Phytoplankton and Bacterial Response to Desert Dust Deposition in the Coastal Waters of the Southeastern Mediterranean Sea: A Four-Year In Situ Survey
Abstract
1. Introduction
2. Material and Methods
3. Results and Discussion
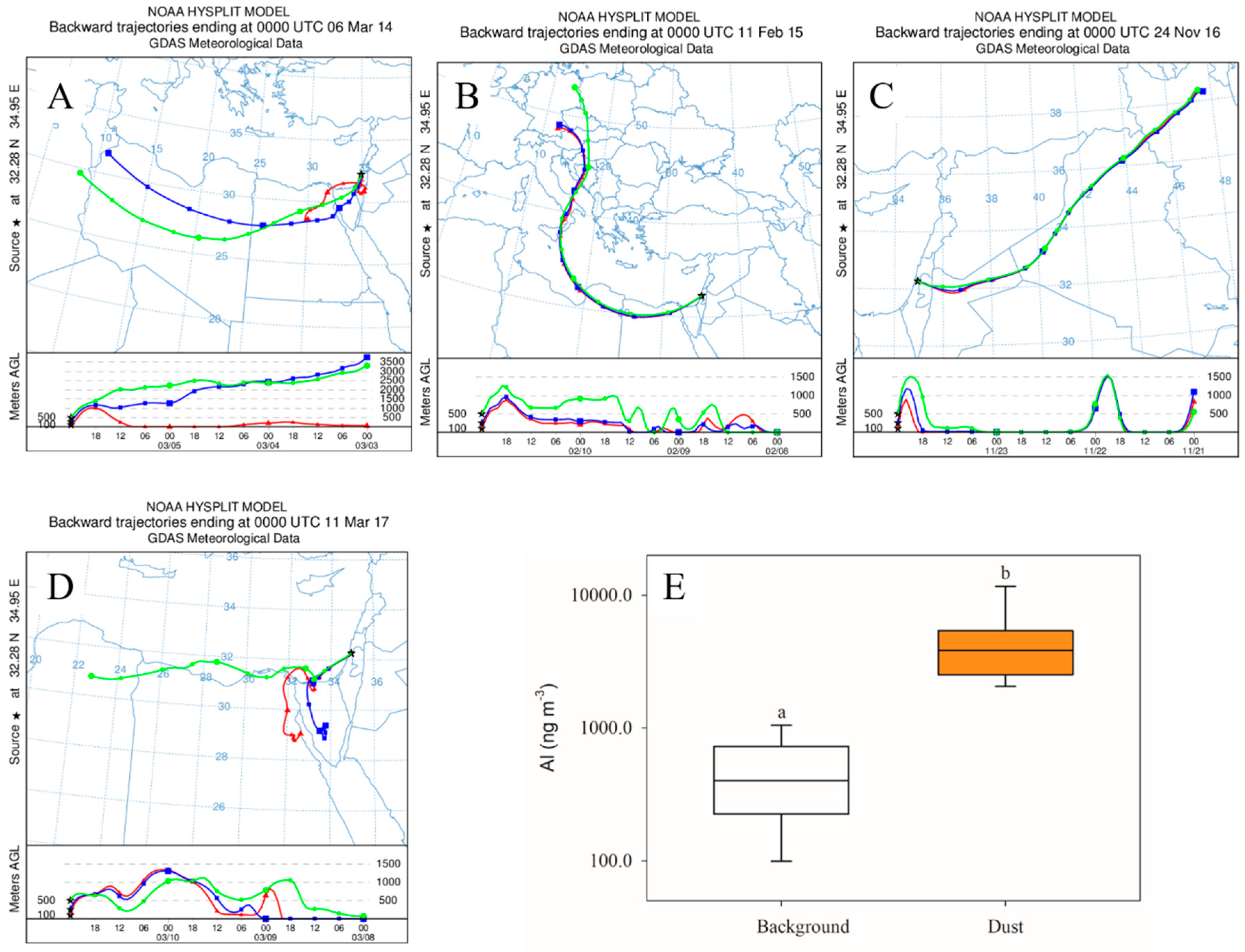
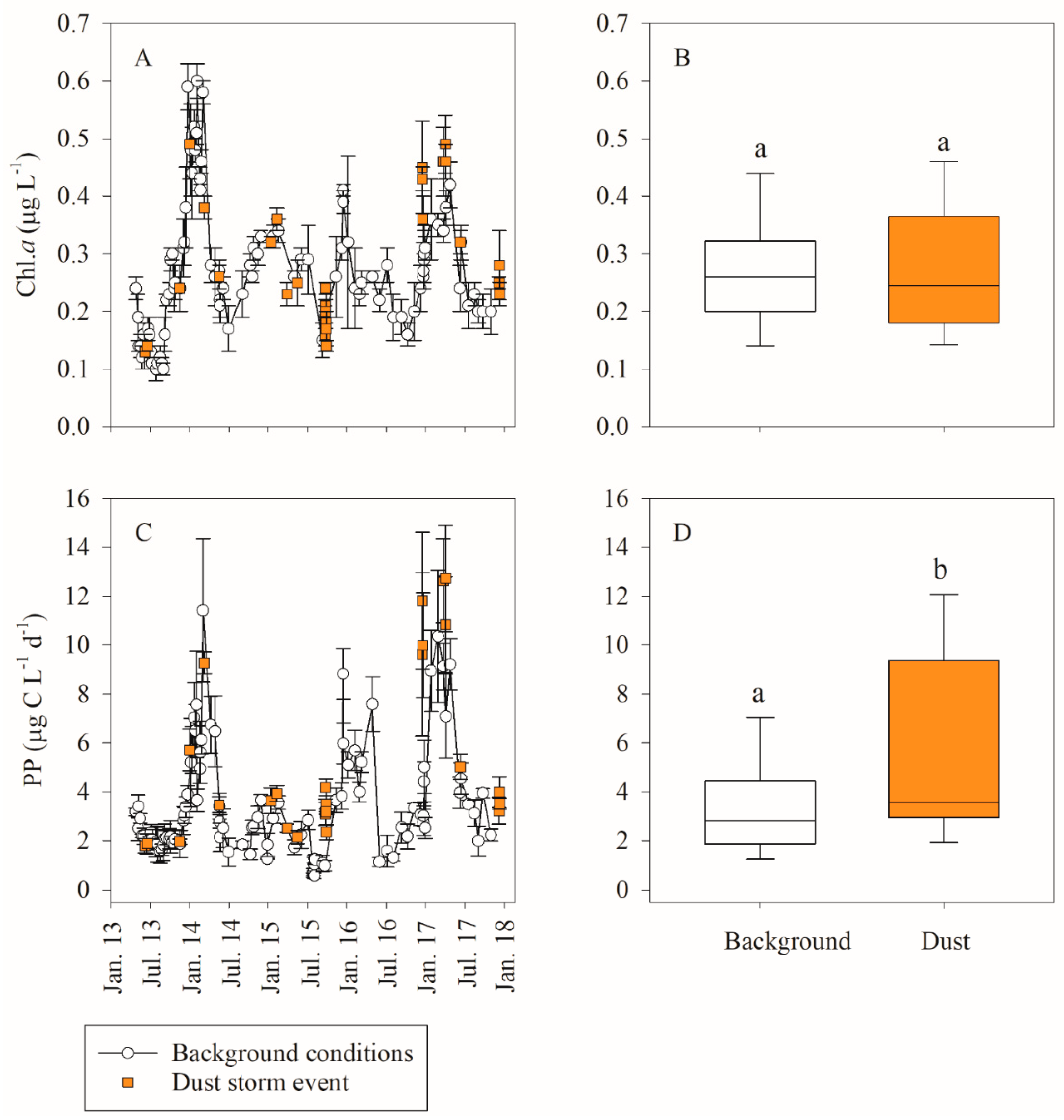
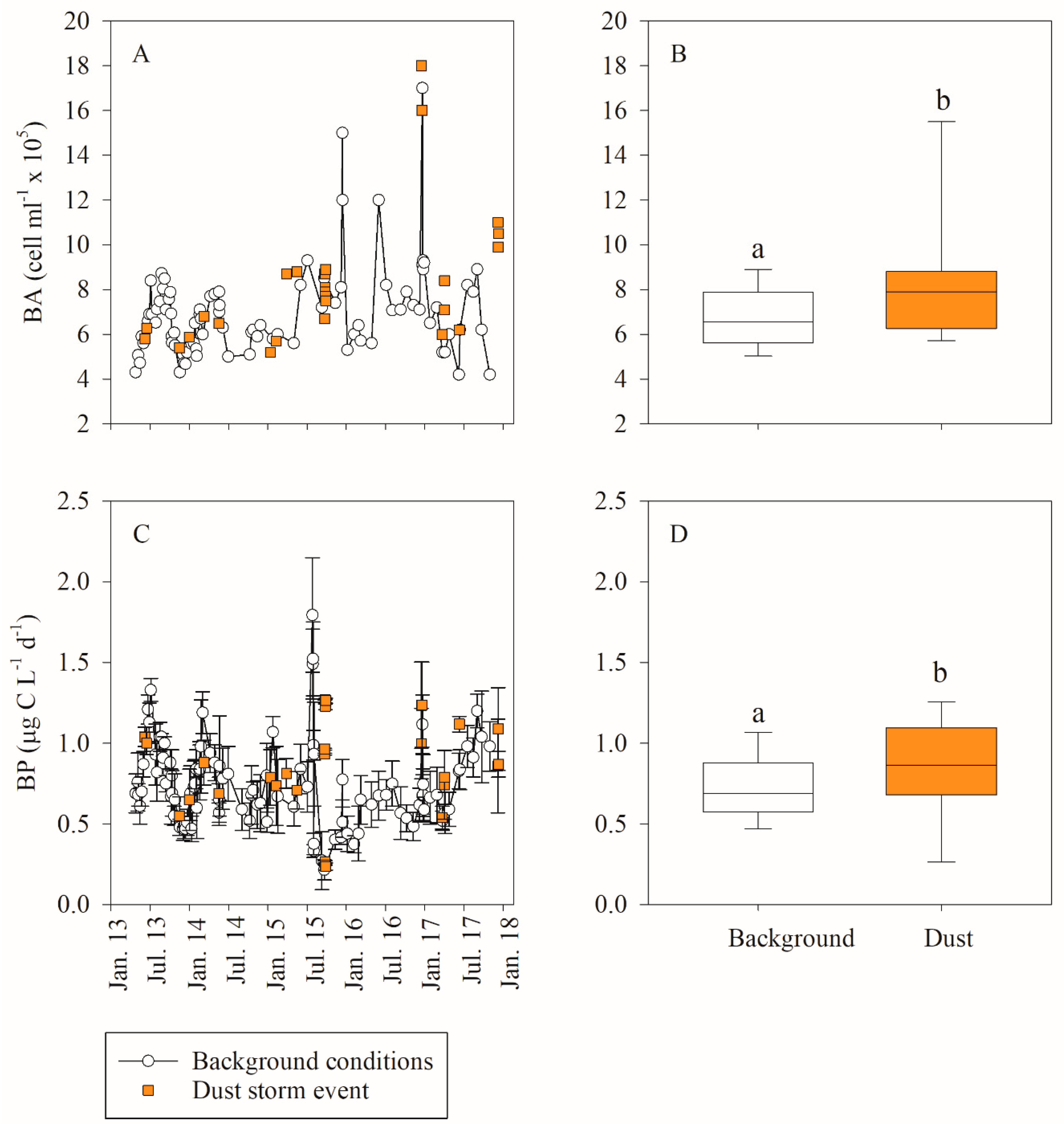
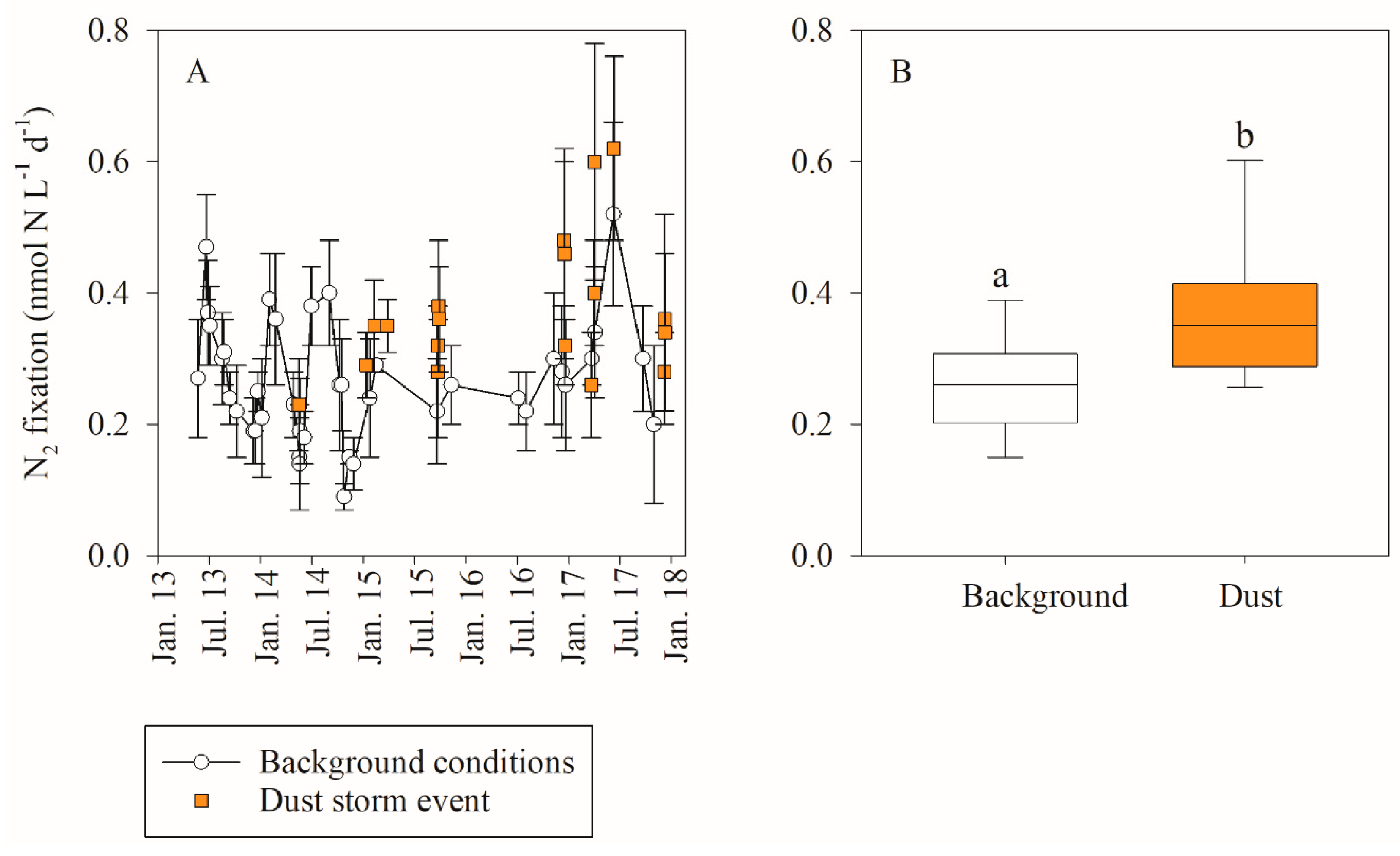
3.1. In Situ Response of Bacteria and Phytoplankton to Dust Storm Events
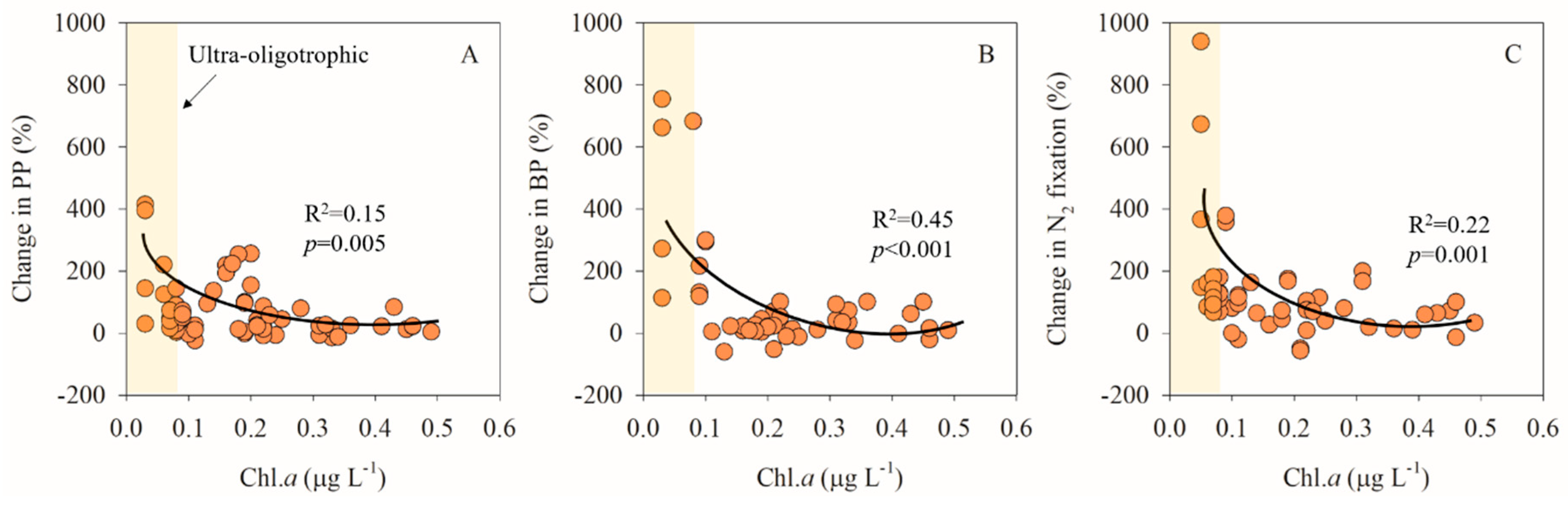
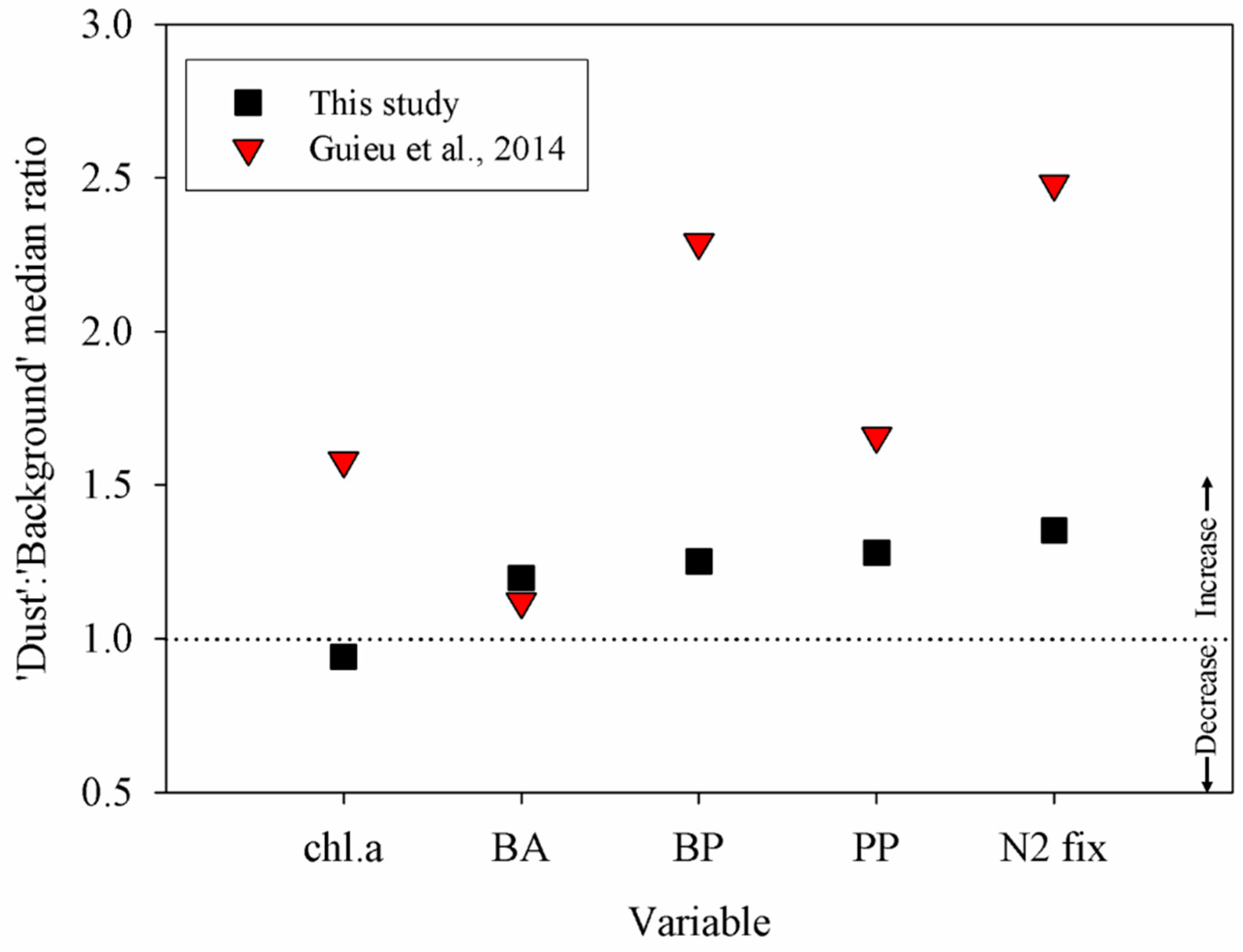
3.2. Relationship between Degree of Oligotrophy and Magnitude of the Metabolic Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duce, R.A.; Liss, P.S.; Merrill, J.T.; Atlas, E.L.; Buat-Menard, P.; Hicks, B.B.; Miller, J.M.; Prospero, J.M.; Arimoto, R.; Church, T.M.; et al. The atmospheric input of trace species to the world ocean. Glob. Biogeochem. Cycles 1991, 5, 193–259. [Google Scholar] [CrossRef]
- Herut, B.; Collier, R.; Krom, M.D. The role of dust in supplying nitrogen and phosphorus to the Southeast Mediterranean. Limnol. Oceanogr. 2002, 47, 870–878. [Google Scholar] [CrossRef]
- Jickells, T.D.; An, Z.S.; Andersen, K.K.; Baker, A.R.; Bergametti, G.; Brooks, N.; Cao, J.J.; Boyd, P.W.; Duce, R.A.; Hunter, K.A.; et al. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, N.; Jickells, T.D.; Baker, A.R.; Artaxo, P.; Benitez-Nelson, C.R.; Bergametti, G.; Bond, T.C.; Chen, Y.; Cohen, D.D.; Herut, B.; et al. Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Glob. Biogeochem. Cycles 2008, 22, 1–19. [Google Scholar] [CrossRef]
- Griffin, D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Favet, J.; Lapanje, A.; Giongo, A.; Kennedy, S.; Aung, Y.-Y.; Cattaneo, A.; Davis-Richardson, A.G.; Brown, C.T.; Kort, R.; Brumsack, H.-J.; et al. Microbial hitchhikers on intercontinental dust: Catching a lift in Chad. ISME J. 2013, 7, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Rahav, E.; Ovadia, G.; Paytan, A.; Herut, B. Contribution of airborne microbes to bacterial production and N2 fixation in seawater upon aerosol deposition. Geophys. Res. Lett. 2016, 43. [Google Scholar] [CrossRef]
- Gat, D.; Mazar, Y.; Cytryn, E.; Rudich, Y. Origin-dependent variations in the atmospheric microbiome community in Eastern Mediterranean dust storms. Environ. Sci. Technol. 2017, 51, 6709–6718. [Google Scholar] [CrossRef] [PubMed]
- Mayol, E.; Arrieta, J.M.; Jiménez, M.A.; Martínez-Asensio, A.; Garcias-Bonet, N.; Dachs, J.; González-Gaya, B.; Royer, S.J.; Benítez-Barrios, V.M.; Fraile-Nuez, E.; et al. Long-range transport of airborne microbes over the global Tropical and Subtropical Ocean. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Katra, I.; Arotsker, L.; Krasnov, H.; Zaritsky, A.; Kushmaro, A.; Ben-Dov, E. Richness and diversity in dust stormborne biomes at the Southeast Mediterranean. Sci. Rep. 2014, 4, 5265. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, A.; Moore, J.K.; Mahowald, N.; Luo, C.; Zender, C.S. Impacts of atmospheric nutrient inputs on marine biogeochemistry. J. Geophys. Res. 2010, 115, G01006. [Google Scholar] [CrossRef]
- Herut, B.; Zohary, T.; Krom, M.D.; Mantoura, R.F.C.; Pitta, P.; Psarra, S.; Rassoulzadegan, F.; Tanaka, T.; Frede Thingstad, T. Response of East Mediterranean surface water to Saharan dust: On-board microcosm experiment and field observations. Deep. Res. Part II Top. Stud. Oceanogr. 2005, 52, 3024–3040. [Google Scholar] [CrossRef]
- Herut, B.; Rahav, E.; Tsagaraki, T.M.; Giannakourou, A.; Tsiola, A.; Psarra, S.; Lagaria, A.; Papageorgiou, N.; Mihalopoulos, N.; Theodosi, C.N.; et al. The potential impact of Saharan dust and polluted aerosols on microbial populations in the East Mediterranean Sea, an overview of a mesocosm experimental approach. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Guieu, C.; Dulac, F.; Desboeufs, K.; Wagener, T.; Pulido-Villena, E.; Grisoni, J.M.; Louis, F.; Ridame, C.; Blain, S.; Brunet, C.; et al. Large clean mesocosms and simulated dust deposition: A new methodology to investigate responses of marine oligotrophic ecosystems to atmospheric inputs. Biogeosciences 2010, 7, 2765–2784. [Google Scholar] [CrossRef]
- Paytan, A.; Mackey, K.R.M.; Chen, Y.; Lima, I.D.; Doney, S.C.; Mahowald, N.; Labiosa, R.; Post, A.F. Toxicity of atmospheric aerosols on marine phytoplankton. Proc. Natl. Acad. Sci. USA 2009, 106, 4601–4605. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.M.; Ridame, C.; Davey, M.; La Roche, J.; Geider, R.J. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 2004, 429, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Marañón, E.; Fernández, A.; Mouriño-Carballido, B.; Martínez-García, S.; Teira, E.; Cermeño, P.; Chouciño, P.; Huete-Ortega, M.; Fernández, E.; Calvo-Díaz, A.; et al. Degree of oligotrophy controls the response of microbial plankton to Saharan dust. Limnol. Oceanogr. 2010, 55, 2339–2352. [Google Scholar] [CrossRef]
- Astrahan, P.; Herut, B.; Paytan, A.; Rahav, E. The impact of dry atmospheric deposition on the sea-surface microlayer in the SE Mediterranean Sea: An experimental approach. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Guieu, C.; Aumont, O.; Paytan, A.; Bopp, L.; Law, C.S.; Mahowald, N.; Achterberg, E.P.; Marañón, E.; Salihoglu, B.; Crise, A.; et al. Global biogeochemical cycles deposition to Low Nutrient Low Chlorophyll regions. Glob. Biogeochem. Cycles 2014, 28, 1179–1198. [Google Scholar] [CrossRef]
- Berman, T.; Townsend, D.; Elsayed, S. Optical transparency, chlorophyll and primary productivity in the Eastern Mediterranean near the Israeli Coast. Oceanol. Acta 1984, 7, 367–372. [Google Scholar]
- Herut, B.; Almogi-Labin, A.; Jannink, N.; Gertman, I. The seasonal dynamics of nutrient and chlorophyll a concentrations on the SE Mediterranean shelf-slope. Oceanol. Acta 2000, 23, 771–782. [Google Scholar] [CrossRef]
- Raveh, O.; David, N.; Rilov, G.; Rahav, E. The temporal dynamics of coastal phytoplankton and bacterioplankton in the Eastern Mediterranean Sea. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Rahav, E.; Raveh, O.; Hazan, O.; Gordon, N.; Kress, N.; Silverman, J.; Herut, B. Impact of nutrient enrichment on productivity of coastal water along the SE Mediterranean shore of Israel−A bioassay approach. Mar. Pollut. Bull. 2018, 127, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Azov, Y. Seasonal patterns of phytoplankton productivity and abundance in nearshore oligotrophic waters of the Levant Basin (Mediterranean). J. Plankton Res. 1986, 8, 41–53. [Google Scholar] [CrossRef]
- Yacobi, Y.Z.Y.; Zohary, T.; Kress, N.; Hecht, A.; Robarts, R.D.; Waiser, M.; Wood, A.M.; Li, W.K.W. Chlorophyll distribution throughout the Southeastern Mediterranean in relation to the physical structure of the water mass. J. Mar. Syst. 1995, 6, 179–190. [Google Scholar] [CrossRef]
- Kimor, B.; Wood, E.J.F. Plankton study in Eastern Mediterranean Sea. Mar. Biol. 1975, 29, 321–333. [Google Scholar] [CrossRef]
- Rahav, E.; Bar-Zeev, E. Sewage outburst triggers Trichodesmium bloom and enhance N2 fixation rates. Sci. Rep. 2017, 7, 4367. [Google Scholar] [CrossRef] [PubMed]
- Rahav, E.; Giannetto, M.J.; Bar-Zeev, E. Contribution of mono and polysaccharides to heterotrophic N2 fixation at the eastern Mediterranean coastline. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.R.; Neff, J.C. The contemporary physical and chemical flux of aeolian dust: A synthesis of direct measurements of dust deposition. Chem. Geol. 2009, 267, 46–63. [Google Scholar] [CrossRef]
- Ganor, E.; Osetinsky, I.; Stupp, A.; Alpert, P. Increasing trend of African dust over 49 years in the Eastern Mediterranean. J. Geophys. Res. Atmos. 2010, 115, 1–7. [Google Scholar] [CrossRef]
- Kocak, M.; Kubilay, N.; Tuğrul, S.; Mihalopoulos, N. Atmospheric nutrient inputs to the northern Levantine basin from a long-term observation: Sources and comparison with riverine inputs. Biogeosciences 2010, 7, 4037–4050. [Google Scholar] [CrossRef]
- Herut, B.; Krom, M.D.; Pan, G.; Mortimer, R. Atmospheric input of nitrogen and phosphorus to the Southeast Mediterranean: Sources, fluxes, and possible impact. Limnol. Ocean. 1999, 44, 1683–1692. [Google Scholar] [CrossRef]
- Guerzoni, S.; Chester, R.; Dulac, F.; Herut, B.; Loÿe-Pilot, M.-D.; Measures, C.; Migon, C.; Molinaroli, E.; Moulin, C.; Rossini, P.; et al. The role of atmospheric deposition in the biogeochemistry of the Mediterranean Sea. Prog. Oceanogr. 1999, 44, 147–190. [Google Scholar] [CrossRef]
- Léon, J.-F.; Augustin, P.; Mallet, M.; Bourrianne, T.; Pont, V.; Dulac, F.; Fourmentin, M.; Lambert, D.; Sauvage, B. Aerosol vertical distribution, optical properties and transport over Corsica (Western Mediterranean). Atmos. Chem. Phys. Discuss. 2015, 15, 9507–9540. [Google Scholar] [CrossRef]
- Rahav, E.; Paytan, A.; Chien, C.; Ovadia, G.; Katz, T.; Herut, B. The impact of atmospheric dry deposition associated microbes on the Southeastern Mediterranean Sea surface water following an intense dust storme. Front. Mar. Sci. 2016, 3, 127. [Google Scholar] [CrossRef]
- Calvo-Díaz, A.; Díaz-Pérez, L.; Suárez, L.Á.; Morán, X.A.G.; Teira, E.; Marañón, E. Decrease in the autotrophic-to-heterotrophic biomass ratio of picoplankton in oligotrophic marine waters due to bottle enclosure. Appl. Environ. Microbiol. 2011, 77, 5739–5746. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.; Kubilay, N.; Herut, B.; Nimmo, M. Dry atmospheric fluxes of trace metals (Al, Fe, Mn, Pb, Cd, Zn, Cu) over the Levantine Basin: A refined assessment. Atmos. Environ. 2005, 39, 7330–7341. [Google Scholar] [CrossRef]
- Herut, B.; Nimmo, M.; Medway, A.; Chester, R.; Krom, M.D. Dry atmospheric inputs of trace metals at the Mediterranean coast of Israel (SE Mediterranean): Sources and fluxes. Atmos. Environ. 2001, 35, 803–813. [Google Scholar] [CrossRef]
- Welschmeyer, N.A. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 1994, 39, 1985–1992. [Google Scholar] [CrossRef]
- Bar-Zeev, E.; Rahav, E. Microbial metabolism of transparent exopolymer particles during the summer months along a eutrophic estuary system. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Steemann-Nielsen, E. On the determination of the activity for measuring primary production. J. Cons. Int. Explor. Mer. 1952, 18, 117–140. [Google Scholar]
- Simon, M.; Alldredge, A.; Azam, F. Bacterial carbon dynamics on marine snow. Mar. Ecol. Prog. Ser. 1990, 65, 205–211. [Google Scholar] [CrossRef]
- Simon, M.; Alldredge, A.; Azam, F. Protein-content and protein-synthesis rates of planktonic marine-bacteria. Mar. Ecol. Prog. Ser. 1989, 51, 201–213. [Google Scholar] [CrossRef]
- Mohr, W.; Großkopf, T.; Wallace, D.W.R.; Laroche, J. Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Rahav, E.; Herut, B.; Mulholland, M.R.; Belkin, N.; Elifantz, H.; Berman-Frank, I. Heterotrophic and autotrophic contribution to dinitrogen fixation in the Gulf of Aqaba. Mar. Ecol. Prog. Ser. 2015, 522. [Google Scholar] [CrossRef]
- D’Ortenzio, F.; Ribera d’Alcalà, M. On the trophic regimes of the Mediterranean Sea: A satellite analysis. Biogeosciences 2009, 6, 139–148. [Google Scholar] [CrossRef]
- Siokou-Frangou, I.; Christaki, U.; Mazzocchi, M.G.; Montresor, M.; Ribera d’Alcalá, M.; Vaqué, D.; Zingone, A. Plankton in the open Mediterranean Sea: A review. Biogeosciences 2010, 7, 1543–1586. [Google Scholar] [CrossRef]
- Mella-Flores, D.; Mazard, S.; Humily, F.; Partensky, F.; Mahe, F.; Bariat, L.; Courties, C.; Marie, D.; Ras, J.; Mauriac, R.; et al. Is the distribution of Prochlorococcus and Synechococcus ecotypes in the Mediterranean Sea affected by global warming? Biogeosciences 2011, 8, 2785–2804. [Google Scholar] [CrossRef]
- Bergamasco, A.; Malanotte-Rizzoli, P. The circulation of the Mediterranean Sea: A historical review of experimental investigations. Adv. Oceanogr. Limnol. 2010, 1, 11–28. [Google Scholar] [CrossRef]
- Kress, N.; Gertman, I.; Herut, B. Temporal evolution of physical and chemical characteristics of the water column in the easternmost Levantine Basin (Eastern Mediterranean Sea) from 2002 to 2010. J. Mar. Syst. 2014, 135, 6–13. [Google Scholar] [CrossRef]
- Krom, M.D.; Kress, N.; Berman-Frank, I.; Rahav, E. Past, present and future patterns in the nutrient chemistry of the eastern Mediterranean. In The Mediterranean Sea: Its History and Present Challenges; Goffredo, S., Dubinsky, Z., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 49–68. ISBN 978-94-007-6703-4. [Google Scholar]
- Ozer, T.; Gertman, I.; Kress, N.; Silverman, J.; Herut, B. Interannual thermohaline (1979–2014) and nutrient (2002–2014) dynamics in the Levantine surface and intermediate water masses, SE Mediterranean Sea. Glob. Planet. Chang. 2017, 151, 60–67. [Google Scholar] [CrossRef]
- Rahav, E.; Herut, B.; Levi, A.; Mulholland, M.R.; Berman-Frank, I. Springtime contribution of dinitrogen fixation to primary production across the Mediterranean Sea. Ocean Sci. 2013, 9, 489–498. [Google Scholar] [CrossRef]
- Hazan, O.; Silverman, J.; Sisma-Ventura, G.; Ozer, T.; Gertman, I.; Shoham-Frider, E.; Kress, N.; Rahav, E. Mesopelagic prokaryotes alter surface phytoplankton production during simulated deep mixing experiments in Eastern Mediterranean Sea waters. Front. Mar. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Herut, B.; Tibor, G.; Yacobi, Y.Z.; Kress, N. Synoptic measurements of chlorophyll-a and suspended particulate matter in a transitional zone from polluted to clean seawater utilizing airborne remote sensing and ground measurements, Haifa Bay (SE Mediterranean). Mar. Pollut. Bull. 1999, 38, 762–772. [Google Scholar] [CrossRef]
- Belkin, N.; Rahav, E.; Elifantz, H.; Kress, N.; Berman-Frank, I. Enhanced salinities as a proxy of seawater desalination discharges impact coastal microbial communities of the Eastern Mediterranean Sea. Environ. Microbiol. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Kress, N.; Galil, B.S. Twenty two years of sewage sludge marine disposal monitoring in the Eastern Mediterranean Sea: Impact on sediment quality and infauna and the response to load reduction. Mar. Pollut. Bull. 2016, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.; Rahav, E.; Bar-Zeev, E. Short-term effects of SWRO desalination brine on benthic heterotrophic microbial communities. Desalination 2017, 417, 52–59. [Google Scholar] [CrossRef]
- Benavides, M.; Bonnet, B.; Hernández, N.; Martínez-Pérez, A.M.; Nieto-Cid, M.; Álvarez-Salgado, X.A.; Baños, I.; Montero, M.F.; Mazuecos, I.P.; Gasol, J.M.; et al. Basin-wide N2 fixation in the deep waters of the Mediterranean Sea. Glob. Biogeochem. Cycles 2016, 30, 952–961. [Google Scholar] [CrossRef]
- Koçak, M.; Kubilay, N.; Mihalopoulos, N. Ionic composition of lower tropospheric aerosols at a Northeastern Mediterranean site: Implications regarding sources and long-range transport. Atmos. Environ. 2004, 38, 2067–2077. [Google Scholar] [CrossRef]
- Pitta, P.; Kanakidou, M.; Mihalopoulos, N.; Christodoulaki, S.; Dimitriou, P.D.; Frangoulis, C.; Giannakourou, A.; Kagiorgi, M.; Lagaria, A.; Nikolaou, P.; et al. Saharan dust deposition effects on the microbial food web in the Eastern Mediterranean: A study based on a mesocosm experiment. Front. Mar. Sci. 2017, 4, 1–19. [Google Scholar] [CrossRef]
- Yogev, T.; Rahav, E.; Bar-Zeev, E.; Man-Aharonovich, D.; Stambler, N.; Kress, N.; Béjà, O.; Mulholland, M.R.; Herut, B.; Berman-Frank, I. Is dinitrogen fixation significant in the Levantine Basin, East Mediterranean Sea? Environ. Microbiol. 2011, 13, 854–871. [Google Scholar] [CrossRef] [PubMed]
- Karl, D.M.; Hebel, D.V.; Björkman, K.; Letelier, R.M. The role of dissolved organic matter release in the productivity of the oligotrophic North Pacific Ocean. Limnol. Oceanogr. 1998, 43, 1270–1286. [Google Scholar] [CrossRef]
- González, N.; Gattuso, J.P.; Middelburg, J.J. Oxygen production and carbon fixation in oligotrophic coastal bays and the relationship with gross and net primary production. Aquat. Microb. Ecol. 2008, 52, 119–130. [Google Scholar] [CrossRef]
- Iluz, D.; Dishon, G.; Capuzzo, E.; Meeder, E.; Astoreca, R.; Montecino, V.; Znachor, P.; Ediger, D.; Marra, J. Short-term variability in primary productivity during a wind-driven diatom bloom in the Gulf of Eilat (Aqaba). Aquat. Microb. Ecol. 2009, 56, 205–215. [Google Scholar] [CrossRef]
- Kirchman, D.L. Processes in Microbial Ecology, 1st ed.; Kirchman, D.L., Ed.; Oxford University Press: Oxford, UK, 2012; ISBN 0199586934. [Google Scholar]
- Laws, E.A. Evaluation of in situ phytoplankton growth rates: A synthesis of data from varied approaches. Ann. Rev. Mar. Sci. 2013, 5, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xia, X.; Pitta, P.; Herut, B.; Rahav, E.; Berman-Frank, I.; Giannakourou, A.; Tsiola, A.; Tsagaraki, T.M.; Liu, H. Shifts in microbial community structure and activity in the ultra-oligotrophic Eastern Mediterranean Sea driven by the deposition of Saharan dust and European aerosols. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Del Giorgio, P.; Cole, J.J.; Cimbleris, A. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 1997, 385, 148–151. [Google Scholar] [CrossRef]
- Pulido-Villena, E.; Wagener, T.; Guieu, C. Bacterial response to dust pulses in the western Mediterranean: Implications for carbon cycling in the oligotropic ocean. Glob. Biogeochem. Cycles 2008, 22, 1–12. [Google Scholar] [CrossRef]
- Joint, I.; Henriksen, P.; Fonnes, G.A.; Bourne, D.; Thingstad, T.F.; Riemann, B. Competition for inorganic nutrients between phytoplankton and bacterioplankton in nutrient manipulated mesocosms. Aquat. Microb. Ecol. 2002, 29, 145–159. [Google Scholar] [CrossRef]
- Thingstad, T.F.; Krom, M.D.; Mantoura, R.F.C.; Flaten, G.A.F.; Groom, S.; Herut, B.; Kress, N.; Law, C.S.; Pasternak, A.; Pitta, P.; et al. Nature of phosphorus limitation in the ultraoligotrophic Eastern Mediterranean. Science 2005, 309, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Pitta, P.; Nejstgaard, J.C.; Tsagaraki, T.M.; Zervoudaki, S.; Egge, J.K.; Frangoulis, C.; Lagaria, A.; Magiopoulos, I.; Psarra, S.; Sandaa, R.-A.; et al. Confirming the “rapid phosphorus transfer from microorganisms to mesozooplankton in the Eastern Mediterranean Sea” scenario through a mesocosm experiment. J. Plankton Res. 2016, 38, 502–521. [Google Scholar] [CrossRef]
- Peter, H.; Hörtnagl, P.; Reche, I.; Sommaruga, R. Bacterial diversity and composition during rain events with and without Saharan dust influence reaching a high mountain lake in the Alps. Environ. Microbiol. Rep. 2014, 6, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Reche, I.; Ortega-Retuerta, E.; Romera, O.; Pulido-Villena, E.; Morales-Baquero, R.; Casamayor, E.O. Effect of Saharan dust inputs on bacterial activity and community composition in Mediterranean lakes and reservoirs. Limnol. Oceanogr. 2009, 54, 869–879. [Google Scholar] [CrossRef]
- Rahav, E.; Paytan, A.; Mescioglu, E.; Galletti, Y.; Rosenfeld, S.; Raveh, O.; Santinelli, C.; Ho, T.-Y.; Herut, B. Airborne microbes contribute to N2 fixation in surface water of the Northern Red Sea. Geophys. Res. Lett. 2018, 45, GL077132. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Gentili, B.; Stramski, D. Phytoplankton class-specific primary production in the world’s oceans: Seasonal and interannual variability from satellite observations. Glob. Biogeochem. Cycles 2010, 24, GB3016. [Google Scholar] [CrossRef]
- Law, C.S.; Woodward, E.M.S.; Ellwood, M.J.; Marriner, A.; Bury, S.J.; Safi, K.A. Response of surface nutrient inventories and nitrogen fixation to a tropical cyclone in the Southwest Pacific. Limnol. Oceanogr. 2011, 56, 1372–1385. [Google Scholar] [CrossRef]
- Moore, C.M.; Mills, M.M.; Milne, A.; Langlois, R.; Achterberg, E.P.; Lochte, K.; Geider, R.J.; La Roche, J. Iron limits primary productivity during spring bloom development in the Central North Atlantic. Glob. Chang. Biol. 2006, 12, 626–634. [Google Scholar] [CrossRef]
- Foster, R.A.; Paytan, A.; Zehr, J.P. Seasonality of N2 fixation and nifH gene diversity in the Gulf of Aqaba (Red Sea). Limnol. Oceanogr. 2009, 54, 219–233. [Google Scholar] [CrossRef]
- Ridame, C.; Le Moal, M.; Guieu, C.; Ternon, E.; Biegala, I.C.; L’Helguen, S.; Pujo-Pay, M. Nutrient control of N2 fixation in the oligotrophic Mediterranean Sea and the impact of Saharan dust events. Biogeosciences 2011, 8, 2629–2657. [Google Scholar] [CrossRef]
- Ternon, E.; Guieu, C.; Ridame, C.; L’Helguen, S.; Catala, P. Longitudinal variability of the biogeochemical role of Mediterranean aerosols in the Mediterranean Sea. Biogeosciences 2011, 8, 1067–1080. [Google Scholar] [CrossRef]
- Ridame, C.; Guieu, C.; L’Helguen, S. Strong stimulation of N2 fixation in oligotrophic Mediterranean Sea: Results from dust addition in large in situ mesocosms. Biogeosciences 2013, 10, 7333–7346. [Google Scholar] [CrossRef]
- Bonnet, S.; Guieu, C.; Chiaverini, J.; Ras, J.; Stock, A. Effect of atmospheric nutrients on the autotrophic communities in a low nutrient, low chlorophyll system. Limnol. Oceanogr. 2005, 50, 1810–1819. [Google Scholar] [CrossRef]
- Archer, D.E.; Johnson, K. A Model of the iron cycle in the ocean. Glob. Biochem. 2000, 14, 269–279. [Google Scholar] [CrossRef]
- Moore, J.K.; Doney, S.C.; Kleypas, J.A.; Glover, D.M.; Fung, I.Y. An intermediate complexity marine ecosystem model for the global domain. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 403–462. [Google Scholar] [CrossRef]
- Torfstein, A.; Teutsch, N.; Tirosh, O.; Shaked, Y.; Rivlin, T.; Zipori, A.; Stein, M.; Lazar, B.; Erel, Y. Chemical characterization of atmospheric dust from a weekly time series in the north Red Sea between 2006 and 2010. Geochim. Cosmochim. Acta 2017, 211, 373–393. [Google Scholar] [CrossRef]
- Hill, P.G.; Zubkov, M.V.; Purdie, D.A. Differential responses of Prochlorococcus and SAR11-dominated bacterioplankton groups to atmospheric dust inputs in the tropical Northeast Atlantic Ocean. FEMS Microbiol. Lett. 2010, 306, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Neuer, S.; Torres-Padrón, M.E.; Gelado-Caballero, M.D.; Rueda, M.J.; Hernández-Brito, J.; Davenport, R.; Wefer, G. Dust deposition pulses to the eastern subtropical north Atlantic gyre: Does ocean’s biogeochemistry respond? Glob. Biogeochem. Cycles 2004, 18, 1–10. [Google Scholar] [CrossRef]
- Womack, A.M.; Bohannan, B.J.M.; Green, J.L. Biodiversity and biogeography of the atmosphere. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3645–3653. [Google Scholar] [CrossRef] [PubMed]
- Polymenakou, P.N. Atmosphere: A source of pathogenic or beneficial microbes? Atmosphere 2012, 3, 87–102. [Google Scholar] [CrossRef]
- Prospero, J.M.; Lamb, P.J. African droughts and dust transport to the Caribbean: Climate change implications. Science 2003, 302, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Polovina, J.J.; Howell, E.A.; Abecassis, M. Ocean’s least productive waters are expanding. Geophys. Res. Lett. 2008, 35, 2–6. [Google Scholar] [CrossRef]
- Steinacher, M.; Joos, F.; Frölicher, T.L.; Bopp, L.; Cadule, P.; Doney, S.C.; Gehlen, M.; Schneider, B.; Segschneider, J. Projected 21st century decrease in marine productivity: A multi-model analysis. Biogeosciences 2010, 7, 979–1005. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahav, E.; Belkin, N.; Paytan, A.; Herut, B. Phytoplankton and Bacterial Response to Desert Dust Deposition in the Coastal Waters of the Southeastern Mediterranean Sea: A Four-Year In Situ Survey. Atmosphere 2018, 9, 305. https://doi.org/10.3390/atmos9080305
Rahav E, Belkin N, Paytan A, Herut B. Phytoplankton and Bacterial Response to Desert Dust Deposition in the Coastal Waters of the Southeastern Mediterranean Sea: A Four-Year In Situ Survey. Atmosphere. 2018; 9(8):305. https://doi.org/10.3390/atmos9080305
Chicago/Turabian StyleRahav, Eyal, Natalia Belkin, Adina Paytan, and Barak Herut. 2018. "Phytoplankton and Bacterial Response to Desert Dust Deposition in the Coastal Waters of the Southeastern Mediterranean Sea: A Four-Year In Situ Survey" Atmosphere 9, no. 8: 305. https://doi.org/10.3390/atmos9080305
APA StyleRahav, E., Belkin, N., Paytan, A., & Herut, B. (2018). Phytoplankton and Bacterial Response to Desert Dust Deposition in the Coastal Waters of the Southeastern Mediterranean Sea: A Four-Year In Situ Survey. Atmosphere, 9(8), 305. https://doi.org/10.3390/atmos9080305