Simulation of Trinitrogen Migration and Transformation in the Unsaturated Zone at a Desert Contaminant Site (NW China) Using HYDRUS-2D
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Study-Area Setting and Model Generalizations
2.1.1. Study-Area Setting
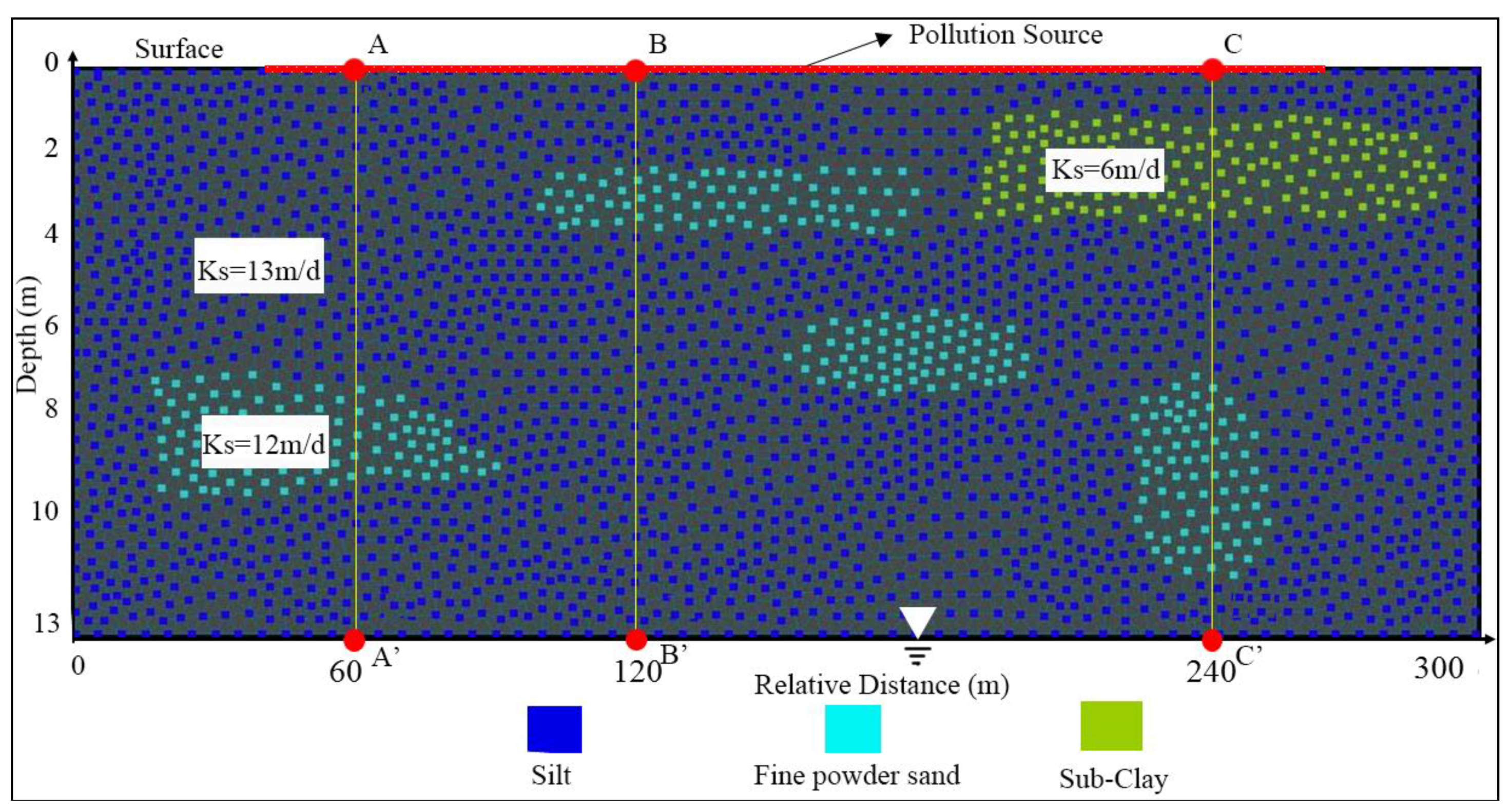
2.1.2. Study-Area Generalization
2.2. Mathematical Model and Numerical Modeling
2.2.1. Soil-Moisture Model
2.2.2. Soil Solute Transport Model
2.2.3. Spatial and Temporal Discretizing
2.2.4. Initial Conditions and Boundary Conditions
2.3. Initial Setting of Parameters
2.4. Model Calibration and Validation
2.4.1. Data Sources
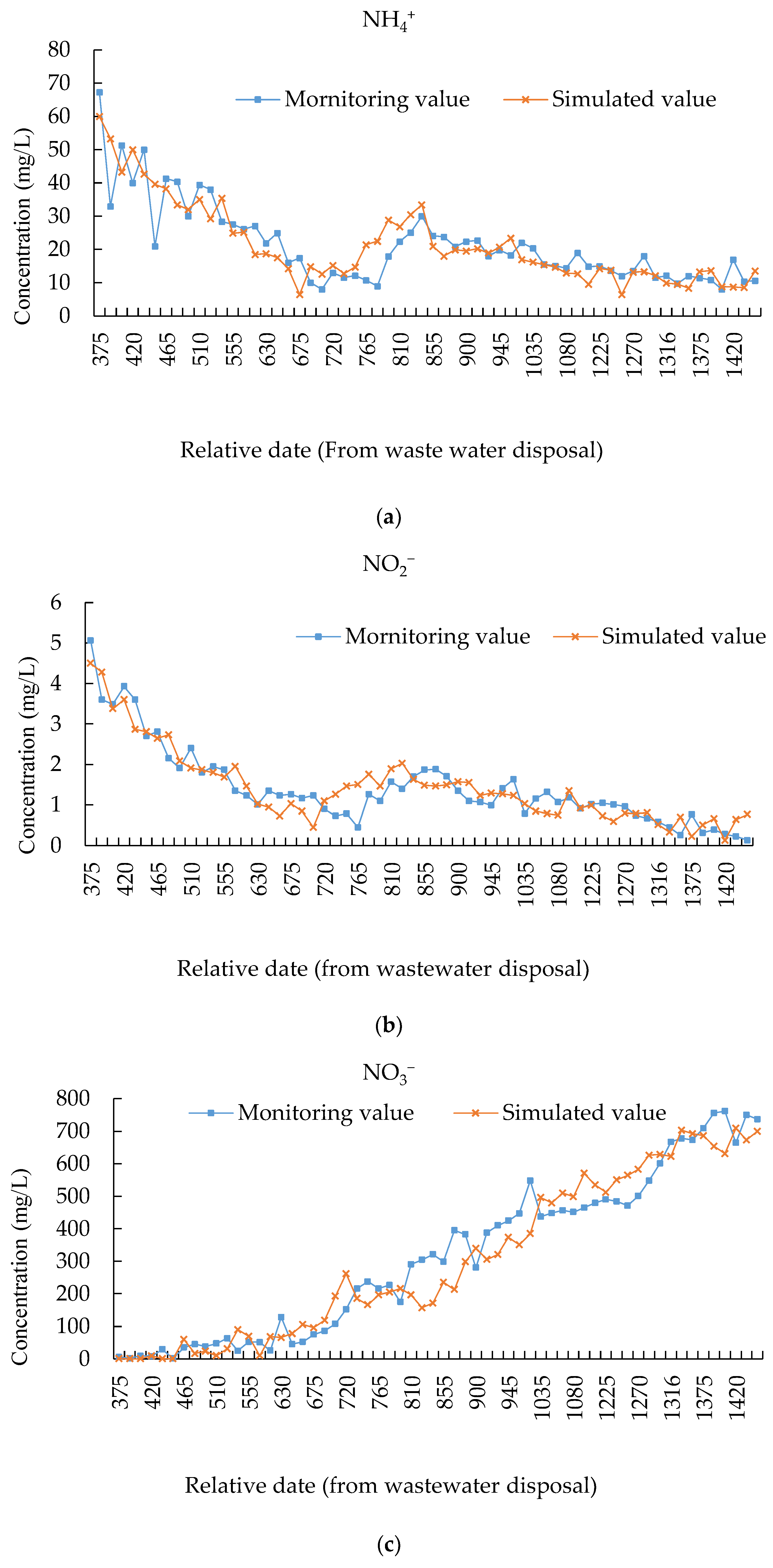
2.4.2. Calibration and Validation
3. Simulation Results
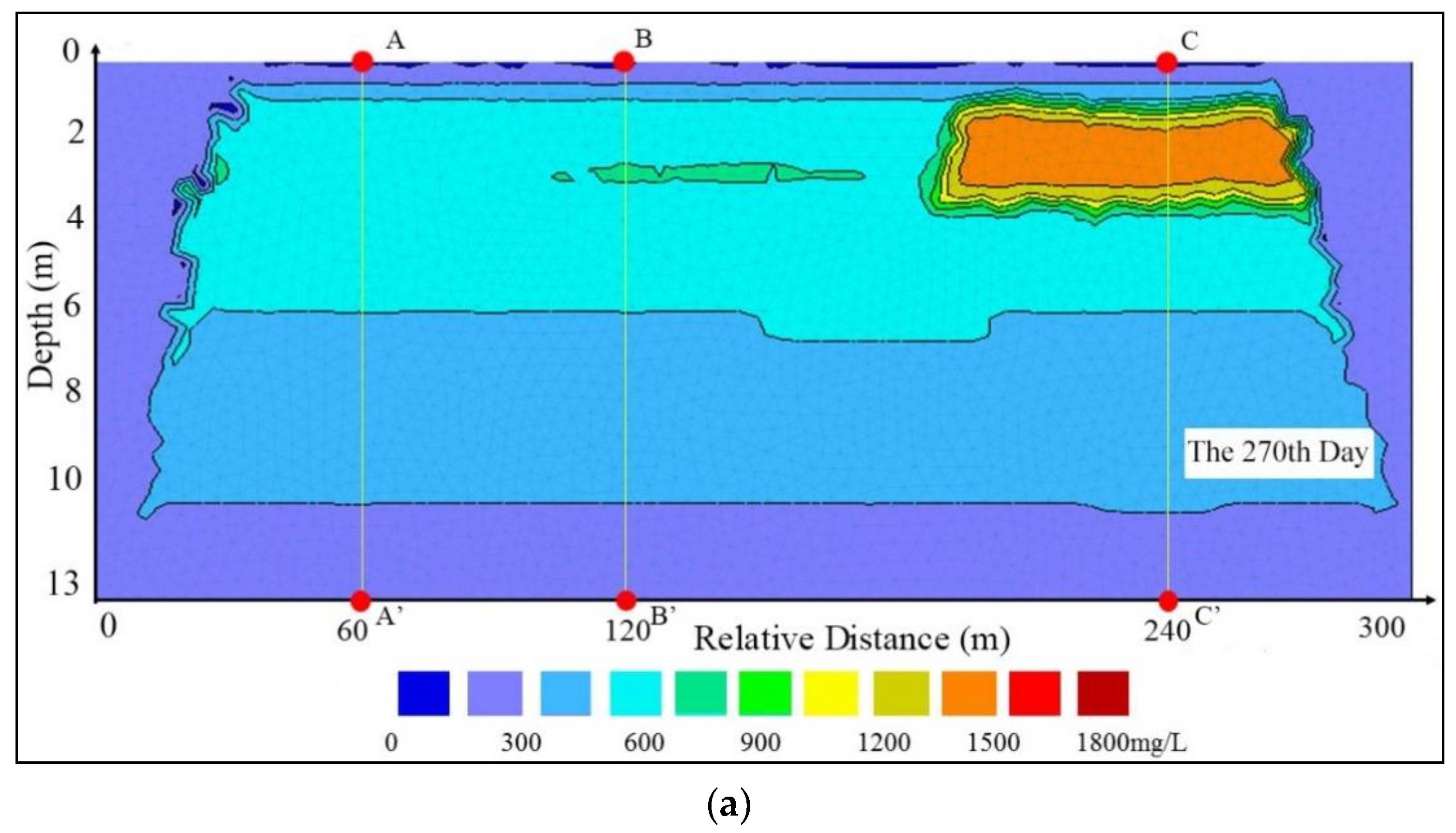
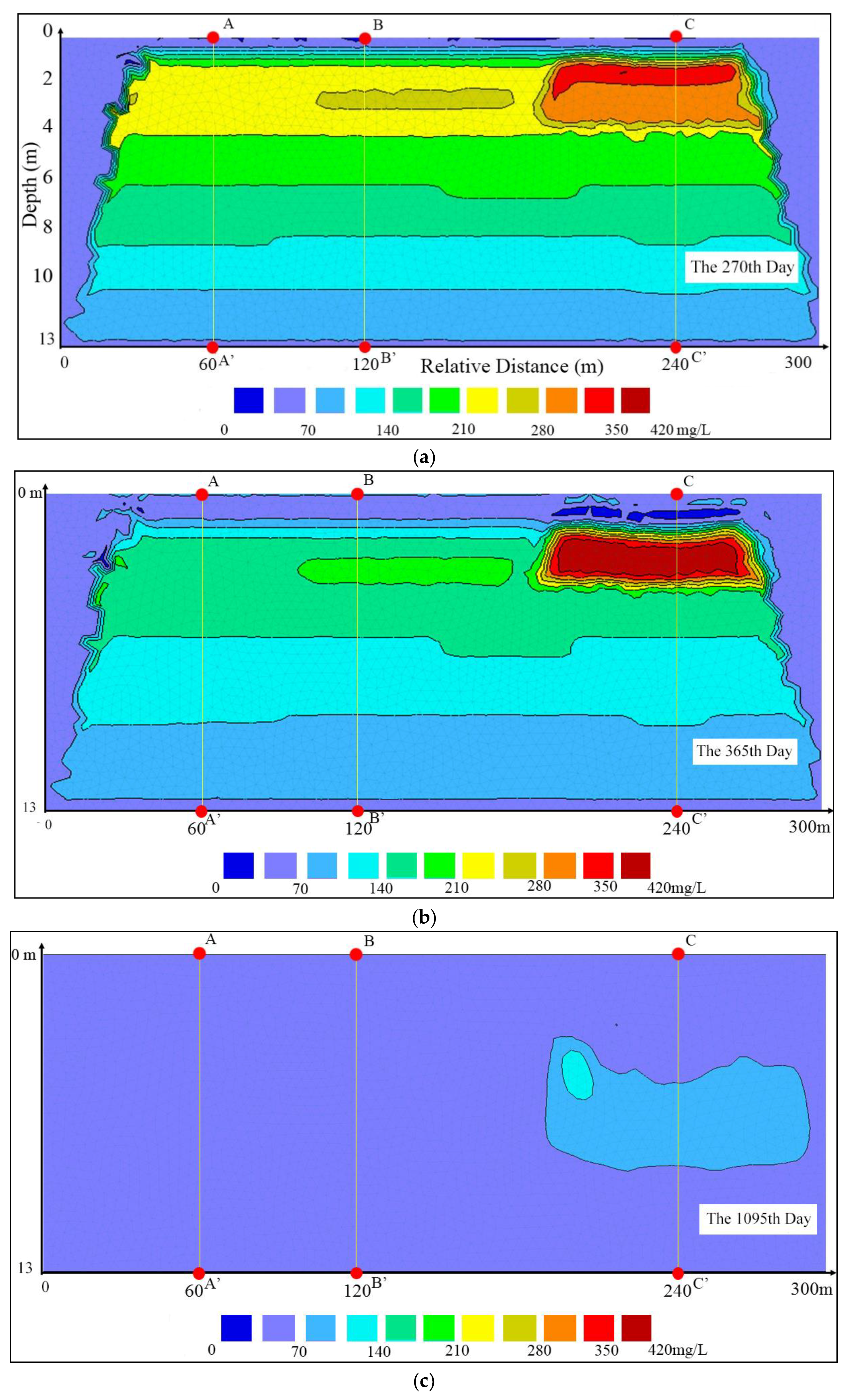
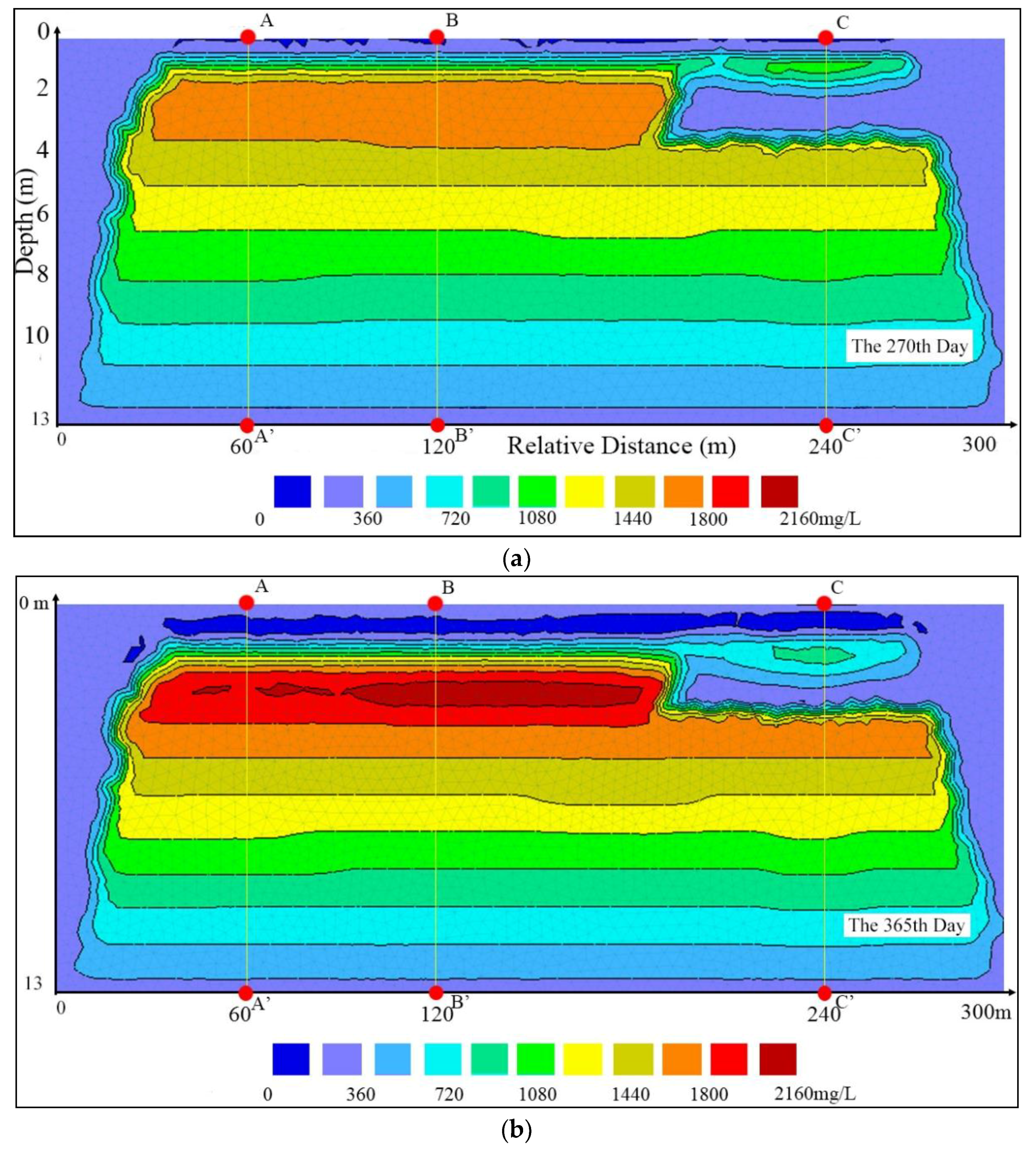
3.1. Vertical Changes in the Profile
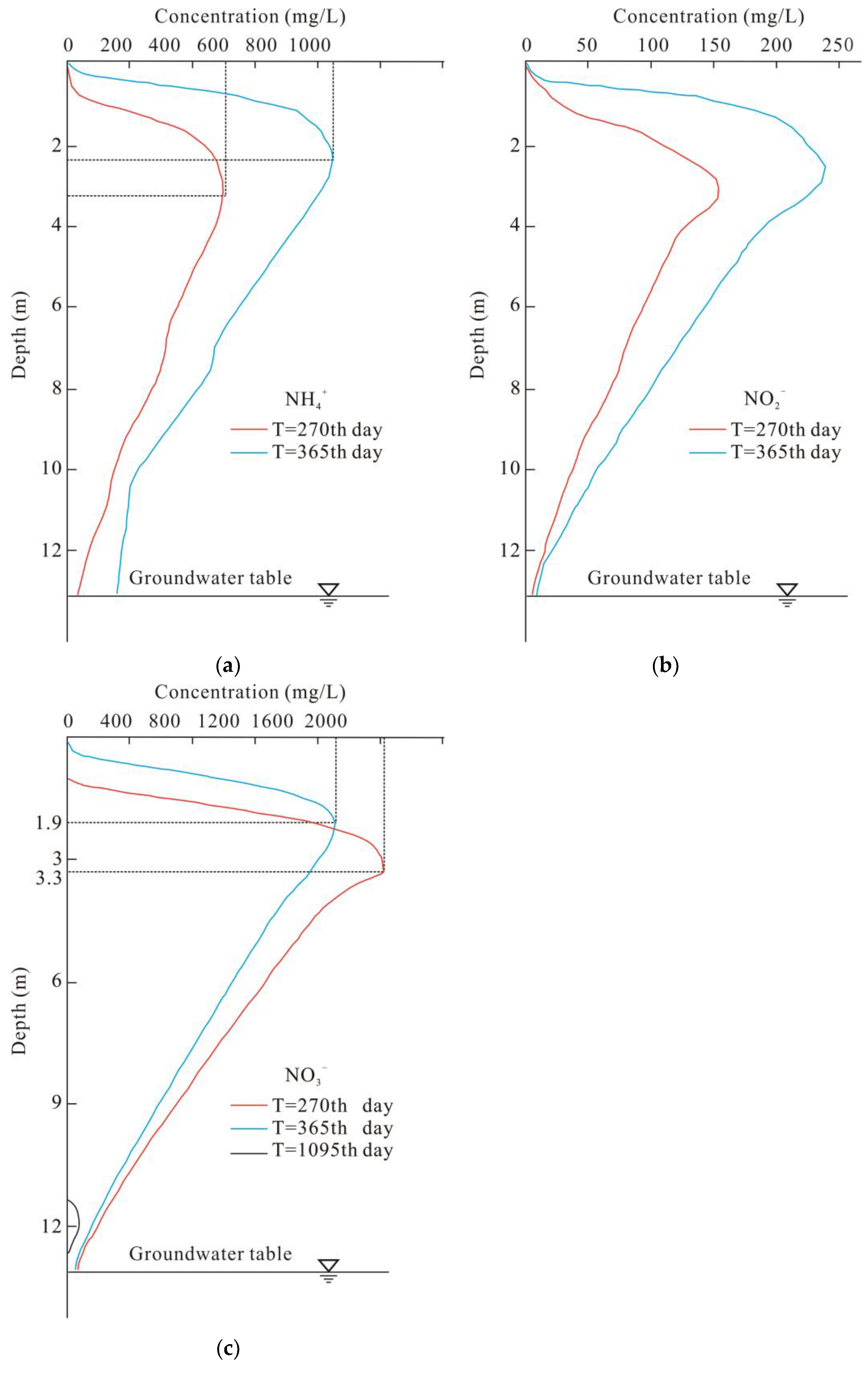
3.2. Detailed Vertical Changes
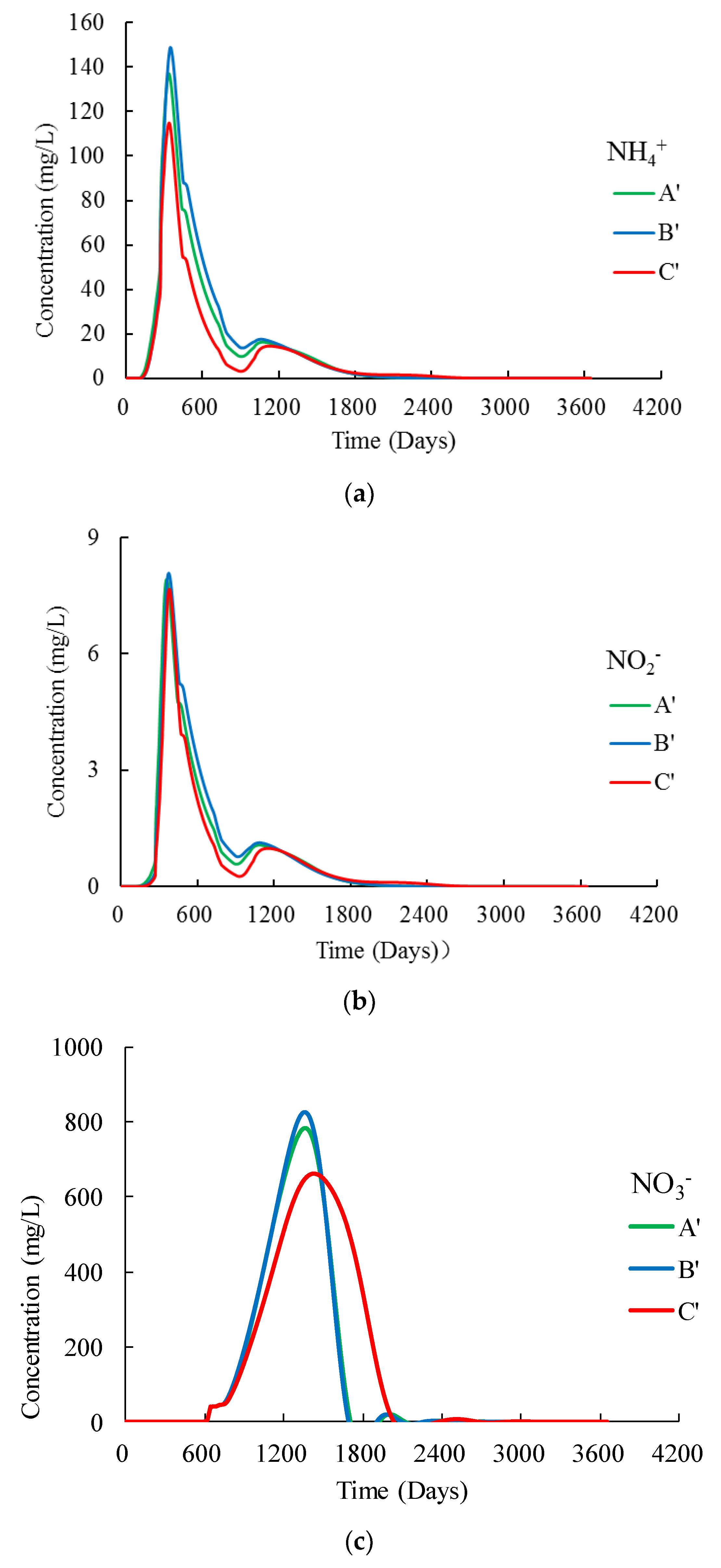
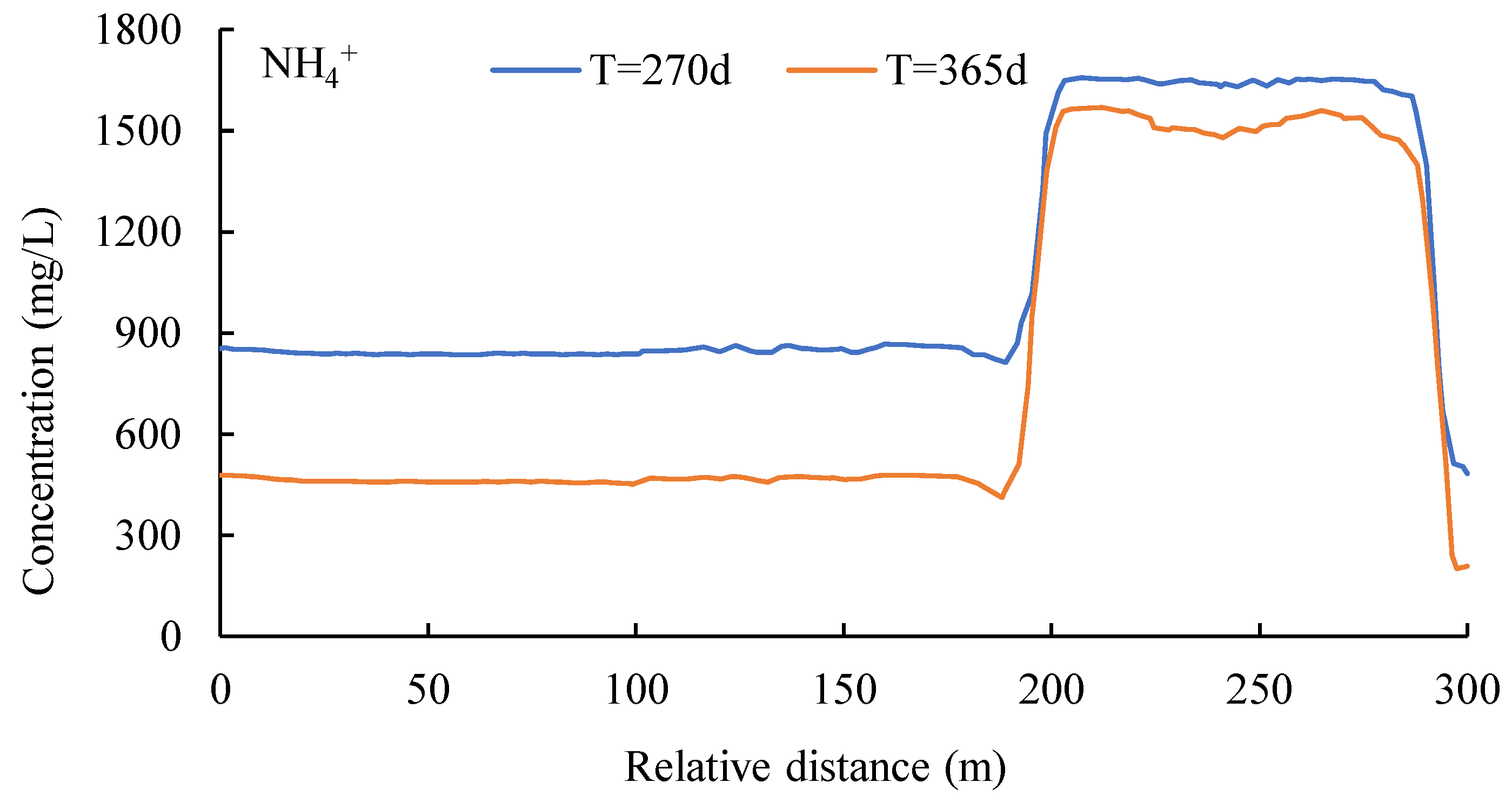
3.3. Nitrogen Concentrations at the Water Table
4. Discussion
4.1. Hysteresis of the Infiltration Process
4.2. Impacts of the Subclay Lenses
4.3. Effectiveness of the Unsaturated Zone for Groundwater Protection
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duan, W.; Takara, K.; He, B.; Luo, P.; Nover, D.; Yamashiki, Y. Spatial and temporal trends in estimates of nutrient and suspended sediment loads in the Ishikari River, Japan, 1985 to 2010. Sci. Total Environ. 2013, 461, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; He, B.; Nover, D.; Yang, G.; Chen, W.; Meng, H.; Zou, S.; Liu, C. Water quality assessment and pollution source identification of the eastern Poyang Lake basin using multivariate statistical methods. Sustainability 2016, 8, 133. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Yin, Z.; Zuo, R.; Wang, J.; Yang, J.; Teng, Y.; Zhai, Y. Migration process simulation of ammonia nitrogen in contaminated site. Chin. Environ. Sci. 2017, 37, 4585–4595. [Google Scholar]
- Lai, L.; Zhou, H.; Lai, B. Heterogeneous degradation of bisphenol A by peroxymonosulfate activated with vanadium-titanium magnetite: Performance, transformation pathways and mechanism. Chem. Eng. J. 2018, 349, 633–645. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; Yao, G.; Bo, L. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024. [Google Scholar] [CrossRef]
- Azizian, M.; Boano, F.; Cook, P.L.M.; Detwiler, R.L.; Rippy, M.A.; Grant, S.B. Ambient groundwater flow diminishes nitrate processing in the hyporheic zone of streams. Water Resour. Res. 2017, 53, 3941–3967. [Google Scholar] [CrossRef]
- Du, Y.; Ma, T.; Deng, Y.M.; Shen, S.; Lu, Z.J. Sources and fate of high levels of ammonium in surface water and shallow groundwater of the Jianghan Plain, Central China. Environ. Sci.-Process. Impacts 2017, 19, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Pasten-Zapata, E.; Ledesma-Ruiz, R.; Harter, T.; Ramirez, A.I.; Mahlknecht, J. Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi-tracer approach. Sci. Total Environ. 2014, 470, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Boningari, T.; Smirniotis, P.G. Impact of nitrogen oxides on the environment and human health: Mn-based materials for the NOx abatement. Curr. Opin. Chem. Eng. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Cao, S.J.; Kong, X.R.; Li, L.Y.; Zhang, W.R.; Ye, Z.P.; Deng, Y.L. An investigation of the PM2.5 and NO2 concentrations and their human health impacts in the metro subway system of Suzhou, China. Environ. Sci.-Process. Impacts 2017, 19, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Boufekane, A.; Saighi, O. Application of Groundwater Vulnerability Overlay and Index Methods to the Jijel Plain Area (Algeria). Groundwater 2018, 56, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, K.; Sartaj, M.; Tsai, F.T.C.; Singh, V.P.; Kazakis, N.; Melesse, A.M.; Prakash, I.; Bui, D.T.; Binh, T.P. A comparison study of DRASTIC methods with various objective methods for groundwater vulnerability assessment. Sci. Total Environ. 2018, 642, 1032–1049. [Google Scholar] [CrossRef] [PubMed]
- Coban, O.; Kuschk, P.; Kappelmeyer, U.; Spott, O.; Martienssen, M.; Jetten, M.S.M.; Knoeller, K. Nitrogen transforming community in a horizontal subsurface-flow constructed wetland. Water Res. 2015, 74, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Y.; Zheng, C.M.; Liu, J.; Cao, G.L.; Xiao, H.L.; Li, H.T.; Li, W.P. Conceptual and numerical models for groundwater flow in an arid inland river basin. Hydrol. Process. 2015, 29, 1480–1492. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, L.; Zhang, S.; Li, Z.; Yin, M.; Zang, Y. The Application of Visual Modflow to the Simulation of Groundwater Nitrate Contamination in Shijiazhuang. Acta Geosci. Sin. 2007, 28, 561–566. [Google Scholar]
- Twarakavi, N.K.C.; Simunek, J.; Seo, S. Evaluating interactions between groundwater and vadose zone using the HYDRUS-based flow package for MODFLOW. Vadose Zone J. 2008, 7, 757–768. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.X.; Wu, W.X.; Shi, D.Z.; Yang, M.; Zhong, Z.K. Evaluation of biochar effects on nitrogen retention and leaching in multi-layered soil columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gasser, M.O.; MacDonald, J.D.; Pelster, D.E.; Bertrand, N. Ammonia volatilization and nitrogen retention: How deep to incorporate urea? J. Environ. Qual. 2013, 42, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, G.; Tsihrintzis, V.A. Environmental Benefits and Control of Pollution to Surface Water and Groundwater by Agroforestry Systems: A. Review. Water Resour. Manag. 2018, 32, 1–29. [Google Scholar] [CrossRef]
- Izbicki, J.A.; Flint, A.L.; O’Leary, D.R.; Nishikawa, T.; Martin, P.; Johnson, R.D.; Clark, D.A. Storage and mobilization of natural and septic nitrate in thick unsaturated zones, California. J. Hydrol. 2015, 524, 147–165. [Google Scholar] [CrossRef]
- Lima, M.L.; Romanelli, A.; Massone, H.E. Assessing groundwater pollution hazard changes under different socio-economic and environmental scenarios in an agricultural watershed. Sci. Total Environ. 2015, 530, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, B.; Gil, A.; Flotats, X.; Sanchez, J.A. Assessment of groundwater vulnerability to nitrates from agricultural sources using a GIS-compatible logic multicriteria model. J. Environ. Manag. 2016, 171, 70–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, S.; Semkuyu, D.J.; Pandey, V.P. Assessment of groundwater vulnerability and risk to pollution in Kathmandu Valley, Nepal. Sci. Total Environ. 2016, 556, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Du, Q.; Zhai, Y.; Yang, J.; Guo, Y.; Zuo, R. Simulation and prediction of leakage incident on migration and transformation of three nitrogens based on HYDRUS-2D software. Environ. Pollut. Control 2017, 39, 1071–1076. [Google Scholar]
- Simunek, J.; van Genuchten, M.T.; Sejna, M. Recent developments and applications of the Hydrus computer software packages. Vadose Zone J. 2016, 15. [Google Scholar] [CrossRef]
- Li, Y.; Simunek, J.; Zhang, Z.; Huang, M.; Ni, L.; Zhu, L.; Hua, J.; Chen, Y. Water flow and nitrate transport through a lakeshore with different revetment materials. J. Hydrol. 2015, 520, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, P.J.; Johnston, P.M.; Gill, L.W. The impact of on-site wastewater from high density cluster developments on groundwater quality. J. Contam. Hydrol. 2015, 182, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Baram, S.; Couvreur, V.; Harter, T.; Read, M.; Brown, P.H.; Kandelous, M.; Smart, D.R.; Hopmans, J.W. Estimating Nitrate Leaching to Groundwater from Orchards: Comparing Crop Nitrogen Excess, Deep Vadose Zone Data-Driven Estimates, and HYDRUS Modeling. Vadose Zone J. 2016, 15, 13. [Google Scholar] [CrossRef]
- Kazakis, N.; Voudouris, K.S. Groundwater vulnerability and pollution risk assessment of porous aquifers to nitrate: Modifying the DRASTIC method using quantitative parameters. J. Hydrol. 2015, 525, 13–25. [Google Scholar] [CrossRef]
- Tran, N.H.; Gin, K.Y.-H.; Ngo, H.H. Fecal pollution source tracking toolbox for identification, evaluation and characterization of fecal contamination in receiving urban surface waters and groundwater. Sci. Total Environ. 2015, 538, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Hanson, B.R.; Simunek, J.; Hopmans, J.W. Evaluation of urea-ammonium-nitrate fertigation with drip irrigation using numerical modeling. Agric. Water Manag. 2006, 86, 102–113. [Google Scholar] [CrossRef]
- Sopilniak, A.; Elkayam, R.; Lev, O. Nitrification in a soil-aquifer treatment system: Comparison of potential nitrification and concentration profiles in the vadose zone. Environ. Sci.-Process. Impacts 2017, 19, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Feng, Q.; Si, J.H.; Chang, Z.Q.; Xi, H.Y.; Liu, W. Progress in the study of models for water and salinity transport in soils. J. Glaciol. Geocryol. 2008, 30, 527–534. [Google Scholar]
- Elmaloglou, S.; Soulis, K.X.; Dercas, N. Simulation of Soil Water Dynamics under Surface Drip Irrigation from Equidistant Line Sources. Water Resour. Manag. 2013, 27, 4131–4148. [Google Scholar] [CrossRef]
- Phogat, V.; Potter, N.J.; Cox, J.W.; Simunek, J. Long-Term Quantification of Stream-Aquifer Exchange in a Variably-Saturated Heterogeneous Environment. Water Resour. Manag. 2017, 31, 4353–4366. [Google Scholar] [CrossRef] [Green Version]
- Mo’allim, A.A.; Kamal, M.R.; Muhammed, H.H.; Yahaya, N.K.E.M.; Zawawe, M.A.B.M.; Man, H.B.C.; Wayayok, A. An Assessment of the Vertical Movement of Water in a Flooded Paddy Rice Field Experiment Using Hydrus-1D. Water 2018, 10, 783. [Google Scholar] [CrossRef]
- De Maet, T.; Hanert, E.; Vanclooster, M. A fully-explicit discontinuous Galerkin hydrodynamic model for variably-saturated porous media. J. Hydrodyn. 2014, 26, 594–607. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Li, Y. Simulation of water and solute transport characteristics in different bioretention tanks using HYDRUS-1D model. Acta Sci. Circums. 2017, 37, 4150–4159. [Google Scholar]
- Li, J.; Zhao, R.; Li, Y.; Chen, L. Modeling the effects of parameter optimization on three bioretention tanks using the HYDRUS-1D model. J. Environ. Manag. 2018, 217, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, R.P.; Chenaf, D. Effects of monitoring and pumping well pipe capacities during pumping tests in confined aquifers. Can. Geotech. J. 2003, 40, 1093–1103. [Google Scholar] [CrossRef]
- Ou, C.-Y.; Chen, S.-H. Performance and analysis of pumping tests in a gravel formation. Bull. Eng. Geol. Environ. 2010, 69, 1–12. [Google Scholar] [CrossRef]
- Salazar, O.; Wesstrom, I.; Youssef, M.A.; Skaggs, R.W.; Joel, A. Evaluation of the DRAINMOD-N II model for predicting nitrogen losses in a loamy sand under cultivation in south-east Sweden. Agric. Water Manag. 2009, 96, 267–281. [Google Scholar] [CrossRef]
- Pickens, J.F.; Grisak, G.E. Scale-dependent dispersion in a stratified granular aquifer. Water Resour. Res. 1981, 17, 1191–1211. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Wang, T. Isotopes tracer research of wet sand layer water sources in Alxa Desert. Adv. Water Sci. 2014, 25, 196–206. [Google Scholar]
- Ma, J.; Li, X.; Huang, T.; Edmunds, W.M. Chemical evolution and recharge characteristics of water resources in the Shiyang River Basin. Resour. Sci. 2005, 27, 117–122. [Google Scholar]
- Ding, Z.; Ma, J.; Zhang, B.; Zhao, X. Analysis on the climate change in the Shiyang River basin since resent 50 years. Arid Zone Res. 2007, 24, 779–784. [Google Scholar]
- Fan, Y.; Zhao, T.; Bai, G.; Liu, W. HYDRUS-2D simulation of soil wetting pattern with horizontal moistube-irrigation and analysis of its influencing factors. Trans. Chin. Soc. Agric. Eng. 2018, 34, 115–124. [Google Scholar]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Hilscher, A.; Knicker, H. Carbon and nitrogen degradation on molecular scale of grass-derived pyrogenic organic material during 28 months of incubation in soil. Soil Biol. Biochem. 2011, 43, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Zuo, R.; Jin, S.; Chen, M.; Guan, X.; Wang, J.; Zhai, Y.; Teng, Y.; Guo, X. In-situ study of migration and transformation of nitrogen in groundwater based on continuous observations at a contaminated desert site. J. Contam. Hydrol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Chiogna, G.; Eberhardt, C.; Grathwohl, P.; Cirpka, O.A.; Rolle, M. Evidence of Compound-Dependent Hydrodynamic and Mechanical Transverse Dispersion by Multitracer Laboratory Experiments. Environ. Sci. Technol. 2010, 44, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Kumar, E.; Sillanpaa, M. Fluoride removal from water by adsorption—A review. Chem. Eng. J. 2011, 171, 811–840. [Google Scholar] [CrossRef]
- Sprenger, M.; Seeger, S.; Blume, T.; Weiler, M. Travel times in the vadose zone: Variability in space and time. Water Resour. Res. 2016, 52, 5727–5754. [Google Scholar] [CrossRef] [Green Version]
- Van Hulle, S.W.H.; Vandeweyer, H.J.P.; Meesschaert, B.D.; Vanrolleghem, P.A.; Dejans, P.; Dumoulin, A. Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem. Eng. J. 2010, 162, 1–20. [Google Scholar] [CrossRef]
- Djaman, K.; Mel, V.C.; Diop, L.; Sow, A.; El-Namaky, R.; Manneh, B.; Saito, K.; Futakuchi, K.; Irmak, S. Effects of Alternate Wetting and Drying Irrigation Regime and Nitrogen Fertilizer on Yield and Nitrogen Use Efficiency of Irrigated Rice in the Sahel. Water 2018, 10, 711. [Google Scholar] [CrossRef]
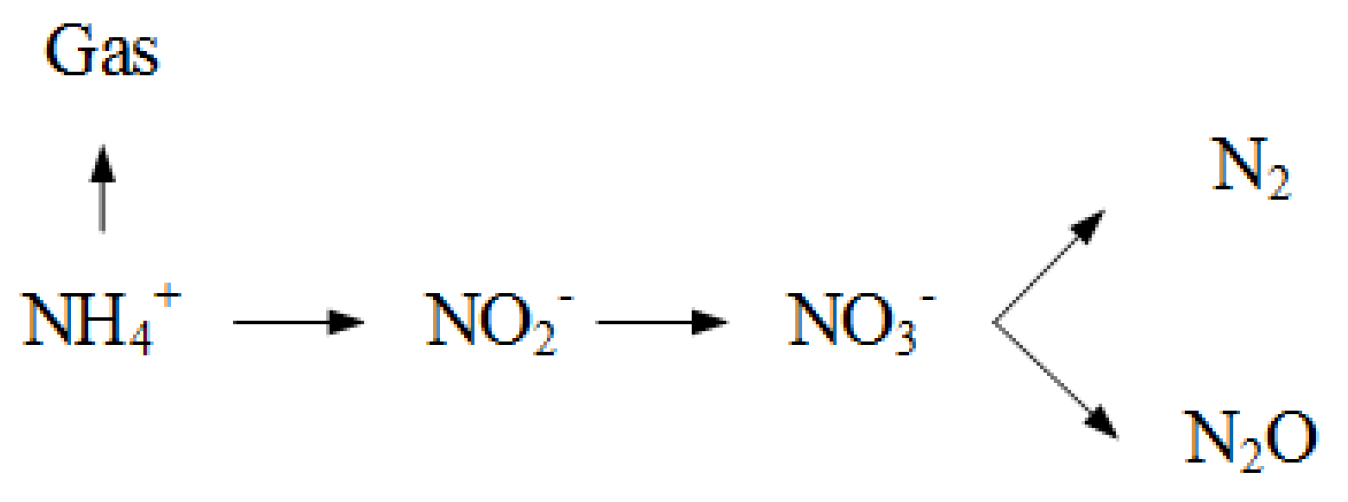


| Parameter | θr | α | n | Ks | l | |
|---|---|---|---|---|---|---|
| Unit | cm3/cm3 | cm3/cm3 | m−1 | / | cm/day | / |
| Initial values | ||||||
| Silt | 0.076 | 0.47 | 0.016 | 1.6 | 1300 | 0.5 |
| Fine powder sand | 0.0089 | 0.44 | 0.01 | 1.24 | 1200 | 0.5 |
| Subclay | 0.034 | 0.45 | 0.02 | 1.4 | 600 | 0.5 |
| Calibrated values | ||||||
| Silt | 0.076 | 0.45 | 0.02 | 1.41 | 1300 | 0.5 |
| Fine powder sand | 0.0089 | 0.43 | 0.01 | 1.23 | 1200 | 0.5 |
| Subclay | 0.034 | 0.46 | 0.016 | 1.37 | 600 | 0.5 |
| Parameter | Bulk.d | Disp.L | Disp.T | Kd | Dw | K1 | K2 | K3 |
|---|---|---|---|---|---|---|---|---|
| Unit | mg/cm3 | cm | cm | cm3/mg | cm2/day | day−1 | day−1 | day−1 |
| Initial values | ||||||||
| Silt | 1360 | 20.4 | 18.2 | 0.098 | 4 | 0.0010 | 0.0200 | 0.00005 |
| Fine powder sand | 1350 | 1.45 | 0.14 | 0.182 | 4 | 0.0082 | 0.0200 | 0.00005 |
| Subclay | 1300 | 0.6 | 0.06 | 0.200 | 4 | 0.0008 | 0.0002 | 0.0006 |
| Calibrated values | ||||||||
| Silt | 1360 | 20.6 | 17.5 | 0.098 | 4 | 0.0012 | 0.0200 | 0.00005 |
| Fine powder sand | 1350 | 1.47 | 0.18 | 0.182 | 4 | 0.0084 | 0.0200 | 0.00005 |
| Subclay | 1300 | 0.54 | 0.06 | 0.500 | 4 | 0.0007 | 0.0016 | 0.0006 |
| Indicator Name | MAE | RMSE | PBAIS (%) | NSE |
|---|---|---|---|---|
| NH4+ | 4.741 | 6.803 | 0.65 | 0.71 |
| NO2− | 0.332 | 0.386 | −1.01 | 0.82 |
| NO3− | 56.916 | 75.315 | 3.35 | 0.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Zhai, Y.; Xia, X.; Yin, Z.; Du, Q.; Zuo, R.; Wang, J.; Teng, Y.; Xu, M. Simulation of Trinitrogen Migration and Transformation in the Unsaturated Zone at a Desert Contaminant Site (NW China) Using HYDRUS-2D. Water 2018, 10, 1363. https://doi.org/10.3390/w10101363
Zheng F, Zhai Y, Xia X, Yin Z, Du Q, Zuo R, Wang J, Teng Y, Xu M. Simulation of Trinitrogen Migration and Transformation in the Unsaturated Zone at a Desert Contaminant Site (NW China) Using HYDRUS-2D. Water. 2018; 10(10):1363. https://doi.org/10.3390/w10101363
Chicago/Turabian StyleZheng, Fuxin, Yuanzheng Zhai, Xuelian Xia, Zhihua Yin, Qingqing Du, Rui Zuo, Jinsheng Wang, Yanguo Teng, and Mo Xu. 2018. "Simulation of Trinitrogen Migration and Transformation in the Unsaturated Zone at a Desert Contaminant Site (NW China) Using HYDRUS-2D" Water 10, no. 10: 1363. https://doi.org/10.3390/w10101363






