Uptake Fluoride from Water by Starch Stabilized Layered Double Hydroxides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Synthesis of Adsorbents
2.2. Characterization Methods
2.3. Experimental Procedure
3. Results and Discussion
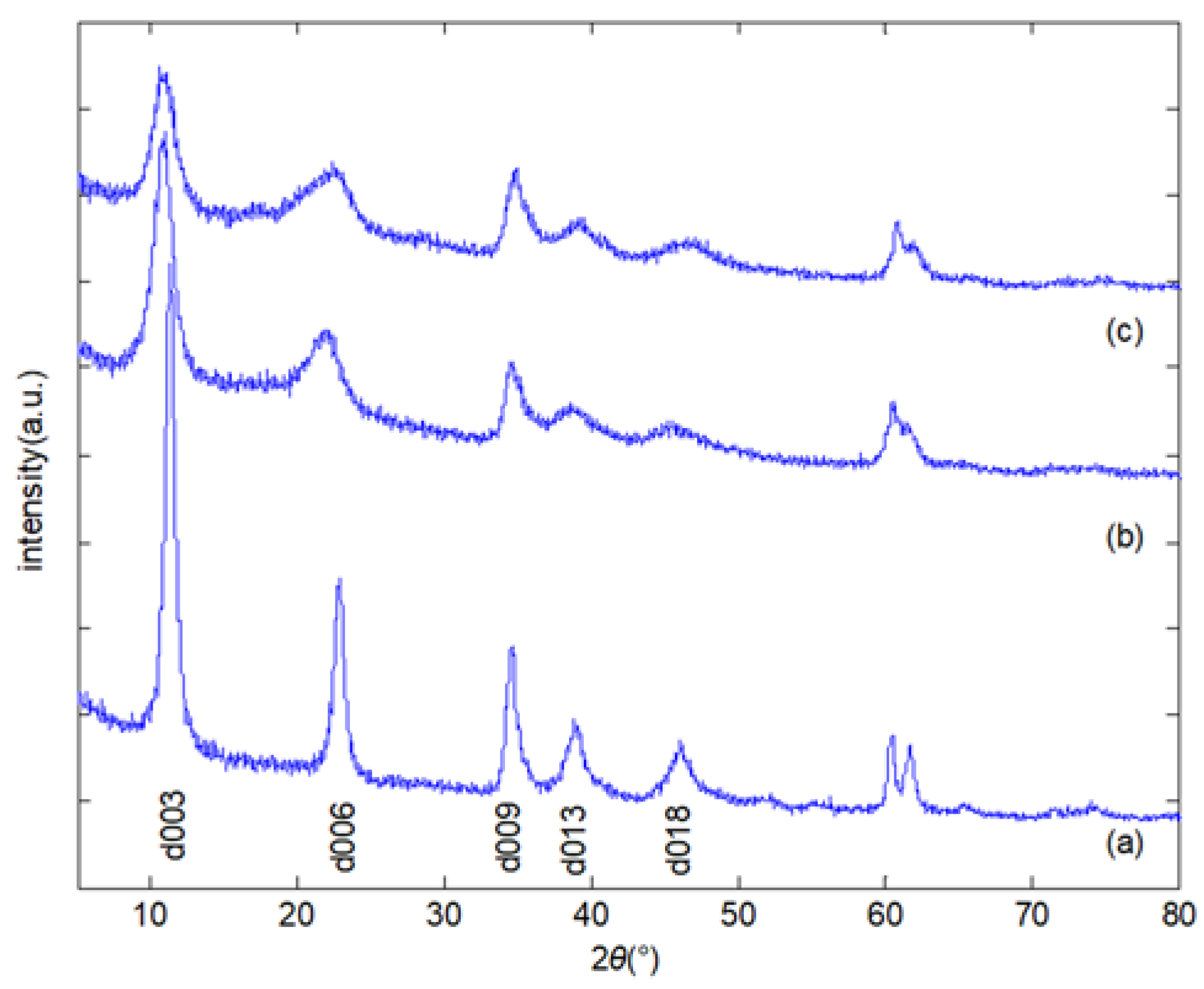
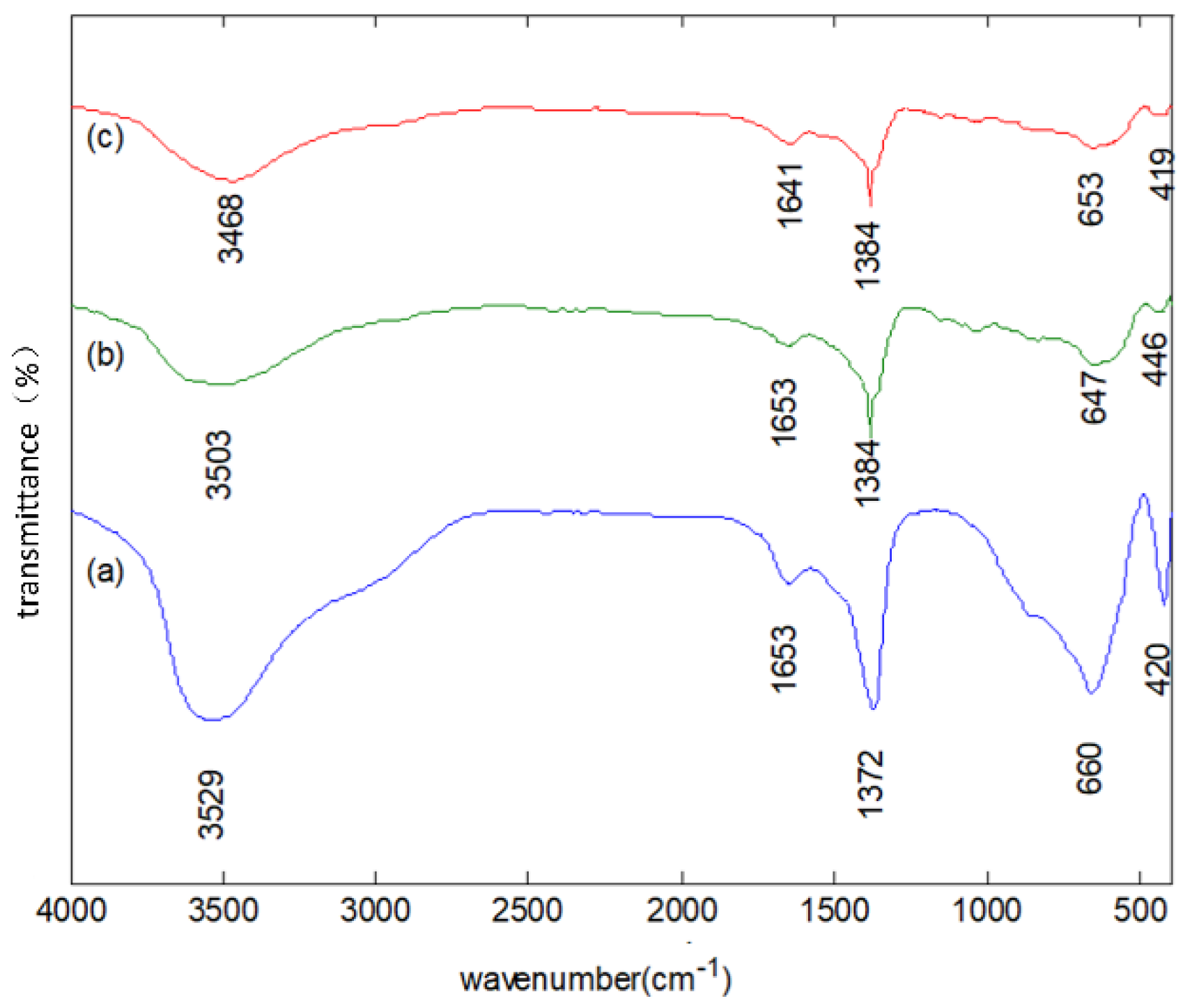
3.1. Characteristics of LDHs
3.2. Adsorption Study
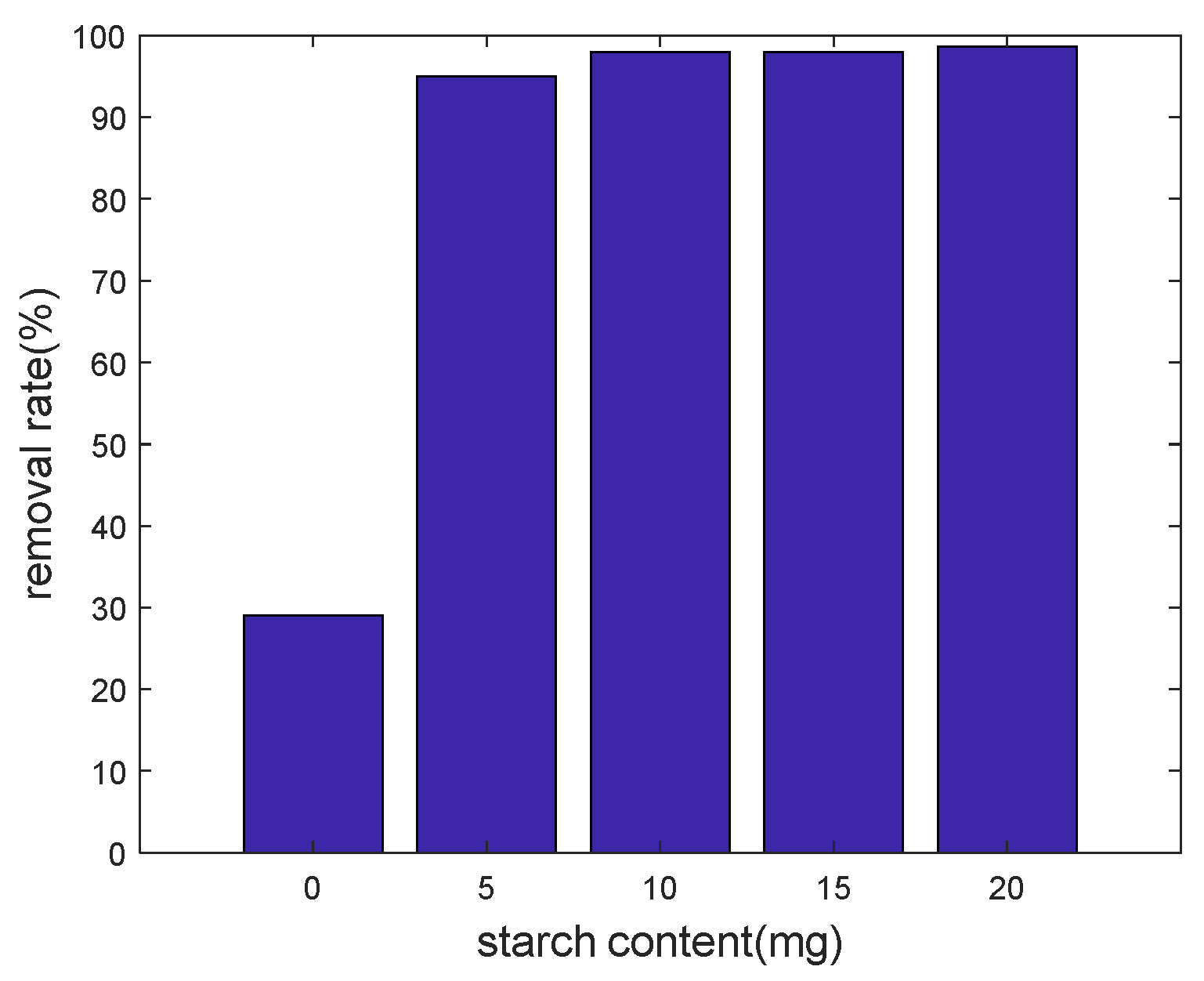
3.2.1. Dosage Effect of Starch Content
3.2.2. Adsorption Isotherm
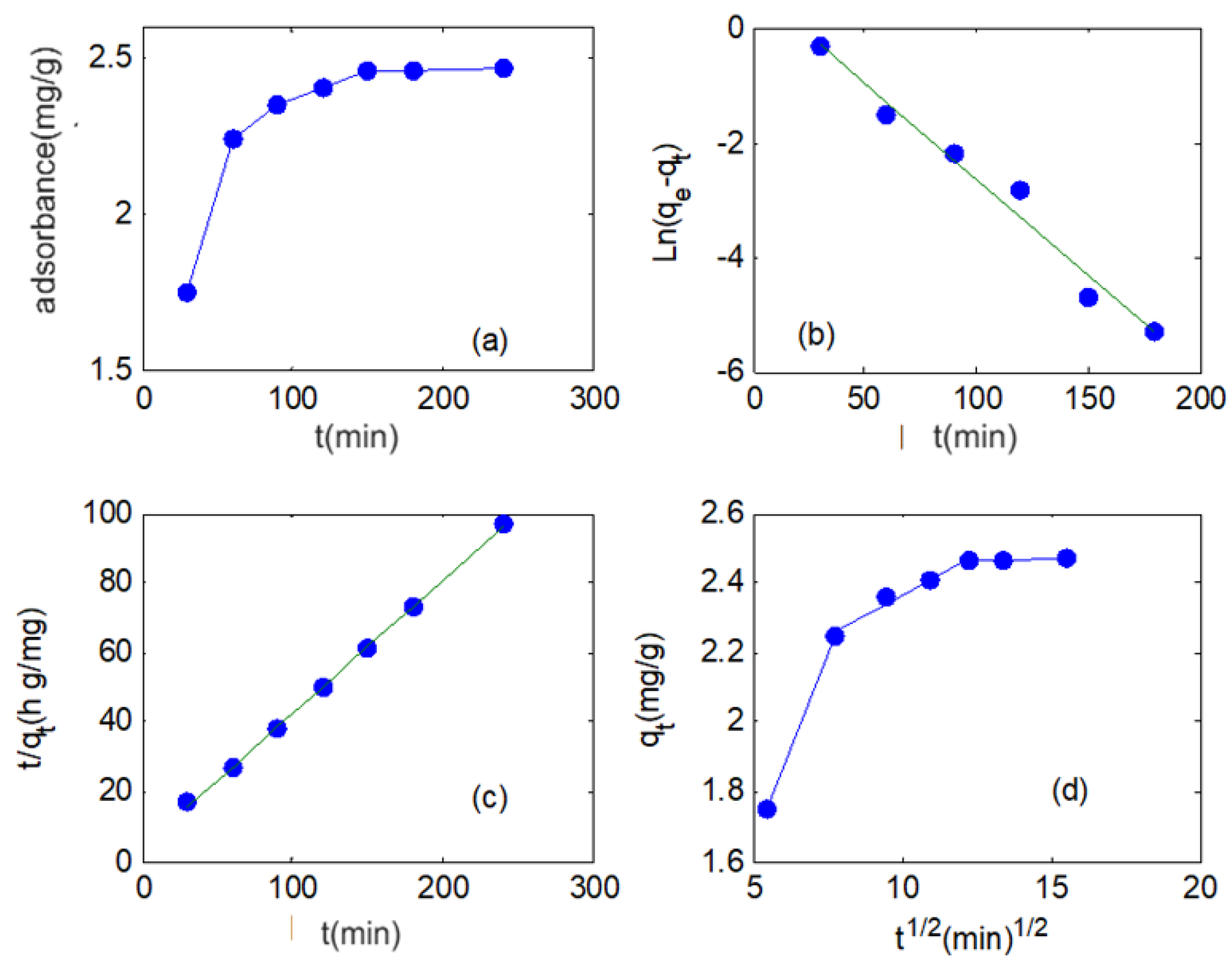
3.2.3. Adsorption Kinetic Studies
3.2.4. Adsorption Thermodynamic Studies
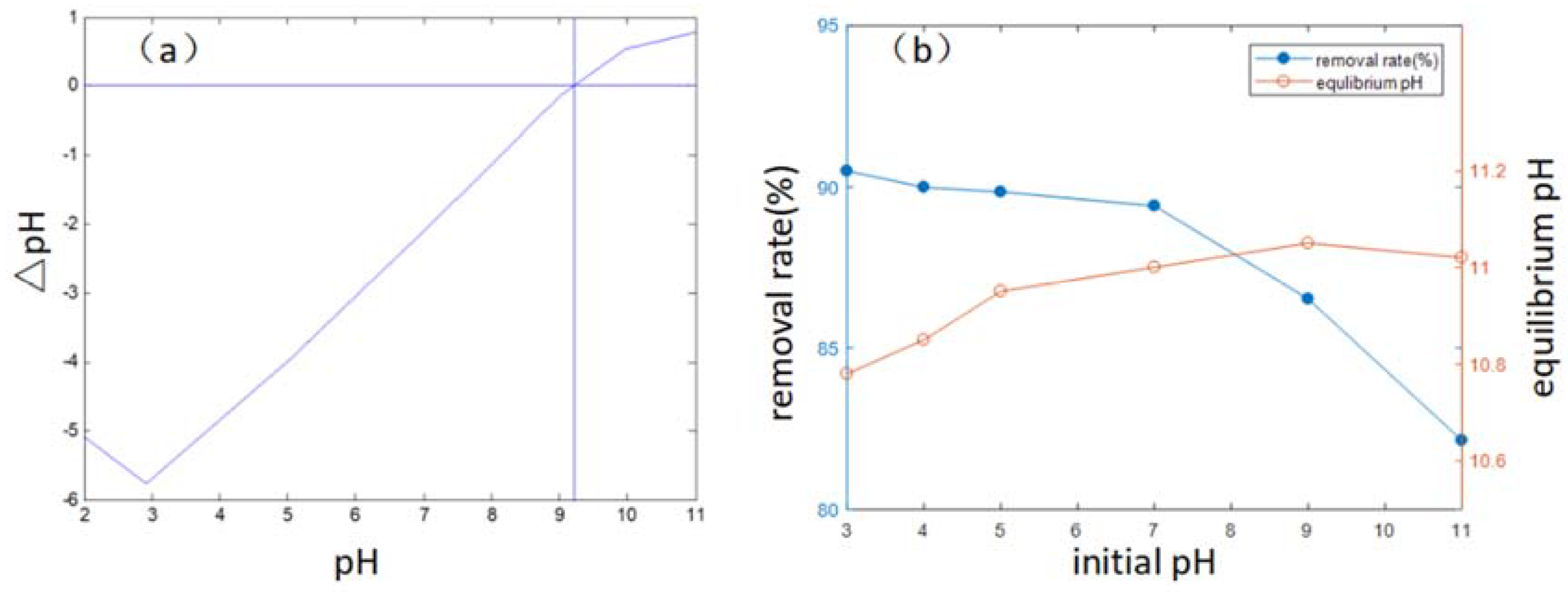
3.3. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Wang, T.; Xiao, Y.; Tian, E.; Wang, W.; Zhao, Y.; Tian, L.; Jiang, H.; Luo, X. Efficient fluoride removal from aqueous solution by synthetic Fe-Mg-La tri-metal nanocomposite and the analysis of its adsorption mechanism. J. Alloy. Compd. 2018, 738, 118–129. [Google Scholar] [CrossRef]
- Theiss, F.L.; Couperthwaite, S.J.; Ayoko, G.A.; Frost, R.L. A review of the removal of anions and oxyanions of the halogen elements from aqueous solution by layered double hydroxides. J. Colloid Interface Sci. 2014, 417, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Sakhare, N.; Lunge, S.; Rayalu, S.; Bakardjiva, S.; Subrt, J.; Devotta, S.; Labhsetwar, N. Defluoridation of water using calcium aluminate material. Chem. Eng. J. 2012, 203, 406–414. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Yan, X. Metal-organic framework-801 for efficient removal of fluoride from water. Microporous Mesoporous Mater. 2018, 259, 163–170. [Google Scholar] [CrossRef]
- Gao, C.; Yu, X.; Luo, T.; Jia, Y.; Sun, B.; Liu, J.; Huang, X. Millimeter-sized Mg–Al-LDH nanoflake impregnated magnetic alginate beads (LDH-n-MABs): A novel bio-based sorbent for the removal of fluoride in water. J. Mater. Chem. A 2014, 2, 2119–2128. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A zirconium-based nanoparticle: Essential factors for sustainable application in treatment of fluoride containing water. J. Colloid Interface Sci. 2014, 416, 227–234. [Google Scholar] [CrossRef] [PubMed]
- WHO World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 371. [Google Scholar]
- Grzegorzek, M.; Majewska-Nowak, K. The influence of organic matter on fluoride removal efficiency during the electrodialysis process. Desalination Water Treat. 2017, 69, 153–162. [Google Scholar]
- Yu, Z.; Xu, C.; Yuan, K.; Gan, X.; Feng, C.; Wang, X.; Zhu, L.; Zhang, G.; Xu, D. Characterization and adsorption mechanism of ZrO2 mesoporous fibers for health-hazardous fluoride removal. J. Hazard. Mater. 2018, 346, 82–92. [Google Scholar] [PubMed]
- Jiang, H.; Zhang, W.; Chen, P.; He, Q.; Li, M.; Tian, L.; Tu, Z.; Xu, Y. One Pot Method to Synthesize a Novel La–Zr Composite with Exceptionally High Fluoride Removal Performance. J. Inorg. Organomet. Polym. 2016, 26, 285–293. [Google Scholar]
- Kang, D.; Yu, X.; Ge, M. Morphology-dependent properties and adsorption performance of CeO2 for fluoride removal. Chem. Eng. J. 2017, 330, 36–43. [Google Scholar] [CrossRef]
- Wan, D.; Liu, Y.; Xiao, S.; Chen, J.; Zhang, J. Uptake fluoride from water by calcined Mg-Al-CO3 hydrotalcite: Mg/Al ratio effect on its structure, electrical affinity and adsorptive property. Colloids Surfaces A 2015, 469, 307–314. [Google Scholar] [CrossRef]
- Liu, S.; Ye, X.; He, K.; Chen, Y.; Hu, Y. Simultaneous removal of Ni(II) and fluoride from a real flue gas desulfurization wastewater by electrocoagulation using Fe/C/Al electrode. J. Water Reuse Desalin. 2017, 7, 288–297. [Google Scholar] [CrossRef]
- Markovski, J.; Garcia, J.; Hristovski, K.D.; Westerhoff, P. Nano-enabling of strong-base ion-exchange media via a room-temperature aluminum (hydr)oxide synthesis method to simultaneously remove nitrate and fluoride. Sci. Total Environ. 2017, 599–600, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Agbaje, T.A.; Al-Gharabli, S.; Mavukkandy, M.O.; Kujawa, J.; Arafat, H.A. PVDF/magnetite blend membranes for enhanced flux and salt rejection in membrane distillation. Desalination 2018, 436, 69–80. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Mageste, A.B.; Dias, A.; Virtuoso, L.S.; Siqueira, K.P.F. Layered double hydroxides for remediation of industrial wastewater containing manganese and fluoride. J. Clean Prod. 2018, 171, 275–284. [Google Scholar] [CrossRef]
- Chubar, N.; Gilmour, R.; Gerda, V.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered double hydroxides as the next generation inorganic anion exchangers: Synthetic methods versus applicability. Adv. Colloid Interface 2017, 245, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, Y.; Lv, F.; Tong, W.; Xin, H.; Meng, Z.; Wang, X.; Chu, P.K. Preparation of layered double hydroxides using boron mud and red mud industrial wastes and adsorption mechanism to phosphate. Water Environ. J. 2017, 31, 145–157. [Google Scholar] [CrossRef]
- Shan, R.; Yan, L.; Yang, K.; Hao, Y.; Du, B. Adsorption of Cd(II) by Mg–Al–CO3- and magnetic Fe3O4/Mg–Al–CO3-layered double hydroxides: Kinetic, isothermal, thermodynamic and mechanistic studies. J. Hazard. Mater. 2015, 299, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Sasaki, K.; Hirajima, T. Effect of calcination temperature on Mg-Al bimetallic oxides as sorbents for the removal of F- in aqueous solutions. Chemosphere 2014, 95, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, G.; Peng, C.; Qiao, H.; Ke, F.; Hou, R.; Li, D.; Cai, H.; Wan, X. Adsorptive removal of fluoride from drinking water using porous starch loaded with common metal ions. Carbohydr. Polym. 2017, 160, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhao, D. Immobilization of arsenate in a sandy loam soil using starch-stabilized magnetite nanoparticles. J. Hazard. Mater. 2014, 271, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lv, L. Defluoridation of drinking water by calcined MgAl-CO3 layered double hydroxides. Desalination 2007, 208, 125–133. [Google Scholar] [CrossRef]
- Swain, S.K.; Patnaik, T.; Singh, V.K.; Jha, U.; Patel, R.K.; Dey, R.K. Kinetics, equilibrium and thermodynamic aspects of removal of fluoride from drinking water using meso-structured zirconium phosphate. Chem. Eng. J. 2011, 171, 1218–1226. [Google Scholar] [CrossRef]
- Sun, Z.; Park, J.; Kim, D.; Shin, C.; Zhang, W.; Wang, R.; Rao, P. Synthesis and Adsorption Properties of Ca-Al Layered Double Hydroxides for the Removal of Aqueous Fluoride. Water Air Soil Pollut. 2017, 228, 23. [Google Scholar]
- Mandal, S.; Mayadevi, S. Cellulose supported layered double hydroxides for the adsorption of fluoride from aqueous solution. Chemosphere 2008, 72, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, Y.; Zhang, W. Rapid and convenient removal of fluoride by magnetic magnesium-aluminum-lanthanum composite: Synthesis, performance and mechanism. Asia Pac. J. Chem. Eng. 2017, 12, 640–650. [Google Scholar] [CrossRef]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.R.; Lakzian, A. Adsorption-desorption characteristics of nitrate, phosphate and sulfate on Mg-Al layered double hydroxide. Appl. Clay Sci. 2013, 80–81, 305–312. [Google Scholar] [CrossRef]
- Vazquez Mejia, G.; Solache-Rios, M.; Martinez-Miranda, V. Removal of fluoride and arsenate ions from aqueous solutions and natural water by modified natural materials. Desalination Water Treat. 2017, 85, 271–281. [Google Scholar]
- El-Said, G.F.; El-Sadaawy, M.M.; Aly-Eldeen, M.A. Adsorption isotherms and kinetic studies for the defluoridation from aqueous solution using eco-friendly raw marine green algae, Ulva lactuca. Environ. Monit. Assess. 2018, 190, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Türker, O.C.; Baran, T. A combination method based on chitosan adsorption and duckweed (Lemna gibba L.) phytoremediation for boron (B) removal from drinking water. Int. J. Phytoremediat. 2018, 20, 175–183. [Google Scholar]
- Ren, G.; Wang, X.; Huang, P.; Zhong, B.; Zhang, Z.; Yang, L.; Yang, X. Chromium (VI) adsorption from wastewater using porous magnetite nanoparticles prepared from titanium residue by a novel solid-phase reduction method. Sci. Total Environ. 2017, 607, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Dayananda, D.; Sarva, V.R.; Prasad, S.V.; Arunachalam, J.; Ghosh, N.N. Preparation of CaO loaded mesoporous Al2O3: Efficient adsorbent for fluoride removal from water. Chem. Eng. J. 2014, 248, 430–439. [Google Scholar] [CrossRef]

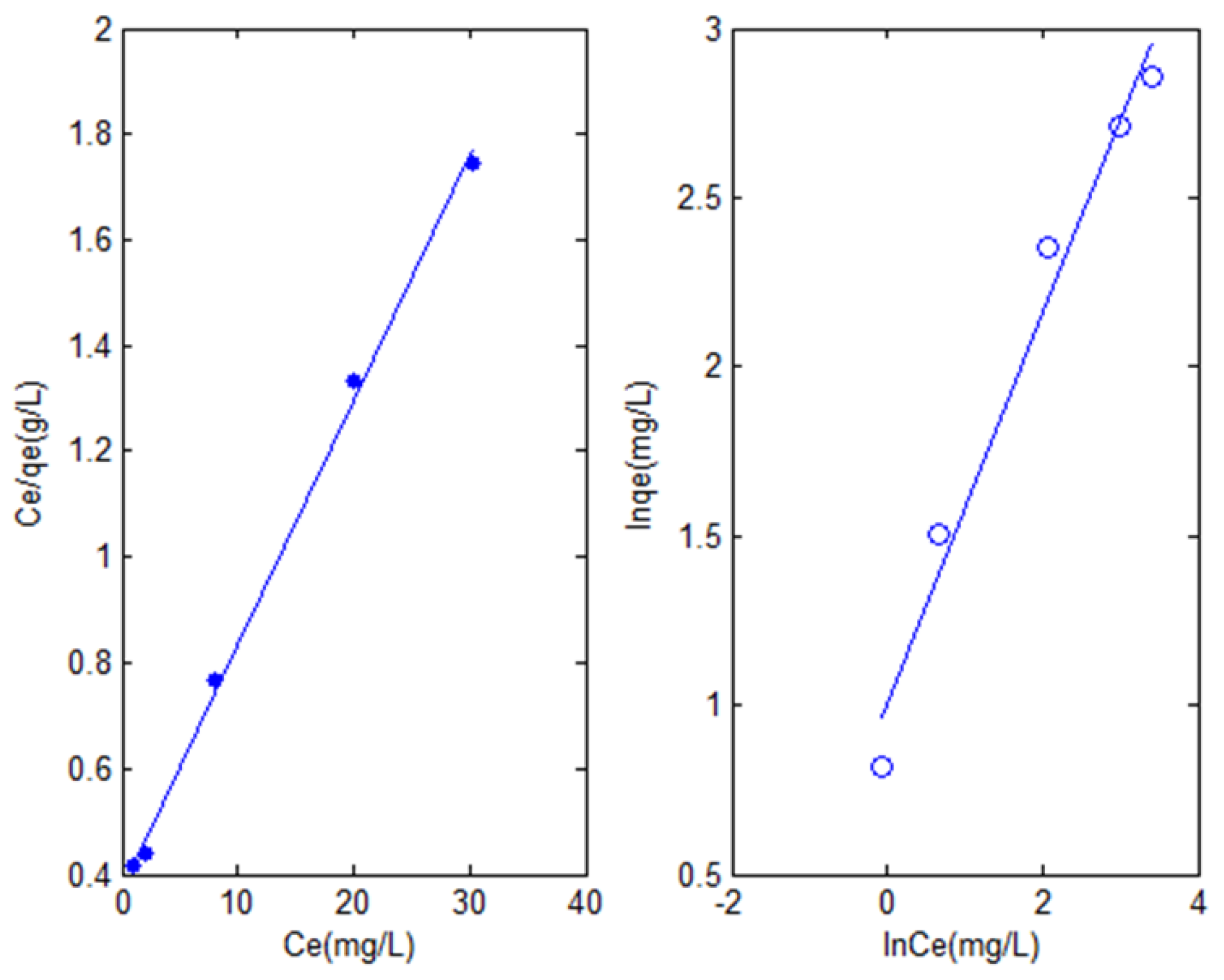
| Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| KL | qm (mg/g) | R2 | RMSE | KF | n | R2 | RMSE |
| 0.123 | 21.72 | 0.9987 | 0.0102 | 2.719 | 1.7393 | 0.9884 | 0.0804 |
| Kinetic Models | Parameter | |||
|---|---|---|---|---|
| Pseudo-first-order | k1 (min−1) | qe (mg/g) | R2 | RMSE |
| 0.0771 | 2.036 | 0.9872 | 0.2401 | |
| Pseudo-second-order | k2 (g/(mg·min)) | qe (mg/g) | R2 | RMSE |
| 0.1467 | 2.6107 | 0.9995 | 0.1194 | |
| intraparticle diffusion | kF (mg/(g·min0.5)) | R2 | RMSE | - |
| 2.4289 | 0.8471 | 0.7321 | - | |
| Temperature (°C) | b | Thermodynamic Parameter | ||
|---|---|---|---|---|
| ΔG0 (KJ/mol) | ΔH0 (KJ/mol) | ΔS0 (J/(mol·K)) | ||
| 20 | 9.73 | −12.44 | 35.63 | 0.0806 |
| 30 | 4.25 | −10.78 | ||
| 40 | 2.67 | −9.93 | ||
| 50 | 2.55 | −10.12 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yue, X.; Lu, X.; Guo, Y. Uptake Fluoride from Water by Starch Stabilized Layered Double Hydroxides. Water 2018, 10, 745. https://doi.org/10.3390/w10060745
Liu J, Yue X, Lu X, Guo Y. Uptake Fluoride from Water by Starch Stabilized Layered Double Hydroxides. Water. 2018; 10(6):745. https://doi.org/10.3390/w10060745
Chicago/Turabian StyleLiu, Jiming, Xiuping Yue, Xinyu Lu, and Yu Guo. 2018. "Uptake Fluoride from Water by Starch Stabilized Layered Double Hydroxides" Water 10, no. 6: 745. https://doi.org/10.3390/w10060745
APA StyleLiu, J., Yue, X., Lu, X., & Guo, Y. (2018). Uptake Fluoride from Water by Starch Stabilized Layered Double Hydroxides. Water, 10(6), 745. https://doi.org/10.3390/w10060745




