Uptake of Sb(V) by Nano Fe3O4-Decorated Iron Oxy-Hydroxides
Abstract
:1. Introduction
2. Materials and Methods
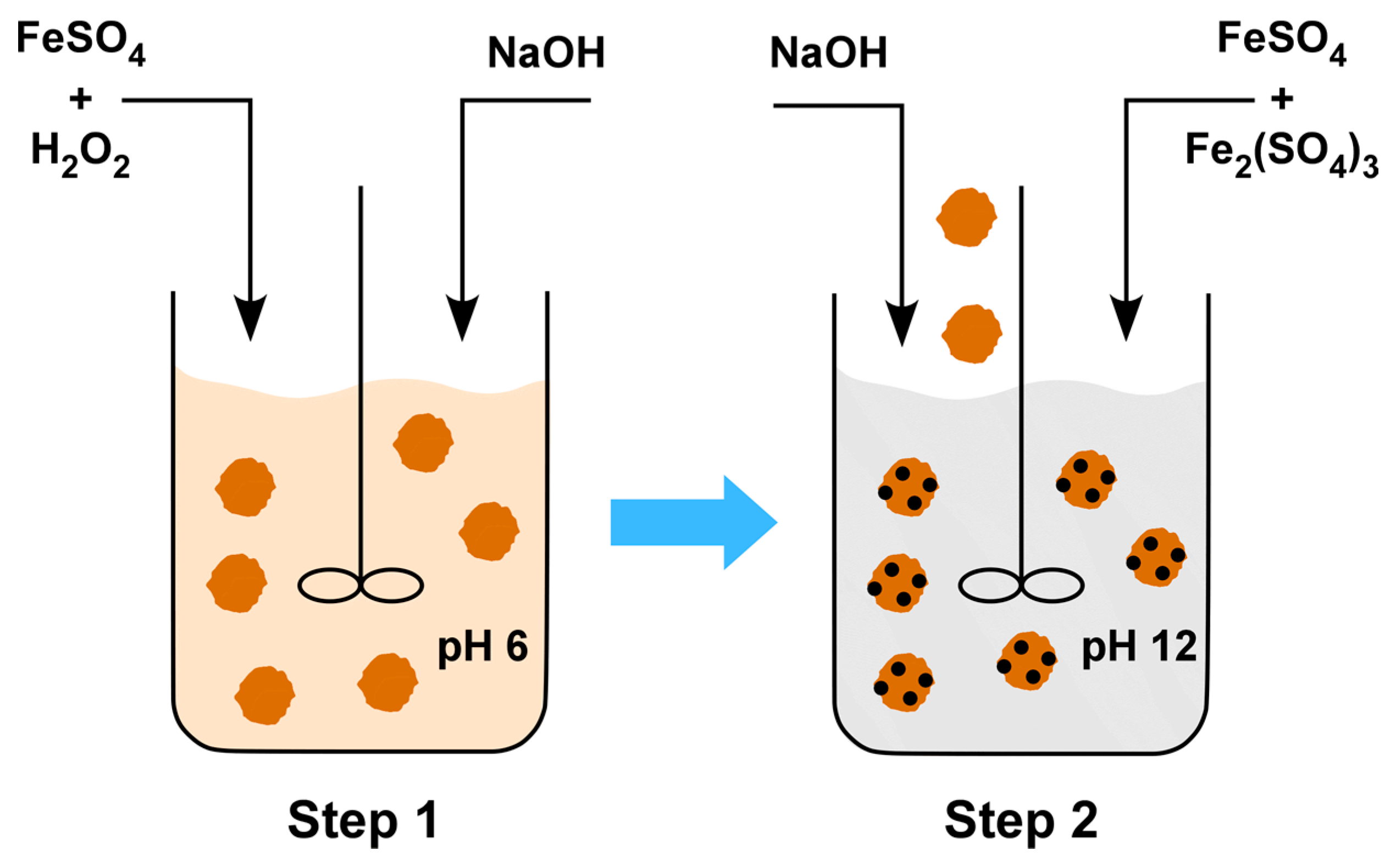
2.1. Synthesis
2.2. Characterization
2.3. Antimony Adsorption
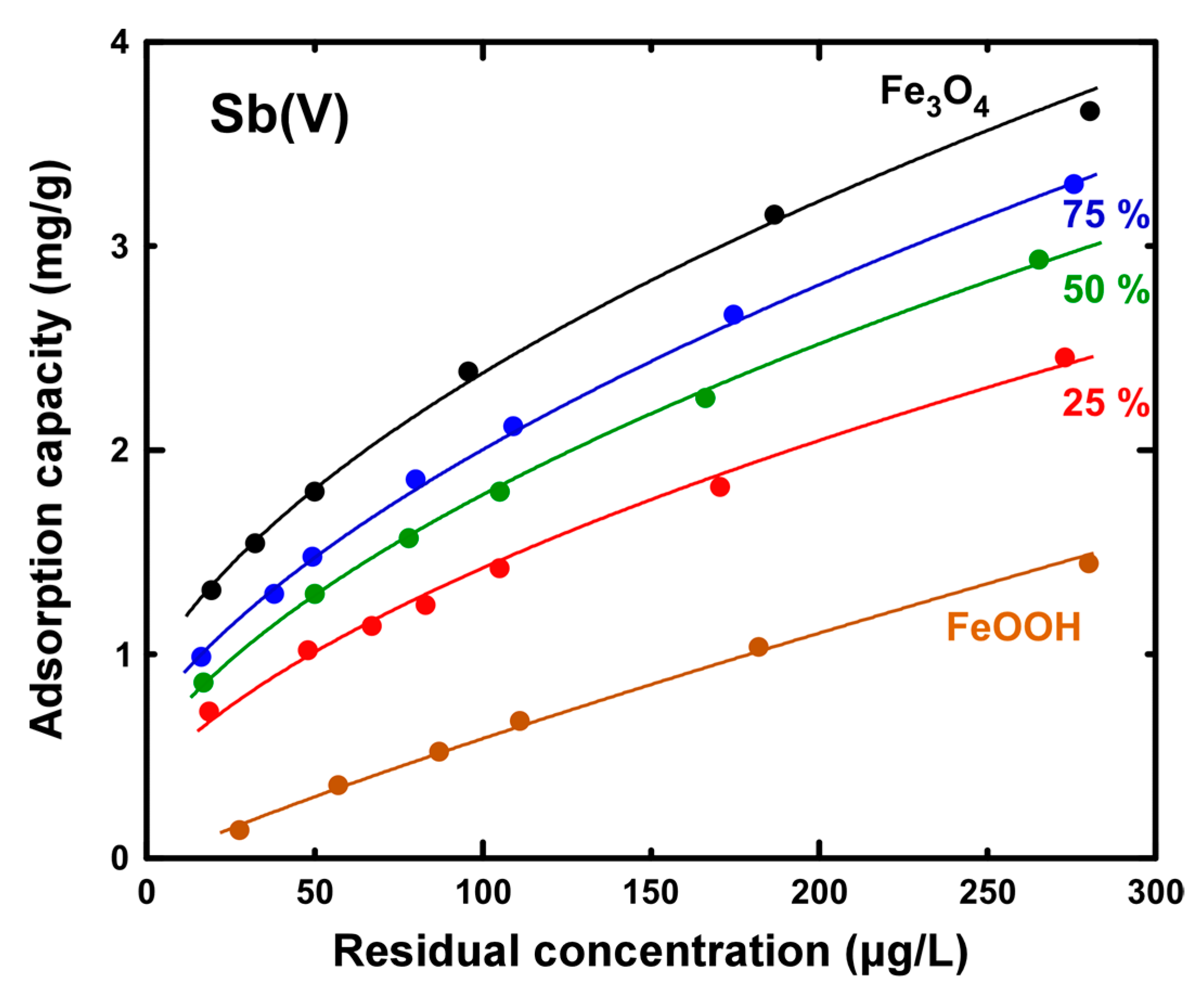
3. Results
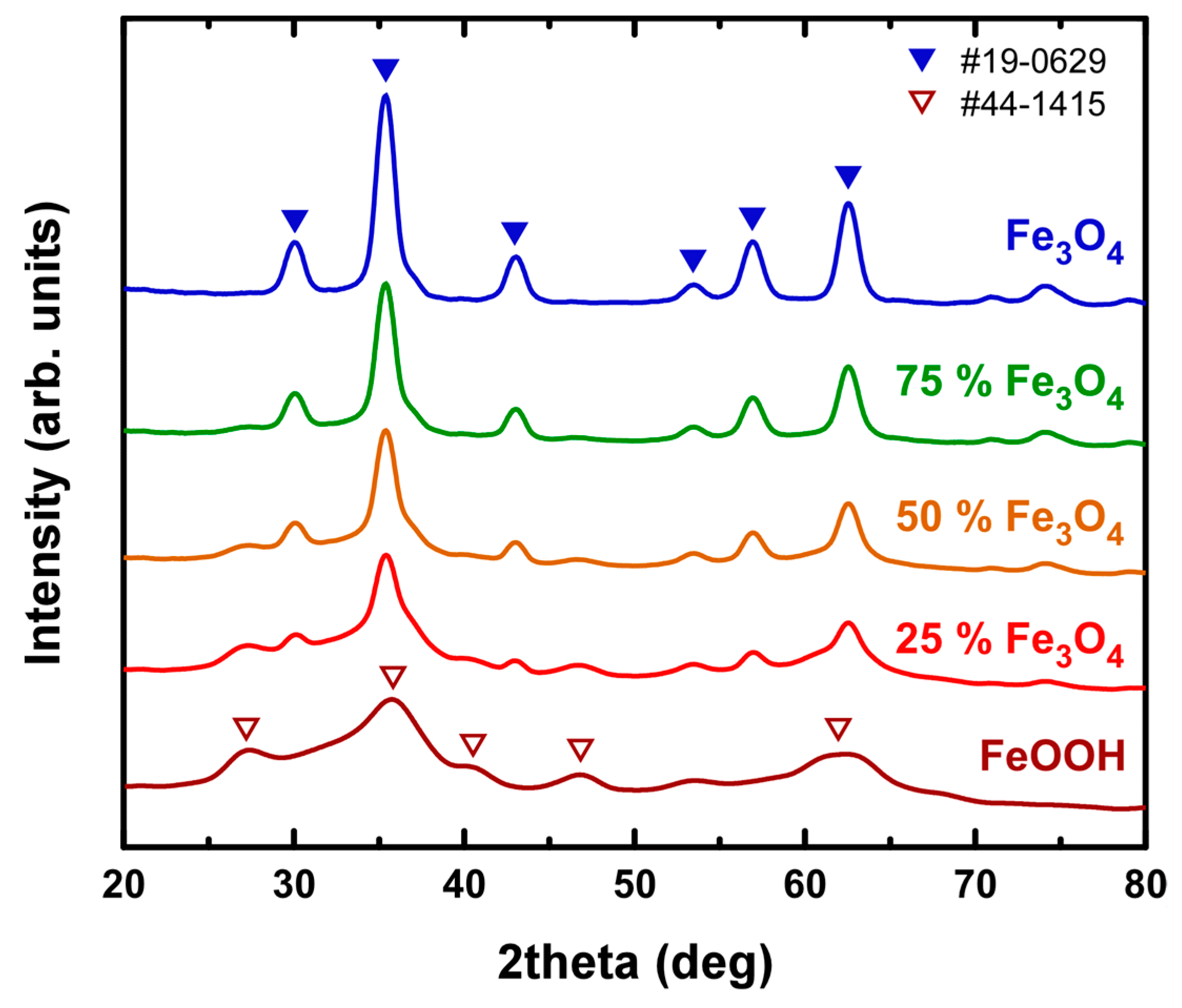
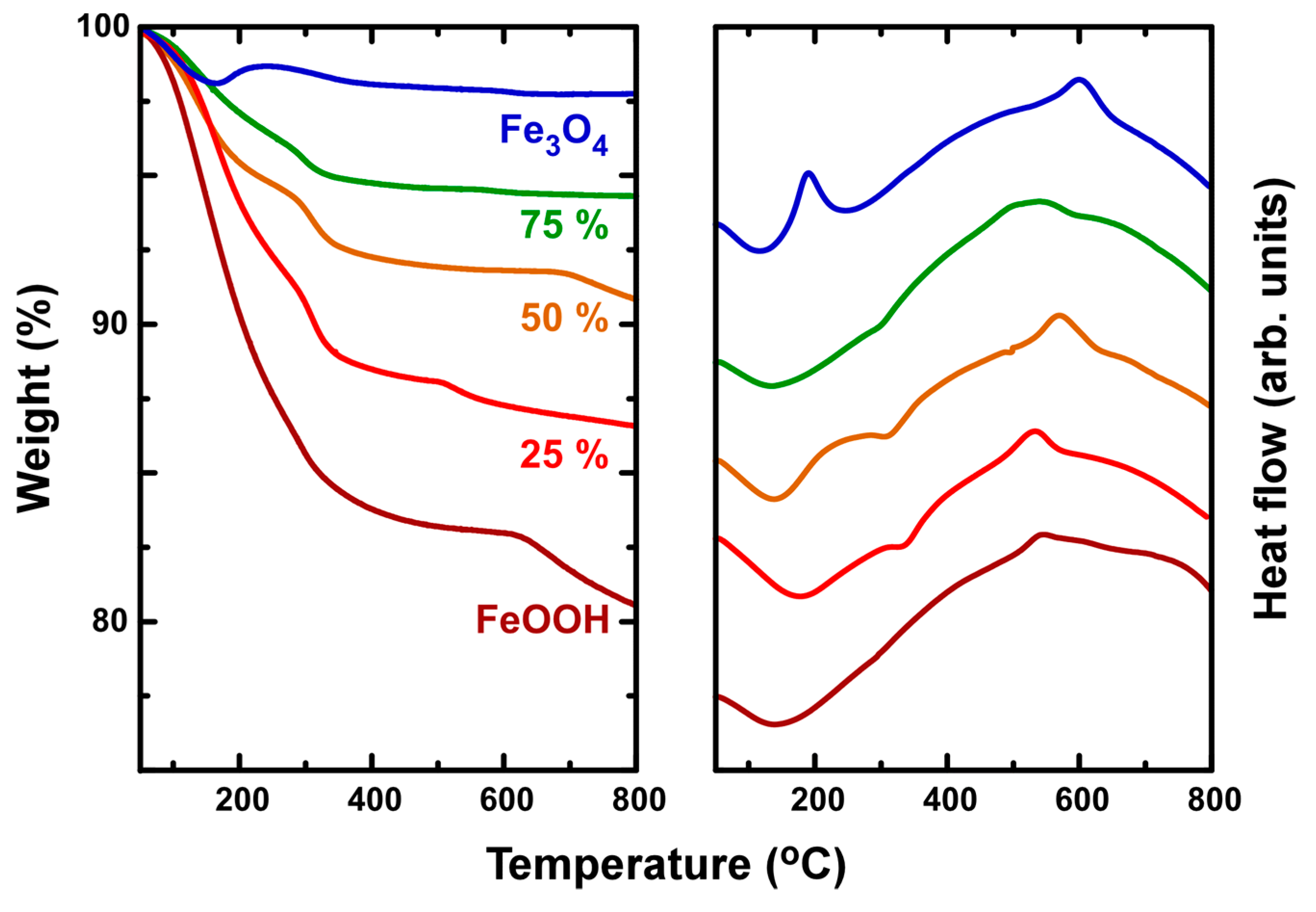
3.1. Material Characterization
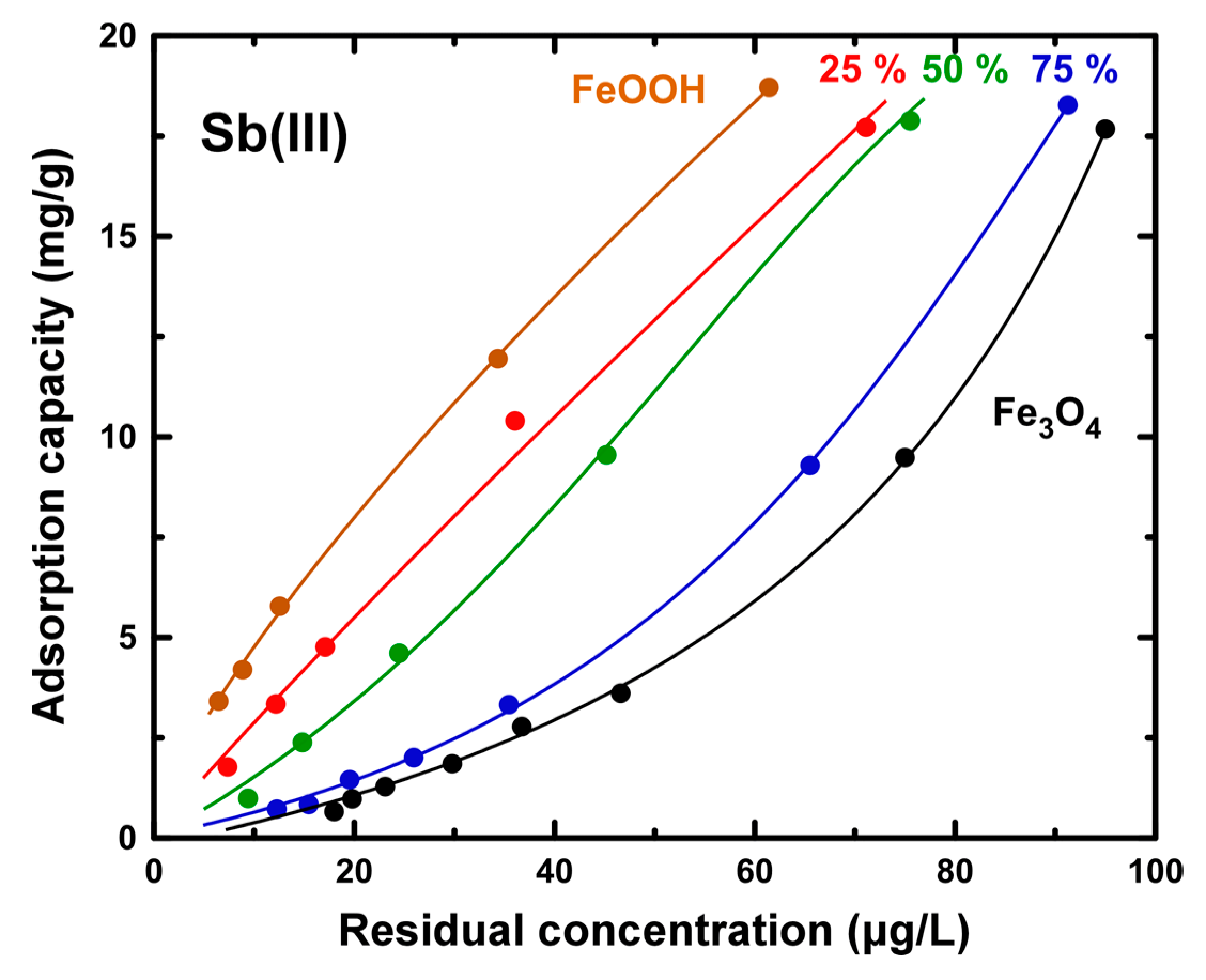
3.2. Evaluation of Sb Removal
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Filella, M.; Belzile, N.; Chen, Y.-W. Antimony in the environment: A review focused on natural waters: I. Occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency. Edition of the Drinking Water Standards and Health Advisories; US Environmental Protection Agency: Washington, DC, USA, 2012.
- Scheinost, A.C.; Rossberg, A.; Vantelon, D.; Xifra, I.; Kretzschmar, R.; Leuz, A.K.; Funke, H.; Johnson, C.A. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 3299–3312. [Google Scholar] [CrossRef]
- Wu, Z.; He, M.; Guo, X.; Zhou, R. Removal of antimony (III) and antimony (V) from drinking water by ferric chloride coagulation: Competing ion effect and the mechanism analysis. Sep. Purif. Technol. 2010, 76, 184–190. [Google Scholar] [CrossRef]
- Li, X.; Dou, X.; Li, J. Antimony(V) removal from water by iron-zirconium bimetal oxide: Performance and mechanism. J. Environ. Sci. 2012, 24, 1197–1203. [Google Scholar] [CrossRef]
- Mitrakas, M.; Mantha, Z.; Tzollas, N.; Stylianou, S.; Katsoyiannis, I.; Zouboulis, A. Removal of Antimony Species, Sb(III)/Sb(V), from Water by Using Iron Coagulants. Water 2018, 10, 1328. [Google Scholar]
- Wu, D.; Sun, S.-P.; He, M.; Wu, Z.; Xiao, J.; Chen, X.D.; Wu, W.D. As(V) and Sb(V) co-adsorption onto ferrihydrite: Synergistic effect of Sb(V) on As(V) under competitive conditions. Environ. Sci. Pollut. Res. 2018, 25, 14585–14594. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Zhu, L.; Lin, S.; Sun, X.; Jiang, Z.; Tratnyek, P.G. Sequestration of antimonite by zerovalent iron: Using weak magnetic field effects to enhance performance and characterize reaction mechanisms. Environ. Sci. Technol. 2016, 50, 1483–1491. [Google Scholar] [CrossRef]
- Leng, Y.; Guo, W.; Su, S.; Yi, C.; Xing, L. Removal of antimony(III) from aqueous solution by graphene as an adsorbent. Chem. Eng. J. 2012, 211–212, 406–411. [Google Scholar] [CrossRef]
- Xu, Y.; Ohki, A. Adsorption and removal of antimony from aqueous solution by an activated alumina. Toxicol. Environ. Chem. 2001, 80, 145–154. [Google Scholar] [CrossRef]
- Wingenfelder, U.; Furrer, G.; Schulin, R. Sorption of antimonate by HDTMA-modified zeolite. Microporous Mesoporous Mater. 2006, 95, 265–271. [Google Scholar] [CrossRef]
- Simeonidis, K.; Papadopoulou, V.; Tresintsi, S.; Kokkinos, E.; Katsoyiannis, I.; Zouboulis, A.; Mitrakas, M. Efficiency of iron-based oxy-hydroxides in removing antimony from groundwater to levels below the drinking water regulation limits. Sustainability 2017, 9, 238. [Google Scholar] [CrossRef]
- Shan, C.; Ma, Z.; Tong, M. Efficient removal of trace antimony(III) through adsorption by hematite modified magnetic nanoparticles. J. Hazard. Mater. 2014, 268, 229–236. [Google Scholar] [CrossRef]
- Ilavský, J.; Barloková, D.; Hudec, P.; Munka, K. Iron-based sorption materials for the removal of antimony from water. J. Water Supply Res. Technol. 2014, 63, 518–524. [Google Scholar] [CrossRef]
- Miao, Y.; Han, F.; Pan, B.; Niu, Y.; Nie, G.; Lv, L. Antimony(V) removal from water by hydrated ferric oxides supported by calcite sand and polymeric anion exchanger. J. Environ. Sci. 2014, 26, 307–314. [Google Scholar] [CrossRef]
- Mishra, S.; Dwivedi, J.; Kumar, A.; Sankararamakrishnan, N. Removal of antimonite (Sb(III)) and antimonate (Sb(V)) using zerovalent iron decorated functionalized carbon nanotubes. RSC Adv. 2016, 6, 95865–95878. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.W.; Wang, Z. Oxidation of antimony (III) by amorphous iron and Manganese oxyhydroxides. Chem. Geol. 2001, 174, 379–387. [Google Scholar] [CrossRef]
- Qi, P.; Pichler, T. Sequential and simultaneous adsorption of Sb(III) and Sb(V) on ferrihydrite: Implications for oxidation and competition. Chemosphere 2016, 145, 55–60. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Zouboulis, A.; Mitrakas, M. Comparative study of As(V) removal by ferric coagulation and oxy-hydroxides adsorption: Laboratory and full-scale case studies. Desalin. Water Treat. 2013, 51, 2872–2880. [Google Scholar] [CrossRef]
- Ebadi, A.; Soltan Mohammadzadeh, J.S.; Khudiev, A. What is the correct form of BET isotherm for modeling liquid phase adsorption? Adsorption 2009, 15, 65–73. [Google Scholar] [CrossRef]
- Powder Diffraction File, 2004th ed.; Joint Center for Powder Diffraction Studies, International Centre for Diffraction Data: Newtown Square, PA, USA, 2004.
- Simeonidis, K.; Kaprara, E.; Samaras, T.; Angelakeris, M.; Pliatsikas, N.; Vourlias, G.; Mitrakas, M.; Andritsos, N. Optimizing magnetic nanoparticles for drinking water technology: The case of Cr(VI). Sci. Total Environ. 2015, 535, 61–68. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, R.; Liu, H.; Qu, J. Adsorption of Sb(III) and Sb(V) on freshly prepared ferric hydroxide (FeOxHy). Environ. Eng. Sci. 2015, 32, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ilgen, A.G.; Trainor, T.P. Sb(III) and Sb(V) sorption onto Al-rich phases: Hydrous Al oxide and the clay minerals kaolinite KGa-1b and oxidized and reduced nontronite NAu-1. Environ. Sci. Technol. 2012, 46, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Rajapaksha, A.U.; Dou, X.; Bolan, N.S.; Yang, J.E.; Ok, Y.S. Surface complexation modeling and spectroscopic evidence of antimony adsorption on iron-oxide-rich red earth soils. J. Colloid Interface Sci. 2013, 406, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Tresintsi, S.; Simeonidis, K.; Estradé, S.; Martinez-Boubeta, C.; Vourlias, G.; Pinakidou, F.; Katsikini, M.; Paloura, E.C.; Stavropoulos, G.; Mitrakas, M. Tetravalent manganese feroxyhyte: A novel nanoadsorbent equally selective for As(III) and As(V) removal from drinking water. Environ. Sci. Technol. 2013, 47, 9699–9705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, F.; Liu, H.; Qu, J.; Liu, R. Respective role of Fe and Mn oxide contents for arsenic sorption in iron and manganese binary oxide: An X-ray absorption spectroscopy investigation. Environ. Sci. Technol. 2014, 48, 10316–10322. [Google Scholar] [CrossRef]


| Species | Fe3O4 (wt %) | Q5 (mg/g) | Freundlich | BET | |||
|---|---|---|---|---|---|---|---|
| KF | 1/n | a | b | c (10−5) | |||
| Sb(III) | 0 | 2.8 | 0.825 | 0.758 | |||
| 25 | 1.5 | 0.354 | 0.917 | ||||
| 50 | 0.72 | 0.136 | −0.011 | 7.5 | |||
| 75 | 0.28 | 0.053 | −0.013 | 6.0 | |||
| 100 | 0.27 | 0.051 | −0.009 | 1.6 | |||
| Sb(V) | 0 | 0.05 | 0.012 | 0.840 | |||
| 25 | 0.29 | 0.123 | 0.530 | ||||
| 50 | 0.38 | 0.164 | 0.517 | ||||
| 75 | 0.40 | 0.177 | 0.510 | ||||
| 100 | 0.45 | 0.202 | 0.505 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeonidis, K.; Kalaitzidou, K.; Kaprara, E.; Mitraka, G.; Asimakidou, T.; Balcells, L.; Mitrakas, M. Uptake of Sb(V) by Nano Fe3O4-Decorated Iron Oxy-Hydroxides. Water 2019, 11, 181. https://doi.org/10.3390/w11010181
Simeonidis K, Kalaitzidou K, Kaprara E, Mitraka G, Asimakidou T, Balcells L, Mitrakas M. Uptake of Sb(V) by Nano Fe3O4-Decorated Iron Oxy-Hydroxides. Water. 2019; 11(1):181. https://doi.org/10.3390/w11010181
Chicago/Turabian StyleSimeonidis, Konstantinos, Kyriaki Kalaitzidou, Efthimia Kaprara, Georgia Mitraka, Theopoula Asimakidou, Lluis Balcells, and Manassis Mitrakas. 2019. "Uptake of Sb(V) by Nano Fe3O4-Decorated Iron Oxy-Hydroxides" Water 11, no. 1: 181. https://doi.org/10.3390/w11010181
APA StyleSimeonidis, K., Kalaitzidou, K., Kaprara, E., Mitraka, G., Asimakidou, T., Balcells, L., & Mitrakas, M. (2019). Uptake of Sb(V) by Nano Fe3O4-Decorated Iron Oxy-Hydroxides. Water, 11(1), 181. https://doi.org/10.3390/w11010181







