Assessment of the Binding of Protons, Al and Fe to Biochar at Different pH Values and Soluble Metal Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Analyses
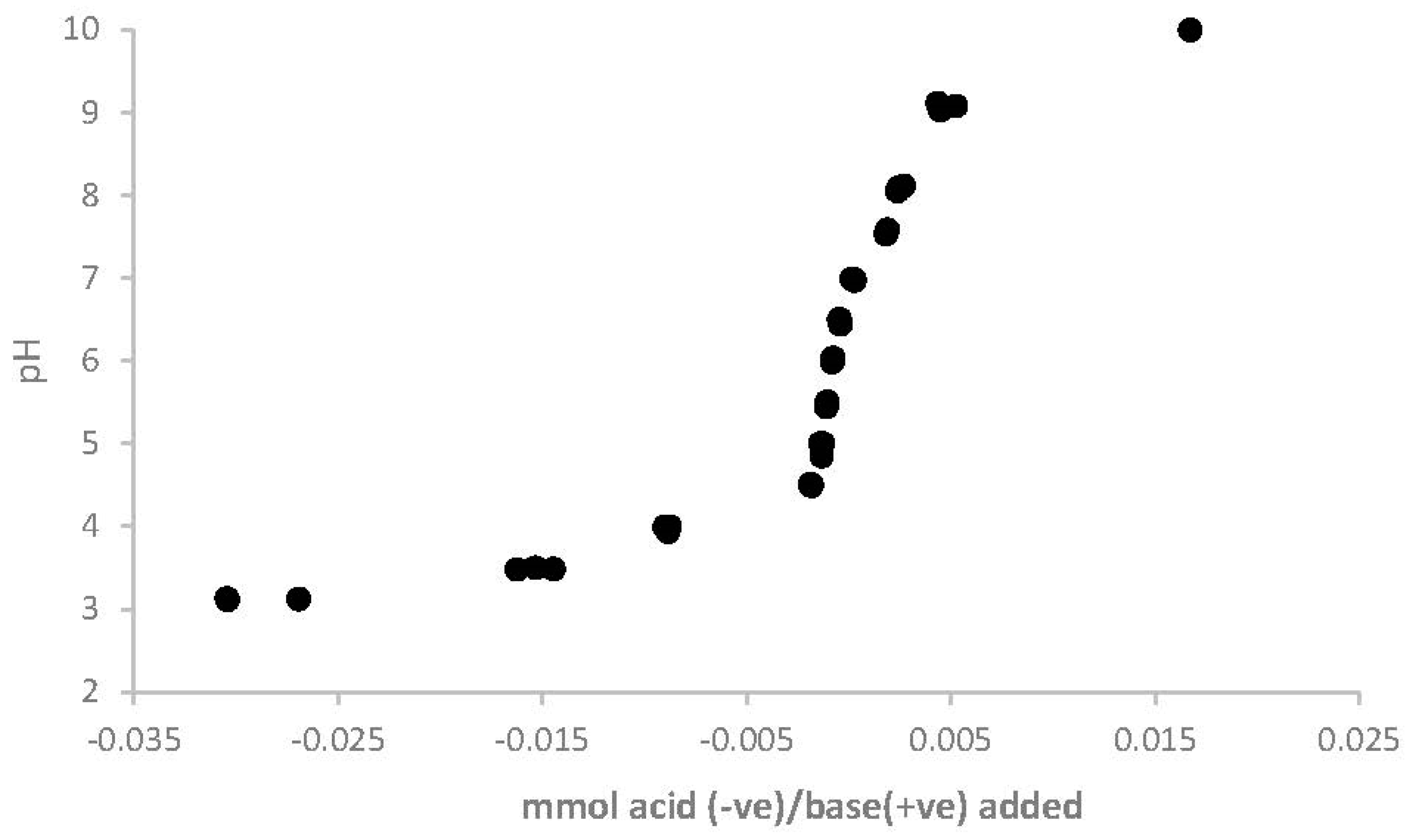
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simpson, S.; Fitzpatrick, R.; Shand, P.; Angel, B.; Spadaro, D.; Merry, R.; Thomas, M. Chapter 3: Acid and Metal Mobilisation Following Rewetting of Acid Sulfate Soils from the River Murray, South Australia: A Rapid Laboratory Method. In Inland Acid Sulfate Soil Systems across Australia; Fitzpatrick, R., Shand, P., Eds.; CRC LEME: Perth, Australia, 2008; pp. 90–97. [Google Scholar]
- Cook, F.J.; Hicks, W.; Gardner, E.A.; Carlin, G.D.; Froggatt, D.W. Export of Acidity in Drainage Water from Acid Sulphate Soils. Mar. Pollut. Bull. 2000, 41, 319–326. [Google Scholar] [CrossRef]
- Sammut, J.; Lines-Kelly, R. An Introduction to Acid Sulphate Soils; Natural Heritage Trust: Sydney, Australia, 2000. [Google Scholar]
- White, I.; Melville, M.D.; Wilson, B.P.; Sammut, J. Reducing acidic discharges from coastal wetlands in eastern Australia. Wetl. Ecol. Manag. 1997, 5, 55–72. [Google Scholar] [CrossRef]
- Macdonald, B.C.T.; White, I.; Åström, M.E.; Keene, A.F.; Melville, M.D.; Reynolds, J.K. Discharge of weathering products from acid sulfate soils after a rainfall event, Tweed River, eastern Australia. Appl. Geochem. 2007, 22, 2695–2705. [Google Scholar] [CrossRef]
- Australian and New Zealand Environment and Conservation Council (ANZECC). Australian and New Zealand Guidelines for Fresh and Marine Water Quality; Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand: Canberra, Australia, 2000. [Google Scholar]
- Hicks, W.; Fitzpatrick, R.; Bowman, G. Managing coatal acid sulfate soils: The East Trinity example. In Advances in Regolith; Roach, I.C., Ed.; CRC LEME Regional: Bentley, WA, USA, 2003; pp. 174–177. [Google Scholar]
- Dang, T.; Mosley, L.M.; Fitzpatrick, R.; Marschner, P. Organic materials retain high proportion of protons, iron and aluminium from acid sulphate soil drainage water with little subsequent release. Environ. Sci. Pollut. Res. 2016, 23, 23582–23592. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Mosley, L.M.; Fitzpatrick, R.; Marschner, P. Addition of organic material to sulfuric soil can reduce leaching of protons, iron and aluminium. Geoderma 2016, 271, 63–70. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Trakal, L.; Bingöl, D.; Pohořelý, M.; Hruška, M.; Komárek, M. Geochemical and spectroscopic investigations of Cd and Pb sorption mechanisms on contrasting biochars: Engineering implications. Bioresour. Technol. 2014, 171, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Elaigwu, S.E.; Rocher, V.; Kyriakou, G.; Greenway, G.M. Removal of Pb2+ and Cd2+ from aqueous solution using chars from pyrolysis and microwave-assisted hydrothermal carbonization of Prosopis africana shell. J. Ind. Eng. Chem. 2014, 20, 3467–3473. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U., Jr. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J.C. Assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Bannon, D.I.; Wartelle, L.H. Retention of Heavy Metals by Carboxyl Functional Groups of Biochars in Small Arms Range Soil. J. Agric. Food Chem. 2012, 60, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Haynes, R.J. Sorption of Heavy Metals by Inorganic and Organic Components of Solid Wastes: Significance to Use of Wastes as Low-Cost Adsorbents and Immobilizing Agents. Crit. Rev. Environ. Sci. Technol. 2010, 40, 909–977. [Google Scholar] [CrossRef]
- Bulut, Y.; Baysal, Z. Removal of Pb(II) from wastewater using wheat bran. J. Environ. Manag. 2006, 78, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bigham, J.; Nordstrom, D.K. Iron and aluminum hydroxysulfates from acid sulfate waters. Rev. Miner. Geochem. 2000, 40, 351–403. [Google Scholar] [CrossRef]
- Simpson, S.; Vardanega, C.R.; Jarolimek, C.; Jolley, D.F.; Angel, B.M.; Mosley, L.M. Metal speciation and potential bioavailability changes during discharge and neutralisation of acidic drainage water. Chemosphere 2014, 103, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Creeper, N.L.; Shand, P.; Hicks, W.; Fitzpatrick, R.W. Porewater Geochemistry of Inland Acid Sulfate Soils with Sulfuric Horizons Following Postdrought Reflooding with Freshwater. J. Environ. Qual. 2015, 44, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Creeper, N.L.; Hicks, W.S.; Shand, P.; Fitzpatrick, R.W. Geochemical processes following freshwater reflooding of acidified inland acid sulfate soils: An in situ mesocosm experiment. Chem. Geol. 2015, 411, 200–214. [Google Scholar] [CrossRef]
- Mosley, L.; Palmer, D.; Leyden, E.; Cook, F.; Zammit, B.; Shand, P.; Baker, A.; Fitzpatrick, R.W. Acidification of floodplains due to river level decline during drought. J. Contam. Hydrol. 2014, 161, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.R.; Eyre, B.D. Radon tracing of groundwater discharge into an Australian estuary surrounded by coastal acid sulphate soils. J. Hydrol. 2011, 396, 246–257. [Google Scholar] [CrossRef]
- Mosley, L.; Fitzpatrick, R.; Palmer, D.; Leyden, E.; Shand, P. Changes in acidity and metal geochemistry in soils, groundwater, drain and river water in the Lower Murray River after a severe drought. Sci. Total Environ. 2014, 485–486, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.G.; Keene, A.F.; Bush, R.T.; Burton, E.D.; Sullivan, L.A.; Isaacson, L.; McElnea, A.E.; Ahern, C.R.; Smith, C.D.; Powell, B. Iron geochemical zonation in a tidally inundated acid sulfate soil wetland. Chem. Geol. 2011, 280, 257–270. [Google Scholar] [CrossRef]
- Fitzpatrick, R.W.; Mosley, L.M.; Raven, M.D.; Shand, P. Schwertmannite formation and properties in acidic drain environments following exposure and oxidation of acid sulfate soils in irrigation areas during extreme drought. Geoderma 2017, 308 (Suppl. C), 235–251. [Google Scholar] [CrossRef]
- Krstic, D.; Djalovic, I.; Nikezic, D.; Bjelic, D. Aluminium in Acid Soils: Chemistry, Toxicity and Impact on Maize Plants. In Plants, Food Production—Approaches, Challenges and Tasks; Aladjadjiyan, A., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Hicks, W.S.; Bowman, G.M.; Fitzpatrick, R.W. Effect of season and landscape position on the aluminium geochemistry of tropical acid sulfate soil leachate. Soil Res. 2009, 47, 137–153. [Google Scholar] [CrossRef]
- Rayment, G.E.; Lysons, D.J. Soil Chemical Methods-Australia; CSIRO Publishing: Victoria, Australia, 2011; Volume 3. [Google Scholar]
- Aitken, R.; Moody, P. The effect of valence and Ionic-strength on the measurement of pH buffer capacity. Soil Res. 1994, 32, 975–984. [Google Scholar] [CrossRef]
- Weber, T.; Allard, T.; Tipping, E.; Benedetti, M.F. Modeling Iron Binding to Organic Matter. Environ. Sci. Technol. 2006, 40, 7488–7493. [Google Scholar] [CrossRef] [PubMed]
- Ahern, C.R.; McElnea, A.E.; Sullivan, L.A. Acid neutralising capacity, carbonate and alkali cation methods. In Acid Sulfate Soils Laboratory Methods Guidelines; Ahern, C.R., McElnea, A.E., Sullivan, L.A., Eds.; Department of Natural Resources, Nines and Energy: Indooroopilly, Australia, 2004. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Zarcinas, B.A.; Mclaughlin, M.J.; Smart, M.K. The effect of acid digestion technique on the performance of nebulization systems used in inductively coupled plasma spectrometry. Commun. Soil Sci. Plant Anal. 1996, 27, 1331–1354. [Google Scholar] [CrossRef]
- McBeath, A.V.; Smernik, R.J.; Krull, E.S.; Lehmann, J. The influence of feedstock and production temperature on biochar carbon chemistry: A solid-state 13C NMR study. Biomass Bioenergy 2014, 60, 121–129. [Google Scholar] [CrossRef]
- Zhang, J.; Lü, F.; Luo, C.; Shao, L.; He, P. Humification characterization of biochar and its potential as a composting amendment. J. Environ. Sci. 2014, 26, 390–397. [Google Scholar] [CrossRef]
- Milne, C.J.; Kinniburgh, D.G.; de Wit, J.C.M.; van Riemsdijk, W.H.; Koopal, L.K. Analysis of proton binding by a peat humic acid using a simple electrostatic model. Geochim. Cosmochim. Acta 1995, 59, 1101–1112. [Google Scholar] [CrossRef]
- Montenegro, A.C.; Orsetti, S.; Molina, F.V. Modelling proton and metal binding to humic substances with the NICA–EPN model. Environ. Chem. 2014, 11, 318–332. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Kinniburgh, D.G.; Milne, C.J.; Benedetti, M.F.; Pinheiro, J.P.; Filius, J.; Koopal, L.K.; Van Riemsdijk, W.H. Metal Ion Binding by Humic Acid: Application of the NICA-Donnan Model. Environ. Sci. Technol. 1996, 30, 1687–1698. [Google Scholar] [CrossRef]
- Kirby, C.S.; Cravotta Iii, C.A. Net alkalinity and net acidity 1: Theoretical considerations. Appl. Geochem. 2005, 20, 1920–1940. [Google Scholar] [CrossRef]
- Mosley, L.; Willson, P.; Hamilton, B.; Butler, G.; Seaman, R. The capacity of biochar made from common reeds to neutralise pH and remove dissolved metals in acid drainage. Environ. Sci. Pollut. Res. 2015, 22, 15113–15122. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, B. Interactions of Aluminum with Biochars and Oxidized Biochars: Implications for the Biochar Aging Process. J. Agric. Food Chem. 2013, 62, 373–380. [Google Scholar] [CrossRef] [PubMed]
| pHw | 7.5 | |
|---|---|---|
| TOC | mg g−1 | 551.6 |
| Total N | mg g−1 | 5.6 |
| C/N | 98 | |
| ANC | % CaCO3 | 3.8 |
| CEC | cmol(+) kg−1 | 39.3 |
| Surface area | m2 g−1 | 2.5 |
| Acid extractable Al | mg g−1 | 3.8 |
| Acid extractable Fe | mg g−1 | 19.8 |
| Chemical functional groups | % C detected | |
| Alkyl | 10.3 | |
| N-Alkyl/Methoxyl | 3.1 | |
| O-Alkyl | 4.5 | |
| Di-O-Alkyl | 3.6 | |
| Aryl | 57.5 | |
| O-Aryl | 13.6 | |
| Amide/Carboxyl | 4.3 | |
| Ketone | 3.2 |
| Added Al | Amount of Soluble Al after Adjusted pH (µg per tube) | ||
|---|---|---|---|
| (mM) | (µg per tube) | pH 4 | pH 7 |
| 0.001 | 0.7 | 0.7 | 0.7 |
| 0.01 | 6.7 | 6.7 | 2.3 |
| 0.1 | 67.5 | 67.5 | 15.9 |
| 1 | 674.5 | 562.6 | 21.3 |
| 5 | 3372.5 | 2177.8 | 29.8 |
| 10 | 6745.0 | 4302.5 | 36.7 |
| Added Fe | Amount of Soluble Fe after Adjusted pH (µg per tube) | ||
|---|---|---|---|
| (mM) | (µg per tube) | pH 4 | pH 7 |
| 0.001 | 1.4 | 1.3 | 1.3 |
| 0.01 | 14.0 | 2.1 | 1.6 |
| 0.1 | 139.6 | 9.1 | 6.7 |
| 1 | 1396.3 | 43.5 | 47.7 |
| 5 | 6981.3 | 229.0 | 339.9 |
| 10 | 13,962.5 | 685.2 | 415.8 |
| pH 4 | pH 7 | ||||
|---|---|---|---|---|---|
| Soluble Added (µg/g) | Binding (µg/g) | Binding (%) | Soluble Added (µg/g) | Binding (µg/g) | Binding (%) |
| 0 | −20 | 0 | 0 | −1 | 0 |
| 3 | −9 | 0 | 3 | 2 | 60.5 |
| 27 | 14 | 51.0 | 9 | 7 | 74.7 |
| 270 | 243 | 90.1 | 64 | 61 | 95.9 |
| 2250 | 2096 | 93.1 | 85 | 80 | 93.9 |
| 17,210 | 16,990 | 98.7 | 147 | 140 | 95.4 |
| pH 4 | pH 7 | ||||
|---|---|---|---|---|---|
| Soluble Added (µg/g) | Binding (µg/g) | Binding (%) | Soluble Added (µg/g) | Binding (µg/g) | Binding (%) |
| 0 | −18 | 0 | 0 | −1 | 0 |
| 5 | −13 | 0 | 5 | 4 | 74.6 |
| 8 | −13 | 0 | 6 | 4 | 64.2 |
| 37 | 31 | 83.7 | 27 | 25 | 93.3 |
| 174 | 153 | 88.0 | 191 | 189 | 98.8 |
| 2741 | 2489 | 90.8 | 1663 | 1438 | 86.5 |
| Soluble Added | Binding | ||||
|---|---|---|---|---|---|
| Al (µg/g) | % | Fe (µg/g) | % | ||
| Fe (µg/g) | Al (µg/g) | ||||
| 191 | 85 | 82.5 | 89.5 | 190.3 | 99.7 |
| 119 | 113.1 | 96.7 | 188.3 | 98.7 | |
| 147 | 140.7 | 99.5 | 188.4 | 98.7 | |
| 1360 | 85 | 83.2 | 97.8 | 1358.2 | 99.9 |
| 119 | 113.4 | 95.3 | 1356.2 | 99.8 | |
| 147 | 120.6 | 97.5 | 1355.5 | 99.7 | |
| 1663 | 85 | 84.9 | 95.7 | 1662.6 | 100 |
| 119 | 115.4 | 94.8 | 1660.7 | 99.9 | |
| 147 | 131.5 | 96.8 | 1658.8 | 99.7 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, T.; Marschner, P.; Fitzpatrick, R.; Mosley, L.M. Assessment of the Binding of Protons, Al and Fe to Biochar at Different pH Values and Soluble Metal Concentrations. Water 2018, 10, 55. https://doi.org/10.3390/w10010055
Dang T, Marschner P, Fitzpatrick R, Mosley LM. Assessment of the Binding of Protons, Al and Fe to Biochar at Different pH Values and Soluble Metal Concentrations. Water. 2018; 10(1):55. https://doi.org/10.3390/w10010055
Chicago/Turabian StyleDang, Tan, Petra Marschner, Rob Fitzpatrick, and Luke M. Mosley. 2018. "Assessment of the Binding of Protons, Al and Fe to Biochar at Different pH Values and Soluble Metal Concentrations" Water 10, no. 1: 55. https://doi.org/10.3390/w10010055
APA StyleDang, T., Marschner, P., Fitzpatrick, R., & Mosley, L. M. (2018). Assessment of the Binding of Protons, Al and Fe to Biochar at Different pH Values and Soluble Metal Concentrations. Water, 10(1), 55. https://doi.org/10.3390/w10010055





