The Testing of Standard and Recyclable Filter Media to Eliminate Hydrogen Sulphide from Sewerage Systems
Abstract
1. Introduction
- in sewerage networks and house drains under adverse technical conditions;
- in transfer manholes with delivery pipe inlets;
- in long pressure pipes;
- in discharges of special industrial wastewater;
- in wastewater reuse by consumers and the resulting minimal flow rates in sewers;
- in sludge storage and sludge treatment facilities (sediments);
- improper operation and maintenance of both sewerage and facilities.
- Reduction of sulphate to sulphide by sulphur-reducing bacteria.
- Decomposition of amino acids containing sulphur.
- Dimethyl sulphide (DMS) generation via oxidation of CH3SH [9].
- Sewage composition: there is a significant influence of sulphide and sulphate concentrations in wastewater and special industrial service water [12]. Oxidation-reduction potential with values suited to anaerobic conditions: −50 mV. Temperature: biological oxygen demand increases with rising temperatures and anaerobic conditions are established in the sewerage system [13]. pH value: in more alkaline water there is a lower risk of hydrogen sulphide formation. Oxygen concentration: where values below 0.5 mg/L result in anaerobic conditions.
- Hydraulic factors: residence time, as longer residence times beyond 8 h result in anaerobic conditions in the sewerage system [12]. Poor technical design of the sewerage, where over-sizing results in low flow rates in the sewerage system, promoting sedimentation and the creation of an anaerobic environment. Velocity gradient, as a high velocity gradient causes considerable release of hydrogen sulphide into the air. Sediments and biofilm, where organic substrate is hydrolysed in anaerobic sediments along with fermentation, sulphate reduction and methane production [14].
- Sewerage facilities and pumping stations, where long switching intervals of wastewater pumping result in an anaerobic environment. Pressurized sewers, causing long retention times in the pipeline and vacuum stations missing biofilters for exhaust air treatment.
2. Materials and Methods
2.1. Parameters of Laboratory Measurement
- input detector: LL-H2S-1000, declared measuring range of 0–1000 ppm with a resolution of 0.5 ppm and detector accuracy of 0.5%,
- output detector: LL-H2S-200, declared measuring range of 0–200 ppm with a resolution of 0.1 ppm and detector accuracy of 1%. Recorded measuring range of 350 ppm with a resolution of 0.1 ppm.
- volume of one tested filtration medium: 0.5 L;
- maximum concentration of hydrogen sulphide at the inlet to the filtration unit: 300 ppm;
- maximum duration of one test: 30 min;
- hydrochloric acid concentration—HCl: 35%;
- dilution ratio with distilled water: 1:8;
- FeS volume: 1 gram;
- air flow rate set by anemometer: 3 L·min−1.
- all filtration products were stored in a dry laboratory environment;
- testing was performed at a constant temperature of 20 °C;
- air humidity was maintained at 55%;
- atmospheric pressure in the room equalled 1013.25 hPa;
- and constant temperature of the filtration media was 20 °C.
2.2. Filtration Media
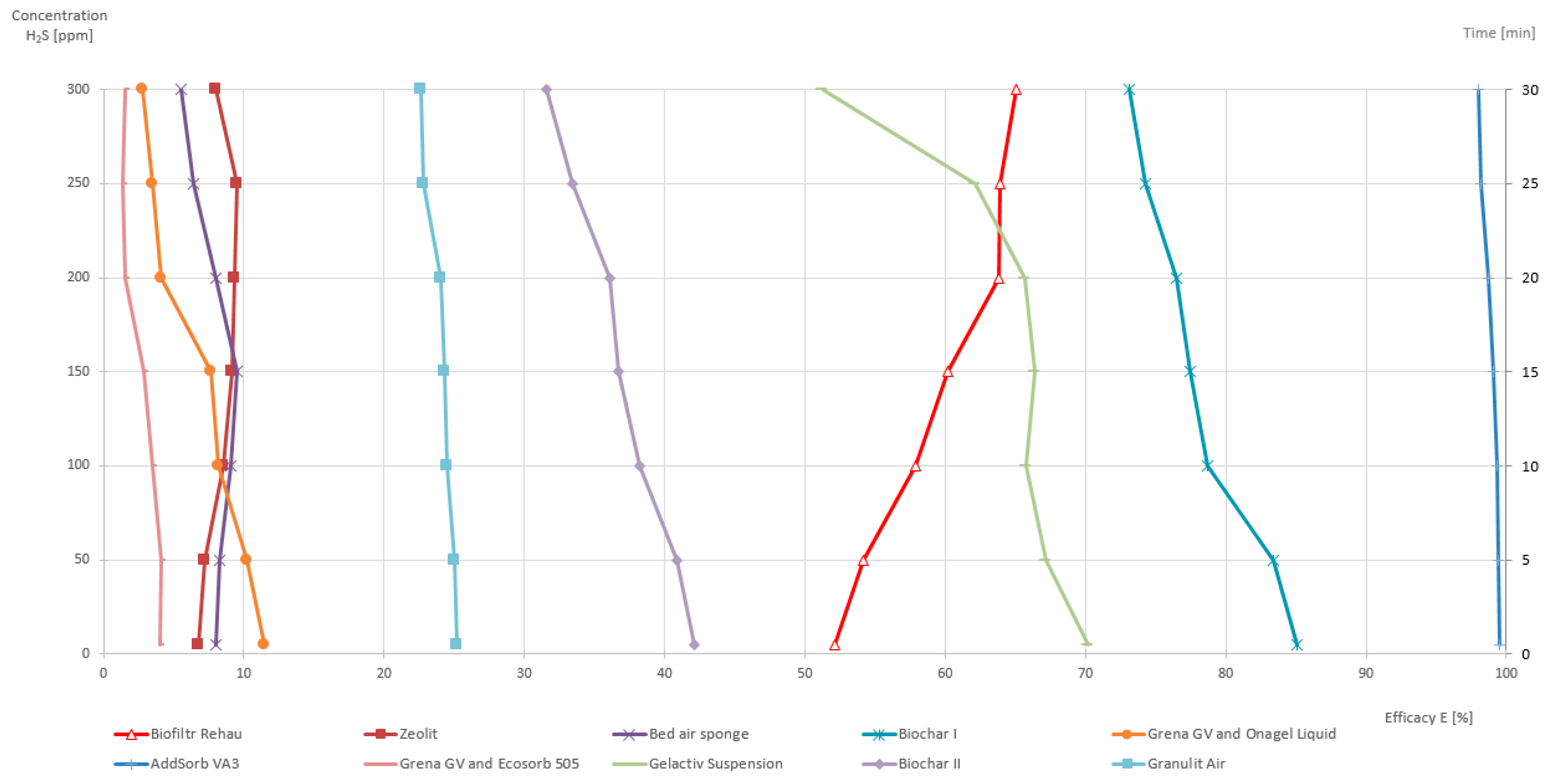
3. Results
- ui—general assessment of i-media;
- vj—rate of importance of j-criterion;
- Cji—sub-assessment of i-media based on j-criterion;
- n—number of criteria;
- p—number of media.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gil, K.; Shin, G.; Im, J. Investigation of odor release from combined sewer. J. Korean Soc. Hazard Mitig. 2010, 10, 185–191. [Google Scholar]
- ČSN EN 12255-9. Wastewater Treatment Plants—Part 9: Odour Control and Ventilation; Czech Office for Standards, Metrology and Testing: Praha, Czech Republic, 2003. [Google Scholar]
- Pandey, S.K.; Kim, K.; Tang, K. A review of sensor-based methods for monitoring hydrogen sulfide. Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Kanakoudis, V.; Tsitsifli, S. Reliability assessment & data classification using discriminant functions & factor analysis. In Proceedings of the 4th IWA Specialized International Conference on Water Loss, Bucharest, Romania, 23–16 September 2007; pp. 836–845. Available online: https://scholar.google.ca/scholar?oi=bibs&cluster=14412914019176863058&btnI=1&hl=en (accessed on 13 March 2016).
- Zeleňáková, M.; Vido, J.; Portela, M.M.; Purcz, P.; Blištán, P.; Hlavatá, H.; Hluštík, P. Precipitation Trends over Slovakia in the Period 1981–2013. Water 2017, 9, 922. [Google Scholar] [CrossRef]
- European Environment Agency. Council 2008/50/EC. On Ambient Air Quality and Cleaner Air for Europe. Directive 2008/50/EC of the European Parliament and of the Council. 2008. Available online: http://data.europa.eu/eli/dir/2008/50/oj (accessed on 14 March 2018).
- European Parliament. Council 2010/75/EU. On Industrial Emissions (Integrated Pollution Prevention and Control). Directive 2010/75/EU of the European Parliament and the Council. 2010. Available online: http://data.europa.eu/eli/dir/2010/75/oj (accessed on 14 March 2018).
- Lomans, B.P.; Pol, A.; Camp, H.J.M. Microbial cycling of volatile organic sulfur compounds. Cell. Mol. Life Sci. 2002, 59, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Yarosz, D.P.; Chen, Y.; Murthy, S. Mechanisms of volatile sulfur compounds and odor production in digested biosolids. In Proceedings of the Water Environment Federation, Baltimore, MD, USA, 19–22 February 2013. [Google Scholar]
- Zhang, L.; Schryver, P.; Gussume, B.; Muynck, W.; Boon, N.; Vestraete, W. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review. Water Res. 2008, 42, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dobrynkin, N.M.; Batygina, M.V.; Noskov, A.S. Water depollution and the odor control by wet air catalytic oxidation of ammonia, sulfides, and mercaptans of industrial wastewater. Chem. Eng. Trans. 2010, 23, 339–344. [Google Scholar]
- ČSN EN 1671. Pressure Sewerage Systems Outside Buildings; Czech Office for Standards, Metrology and Testing: Praha, Czech Republic, 1998. [Google Scholar]
- Sekyiamah, K.; Kim, H.; McConnell, L.L.; Torrents, A.; Ramirez, M. Identification of seasonal variations in volatile sulfur compound formation and release from secondary treatment system at a large wastewater treatment plant. Water Environ. Res. 2008, 80, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Kim, K.; Yoon, H. Effect of incineration on the removal of key offensive odorants released from a Landfill Leachate Treatment Station (LLTS). Chemosphere 2012, 87, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, M.; Schiavi, R.; Santos, F. Odour Control: A Successful Experience in Sorocaba City—Brazil; IWA Publishing: London, UK, 2018. [Google Scholar] [CrossRef]
- Vilmain, J.B.; Courousse, V. Kinetic study of hydrogen sulfide absorption in aqueous chlorine solution. Chem. Eng. Res. Des. 2014, 92, 191–204. [Google Scholar] [CrossRef]
- Cuevasanta, E.; Carballal, S.; Zeida, A.; Alvarez, B. Insights into the mechanism of the reaction between Hydrogen sulfide and peroxynitrite. Free Radic. Biol. Med. 2014, 80, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Willey, B.F.; Jennings, H.; Muroski, F. Removal of Hydrogen Sulfide with Potassium Permanganate; American Water Works Association: Denver, CO, USA, 1964; Available online: http://www.jstor.org/stable/41264210 (accessed on 12 March 2018).
- Zhang, D.; Du, J.; Tang, C.; Huang, Y.; Jin, H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.J. Hydrogen Sulfide: Human Health Aspects; World Health Organization: Geneva, Switzerland, 2003; ISBN 9241530537. [Google Scholar]
- Aempfer, H.W.; Berndt, M.; Voigtlaender, G. Estimation of Residual Service Life for Existing Sewerage Systems. 2002. Available online: http://www.irbnet.de/daten/iconda/CIB9270.pdf (accessed on 12 March 2018).
- Zaldivar, E.C. Settlement Agreement Final Order—Civil Action No. 01-191-RSWL and Civil Action No. 98-9039-RSWL Consolidated—2011 Sewer Odor Master Plan; Wastewater Engineering Services Division, Bureau of Sanition: Los Angeles, CA, USA, 2011.
- Bioteg.com. Biofilter and Odor Control Systems Products and Solutions. Available online: http://www.bioteg.com/biofilter/ (accessed on 10 March 2018).
- Talaiekhozani, A.; Bagheri, M.; Goli, A. An overview of principles of odor production, emission, and control methods in wastewater collection and treatment systems. J. Environ. Manag. 2016, 170, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Jacobi Carbons. A Story of Success. Available online: https://www.jacobi.net/ (accessed on 14 March 2018).
- Bionic Fuel Technologies. Available online: http://bionic-enterprises.com/bionic-fuel-technologies/ (accessed on 14 March 2018).
- Rehau, Unlimited Polymer Solutions. Available online: https://www.rehau.com/group-en/ (accessed on 14 March 2018).
- Biothys, Olfactive Silence. Available online: http://www.biothys.co.kr/ (accessed on 14 March 2018).
- Subio Eko. Available online: http://www.subio.cz/sekce-5/ (accessed on 14 March 2018).
- Grena. Available online: http://www.grena.cz/cz/ (accessed on 14 March 2018).
- Industrial Strength Odor Control. Earth-friendly Odor Solutions. Available online: http://odormanagement.com/ (accessed on 14 March 2018).
- Neutralizes Odors, Naturally. Available online: http://onaonline.com/ (accessed on 14 March 2018).
- Mateson Chemical Corporation. Available online: http://badairsponge.com/ (accessed on 14 March 2018).
- Biothys GmBH. Available online: http://www.biothys.com/home/ (accessed on 14 March 2018).
- ČSN EN 752. Drain and Sewer Systems Outside Buildings—Sewerage Pipelines; Czech Office for Standards, Metrology and Testing: Praha, Czech Republic, 2017. [Google Scholar]
| Iron salts precipitation | Iron can restrict the formation of sulphide in its divalent and trivalent forms. Divalent iron and sulphide form a black precipitate of ferrous sulphide. Trivalent iron can cause sulphate to be eliminated by producing elemental sulphur and oxidising to divalent iron. These iron salts used include ferrous chloride (FeCl2), ferrous sulphate heptahydrate (FeSO4·7H2O) and iron chloride sulphate (FeClSO4) [15]. |
| Chlorination | Chlorine is dosed into wastewater as a solution of gas that removes sulphide ions. The efficiency of sulphide reduction is limited due to the ability of chlorine to oxidise with organic and inorganic compounds. Adverse substances formed during the reaction of chlorine and certain organic substances with wastewater are trihalomethanes [16]. |
| Oxidation by hydrogen peroxide | Hydrogen peroxide can oxidise sulphide to produce water and oxygen. In addition, by producing oxygen, hydrogen peroxide can contribute to maintaining aerobic conditions in wastewater and achieve high removal rates [17]. |
| Oxidation using potassium permanganate | Potassium permanganate (KMnO4) is a strong agent that is capable of oxidising sulphide to sulphate. Typically, potassium permanganate is distributed in the dry state and it is therefore necessary to obtain an aqueous solution before application [18]. |
| Biological oxidation of sulphide | The sulphide inhibition process also eliminates two types of bacteria—Thiomicrospira denitrificans and Thiobaciluus denitrificans. In the presence of nitrates, these bacteria are capable of oxidising sulphides and sulphur. Necessary electrons are taken from nitrates, which results in their reduction [19]. |
| Restriction of anaerobic conditions using nitrate solution | Nitrate addition can establish anoxic conditions in wastewater. Nitrates are more easily reducible than sulphate in this environment, hereby reducing sulphide formation. |
| Compressed air or pure oxygen | Wastewater aeration is most often applied in pressure pipes or wet pits. Pure oxygen is five times more concentrated than oxygen contained in the air and, therefore, it is possible to achieve higher dissolved oxygen values in wastewater using oxygen 5 to 7 mg·L−1 than using air 3 to 5 mg·L−1. |
| Air scrubber, oxidation and absorption | This technology can be used to treat practically all water-soluble contaminants. Liquid in the washer is sprinkled and creates artificial fog. The harmful substances are entrapped by the fog and dissolved from their gaseous phase into aqueous chemical solutions. The reaction mechanism is purely chemical and stable, resisting changes of input substances and external conditions [20]. |
| Bio-air scrubbers | The bio-scrubber is fitted with an artificial carrier designed to have the largest possible surface area. Biofilm is then formed on the surface by sprinkled medium and substances separated from the air. There are two processes taking place in the biofilm, both physical and biochemical. As part of the physical process, gas molecules enter the sprinkled liquid and in the biochemical process the pollution is directly degraded in the liquid with the help of microorganisms [21,22]. |
| Biological filters | This principle is similar to the bio-scrubber. The difference is that, instead of the biofilm medium, the filter medium is firm and the odorous substances are absorbed and degraded by microbiological organisms. |
| Biofilters with forced dry matter extraction | This biological filter technology can be used to treat various biodegradable water-soluble contaminants. Harmful substances from the air are dissolved in the filter from the gaseous to aqueous phase on the surface of an organic medium. For example, peat, tree bark, modified root and coconut fibres, as well as compost are used. Odour molecules are then degraded by the bacterial population in this medium [23]. |
| Biofilters without forced dry matter extraction | Due to the absence of a ventilator and without the need for power supply for the ventilator, this type of filter can be directly fitted into the sewer manhole under the cover. The filter medium and its fraction must be selected so that the air can freely escape from the sewer without causing a significant pressure loss in the air flow [24]. |
| Adsorption on filter material | When a filter material is used, the air flow passes through an adsorbent layer. The odour-causing compounds are attracted to the surface of the adsorbent. This is the simplest of the aforementioned odour-removal technologies. There is no need for any chemicals to be dosed into the system and there is no need to control biological processes that might be disturbed. |
| Photooxidation, ozonisation, ionization | To remove harmful substances from the air, ozone and hydroxyl ions are also used that are supplied into the air using strong electric discharges or short-wave ultraviolet (UV) radiation. This technology is preceded by a particulate filter so as not to damage UV lamps and electrodes. |
| Neutralization, compensation and masking | These methods only mask odours and the harmful substances lose their noticeable organoleptic properties. However, individuals are still in contact with dangerous substances and this makes it all the more dangerous because the odour loses its warning property. Essential oils and various natural and synthetic substances are used for this purpose. There are no chemical processes in contact with the air. |
| Fresh air dilution | Dilution of odorous substances is important in those cases where the operator wants to protect the pipeline from biogenic sulphide corrosion and it is possible to vent out large volumes of foul-smelling air into rural areas. The concentration of odorous substances in sewerage is significantly reduced by using a ventilator located in the pipeline. |
| Filtration Medium | Description | Manufacturer | Structure | Photo Documentation |
|---|---|---|---|---|
| Activated carbon extruded Identification: AddSorb VA3 | Intended for gaseous phase based on graphite plates with a large internal surface. | Jacobi Carbons Parent Company [25] Made in: Japan | Bulk material Granulometry 4–8 mm |  |
| Biochar I | Product of spruce wood microwave pyrolysis unit. | Bionic E & M, s.r.o. Brno University of Technology, AdMaS Center [26] Made in: Czech Republic | Bulk material Granulometry 2–10 mm |  |
| Biochar II | Product of sewage sludge microwave pyrolysis unit. | |||
| Product Identification: Biofilter Rehau | Recyclable old paper without black ink. | Rehau company [27] Made in: Switzerland | Bulk material Granulometry 5–30 mm |  |
| Filtration Medium | Description | Manufacturer | Structure | Photo Documentation |
|---|---|---|---|---|
| Clayey granulate Identification: Granulit Air | Strong deodorizing agent. Porous granulate, excellent decomposition of organic substances. | Biothys asia co., Ltd. [28] Made in: Korea | Bulk material Granulometry 4–8 mm |  |
| Natural mineral Zeolite | Alkali metal aluminosilicate crystalline and alkaline earth metals. | Subio, s.r.o. [29] Made in: Czech Republic | Bulk material Granulometry 2.5–5 mm |  |
| Natural mineral Identification: Grena GV Vermicularis | Mineral-phyllosilicates. This serves as a carrier layer for gels that soak into GV and do not cause their loss. | Grena, a.s. [30] Made in: Czech Republic | Bulk material Granulometry 0–5 mm |  |
| Filtration Medium | Description | Manufacturer | Structure | Photo Documentation |
|---|---|---|---|---|
| Milky white gel Identification: Ecosorb 505 | Mixture of natural essential oils and food grade emulsifiers. | OMI Industries, co. [31] Made in: USA, Illinois | Gel form |  |
| Milky white gel Identification: Ona Gel Liquid | Mixture of hydrocarbons of plant origin and chemical compounds for odour neutralization. | Odorchem Manufacturing co. [32] Made in: Canada | Gel balls |  |
| Blue gel Identification: Bad Air Sponge | Gel containing activated carbon, wetting and neutralizing agents. | Mateson Chemical Corporation [33] Made in: USA, Philadelphia | Semi-solid gel |  |
| Orange gel Identification: Gelactiv Suspension | Gel plate containing active substances in the neutralization process. | Biothys GmBH [34] Made in: Germany | Gel plate |  |
| Group of Criteria—Indicator Weight | Category Description Criterion Weight | Classification at the Level | |||
|---|---|---|---|---|---|
| CI | CII | CIII | CIV | ||
| 0.1 | 0.15 | 0.25 | 0.5 | ||
| Category C1—0.70 | Filtration medium effectiveness | >90% | 70–90% | 70–50% | <50% |
| Category C2—0.15 | Filtration medium sorption capacity | >12 months | 6–12 months | 3–6 months | <3 months |
| Category C3—0.10 | Filtration medium preparation | Excellent | Good | Sufficient | Insufficient |
| Category C4—0.05 | Residual odour perception | None | Mild | Strong | Extreme |
| Final Order | Tested Medium | Filtration Medium Effectiveness | Category | General Assessment Media | |||||
|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Average | C1 | C2 | C3 | C4 | |||
| 1. | Activated Coal AddSorb VA3 | 98.0 | 99.5 | 98.9 | CI | CI | CI | CI | 0.100 |
| 2. | Biochar I | 85.1 | 73.1 | 78.4 | CII | CII | CI | CI | 0.138 |
| 3. | Biofilm Rehau | 52.1 | 65.1 | 59.6 | CIII | CI | CI | CII | 0.213 |
| 4. | Gelactiv Suspension | 51.2 | 70.2 | 64.1 | CIII | CII | CIV | CII | 0.255 |
| 5. | Biochar II | 31.6 | 42.1 | 37.0 | CIV | CII | CI | CII | 0.388 |
| 6. | Grena GV Vermicularis and Ecosorb 505 | 1.4 | 4.1 | 2.7 | CIV | CII | CII | CIII | 0.410 |
| 7. | Zeolite | 6.8 | 9.3 | 8.4 | CIV | CI | CI | CIV | 0.440 |
| 8. | Grena GV Vermicularis and Ona Gel Liquid | 2.8 | 11.5 | 6.9 | CIV | CII | CII | CIV | 0.448 |
| 9. | Granulit Air | 22.6 | 25.2 | 24.1 | CIV | CIV | CIV | CIII | 0.463 |
| 10. | Bad Air Sponge | 9.5 | 5.5 | 7.8 | CIV | CIV | CIV | CIV | 0.500 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hluštík, P.; Novotný, J. The Testing of Standard and Recyclable Filter Media to Eliminate Hydrogen Sulphide from Sewerage Systems. Water 2018, 10, 689. https://doi.org/10.3390/w10060689
Hluštík P, Novotný J. The Testing of Standard and Recyclable Filter Media to Eliminate Hydrogen Sulphide from Sewerage Systems. Water. 2018; 10(6):689. https://doi.org/10.3390/w10060689
Chicago/Turabian StyleHluštík, Petr, and Jiří Novotný. 2018. "The Testing of Standard and Recyclable Filter Media to Eliminate Hydrogen Sulphide from Sewerage Systems" Water 10, no. 6: 689. https://doi.org/10.3390/w10060689
APA StyleHluštík, P., & Novotný, J. (2018). The Testing of Standard and Recyclable Filter Media to Eliminate Hydrogen Sulphide from Sewerage Systems. Water, 10(6), 689. https://doi.org/10.3390/w10060689




