signal measurement time: 1 min.
Figure 5 shows that the miniaturized sensing system is able to detect the presence of Hg2+ traces in water. SiPM optical detection revealed that during the first 100 s the current signal, transduced by the incoming induced luminescence is stable and there is a correlation between the bioluminescence intensity and the mercury concentration in water. The signal decrease after 100 s may be due to the static conditions maintained (no agitation) in the SiPM during measurements, which may reduce oxygen diffusion, as described above. The lowest detectable Hg2+ concentration was 0.25 µg/L as shown by the current value (yellow line in figure) that overpasses the blank sample (black line). The limit of detection (LoD) was calculated by extrapolating the calibration curve and considering the device dark current (LoD is three times the dark current). The result was 0.15 ug/L, below the lowest concentration measured.
The device performances were compared with a standard method for Hg2+ detection. The same experiment was repeated by using a conventional TriStar luminometer for measuring the reporter cell’s bioluminescence (Figure 6; red squares, referred to the left axis). In the same figure, the results obtained by the SiPM-based system on the same samples are reported (Figure 6, blue squares referred to the right axis). The values measured are the same, within the experimental errors, confirming our system can efficiently detect Hg2+ contamination. The lowest measurable concentration (0.25 µg/L of Hg2+) was the same for the two measurement systems.
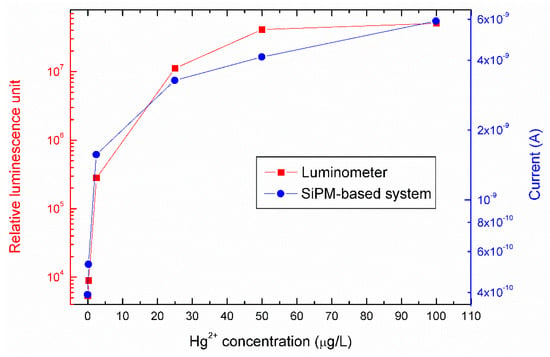
Figure 6.
Data comparison between the mercury-induced bioluminescence output of the E. coli reporter cells measured in the SiPM-based miniaturized system (blue curve, referred to the right axis) and by a conventional luminometer (red curve, referred to the left axis). The SiPM-based system current data were taken at 10 s of acquisition.
4. Discussion
Among heavy metals, mercury is considered one of the major environmental pollutants. It is strongly recommended that the use of mercury products in industry and medicine should be eliminated, where possible, as a preventive measure for public health. In fact, even though overt mercury poisoning is rare, the risk to human health remains a major public concern due to the continuous contamination in the environment. Thus, its control in water intended for drinking and contact recreation is important and many worldwide institutions such as the World Health Organization (WHO), the Council of the European Union and the Environmental Protection Agency (EPA) have imposed a common limit of 1 µg/L [2,5,47,48].
A number of analytical methods can be used for the detection and quantification of low concentrations of mercury in water, including cold-vapor atomic fluorescence spectrometry (CV-AFS), cold-vapor atomic absorption spectrometry (CV-AAS) [49], and flow injection-inductively coupled plasma optical emission spectrometry (FI-ICP-OES) [50]. These methods are generally time-consuming because mercury must be reduced to the elemental state and preconcentrated prior to the analysis [51,52]. Finally, such conventional analytical methods are generally expensive and require dedicated laboratories.
More recently, alternative strategies based on whole cell biosensors have been developed in order to face these drawbacks. In this work, we introduce a whole-cell based miniaturizable system for bioluminescent inorganic mercury detection in water. The main advancement of the proposed technology is the miniaturizability itself, which means the possibility to perform on-site, real-time and cheap (low reactant consumption) measurements.
Bioluminescence detection is the best approach since it excludes any external laser/light source of excitation thus minimizing the signal noise and the risk of photobleaching, typical issues in fluorescent sensors [53].
The sensor is composed by a cuvette containing a suspension of the E. coli reporter bacteria, and a micro-sized silicon photomultiplier, SiPM, detector, both integrated in a miniaturized dark box. The SiPM is an optical detector widely used in biosensing applications for healthcare and environmental monitoring [54,55,56,57].
The principle of the measurement bases on the induction of luciferase production in the E. coli reporter cells in response to detected Hg2+ in the water, entering the cells. The luciferase spontaneously emits bioluminescence without any further need of externally added cosubstrates. The SiPM acquires and transduces the 490 nm bioluminescence light into a current signal that is processed for a qualitative and quantitative analysis (complete technology is described in Section 2). The higher is the influx of Hg2+ into the cells, the higher is the luciferase production and the resulting bioluminescence, up to a saturation level.
The increasing bioluminescence as a function of mercury concentration to which the cells are exposed was easily seen in a simple photographic reproduction of the reporter induction kinetics. This analysis also showed the importance of oxygen as a cofactor for the luciferase reaction (Figure 3 and Figure 4). Agitation of the cells enhances the oxygen uptake thus leading to an increase of luminescence intensity, as proved by the image in Figure 4b acquired from a mixed 100 µg/L Hg2+ treated sample.
In a second phase, the sensitivity and performance of the proposed miniaturizable detection system for the cellular bioluminescence were evaluated. The limit of detection in the SiPM-based detector was 0.25 µg/L of Hg2+, which is lower than the necessary regulatory thresholds for Hg2+ in water intended for human consumption. The bioluminescence increased in a sigmoidal trend as a function of Hg2+ concentration, increasing from 0.25 to 25 µg/L of Hg2+, and then reaching a plateau between 50 and 100 µg/L. Similar trends were seen when bioreporter assays were measured in a conventional luminometer (Figure 6). The SiPM device is thus an accurate replacement for the detection of the cellular bioluminescence.
Both technologies imply small volumes of reagent solutions (no more than 200 µL) and 1h of reporter induction, thus reducing the time of operation and materials. However, the proposed sensor brings an additional advantage of integration of both sensing element and detector and miniaturizability of the entire sensing system. This is perfectly consistent with the idea of a portable analyzer for the in situ surveillance of water quality and safety.
The presented results are intended as a first characterization of the system and the aim was not to present a ready-to-use analytical method that can substitute the gold standard, considering that measurement parameters were varied, no interferences were checked, and no real water samples were tested. For these reasons, further experiments are in progress in order to better define the limitation of the proposed technology in real case scenarios. Moreover, the specificity of this type of bioassay, already documented for similar bioreporters sensitive to other priority chemicals, will also be evaluated [58,59].
5. Conclusions
The whole-cell based miniaturizable sensing system we proposed (based on a small-size silicon photomultiplier) is able to reveal the presence of mercury in water at 0.25 µg/L, providing a good sensitivity within the micro-sized detection system, comparable to the last generation TriStar² S LB 942 Modular Monochromator Multimode Reader luminometer. The dynamic range was from 0.25 to 25 µg/L Hg2+, covering the most relevant environmental concentrations and detecting below the worldwide regulatory thresholds of 1 µg/L established for mercury accumulation in water. Moreover, the proposed system requires no additional reagents to perform the analysis, leading to important cost savings. The small dimensions and the miniaturizability of the entire system pave the basis for a portable detection technology that could be easily extended to other E. coli strains targeting different priority chemicals. Finally, the proposed technology can find potential extensions also in SPA pools or in regulating natural ponds, when considering specific SPA waters from natural thermal springs and other chemical elements [60].
Author Contributions
Conceptualization, E.L.S., M.A.C. and S.L.; methodology, J.R.v.d.M., E.L.S. and S.L.; investigation and data curation, E.L.S., D.C. and S.L.; writing—original draft preparation, E.L.S. and M.A.C., writing—review and editing, D.C., J.R.v.d.M., F.A., S.L. and M.A.C.; SiPM production, packaging and characterization, F.A. and A.G.; supervision, J.R.v.d.M., S.L. and M.A.C., project administration, S.L. and M.A.C.
Funding
E.L.S. acknowledges the regional project “Sviluppo ed applicazione di tecnologie biosensoristiche in genomica” [CIP 2014.IT.05.SFOP.014/3/10.4/9.2.10/0008, CUP G67B17000170009] for funding the time spent on this activity.
Acknowledgments
The authors acknowledge G. Faro for her precious help in sample preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Texas Water Quality Standards. Criteria for Recreation; TCEQ Staff: Austin, TX, USA, 2007.
- World Health Organization. Coastal and Fresh Waters. In Guidelines for Safe Recreational Water Environments; WHO: Geneva, Switzerland, 2003; Volume 1, ISBN 92 4 154580. [Google Scholar]
- Valeriani, F.; Protano, C.; Vitali, M.; Romano Spica, V. Swimming attendance during childhood and development of asthma: Meta-analysis. Pediatr. Int. 2017, 59, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Water Quality: Ambient Water Quality Guidelines for Mercury Overview Report—First Update; Ministry of Environment: Victoria, BC, Canada, 1981.
- World Health Organization. Recommendations. In Guidelines for Drinking-Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2004; Volume 1, ISBN 92 4 154638 7. [Google Scholar]
- Lepak, J.M.; Shayler, H.A.; Kraft, C.E.; Knuth, B.A. Mercury contamination in sport fish in the Northeastern United States: Considerations for future data collection. BioScience 2009, 59, 174–181. [Google Scholar] [CrossRef]
- Taylor, D.R.; Williamson, P.R. Mercury contamination in Southern New England coastal fisheries and dietary habits of recreational anglers and their families: Implications to human health and issuance of consumption advisories. Mar. Pollut. Bull. 2017, 114, 144–156. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. 2003. Available online: https://www.who.int/ipcs/publications/cicad/en/cicad50.pdf (accessed on 4 June 2019).
- CDC. Environmental Health. Mercury; 2009. Available online: https://www.cdc.gov/biomonitoring/pdf/Mercury_FactSheet.pdf (accessed on 12 June 2019).
- Environmental Protection Agency. Mercury: Basic Information. Available online: http://www.epa.gov/mercury/about.htm (accessed on 12 June 2019).
- National Research Council. Toxicological Effects of Methylmercury; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Mergler, D.; Anderson, H.A.; Chan, L.H.M.; Mahaffey, K.R.; Murray, M.; Sakamoto, M.; Stern, A.H. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio 2007, 36, 3–11. [Google Scholar] [CrossRef]
- Yu, Y.L.; Wang, J.H. Recent advances in flow-based sample pretreatment for the determination of metal species by atomic spectrometry. Chin. Sci. Bull. 2013, 58, 1992–2002. [Google Scholar] [CrossRef]
- Suvarapu, L.N.; Seo, Y.-K.; Baek, S.-O. Speciation and determination of mercury by various analytical techniques. Rev. Anal. Chem. 2013, 32, 225–245. [Google Scholar] [CrossRef]
- Popp, M.; Hann, S.; Koellensperger, G. Environmental application of elemental speciation analysis based on liquid or gas chromatography hyphenated to inductively coupled plasma mass spectrometry—A review. Anal. Chim. Acta 2010, 668, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Turker, A.R. Separation, preconcentration and speciation of metal ions by solid phase extraction. Sep. Purif. Rev. 2012, 41, 3169–3206. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Lemos, V.A.; Silva, L.O.B.; Queiroz, A.F.S.; Souza, A.S.; da Silva, E.G.P.; dos Santos, W.N.L.; das Virgens, C.F. Analytical strategies of sample preparation for the determination of mercury in food matrices—A review. Microchem. J. 2015, 121, 227–236. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, Z.; Long, Z.; Wu, P.; Zheng, C.; Hou, X. Determination and speciation of mercury in environmental and biological samples by analytical atomic spectrometry. Microchem. J. 2012, 103, 1–14. [Google Scholar] [CrossRef]
- Chung, S.W.; Chan, B.T. A reliable method to determine methylmercury and ethylmercury simultaneously in foods by gas chromatography with inductively coupled plasma mass spectrometry after enzymatic and acid digestion. J. Chrom. A 2011, 9, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Kodamatani, H.; Kanzaki, R.; Tomiyasu, T.; Saito, K.; Kono, Y. Determination of organic and inorganic mercury species as emetine dithiocarbamate complexes by high-performance liquid chromatography with electrogeneratedtris (2,20-bipyridine) ruthenium(iii) chemiluminescence detection. Anal. Lett. 2011, 44, 2769–2779. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Saber-Tehrani, M. Sensitive mercury speciation by reversed phase column high-performance liquid chromatography with UV-visible detection after solid-phase extraction using 6-mercaptopurine and dithizone. J. AOAC Int. 2008, 6, 1453–1458. [Google Scholar]
- Rodrigues, J.L.; de Souza, S.S.; de Oliveira Souza, V.C.; Barbosa, F., Jr. Methylmercury and inorganic mercury determination in blood by using liquid chromatography with inductively coupled plasma mass spectrometry and a fast sample preparation procedure. Talanta 2010, 3, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Dressler, V.L.; Santos, C.M.M.; Antes, F.G.; Bentlin, F.R.S.; Pozebon, D.; Flores, E.M.M. Total mercury, inorganic mercury and methyl mercury determination in red wine. Food Anal. Methods 2012, 5, 505–511. [Google Scholar] [CrossRef]
- Zhu, Z.Q.; Su, Y.; Li, J.; Li, D.; Zhang, J.; Song, S.; Zhao, Y.; Li, G.; Fan, C. Highly sensitive electrochemical sensor for mercury(II) ions by using a mercury-specific oligonucleotide probe and gold nanoparticle-based Amplification. Anal. Chem. 2009, 81, 7660–7666. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.C.; Chen, X.; Guo, Z.; Xie, C.G.; Kong, L.T.; Liu, J.H.; Huang, X.J. Stripping voltammetric detection of mercury(II) based on a surface ion imprinting strategy in electropolymerized microporous poly(2-mercaptobenzothiazole) films modified glassy carbon electrode. Anal. Chim. Acta 2011, 685, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Chen, X.; Yang, Q.; Liu, J.H.; Huang, X.J. Selective adsorption toward toxic metal ions results in selective response: Electrochemical studies on a polypyrrole/reduced graphene oxide nanocomposite. Chem. Comm. 2012, 48, 2180–2182. [Google Scholar] [CrossRef]
- Fu, X.C.; Wu, J.; Nie, L.; Xie, C.G.; Liu, J.H.; Huang, X.J. Electropolymerized surface ion imprinting films on a gold nanoparticles/single-wall carbon nanotube nanohybrids modified glassy carbon electrode for electrochemical detection of trace mercury(II) in water. Anal. Chim. Act. 2012, 720, 29–37. [Google Scholar] [CrossRef]
- Gao, C.; Huang, X.J. Voltammetric determination of mercury(II). TrAC Trends Anal. Chem. 2013, 51, 1–12. [Google Scholar] [CrossRef]
- Guerrini, L.; Rodriguez-Loureiro, I.; Correa-Duarte, M.A.; Lee, Y.H.; Ling, X.Y.; García de Abajo, F.J.; Alvarez-Puebla, R.A. Chemical speciation of heavy metals by surface-enhanced Raman scattering spectroscopy: Identification and quantification of inorganic- and methyl-mercury in water. Nanoscale 2014, 6, 8368–8375. [Google Scholar] [CrossRef] [PubMed]
- Pujol, L.; Evrard, D.; Groenen-Serrano, K.; Freyssinier, M.; Ruffien-Cizsak, A.; Gros, P. Electrochemical sensors and devices for heavy metals assay in water: The French groups’ contribution. Front. Chem. 2014, 2, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R.; Patel, V.B. Biosensors and Environmental Health; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Dzantiev, B.B.; Zherdev, A.V. Antibody-Based Biosensors. In Portable Biosensing of Food Toxicants and Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2013; pp. 161–196. [Google Scholar]
- Gu, M.B.; Mitchell, R.J.; Kim, B.C. Whole-cell-based biosensors for environmental biomonitoring and application. Adv. Biochem. Eng. Biotechnol. 2004, 87, 269–305. [Google Scholar] [PubMed]
- Gui, Q.; Lawson, T.; Shan, S.; Yan, L.; Liu, Y. The application of whole cell-based biosensors for use in environmental analysis and in medical diagnostics. Sensors 2017, 17, 1623. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, K.H.; Giovangrandi, L.; Whittington, R.H.; Kovacs, G.T. Sensitivity of cell-based biosensors to environmental variables. Biosens. Bioelectr. 2005, 20, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Hakkila, K.; Green, T.; Leskinen, P.; Ivask, A.; Marks, R.; Virta, M. Detection of bioavailable heavy metals in EILA Tox-Oregon samples using whole-cell luminescent bacterial sensors in suspension or immobilized onto fibre-optic tips. J. App. Toxicol. 2004, 24, 333–342. [Google Scholar] [CrossRef]
- Van der Meer, J.R.; Belkin, S. Where microbiology meets microengineering: Design and applications of reporter bacteria. Nat. Rev. Microbiol. 2010, 8, 511–522. [Google Scholar] [CrossRef]
- Schaefer, J.K.; Letowski, J.; Barkay, T. Mer-Mediated Resistance and Volatilization of Hg(II) Under Anaerobic Conditions. Geomicrobiol. J. 2002, 19, 87–102. [Google Scholar] [CrossRef]
- Barkay, T.; Miller, S.M.; Summers, A.O. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol. Rev. 2003, 27, 355–384. [Google Scholar] [CrossRef]
- Brown, N.L.; Stoyanov, J.V.; Kidd, S.P.; Hobman, J.L. The MerR family of transcriptional regulators. FEMS Microbiol. Rev. 2003, 27, 145–163. [Google Scholar] [CrossRef]
- Condee, C.W.; Summers, A.O. A mer-lux Transcriptional Fusion for Real-Time Examination of In Vivo Gene Expression Kinetics and Promoter Response to Altered Superhelicity. J. Bacteriol. 1992, 174, 8094–8101. [Google Scholar] [CrossRef][Green Version]
- Tinikul, R.; Chaiyen, P. Structure, Mechanism, and Mutation of Bacterial Luciferase. In Bioluminescence: Fundamentals and Applications in Biotechnology—Volume 3. Advances in Biochemical Engineering/Biotechnology; Thouand, G., Marks, R., Eds.; Springer: Berlin, Germany, 2014; Volume 154. [Google Scholar]
- Cortés-Salazar, F.; Beggah, S.; van der Meer, J.R.; Girault, H.H. Electrochemical As(III) whole-cell based biochip sensor. Biosens. Bioelectr. 2013, 47, 237–242. [Google Scholar] [CrossRef]
- Mazzillo, M.; Ronzhin, A.; Los, S.; Abisso, S.; Sanfilippo, D.; Valvo, G.; Carbone, B.; Piana, A.; Fallica, G.; Albrow, M.; et al. Electro-optical performances of p on n and n on p Silicon Photomultipliers. IEEE Trans. Elctr. Dev. 2012, 59, 3419–3425. [Google Scholar] [CrossRef]
- Pagano, R.; Valvo, G.; Sanfilippo, D.; Libertino, S.; Corso, D.; Fallica, P.G.; Lombardo, S. Silicon photomultiplier device architecture with dark current improved to the ultimate physical limit. Appl. Phys. Lett. 2013, 102, 183502. [Google Scholar] [CrossRef]
- Acerbi, F.; Paternoster, G.; Gola, A.; Regazzoni, V.; Zorzi, N.; Piemonte, C. High-Density Silicon Photomultipliers: Performance and Linearity Evaluation for High Efficiency and Dynamic-Range Applications. IEEE J. Quantum Electron. 2018, 54, 4700107. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Communities 1998, 41, 32–54. [Google Scholar]
- Mercury and Air Toxics Standards (MATS). Available online: https://www.epa.gov/mats (accessed on 24 May 2019).
- Maranhao, T.A.; Silva, J.S.A.; de Andrade, R.M.; Bascunan, A.F.; de Oliveira, F.J.S.; Curtius, A.J. Determination of As and Hg in acetic acid extract by vapor generation coupled to atomic spectrometry for solid waste classification. Microchem. J. 2013, 106, 139. [Google Scholar] [CrossRef]
- Santos, E.J.; Herrmann, A.; Vieira, A.M.; Frescura, A.V.L.; Curtius, A.J. Evaluation of slurry preparation procedures for the determination of mercury by axial view inductively coupled plasma optical emission spectrometry using on-line cold vapor generation. Spectrochim. Acta B At. Spectrosc. 2005, 60, 659–665. [Google Scholar] [CrossRef]
- Leopold, K.; Foulkes, M.; Worsfold, P. Methods for the determination and speciation of mercury in natural waters. A review. Anal. Chim. Acta 2010, 663, 127. [Google Scholar] [CrossRef]
- Dinu, C.; Vasile, G.G.; Cruceru, L. Advanced analytical methods for mercury determination in slightly contaminated water samples. J. Environ. Prot. Ecol. 2013, 14, 1515–1524. [Google Scholar]
- Sciuto, E.L.; Villaggio, G.; Santangelo, M.F.; Laudani, S.; Federico, C.; Saccone, S.; Sinatra, F.; Libertino, S. Study of a Miniaturizable System for Optical Sensing Application to Human Cells. Appl. Sci. 2019, 9, 975. [Google Scholar] [CrossRef]
- Libertino, S.; Conoci, S.; Santangelo, M.F.; Pagano, R.; Sciuto, E.L.; Sinatra, F.; Sanfilippo, D.; Fallica, G.; Lombardo, S. Optical and electrical Si-based biosensors: Fabrication and trasduction issues. J. Anal. Bioanal. Tech. 2014, 12, 1. [Google Scholar]
- Santangelo, M.F.; Sciuto, E.L.; Lombardo, S.A.; Busacca, A.C.; Petralia, S.; Conoci, S.; Libertino, S. Si photomultipliers for bio-sensing applications. IEEE J. Sel. Top. Quant. Electron. 2015, 22, 335–341. [Google Scholar] [CrossRef]
- Santangelo, M.F.; Libertino, S.; Turner, A.P.F.; Filippini, D.; Mak, W.C. Integrating printed microfluidics with silicon photomultipliers for miniaturised and highly sensitive ATP bioluminescence detection. Biosens. Bioelectron. 2018, 99, 464–470. [Google Scholar] [CrossRef]
- Petralia, S.; Sciuto, E.L.; Santangelo, M.; Libertino, S.; Messina, M.A.; Conoci, S. Sulfide species optical monitoring by a miniaturized silicon photomultiplier. Sensors 2018, 18, 727. [Google Scholar] [CrossRef]
- Buffi, N.; Beggah, S.; Truffer, F.; Geiser, M.; van Lintel, H.; Renauda, P.; van der Meer, J.R. An automated microreactor for semi-continuous biosensor measurements. Lab Chip 2016, 16, 1383–1392. [Google Scholar] [CrossRef][Green Version]
- Durand, M.J.; Hua, A.; Jouanneau, S.; Cregut, M.; Thouand, G. Detection of Metal and Organometallic Compounds with Bioluminescent Bacterial Bioassays. In Bioluminescence: Fundamentals and Applications in Biotechnology—Volume 3. Advances in Biochemical Engineering/Biotechnology; Thouand, G., Marks, R., Eds.; Springer: Berlin, Germany, 2015; Volume 154. [Google Scholar]
- Giampaoli, S.; Garrec, N.; Donzé, G.; Valeriani, F.; Erdinger, L.; Romano Spica, V. Regulations concerning natural swimming ponds in Europe: Considerations on public health issues. J. Water Health 2014, 12, 564–572. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).