Understanding the Burial and Migration Characteristics of Deep Geothermal Water Using Hydrogen, Oxygen, and Inorganic Carbon Isotopes
Abstract
:1. Introduction
2. Materials and Methods
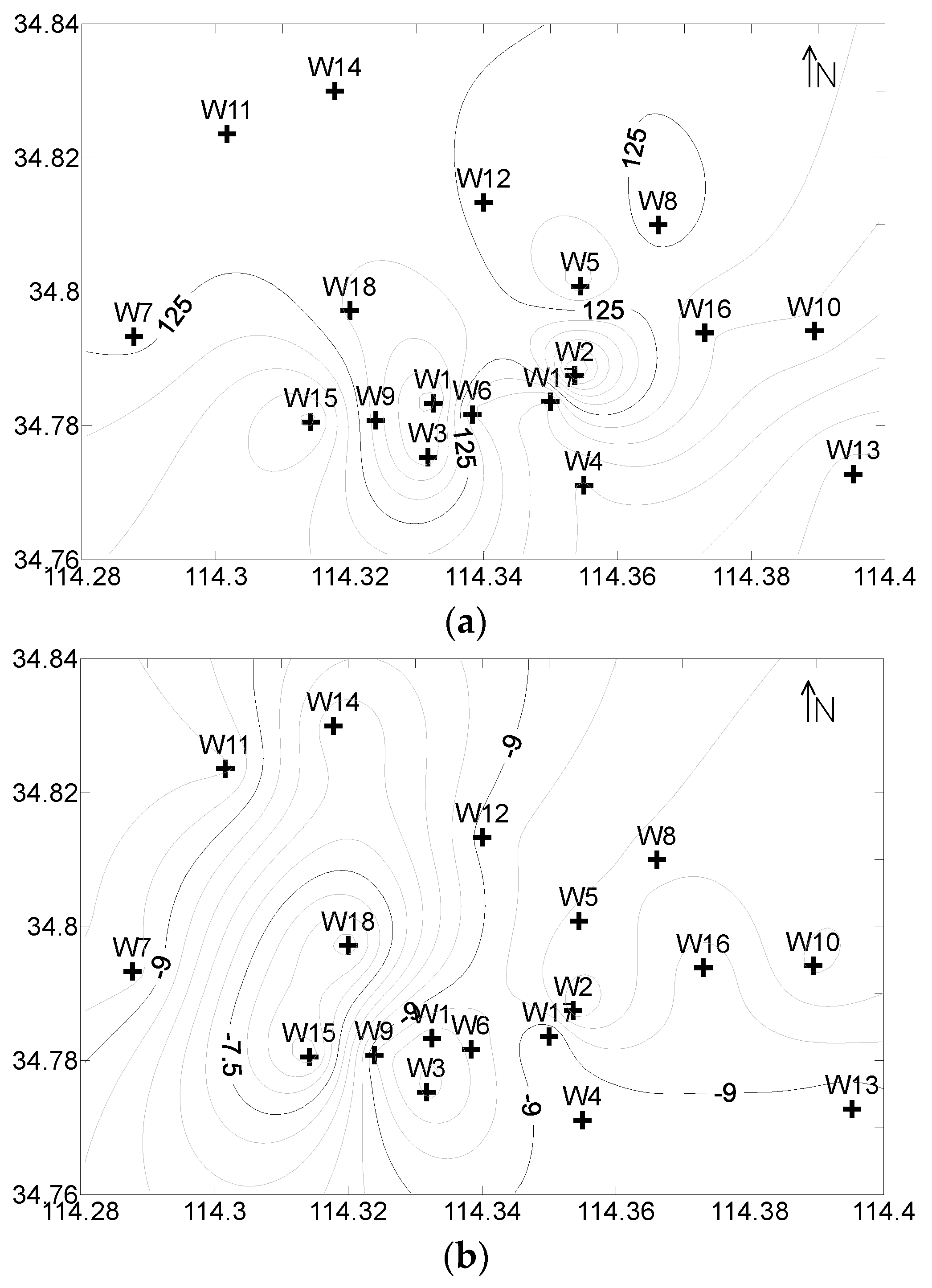
2.1. Hydrogeological Characteristics
2.2. Sample Collection and Testing
3. Results and Discussion
3.1. The Chemical Characteristics of Water
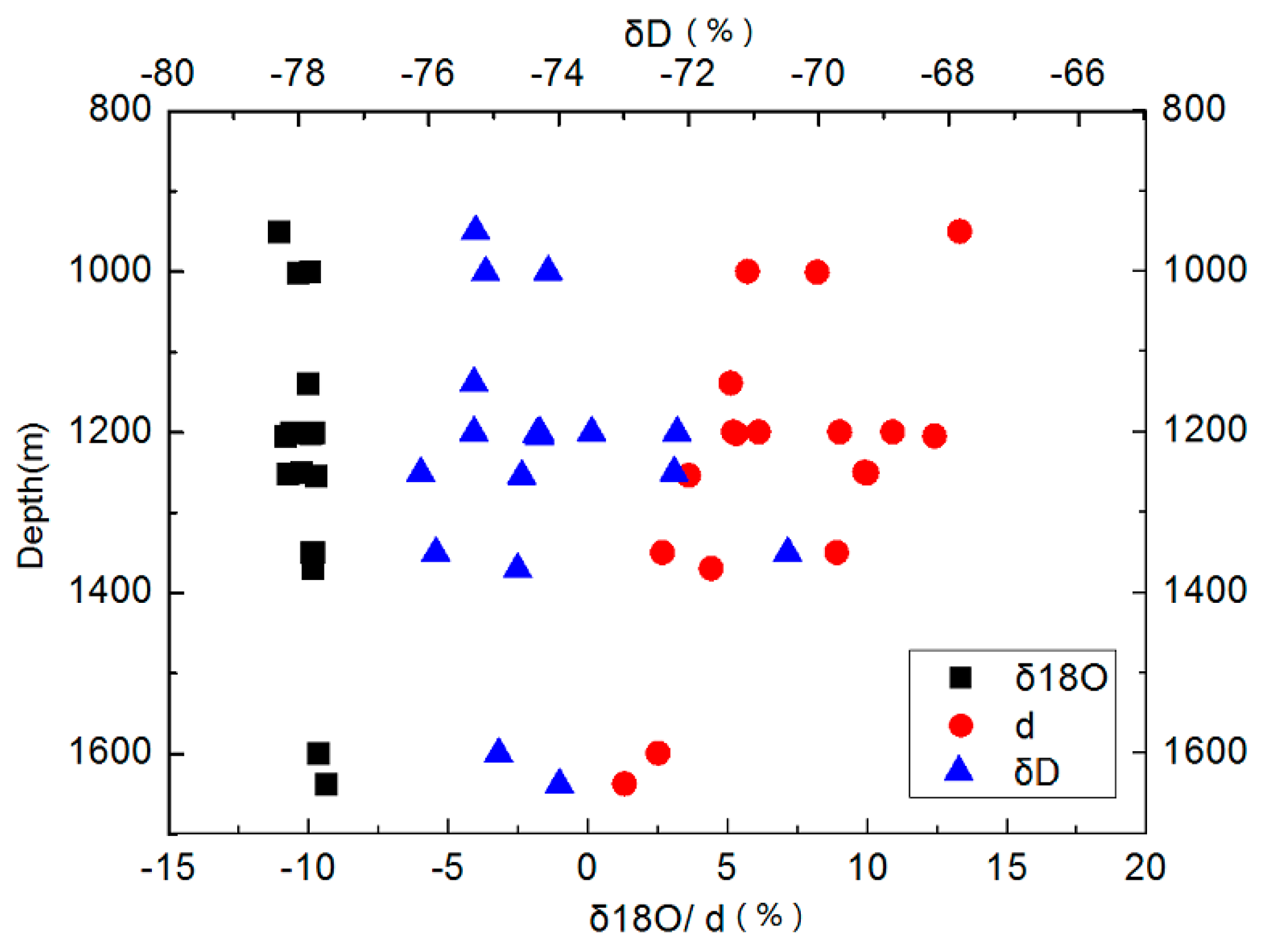
3.2. Isotope Characteristics of δD-δ18O
3.3. D Value Characteristics
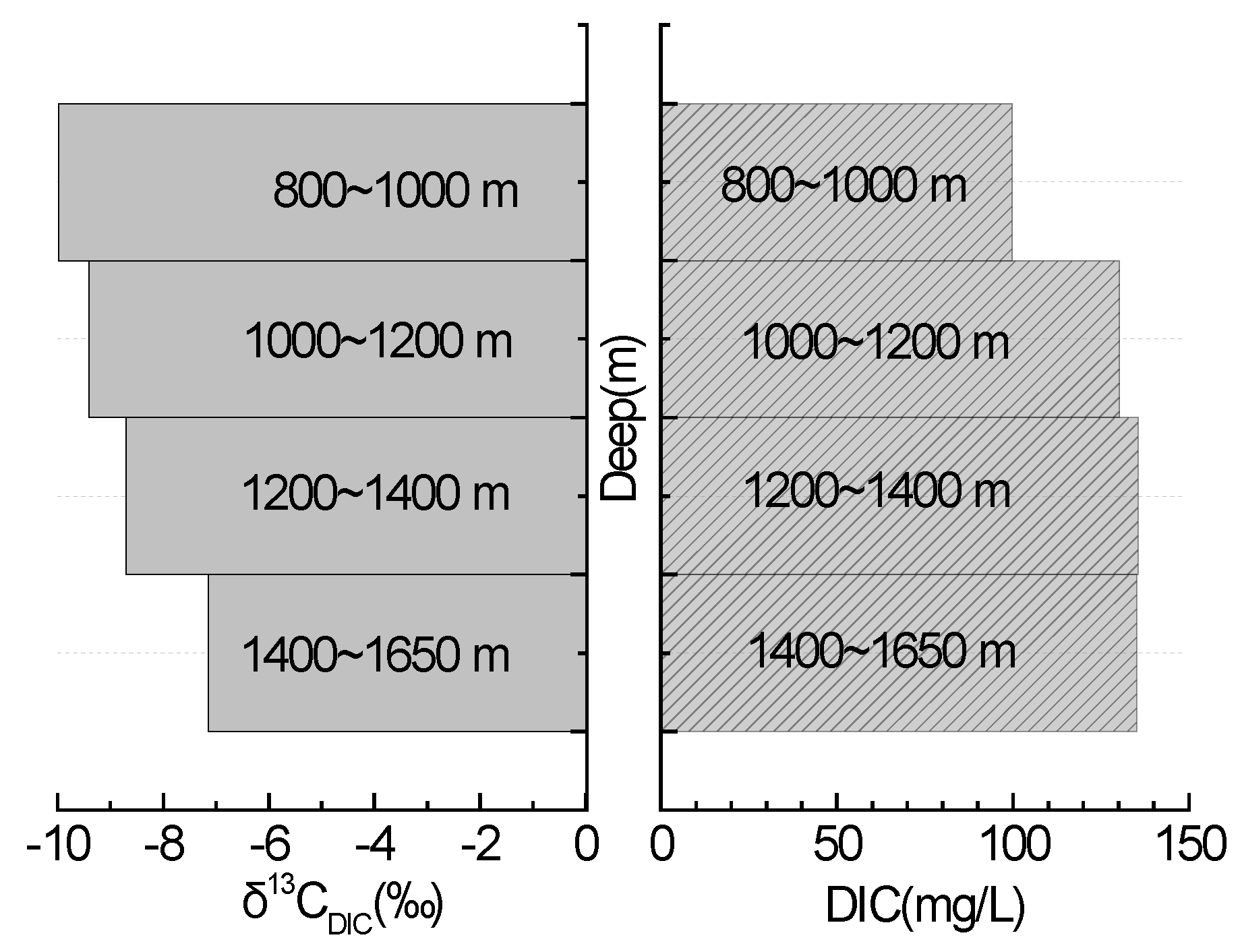
3.4. DIC and δ13CDIC Characteristics
4. Composition of DIC Sources
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marques, J.M.; Graça, H.; Eggenkamp, H.G.; Neves, O.; Carreira, P.M.; Matias, M.J.; Mayer, B.; Nunes, D.; Trancoso, V.N. Isotopic and hydrochemical data as indicators of recharge areas, flow paths and water–rock interaction in the caldas da rainha–quinta das janelasthermomineral carbonate rock aquifer (central Portugal). J. Hydrol. 2013, 476, 302–313. [Google Scholar] [CrossRef]
- Cervi, F.; Borgatti, L.; Dreossi, G.; Marcato, G.; Michelini, M.; Stenni, B. Isotopic features of precipitation and groundwater from the eastern alps of Italy: Results from the MT. Tinisa hydrogeological system. Environ. Earth Sci. 2017, 76, 410. [Google Scholar] [CrossRef]
- Deiana, M.; Mussi, M.; Ronchetti, F. Discharge and environmental isotope behaviours of adjacent fractured and porous aquifers. Environ. Earth Sci. 2017, 76, 595. [Google Scholar] [CrossRef]
- Mussi, M.; Nanni, T.; Tazioli, A.; Vivalda, P.M. The MT conero limestone ridge: The contribution of stable isotopes to the identification of the recharge area of aquifers. Ital. J. Geosci. 2017, 136, 186–197. [Google Scholar] [CrossRef]
- Cervi, F.; Ronchetti, F.; Doveri, M.; Mussi, M.; Marcaccio, M.; Tazioli, A. The use of stable water isotopes from rain gauges network to define the recharge areas of springs: Problems and possible solutions from case studies from the northern Apennines. Geam. Geoing. Ambient. Min. 2016, 149, 19–26. [Google Scholar]
- Peng, T.-R.; Wang, C.-H.; Huang, C.-C.; Fei, L.-Y.; Chen, C.-T.A.; Hwong, J.-L. Stable isotopic characteristic of taiwan’s precipitation: A case study of western pacific monsoon region. Earth Planet. Sci. Lett. 2010, 289, 357–366. [Google Scholar] [CrossRef]
- Schiavo, M.; Hauser, S.; Povinec, P. Stable isotopes of water as a tool to study groundwater–seawater interactions in coastal south-eastern Sicily. J. Hydrol. 2009, 364, 40–49. [Google Scholar] [CrossRef]
- Dotsika, E.; Lykoudis, S.; Poutoukis, D. Spatial distribution of the isotopic composition of precipitation and spring water in Greece. Glob. Planet. Chang. 2010, 71, 141–149. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Liu, X.; Lili, A.; Zhang, Y. Temperature effect on the transport of nitrate and ammonium ions in a loose-pore geothermal reservoir. J. Geochem. Exp. 2013, 124, 59–66. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Zhang, Q.; Zhang, Y. Study on the transformation mechanism of nitrate in a loose-pore geothermal reservoir: Experimental results and numerical simulations. J. Geochem. Exp. 2014, 144, 208–215. [Google Scholar] [CrossRef]
- Huang, S.; Tian, T. Sustainable development of geothermal resource in China and future projects. World Geotherm. Congr. 2005, 4, 24–29. [Google Scholar]
- Haiyan, H. Environmental Impact of Geothermal Development in Henan Province, China. Available online: http://www.os.is/gogn/unu-gtp-report/UNU-GTP-2003-11.pdf (accessed on 19 December 2017).
- Schwertl, M.; Auerswald, K.; Schäufele, R.; Schnyder, H. Carbon and nitrogen stable isotope composition of cattle hair: Ecological fingerprints of production systems? Agricult. Ecosyst. Environ. 2005, 109, 153–165. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin, Germany, 1997; Volume 201. [Google Scholar]
- Craig, H. Isotopic composition and origin of the red sea and salton sea geothermal brines. Science 1966, 154, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Pang, Z.; Wang, X. Characteristics of chemistry and stable isotopes in groundwater of the chaobai river catchment, Beijing. Proced. Earth Planet. Sci. 2013, 7, 487–490. [Google Scholar] [CrossRef]
- West, A.; February, E.; Bowen, G.J. Spatial analysis of hydrogen and oxygen stable isotopes (“isoscapes”) in ground water and tap water across South Africa. J. Geochem. Exp. 2014, 145, 213–222. [Google Scholar] [CrossRef]
- Koh, D.-C.; Ha, K.; Lee, K.-S.; Yoon, Y.-Y.; Ko, K.-S. Flow paths and mixing properties of groundwater using hydrogeochemistry and environmental tracers in the southwestern area of jeju volcanic island. J. Hydrol. 2012, 432, 61–74. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Jouzel, J.; Landais, A.; Stievenard, M.; Johnsen, S.J.; White, J.; Werner, M.; Sveinbjornsdottir, A.; Fuhrer, K. Grip deuterium excess reveals rapid and orbital-scale changes in greenland moisture origin. Science 2005, 309, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Vimeux, F.; Masson, V.; Jouzel, J.; Petit, J.; Steig, E.; Stievenard, M.; Vaikmae, R.; White, J. Holocene hydrological cycle changes in the southern hemisphere documented in east antarctic deuterium excess records. Clim. Dyn. 2001, 17, 503–513. [Google Scholar] [CrossRef]
- Uemura, R.; Matsui, Y.; Yoshimura, K.; Motoyama, H.; Yoshida, N. Evidence of deuterium excess in water vapor as an indicator of ocean surface conditions. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- O’neil, J.; Shaw, S.; Flood, R. Oxygen and hydrogen isotope compositions as indicators of granite genesis in the New England batholith, Australia. Contrib. Miner. Petrol. 1977, 62, 313–328. [Google Scholar] [CrossRef]
- Deines, P. The carbon isotope geochemistry of mantle xenoliths. Earth Sci. Rev. 2002, 58, 247–278. [Google Scholar] [CrossRef]
- Li, F.; Pan, G.; Tang, C.; Zhang, Q.; Yu, J. Recharge source and hydrogeochemical evolution of shallow groundwater in a complex alluvial fan system, southwest of north China plain. Environ. Geol. 2008, 55, 1109–1122. [Google Scholar] [CrossRef]
- Warner, N.R.; Kresse, T.M.; Hays, P.D.; Down, A.; Karr, J.D.; Jackson, R.B.; Vengosh, A. Geochemical and isotopic variations in shallow groundwater in areas of the fayetteville shale development, north-central Arkansas. Appl. Geochem. 2013, 35, 207–220. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Lin, X.; Liao, Z. Geothermal field characteristics in the Kaifeng city. Adv. Water Sci. 2002, 13, 191–196. [Google Scholar]
- Zhu, H.L.; Liu, X.M.; Yang, F.; Yang, H.J.; Wang, X.Y. Analysis and study on geothermal reijection test of deep groundwater in Kaifeng. J. Henan Polytech. Univ. (Nat. Sci.) 2011, 30, 215–219. [Google Scholar]
- Lin, X.; Tabouré, A.; Wang, X.; Liao, Z. Use of a hydrogeochemical approach in determining hydraulic connection between porous heat reservoirs in Kaifeng area, Henan, China. Appl. Geochem. 2007, 22, 276–288. [Google Scholar] [CrossRef]
- Dong, W.-H.; Kang, B.; Du, S.-H.; Shi, X.-F. Estimation of shallow groundwater ages and circulation rates in the Henan plain, China: CFC and deuterium excess methods. Geosci. J. 2013, 17, 479–488. [Google Scholar] [CrossRef]
- Wang, X.; Han, P.; Liao, Z.; Lin, X. The geochemical method for studying hydraulic relationships among porous medium reservoirs. J. Hydraul. Eng. 2001, 32, 75–79. [Google Scholar]
- Li, M.; Gao, S.; Li, K. Features of hydrogen and oxygen isotopes of quaternary groundwater in Henan plain and the recharge analysis. Geotech. Investig. Surv. 2010, 38, 42–47. [Google Scholar]
- Vodila, G.; Palcsu, L.; Futo, I.; Szántó, Z. A 9-year record of stable isotope ratios of precipitation in eastern Hungary: Implications on isotope hydrology and regional palaeoclimatology. J. Hydrol. 2011, 400, 144–153. [Google Scholar] [CrossRef]
- Stumpp, C.; Klaus, J.; Stichler, W. Analysis of long-term stable isotopic composition in german precipitation. J. Hydrol. 2014, 517, 351–361. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Caballero, E.; Huertas, F.; Romanek, C. Chemical, mineralogical and isotope behavior, and phase transformation during the precipitation of calcium carbonate minerals from intermediate ionic solution at 25 °C. Geochim. Cosmochim. Acta 2001, 65, 3219–3231. [Google Scholar] [CrossRef]
- Li, S.-L.; Calmels, D.; Han, G.; Gaillardet, J.; Liu, C.-Q. Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC: Examples from southwest China. Earth Planet. Sci. Lett. 2008, 270, 189–199. [Google Scholar] [CrossRef]
- Francey, R.; Allison, C.; Etheridge, D.; Trudinger, C.; Enting, I.; Leuenberger, M.; Langenfelds, R.; Michel, E.; Steele, L. A 1000-year high precision record of δ13C in atmospheric CO2. Tellus B 1999, 51, 170–193. [Google Scholar] [CrossRef]
- Langenfelds, R.; Francey, R.; Pak, B.; Steele, L.; Lloyd, J.; Trudinger, C.; Allison, C. Interannual growth rate variations of atmospheric CO2 and its δ13C, H2, CH4, and CO between 1992 and 1999 linked to biomass burning. Glob. Biogeochem. Cycles 2002, 16. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Domanski, G. Carbon input by plants into the soil. Review. J. Plant Nutr. Soil Sci. 2000, 163, 421–431. [Google Scholar] [CrossRef]
- Carlson, C.A.; Hansell, D.A.; Nelson, N.B.; Siegel, D.A.; Smethie, W.M.; Khatiwala, S.; Meyers, M.M.; Halewood, E. Dissolved organic carbon export and subsequent remineralization in the mesopelagic and bathypelagic realms of the north atlantic basin. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 1433–1445. [Google Scholar] [CrossRef]

| Geothermal Well | Depth (m) | Stratum  | Temperature (°C) | pH | Na+ (mg/L) | K+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Cl− (mg/L) | SO42− (mg/L) | HCO3− (mg/L) | NO3− (mg/L) | H2SiO3 (mg/L) | DIC (mg C/L) | δ13CDIC (‰) | δD (‰) | δ18O (‰) | d (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W1 | 950 | Nm | 48 | 8.22 | 209.80 | 2.93 | 4.81 | 1.46 | 29.42 | 28.34 | 493.65 | 5.04 | 28.6 | 102.45 | −9.79 | −75.28 | −11.08 | 13.30 |
| W2 | 1000 | Nm | 40 | 8.18 | 199.70 | 2.18 | 4.81 | 2.92 | 34.39 | 28.34 | 454.60 | 1.09 | 27.3 | 91.52 | −10.16 | −74.16 | −9.98 | 5.70 |
| W3 | 1001 | Nm | 48 | 8.19 | 199.00 | 2.81 | 4.81 | 2.92 | 29.42 | 28.34 | 479.62 | 4.53 | 27.3 | 105.41 | −9.99 | −75.12 | −10.41 | 8.20 |
| W4 | 1139 | Nm | 57 | 7.58 | 302.20 | 4.59 | 4.81 | 2.92 | 41.12 | 90.78 | 663.90 | 0.22 | 29.9 | 145.27 | −8.78 | −75.30 | −10.05 | 5.10 |
| W5 | 1200 | Nm | 50.5 | 8.04 | 236.40 | 3.35 | 4.81 | 2.92 | 34.39 | 45.63 | 560.77 | 1.17 | 28.6 | 138.30 | −9.65 | −75.30 | −10.18 | 6.10 |
| W6 | 1200 | Nm | 52 | 8.12 | 219.00 | 3.31 | 4.81 | 2.92 | 29.42 | 34.10 | 546.74 | 1.40 | 23.4 | 135.86 | −9.48 | −74.29 | −10.65 | 10.90 |
| W7 | 1200 | Nm | 54 | 7.91 | 226.40 | 4.29 | 11.82 | 8.63 | 44.67 | 73.97 | 524.77 | 2.02 | 26.0 | 123.12 | −9.44 | −72.18 | −10.15 | 9.00 |
| W8 | 1200 | Nm | 55 | 8.08 | 254.30 | 3.68 | 4.81 | 2.92 | 32.61 | 51.39 | 596.78 | <0.01 | 32.5 | 121.99 | −9.64 | −73.49 | −9.84 | 5.20 |
| W9 | 1202 | Nm | 51 | 8.18 | 229.80 | 3.54 | 4.81 | 2.92 | 29.42 | 45.63 | 549.79 | 0.88 | 28.6 | 117.31 | −9.42 | −74.33 | −9.96 | 5.30 |
| W10 | 1205 | Ng | 52.5 | 8.21 | 224.20 | 2.91 | 4.81 | 2.92 | 29.42 | 45.63 | 524.77 | <0.01 | 31.2 | 135.77 | −9.98 | −74.29 | −10.84 | 12.40 |
| W11 | 1250 | Ng | 53 | 8.19 | 184.00 | 2.32 | 4.81 | 2.92 | 36.16 | 51.39 | 418.60 | 0.38 | 19.5 | 124.40 | −9.64 | −72.22 | −10.27 | 9.90 |
| W12 | 1251 | Nm | 53 | 8.18 | 274.20 | 3.85 | 4.81 | 2.92 | 30.84 | 62.44 | 619.35 | 0.15 | 31.2 | 126.29 | −9.15 | −76.12 | −10.76 | 9.98 |
| W13 | 1254 | Nm | 53.5 | 8.10 | 272.60 | 4.10 | 4.81 | 2.92 | 36.16 | 85.49 | 610.81 | 0.09 | 31.2 | 156.97 | −8.81 | −74.57 | −9.77 | 3.60 |
| W14 | 1350 | Ng | 50 | 9.33 | 247.70 | 5.03 | 4.81 | 2.92 | 41.12 | 102.30 | 404.56 | 12.44 | 20.8 | 124.15 | −7.85 | −70.48 | −9.93 | 8.90 |
| W15 | 1350 | Ng | 66 | 7.92 | 573.80 | 12.46 | 19.04 | 8.63 | 60.23 | 119.59 | 538.20 | 0.40 | 37.7 | 146.56 | −6.39 | −75.89 | −9.82 | 2.66 |
| W16 | 1370 | Ng | 57 | 8.11 | 270.00 | 4.34 | 4.81 | 2.92 | 32.61 | 68.20 | 624.84 | 0.21 | 28.6 | 134.48 | −9.09 | −74.63 | −9.89 | 4.40 |
| W17 | 1600 | Ng | 64 | 7.90 | 282.50 | 4.50 | 4.81 | 2.92 | 32.61 | 85.49 | 644.37 | 0.15 | 29.9 | 140.24 | −8.65 | −74.92 | −9.67 | 2.50 |
| W18 | 1638 | Ng | 65 | 7.43 | 300.50 | 5.21 | 4.81 | 2.92 | 34.39 | 102.30 | 680.98 | 0.09 | 35.1 | 118.78 | −6.43 | −73.99 | −9.41 | 1.30 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Qiao, W.; Chen, J.; Liu, X.; Yang, F. Understanding the Burial and Migration Characteristics of Deep Geothermal Water Using Hydrogen, Oxygen, and Inorganic Carbon Isotopes. Water 2018, 10, 7. https://doi.org/10.3390/w10010007
Wang X, Qiao W, Chen J, Liu X, Yang F. Understanding the Burial and Migration Characteristics of Deep Geothermal Water Using Hydrogen, Oxygen, and Inorganic Carbon Isotopes. Water. 2018; 10(1):7. https://doi.org/10.3390/w10010007
Chicago/Turabian StyleWang, Xinyi, Weifang Qiao, Jing Chen, Xiaoman Liu, and Fang Yang. 2018. "Understanding the Burial and Migration Characteristics of Deep Geothermal Water Using Hydrogen, Oxygen, and Inorganic Carbon Isotopes" Water 10, no. 1: 7. https://doi.org/10.3390/w10010007
APA StyleWang, X., Qiao, W., Chen, J., Liu, X., & Yang, F. (2018). Understanding the Burial and Migration Characteristics of Deep Geothermal Water Using Hydrogen, Oxygen, and Inorganic Carbon Isotopes. Water, 10(1), 7. https://doi.org/10.3390/w10010007




