Abstract
Central Yakutia is in one of the most northern agricultural centers of the world. In this territory a notable area of arable land was made by removing the boreal Taiga with the primary purpose of crop cultivation. Such a method of cultivation significantly changes soil total carbon (STC, soil organic carbon + soil carbonate carbon) balance, because of the destroyed upper humus horizon. Soil organic carbon (SOC) of cultivated arable lands is almost a half of that in forest. In abandoned arable lands with grass vegetation, the recovery of SOC has increased to 30% in comparison with cultivated arable lands. On arable lands recovering with new growth of trees, the SOC is related to the abandonment period. Soil carbonates carbon (SCC) content was significantly lower than SOC and showed significant difference among abandoned and other types of arable lands. Objectives of this study are to identify how STC stocks change in response to conversion of the forests to agricultural land and to analyze the arable land system’s recovery process after abandonment. Furthermore, after transformation of forest to arable land, a significant decrease of STC was observed, primarily due to mechanical loss after plant residue removal. It was also identified that the restoration and self-recovery of STC in abandoned arable lands of Central Yakutia continuously and slightly increase. Grass vegetation regenerates STC for 20 years. While the difference of average STC of forests and cultivated arable lands reached 41%, a new growth of forest on some abandoned arable land follows the tendency of STC decrease due to a low productivity level and suppressing effect on grass vegetation.
1. Introduction
Soil organic carbon (SOC), the largest carbon pool on land, plays an important role in the global carbon cycle [1,2]. Thus, small changes in the SOC stock may significantly impact atmospheric CO2 concentration. In terrestrial ecosystems, forest soil stores about 40% of the total organic carbon (C) [3]. Furthermore, boreal forest occupies 33% of the circumpolar region’s total area and soils here are the reservoir for long-term C stock which takes a significant part of global C storage [4]. It has been reported that boreal forest soils keep more C than above-ground biomass of forest [5,6,7,8]. Thus, up to 85% of total biome C is stored in soils [9].
Soil carbon dynamics is determined by the balance between inputs (e.g., addition of plant residues) and outputs (e.g., SOC decomposition), which is influenced by many factors such as climate conditions, soil properties, and land use [2,10]. Any changes in the system of land use always lead to changes in the SOC storage [11]. The greatest C fluxes caused by land use change are attributed to conversion of croplands to native vegetation and vice versa [12]. Conversion of natural forests to arable land results in the mineralization of soil organic matter (SOM), which leads to decrease of SOC stocks and increasing emission of CO2 to the atmosphere [13,14]. In contrast, the abandonment of arable land (AL) and restoration of forest lead to SOC accumulation [15,16,17,18]. The analysis made by Guo and Gifford [15] showed that in the conversion of forest and pastures to croplands, the carbon accumulation in soil decreased to 42% and 59%, respectively. Post and Kwon [19] reported that the rate of the SOC accumulation due to the conversion of former croplands to grassland ecosystems and pastures varied from 0.03 to 1.14 Mg C ha−1 year−1 or on average, and amounted to 0.33 Mg C ha−1 year−1. In the forestation of former croplands, the average rate of carbon accumulation in the soils was also 0.34 Mg C ha−1 year−1. The decrease and accumulation in SOC followed by land use change is difficult to predict due to variations in the factors that influence SOC mineralization, e.g., location of site, forest type, climate, and soil properties [3].
Central Yakutia is in one of the most northern agricultural centers in the world. For crop cultivation in this territory, a notable area of arable lands was made by removing the boreal Taiga forest. Such a method of cultivation significantly changes SOC balance, because of upper humus horizon destruction. During the second half of the 20th century the Soviet government began the gradual establishment of the state farm system with more collective farms amalgamated into large agricultural enterprises [20]. In the early 1990s, after the collapse of the Soviet collective farming system, the farmland area in Russia decreased drastically [21]. According to Lyuri et al. [18], about 45.5 million ha of arable lands between 1990 and 2007 were abandoned. This area remained without change up to 2011 (coefficient of variation = 1.3% for 2005–2011). This was the most widespread and abrupt land use change in the 20th century in the northern hemisphere [18,22]. This cropland abandonment in Russia substantially shifted the total C balance of country [22,23].
Most arable lands are in the central part of Yakutia and comprise the base of the republic’s agriculture. According to governmental reports, the intensive processes of humus reducing, increasing of salinity, imbalance of nutrients and water, and cryogenic processes causing subsidence of soil surface take place in Central Yakutia. Also, there was a short-term increase of agricultural productivity in the newly made arable lands. For years, a long-term decrease was observed, due not to soil impoverishment but rather to the compaction of sensitive soils [24]. It is important to consider that the soil of arable lands has high level of salinity and most saline soils are in Central Yakutia [25]. Elovskaya’s works showed movement of water-soluble salts to the front of evaporation which is clearly visible on the top soil near the surface. Soils of cryolithozone have no drainage due to the presence of a permafrost table beneath. In these soils, accumulation processes and vertical movement of substances prevail within the active layer. Horizontal run-off of soil water is a little weaker because soils have silt loam and loamy clay composition. Therefore, Elovskaya [25] proved that in cryolithozone soils, water-soluble salts move upward to the evaporation and freezing front, and downward to the freezing front of the permafrost table. Because of this, the middle part of the arable land soil profile has become desalted in dry mid-summer periods and autumn freezing periods.
Generally, the conversion of forest to arable land destroys the soil aggregate structure, which enhances organic matter mineralization and CO2 emission. SOC stocks decrease rapidly and then stabilize after a land use change [13,26]. Because of a lack of study regarding changes in soil C content during the forest conversion to arable land and its functioning in the boreal forest region of Central Yakutia, the objective of this study is to show how soil C stocks change in response to the conversion of forests to arable land and to show how the arable land system is recovering after the abandonment.
2. Materials and Method
2.1. Study Site and Soil Sampling
In Yakutia, the area of cultivated arable lands was 115.0 thousand ha in 1940, but this area decreased to 60.0 thousand ha by 2000 [27]. Till 2008, the area of arable lands decreased up to 38.6 thousand ha, and from 2006 there is a slight increase up to 46 thousand ha [28] (Figure 1). This trend has been observed all over Yakutia, including our study area.
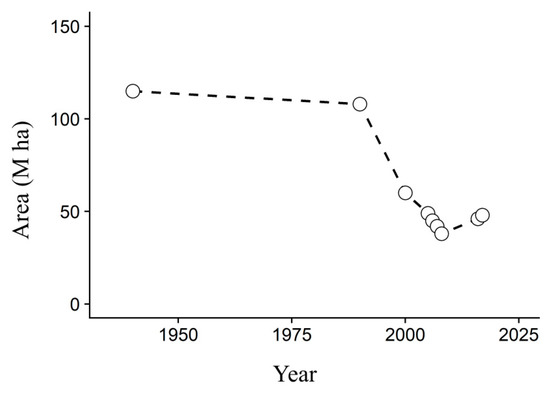
Figure 1.
Area of arable lands in Yakutia from 1940 to 2017 [28].
The studied area is in Central Yakutia, the most populated region in Yakutia (Figure 2). The climate of Central Yakutia is extra continental, characterized by severe, dry winters and hot summers [29]. Average annual temperature has been −9.65 °C for last 100 years and annual precipitation has been 235 mm during last 50 years (The Global Historical Climatology Network-Monthly (GHCN-M) data; ftp://ftp.ncdc.noaa.gov/pub/data/ghcn/v3/). The territory has very high annual amplitude of temperature. Therefore, the recorded minimum and maximum of temperature is −63 °C in January and 38.3 °C in July, respectively. The warm period lasts from May to September, reaching highest temperatures in July (long-term average −19 °C). The coldest month is January, with long-term average of −39.6 °C. About 70% of precipitation falls during the warm period. The maximum monthly average of precipitation is in July and August and amounts to 39 mm for both months. The minimum is in February and March—8 and 6 mm, respectively (GHCN-M data). Thus, climatic conditions of the considered area are severe and do not allow high productivity and high SOC input. Another factor is low decomposition rate of organic matter during the short summer. It is the limiting factor for the fast turnover of C in this area.
Figure 2.
Study area between Lena and Aldan rivers.
Intact forest soils under the larch Taiga were chosen in this study, as the initial state of natural environment before creating arable land. Cultivated arable lands were chosen to consider the present state of STC content and stock. Arable lands with different abandonment age (5, 20, 36 years) were also chosen. Typical soil for all Central Yakutia is the Permafrost Pale (World Reference Base for Soil resources classification; Cambic Turbic Cryosol) soils developed under the Larch forest (Larix Gmelinii). Maximum of C is concentrated in the upper humus-accumulative horizon; in deeper layers the content of C is decreasing [30].
Soil sampling was conducted in summer 2014. For soil sampling, we used the following criteria: the cultivated plots must be converted from primary forest, the cultivated and forest plots must be adjacent and similar by pedological conditions and the time of forest conversion must be known or derivable [31]. Five sites of intact forest soil located in different places were selected: three sites of the cultivated arable land (cultivated AL), five sites of abandoned arable land (abandoned AL) and three sites of arable land with new growth of larch forest i.e., postagrogenic recovery sites (recovered AL) (Table 1). At the point of sampling, among cultivated AL the site NEM-3-W had a 50–60 cm oats stand (Avena satíva), other sites (TG-W, UNA-W) had seedlings of oat with height 20–30 cm. During self-restoration of abandoned AL without direct human impact, the vegetation developed towards a steppe, with dominance of Stipa capillata, Chenopodium album and Lappu lasquarossa. The height of grass vegetation was 40 cm on average. On the sites with new forest, only Larix gmelinii is densely growing. Soil surface almost has no grass vegetation, with a tree diameter up to 5–6 cm and height up to 3–4 m.

Table 1.
Cultivation and abandonment age in the different type of arable lands.
Both mixed and undisturbed (using 100 mL of steel cylinder) samples were taken by the depth of 30 cm (according Food and Agriculture Organization of UN) at three replicates. Organic horizon was also collected at intact forest and postagrogenic recovery sites. According to interviews with local people, the end of cultivation time was clarified for all chosen arable lands (Table 1).
2.2. Sample Analysis and Data Calculation
All soil samples were brought to the laboratory for analysis of soil physics-chemical properties. Composite samples were air-dried immediately and passed through a 1-mm sieve to remove any stones, roots, and other plant materials. Samples for C content estimation were further sieved by using a 0.5 mm sieve. Undisturbed samples were used for determination of soil bulk density (BD). The cores were oven dried at 95 °C for 48 h. BD was calculated by dividing the weight of the dried soil by the volume of the core used. Composite samples were used to determine the soil pH, electric conductivity (EC), and soil texture.
Soil pH and EC were measured in soil water suspensions (1:5) by using a pH meter (F-8 pH meter; Horiba, Kyoto, Japan) and EC meter (B-173 conductivity meter, Horiba, Kyoto, Japan) respectively. Exchangeable cations were determined by extraction with 1 M ammonium acetate (pH 7). The soil texture was determined by pipette method.
Air-dried samples which were sieved through a 0.5 mm sieve were used to analyze the SOC content of both mineral and organic soils. To determine the SOC content of mineral soils, wet oxidation method with K2Cr2O7 were used. Loss on ignition method (weight %) at 550 °C (for 6 h) was used to determine SOM content of organic horizon. “Van Bemmelen factor” of 1.724, based on the assumption that organic matter is 58% carbon, was used for conversion of measured SOM into estimated of SOC. Loss on ignition method has potential overestimation of SOC compared with dry combustion method. Hence, 15 organic samples were analyzed by both loss on ignition and dry combustion method (Flash 2000 NC-soil, Thermo Fisher Scientific, Waltham, MA, USA).
Soil carbonate carbon (SCC) was calculated from the quantity of the CO2 produced by 10% HCl (wt/wt) addition to the sample. Volume of the produced CO2 was measured using an aqueous manometer. The weight of CO2 is calculated from its volume at standard temperature and pressure and is used to calculate the carbonate. Then soil total carbon (STC) was determined as a sum of both SOC and SCC.
The total profile of carbon stock at each site and at each sampling site was calculated as the sum of the component soil horizon SOC stocks for a depth of 0–30 cm.
The SOC stock (Mg C ha−1) of each horizon was calculated as follows:
where BD is soil bulk density (Mg m−3), SOC is soil organic carbon content (mg C kg−1), and D is soil sampling depth (cm), 10 is a factor to adjust units. The SCC stock was calculated using the same method.
SOC stock = BD × SOC × D × 10
The annual average rate of C stock loss (ΔC, Mg C ha−1 year−1) after conversion of intact forest to cultivated AL was calculated as follows:
where CIF and Car are C stocks (Mg C ha−1) in the upper 30 cm in intact forest and abandoned AL plots, respectively.
∆Closs = (CIF − Car)
The annual average rate of C stock recovery (Mg C ha−1 year−1) over a period (T) after abandonment of AL was calculated as follows:
where Cab and Car are C stocks (Mg C ha−1) in the upper 30 cm in abandoned AL and cultivated AL plots, respectively; T years after cultivations [32].
∆Crec = (Cab − Car)/T
2.3. Statistical Analysis
Statistical analyses were performed with R (R Development Core Team 2014; version 3.1.0, Vienna, Austria). The differences in carbon stocks and other soil physico-chemical properties among the different land use were analyzed with a one-way factorial analysis of variance (ANOVA), and a Tukey-Kramer test. The Smirnoff-Grubbs test was used for detection of outlier values.
3. Results
Arable lands were divided up based on time of establishment. As shown in Table 1 there are two main periods of stubbing the forest and cultivation start. In 1965 there were three abandoned AL. They had been cultivated over 13 years and the cultivation was stopped in 1978 except for site NEM-3-A which was cultivated over 29 years. Therefore, abandonment age is 36 years for NEM-1A, NEM-2-A and 20 years for NEM-3-A site. The second period of arable land making was in 1985. These arable lands were cultivated for 29 years and two of them were abandoned in1994 (UNA-A, UNA-N). One site, TG-A, was abandoned in 2009 after 24 years of cropping. Therefore, all sites are divided by abandonment age for three time chronosequences, i.e., 5, 20 and 36 years after abandonment (Table 1).
Most changes after conversion of forest to arable lands have soil moisture that is shown as the result of this work. Soil moisture in forest sites is higher and amounts to 11.9 ± 3.5 % kg kg−1 on average for all sampling sites. Curiously enough, cultivated AL have a little higher soil moisture than abandoned AL. Cultivated AL moisture ranges from 7.1 ± 0.4 to 11.5 ± 0.6 % kg kg−1 with average of 9.4 ± 2.0 % kg kg−1, while abandoned and recovered by forest AL soil moisture average as 9.2 ± 1.6 % kg kg−1. However, AL with new growth of larch forest has less soil moisture, with 7.9 ± 2.4 % kg kg−1 on average.
BD of the initial soils under Taiga forest have significant differences which varies from 0.99 ± 0.18 to 1.27 ± 0.10 Mg m−3. After conversion of forest to the arable land the BD increases from 1.28 ± 0.06 to 1.42 ± 0.08 Mg m−3 on the cultivated AL. After abandonment of arable lands depending on age, the 36- and 20-year plots have less difference of BD. On average, it is almost the same and totals 1.32 ± 0.11 and 1.31 ± 0.09 Mg m−3, respectively. BD of AL abandoned 5 years ago is still high and the same with the average of cultivated AL (1.35 ± 0.09 Mg m−3). In new growth sites BD shows increased values 1.38 for the 36 years and 1.31 ± 0.07 Mg m−3 for AL abandoned 20 years ago. The average value of all four locations shows clear differences of soil compactness which starts from 1.17 ± 0.17 in the forest soil and increases to 1.36 ± 0.08 Mg m−3 in cultivated arable land (Table 2). Thus, BD of forest soils is significantly lower compared with arable lands soils, while the difference among arable land soils is not significant (Figure 3).

Table 2.
Parameters of studied locations in different land use.
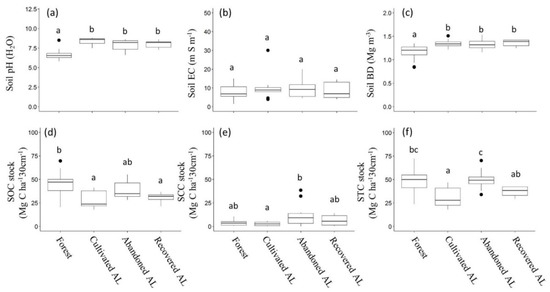
Figure 3.
Distribution of soils parameters among land use. (a) pH, (b) EC, (c) BD, (d) SOC, (e) SCC, (f) STC. Different lowercase letters indicate significant differences in the soils parameters between land use types at a significance level of 5%.
Initial average pH in the forest soil is 6.64 ± 0.65, but after conversion to the cultivated AL it increases to 8.32 ± 0.44. Abandonment of arable lands reduces the pH to 7.92 ± 0.77 without big variations depending on the time of abandonment. The pH of plots with recovered AL is higher than abandoned AL amounts, on average 8.00 ± 0.49 (Table 2). Forest soils have significantly lower pH compared with arable land soils pH (Figure 3).
Soil salinity has a clear trend to decrease after the abandonment of arable land. There is no significant difference between different types of land use. However, the highest EC values are observed in cultivated AL (Table 2, Figure 3). An increase of pH corresponds with EC value.
Average SOC stock within top 30 cm of forest soil is the highest and amounts 47.2 ± 13.9 Mg C ha−1. The variation of SOC stock is big, and ranges from 31.6 ± 15.2 to 58.2 ± 8.4 Mg C ha−1 depending on location of forest. SOC of cultivated AL is almost a half of that in forest (average 27.8 ± 8.7 Mg C ha−1) and has a significant difference between sites (Figure 3). Thus, forest conversion to arable land in cryolithozone promotes significant C loss of soil (Table 2, Figure 3). In abandoned AL with grass vegetation, the recovery of SOC is clearly traced. In comparison with cultivated AL, it has increased by 30% and totals 38.8 ± 8.7 Mg C ha−1, on average. The age of abandonment has no direct relation to SOC. AL abandoned 36 years ago have lower C stock than 20-year-old ones, with average values of 34.5 ± 3.9 and 44.9 ± 4.8 Mg C ha−1, respectively. Five-year-old abandoned AL have 35.3 ± 9.1 Mg C ha−1 of SOC. On the other hand, on tree-recovered AL the SOC is closely related to the age of abandonment, and older recovered AL have higher SOC stock (Table 2). The difference of SOC between the AL is not significant, according to ANOVA analysis (Figure 3).
SCC is significantly lower than SOC and shows significant difference among cultivated and abandoned AL (Figure 3). In the forest soils the average content is 4.6 ± 1.1 Mg C ha−1 which decreases to 2.6 ± 2.1 Mg C ha−1 in the soils of cultivated AL. The highest content of carbonate C is observed in the abandoned AL, where the average is 11.1 ± 11.2 Mg C ha−1. There is no relation of SCC on the age of abandoned AL, and they show high variation.
Combining SOC and SCC, soil total C (STC) was obtained. STC is significantly lower in cultivated AL than in forest and abandoned AL (p < 0.01, Figure 3). Abandoned AL show stable increases in STC depending on the age. On the other hand, lands with recovered AL show comparatively low STC (Table 2).
The rate of STC accumulation in abandoned AL was estimated as 2.29 Mg C ha−1 year−1 during the first five years. In the subsequent 20 and 36 years, the rate of STC accumulation decreased to an average of 1.01 and 0.64 Mg C ha−1 year−1, respectively. SOC restoration is higher in abandoned AL than in recovered AL. In 20 years abandonment, SOC has a significant difference and noticeably lower in recovered AL than in abandoned AL. However, with the increase of abandonment period (36 years) the rate of SOC input in abandoned and recovered ALs turned out to be the same. SCC accumulation rate was higher at the beginning of abandonment but after 20 years slightly decreased without big differences between abandoned and recovered ALs. Generally, the SCC accumulation rate has no significant differences among the time of abandonment.
4. Discussion
The Boreal forest area is resistant and better able to adapt its regions under the climate change [33]. However, the anthropogenic impact on the local sites such as agriculture can significantly change the area [34]. In Central Yakutia, the most northern center where cropping agriculture is developed, arable lands make up a notable area of the territory. The arable lands in this territory are made by the stubbing method which is damaging, removing the uppermost richest organic matter horizon of soil. Consequently, conversion of forest to the arable land changes physical and chemical parameters of soils. From the point of view of soil science, one of the key parameters which can be found in these permafrost-affected soils is BD.
Soil compaction in arable land, caused by heavy machinery constitutes a major threat to agricultural soils. Structurally inert soils that contain little organic matter and predominantly low-activity clay are most prone to compaction. In the studied cultivated AL, the BD significantly increased in comparison with initial forest soils (Table 2, Figure 3). This may occur because of damaged soil structure on the cultivated AL because of long-term use without any management. This corresponds well with the work of Pranagal et al. [35] which showed BD increase during 10 years of arable land. Irrespective of the use type, most frequently BD assumed values that exceed the range accepted by Drewry et al. [36] and Olness et al. [37] as the optimum (0.90–1.20 Mg m−3) for the attainment of maximum yields. Thus, arable lands in this territory after conversing of forest do not have suitable BD. Also, the hypotheses that BD will decrease after afforestation is not supported by our results (recovered AL), and contradicts the conclusions from other studies [38,39], but agrees with the work of Vopravil et al. [40] which found that previously arable horizon characteristics can persist for a long time. There is also probability that in this study, the abandonment age is too short to see the gradual restoration of BD after afforestation which is shown in [41]. The restoration time of BD in the southern Taiga region to initial values lasts about 200 years. Thus, EC increase in cultivated AL can be explained by increasing rate of evaporation from the soil surface. Soil condition with poor infiltration and drainage because of permafrost presence, saturated soil, and compaction leads to the capillary rise of soil moisture with water-soluble salts, which increase soil EC and pH (Table 2). On the abandoned AL there was residue remaining on the surface can limit the rate of evaporation from the soil surface, thereby reducing the potential to deposit salts and spring time increasing snow trapping that can serve as important source of water for leaching salts [42,43,44]. Therefore, similar to salt concentration, the value of EC is also directly proportional to the salinity and highly saline soil always has higher EC. Similar types of findings were reported by Smith and Doran [45], Anderson Cook et al. [46] and Ezatti and Karimi [47] on the deserts of mid-Asia. Usually in areas without permafrost, where the water table is within a few meters of the soil surface, the capillary rise of saline groundwater will transport salts to the soil surface, salinizing all soil profile. This process is more intense in arid regions which are shown in the work of Sinegani et al. [48], because of high levels of water evaporation [49,50]. However, in permafrost areas with limited active layer and absence of groundwater, the pattern of salt migration is completely different. Soil moisture is moving to evaporation or freezing front on the surface and the bottom of the profile. Therefore, in the summer without rainfall the desalinization of the middle of the profile occurs. However, after rains in autumn time salts are almost spread evenly within soil profile [27]. Lateral run-off in these soils occurs over the permafrost table and functions mostly at the end of summer. Thus, soils of the permafrost area within warm season can be either salinized or desalinized.
In the work of Sorokina [51] there is a good example of a forest-steppe ecosystem under restoration and anthropogenic effect in West Siberia. The method to make arable lands in the Central Yakutia and Middle Priangarye is the same, using bulldozers to uproot (stubbing) the Taiga forest. Thus, some of the organic matter during the construction of arable land can be mechanically lost by removing plant residues. A climatic condition in Priangarye is also continental but characterized by smooth winters and more precipitation. Under short and hot climates during the summer, the reduction of humus reserves during development and agricultural use of soils can be explained by more vigorous mineralization of organic matter, reduction of the supply of organic residues to the soil “dilution” of the initial amount of humus in the organic horizon by the subsoil horizon [51]. In the first years of AL functioning, a sharp decrease in the content and reserves of humus, a deterioration of the structural-aggregate state, soil density and the formation of alluvial Agric horizon occur. With increasing duration of agricultural unsystematic use of arable land soils, the activity of mineralization processes decreases, and losses of humus decrease and stabilize [51,52], but still do not fully compensate and recover the loss of carbon due to forest transformation to the arable land.
Self-recovery of soil C after abandonment of arable land is well correlated with time. We found that after 20 years soil C stock is almost recovered to the level of initial forest soil STC stock. However, because of the northern location this rate is slower even than as reported by Kalinina et al. [16] in dry Calcisol and Solonetz soils with low productivity. Her report showed that SOC stocks of the Calcisols increased slowly, comprising 64% of the natural soil, and SOC stocks of the Solonetzes almost reached the level of natural soil after restoration for 12 years.
The dynamics of vegetation and soils are closely related [53]. Work made in southern Taiga area showed that meadow succession of arable land restoration inputs more C into the soils than young forest succession (AL abandoned 20 years ago); old forests with an age of 45 years have lowest input. Our work data of STC stock in abandoned AL with meadows coincides with these data but in new forest growth sites shows the opposite C stock. It is related to the low biomass productivity of new forest growth sites in Central Yakutia. Similarly, low productivity on the sites with dry meadow promotes an increase of SOM with duration of succession [54]. Our work has a similar C accumulation trend with the Chinese cropland conversion and afforestation data reported by Deng et al. [55]. It shows higher C accumulation in soils, at 6–10 and 11–30 years after restoration to the forest. Thus, the restoration age is an important factor to consider when estimating soil C stocks after cropland conversion, and soil C sequestration was significantly and positively correlated with restoration age in the long term [55]. In the case of boreal arable land restoration, vegetation type plays a larger role for C input in the soil. Thus, abandoned AL with grassland vegetation significantly increases organic C stock in topsoil (Figure 3). This situation occurs due to grassland vegetation with more root biomass and more labile residues of biomass than new tree growth. Litter decomposition has more long-term and dense stands of young forest, which suppresses grassland vegetation that cannot grow inside young forest. Sorokina [51] proved that the growing of pine forest on abandoned AL sharply reduces the content of humus and gross nitrogen, and humus acquires a fulvate character. In addition, the growing of the mixed-grass vegetation enhances the accumulation of humus and nitrogen in the upper part of the profile, optimizes the agrophysical properties, and leads to the revival of the sod-accumulative process [51].
Another reason is that as abandonment time increases, the increase of the C inputs quantity is observed, accompanied by a new microclimatic regime, and enhanced organic matter protection promotes soil C accumulation [56]. Therefore, our work shows lower soil C stock on the abandoned AL with new growth of larch forest than on the grass-vegetated plots. This phenomenon of soil C decrease is probably due to the lower productivity of new forest in its earlier years [56,57,58,59]. One more probable reason is a time lag between plant production and soil C accumulation [60]. As it was noticed in North Carolina, the increase of all ecosystems C for 30 years was observed in restored forests [61]. The same pattern was observed by Paul et al. [62]. C stock in early years of forest growth on the area converted to forest from cropland was low, while conversion to shrublands and grasslands from cropland always showed increase of soil C in the same period. However, in the long-term scale, soil C stocks had significantly decreased in shrublands and grassland after cropland was converted to grassland for 30 years [63].
Average stock of SOC in forest (47.2 ± 13.9 Mg C ha−1), cultivated AL (27.8 ± 8.7 Mg C ha−1), abandoned AL (38.8 ± 8.7 Mg C ha−1) and recovered AL (31.1 ± 4.6 Mg C ha−1) in this study were slightly lower than in the southern Taiga region. In that region, cultivated AL soils, SOC at a depth of 0–50 cm is 30.6 Mg C ha−1, which corresponds with our data [53]. In addition, their AL abandoned 7 years ago with grass vegetation has more SOC content (37.3 Mg C ha−1) in comparison with Central Yakutia (35.3 ± 9.1 Mg C ha−1). AL abandoned 20 years old with new growth of forest also shows higher value (45.5 Mg C ha−1) than our study (44.9 ± 0.2 Mg C ha−1). In our study, high value of STC was shown abandoned AL with grass vegetation where the average STC was 41.8 ± 7.9, 50.56 ± 0.2 and 53.4 ± 9.7 Mg C ha−1 for 5, 20, and 36 years of abandonment, respectively. The same pattern of STC recovery over the long term were shown in the works of Morris et al., [64], Mao et al., [65] and Deng et al. [66].
During first 5 years, the rate of C accumulation in abandoned AL amounts to 2.29 Mg C ha−1 year−1 in this study. AL abandoned 20 and 36 years ago show reducing rate of C accumulation with average 1.01 and 0.64 Mg C ha−1 year−1, respectively. These values are higher than total Russia data, which was reported by Kurganova et al. [32]. Her estimated average C accumulation rate in the upper 20 cm of mineral soil was 0.96 Mg C ha−1 year−1 for the first 20 years after abandonment, and 0.19 Mg C ha−1 year−1 during the next 30 years of postagrogenic evolution and natural vegetation establishment.
Also interesting is that abandonment of arable lands recovered either as grassland or new forest. This phenomenon is very difficult to explain because the main parameters of soils in grassland and forest vegetated sites are almost the same.
5. Conclusions
After transformation of forest to arable lands, significant increase of C loss is observed, primarily due to mechanical loss after removing plant residues. The difference between average STC of forest and cultivated arable land reaches 41%. The restoration and self-recovery of plants in abandoned arable lands in Central Yakutia is almost at the same rate as other Russian regions. Grass vegetation regenerates STC for 20 years. However, new growth of forest growing on some abandoned AL has a tendency to decrease STC, because of low productivity and suppression effect on grass vegetation.
Author Contributions
Data curation, S.I.; Supervision, R.V.D. and R.H.; Writing–original draft, A.R.D.
Funding
The work was done under the project No. 0376-2014-0004. Subject 54.1.2. “Mechanisms of transformation and rules of cryolithozone soil functioning in the context of global change: factors, current state and forecast”. Direction 54. “Soils as a component of the biosphere (the formation, evolution, ecological functions)” of the program of fundamental scientific research of the state academies of sciences for 2013–2020.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Janzen, H.H. Carbon cycling in earth systems—A soil science perspective. Agric. Ecosyst. Environ. 2004, 104, 399–417. [Google Scholar] [CrossRef]
- Jobbagy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- The Intergovernmental Panel on Climate Change. The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007.
- Jones, A.; Stolbovoy, V.; Tarnocai, C.; Broll, G.; Spaargaren, O.; Montanarella, L. Soil Atlas of the Northern Circumpolar Region; European Commission, Office for Official Publications of the European Communities: Luxembourg, 2009. [Google Scholar]
- Havas, P.; Kubin, E. Structure, growth and organic matter content in the vegetation cover of an old spruce forest in Northern Finland. Ann. Bot. Fennici 1983, 20, 115–149. [Google Scholar]
- Gower, S.T.; Vogel, J.G.; Norman, J.M.; Kucharik, C.J.; Steele, S.J.; Stow, T.K. Carbon distribution and aboveground net primary production in aspen, jack pine, and black spruce stands in Saskatchewan and Manitoba, Canada. J. Geophys. Res. 1997, 102, 29029–29041. [Google Scholar] [CrossRef]
- Schulze, E.D.; Lloyd, J.; Kelliher, F.M.; Wirth, C.; Rebmann, C.; Lühker, B.; Mund, M.; Knohl, A.; Milyukova, I.M.; Schulze, W.; et al. Productivity of forests in the Eurosiberian boreal region and their potential to act as a carbon sink—A synthesis. Glob. Chang. Biol. 1999, 5, 703–722. [Google Scholar] [CrossRef]
- Martin, J.L.; Gower, S.T.; Plaut, J.; Holmes, B. Carbon pools in a boreal mixed wood logging chronosequence. Glob. Chang. Biol. 2005, 11, 1883–1894. [Google Scholar]
- Malhi, Y.; Baldocchi, D.D.; Jarvis, P.G. The carbon balance of tropical, temperate, and boreal forests. Plant Cell Environ. 1999, 22, 715–740. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.J.; Qiu, Y.; Chen, L.D. Soil nutrients in relation to land use and landscape position in the semi-arid small catchment on the loess plateau in China. J. Arid Environ. 2001, 48, 537–550. [Google Scholar] [CrossRef]
- Kurganova, I.N.; Lopes de Gerenyu, V.O.; Shvidenko, A.Z.; Sapozhnikov, P.M. Changes in the organic carbon pool of abandoned soils in Russia (1990–2004). Eurasian Soil Sci. 2010, 43, 333–340. [Google Scholar] [CrossRef]
- Houghton, R.A.; Goodale, C.L. Effects of land-use change on the carbon balance of terrestrial ecosystems. Ecosyst. Land Use Chang. 2004, 153, 85–98. [Google Scholar]
- Don, A.; Schumacher, J.; Freibauer, A. Impact of tropical land-use change on soil organic carbon stocks: A meta analysis. Glob. Chang. Biol. 2011, 17, 1658–1670. [Google Scholar] [CrossRef]
- Harris, N.L.; Brown, S.; Hagen, S.C.; Saatchi, S.S.; Petrova, S.; Salas, W.; Hansen, M.C.; Potapov, P.V.; Lotsch, A. Baseline map of carbon emissions from deforestation in tropical regions. Sciences 2012, 336, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Kalinina, O.; Barmin, A.N.; Chertov, O.; Dolgikh, A.V.; Goryachkin, S.V.; Lyuri, D.I.; Giani, L. Self-restoration of post-agrogenic soils of Calcisol–Solonetz complex: Soil development, carbon stock dynamics of carbon pools. Geoderma 2015, 237, 117–128. [Google Scholar] [CrossRef]
- Kurganova, I.N.; Lopes de Gerenyu, V.O. The stock of organic carbon in soils of the Russian Federation: Updated estimation in connection with land use changes. Doki. Biol. Sci. 2009, 426, 219–221. [Google Scholar] [CrossRef]
- Lyuri, D.I.; Goryachkin, S.V.; Karavaeva, N.A.; Denisenko, E.A.; Nefedova, T.G. Dynamics of Agricultural Lands of Russia in XX Century and Postagrogenic Restoration of Vegetation and Soils; GEOS: Moscow, Russia, 2010. [Google Scholar]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Takakura, H. Arctic Pastoralist Sakha: Ethnography and Micro-Adaptation in Siberia; Trans Pacific Press: Melbourne, Australia, 2015. [Google Scholar]
- Ioffe, G.; Nefedova, T. Marginal farmland in European Russia. Eurasian Geogr. Econ. 2004, 45, 45–59. [Google Scholar] [CrossRef]
- Henebry, G.M. Carbon in idle croplands. Nature 2009, 457, 1089. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A. Carbon cycle: A return to Soviet soils. Nat. Geosci. 2008, 1, 810. [Google Scholar] [CrossRef]
- Ministry of Nature Protection of the Republic of Sakha. State Report on the State and Protection of the Environment of the Republic of Sakha (Yakutia) for 2000/Ministry of Nature Protection of the Republic of Sakha (Yakutia); Sahapoligrafizdat: Yakutsk, Russia, 2015; 164p. [Google Scholar]
- Elovskaya, L.G.; Konorovskiy, A.K.; Savvinov, D.D. Permafrost Saline Soils of Central Yakutia; Nauka: Moscow, Russia, 1966; p. 274. [Google Scholar]
- Davidson, E.A.; Ackerman, I.L. Changes in soil carbon inventories following cultivation of previously untilled soils. Biogeochemistry 1993, 20, 161–193. [Google Scholar] [CrossRef]
- Egorov, E.G.; Nikiforov, M.M. About the state of land use and crop production in the Republic of Sakha (Yakutia). In Regional Economy: Theory and Practice; Yakutsk Science Center: Yakutsk, Russia, 2009. [Google Scholar]
- Ministry of Nature Protection of the Republic of Sakha. State Report on the State and Protection of the Environment of the Republic of Sakha (Yakutia) for 2014/Ministry of Nature Protection of the Republic of Sakha (Yakutia); Sahapoligrafizdat: Yakutsk, Russia, 2016. [Google Scholar]
- Gavrilova, M.K. Climate in Central Yakutia. Yakutsk Book Press: Yakutsk, Russia, 1973. [Google Scholar]
- Desyatkin, R.V. Content and Composition of Humus in Lena-Amga Interfluve’s Alas soils. In Vesti; Leningrad univ No. 6; LSU Press: Leningrad, Russia, 1981; pp. 75–82. [Google Scholar]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global Pattern of Soil Carbon Losses Due to the Conversion of Forests to Agricultural Land. Scientific Rep. 2014, 4, 4062. [Google Scholar] [CrossRef] [PubMed]
- Kurganova, I.; Lopes de Gerenyu, V.; Six, J.; Kuzyakov, Y. Сarbon cost of collective farming collapse in Russia. Glob. Chang. Biol. 2014, 20, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.Z. Boreal forest health and global change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Desyatkin, R.V. Soil Formation in Thermokarst Depression—Alases of Cryolithozone; Nauka: Novosibirsk, Russia, 2008; p. 324. [Google Scholar]
- Pranagal, J.; Podstawka-Chmielewska, E. Physical properties of a RendzicPhaeozem during a ten-year period of fallowing under the conditions of south-eastern Poland. Geoderma 2012, 189, 262–267. [Google Scholar] [CrossRef]
- Drewry, J.J.; Cameron, K.C.; Buchan, G.D. Pasture yield and soil physical property responses to soil compaction from treading and grazing—A review. Soil Res. 2008, 46, 237–256. [Google Scholar] [CrossRef]
- Olness, A.; Clapp, C.E.; Liu, R.; Palazzo, A.J. Biosoil and their effect on soil properties. In Handbook of Soil Conditioners; Wallace, A., Terry, R.E., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 141–165. [Google Scholar]
- Reiners, W.A.; Bouwman, A.F.; Parsons, W.F.J.; Keller, M. Tropical rain forest conversion to pasture: Changes in vegetation and soil properties. Ecol. Appl. 1994, 4, 363–377. [Google Scholar] [CrossRef]
- Celik, I. Land-use effects on organic matter and physical properties of soil in a southern Mediterranean highland of Turkey. Soil Tillage Res. 2005, 83, 270–277. [Google Scholar] [CrossRef]
- Vopravil, J.; Podrázský, V.; Khel, T.; Holubík, O. Effect of afforestation of agricultural soils and tree species composition on soil physical characteristics changes. Ekológia 2014, 33, 67–80. [Google Scholar] [CrossRef]
- Litvinovich, A.V.; Drichko, V.F.; Pavlova, O.Y.; Chernov, D.V.; Shabanov, M.V. Changes in the Acid-Base Properties of Cultivated Light-Textured Soddy-Podzolic Soils in the Course of Postagrogenic Transformation. Eurasian Soil Sci. 2009, 42, 629–635. [Google Scholar] [CrossRef]
- Van Donk, S.J.; Norman, L.K. Tillage and crop residue removal effects on evaporation, irrigation requirements, and yield. In Proceedings of the 24th 151 Annual Central Plains Irrigation Conference, Colby, CA, USA, 21–22 February 2012. [Google Scholar]
- Moody, J.W.; Jones, J.N.; Lillard, J.H. Influence of straw mulch on soil moisture, soil temperature and the growth of corn. Soil Sci. Soc. Am. J. 1963, 27, 700–703. [Google Scholar] [CrossRef]
- Dose, H.L.; DeSutter, T.M.; Casey, F.X.M.; Brueggeman, R.; Clay, D.E. Naturally occurring soil salinity does not reduce N-transforming enzymes or organisms. Can. J. Soil Sci. 2017, 97, 339. [Google Scholar] [CrossRef]
- Smith, J.L.; Doran, J.W. Measurement and use of pH and electrical conductivity for soil quality analysis. In Methods for Assessing Soil Quality; Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 169–185. [Google Scholar]
- Anderson-Cook, C.M.; Alley, M.M.; Roygard, J.K.F.; Khosla, R.; Noble, R.B.; Doolittle, J.A. Differentiating soil types using electromagnetic conductivity and crop yield maps. Soil Sci. Soc. Am. J. 2002, 66, 1562–1570. [Google Scholar] [CrossRef]
- Ezzati, R.; Karimi, M. Effect of various soil salinity level on the antioxidant and physiological properties of corn plant (Zea mays). J. Exp. Biol. Agric. Sci. 2015, 3, 448–452. [Google Scholar] [CrossRef]
- Sinegani, M.S.; Sinegani, A.A.S.; Hadipour, M. Spatial distribution of total phosphorus and organic carbon in the salt-affected soils in the Meyghan Playa, Iran. Spanish J. Soil Sci. 2017, 7, 149–163. [Google Scholar]
- Ghadimi, F.; Ghomi, M. Assessment of the effects of municipal wastewater on the heavy metal pollution of water and sediment in Arak Mighan lake, Iran. J. Tethys 2013, 1, 205–214. [Google Scholar]
- Ghadimi, F. Assessment of the sources of chemical elements in sediment from Arak Mighan lake. Int. J. Sediment Res. 2014, 29, 159–170. [Google Scholar] [CrossRef]
- Sorokina, O.A. Transformation of Gray Soils Under Forest and Agrogenic Effects in Siberia. Ph.D. Thesis, Federal Agency of Agriculture, Krasnoyarsk State Agrary University, Krasnoyarsk, Russia, October 2006. [Google Scholar]
- Pesterev, А.P. Change of agrophysical properties pale-yellow soils. In Proceedings of the III International Forest Soil Science Conference Productivity and Resistance of Forest Soils, Petrozavodsk, Russia, 7–11 September 2009; pp. 287–288. [Google Scholar]
- Ryzhova, I.M.; Erokhova, A.A.; Podvezennaya, M.A. Dynamics and Structure of Carbon Storage in the Postagrogenic Ecosystems of the Southern Taiga. Eurasian Soil Sci. 2014, 47, 1207–1215. [Google Scholar] [CrossRef]
- Boecker, D.; Centeri, C.; Welp, G.; Möseler, B.D. Parallels of secondary grassland succession and soil regeneration in a chronosequence of central-Hungarian old fields. Folia Geobotanica 2015, 50, 91–106. [Google Scholar] [CrossRef]
- Deng, L.; Liu, G.B.; Shangguan, Z.P. Land-use conversion and changing soil carbon stocks in China’s “Grain-for-Green” Program: A synthesis. Glob. Chang. Biol. 2014, 20, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Laganiere, J.; Angers, D.A.; Pare, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2010, 16, 439–453. [Google Scholar] [CrossRef]
- Vesterdal, L.; Ritter, E.; Gundersen, P. Change in soil organic carbon following afforestation of former arable land. For. Ecol. Manag. 2002, 169, 137–147. [Google Scholar] [CrossRef]
- Don, A.; Rebmann, C.; Kolle, O.; Schere-Lorenzen, M.; Schulze, E.D. Impact of afforestation-associated management changes on the carbon balance of grassland. Glob. Chang. Biol. 2009, 15, 1990–2002. [Google Scholar] [CrossRef]
- Zhang, K.; Dang, H.; Tan, S.; Cheng, X.; Zhang, Q. Change in soil organic carbon following the ‘Grain-for-Green’ programme in China. Land Degrad. Dev. 2010, 21, 13–23. [Google Scholar] [CrossRef]
- Li, D.; Niu, S.; Luo, Y. Global patterns of the dynamics of soil carbon and nitrogen stocks following afforestation: A meta-analysis. N. Phytol. 2012, 195, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Compton, J.E.; Boone, R.D. Long-term impacts of agriculture on soil carbon and nitrogen in New England forests. Ecology 2000, 81, 2314–2330. [Google Scholar] [CrossRef]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in soil carbon following afforestation. For. Ecol. Manag. 2002, 168, 241–257. [Google Scholar] [CrossRef]
- Chang, R.Y.; Fu, B.J.; Liu, G.H.; Liu, S.G. Soil carbon sequestration potential for “Grain for Green” Project in Loess Plateau. China. Environ. Manag. 2011, 48, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Bohm, S.; Haile-Mariam, S.; Paul, E. Evaluation of carbon accrual in afforested agricultural soils. Glob. Chang. Biol. 2007, 13, 1145–1156. [Google Scholar] [CrossRef]
- Mao, R.; Zeng, D.H.; Hu, Y.L.; Li, L.J.; Yang, D. Soil organic carbon and nitrogen stocks in an age-sequence of poplar stands planted on marginal agricultural land in Northeast China. Plant Soil 2010, 332, 277–287. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.B.; Chen, M.L.; Shangguan, Z.P.; Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. Catena 2013, 110, 1–7. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).