Estimation of the Personal Deposited Dose of Particulate Matter and Particle-Bound Metals Using Data from Selected European Cities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Sampling Sites and Experimental Data
2.3. Exposure Dose Model 2 (ExDoM2)
2.4. Input Data
3. Results and Discussion
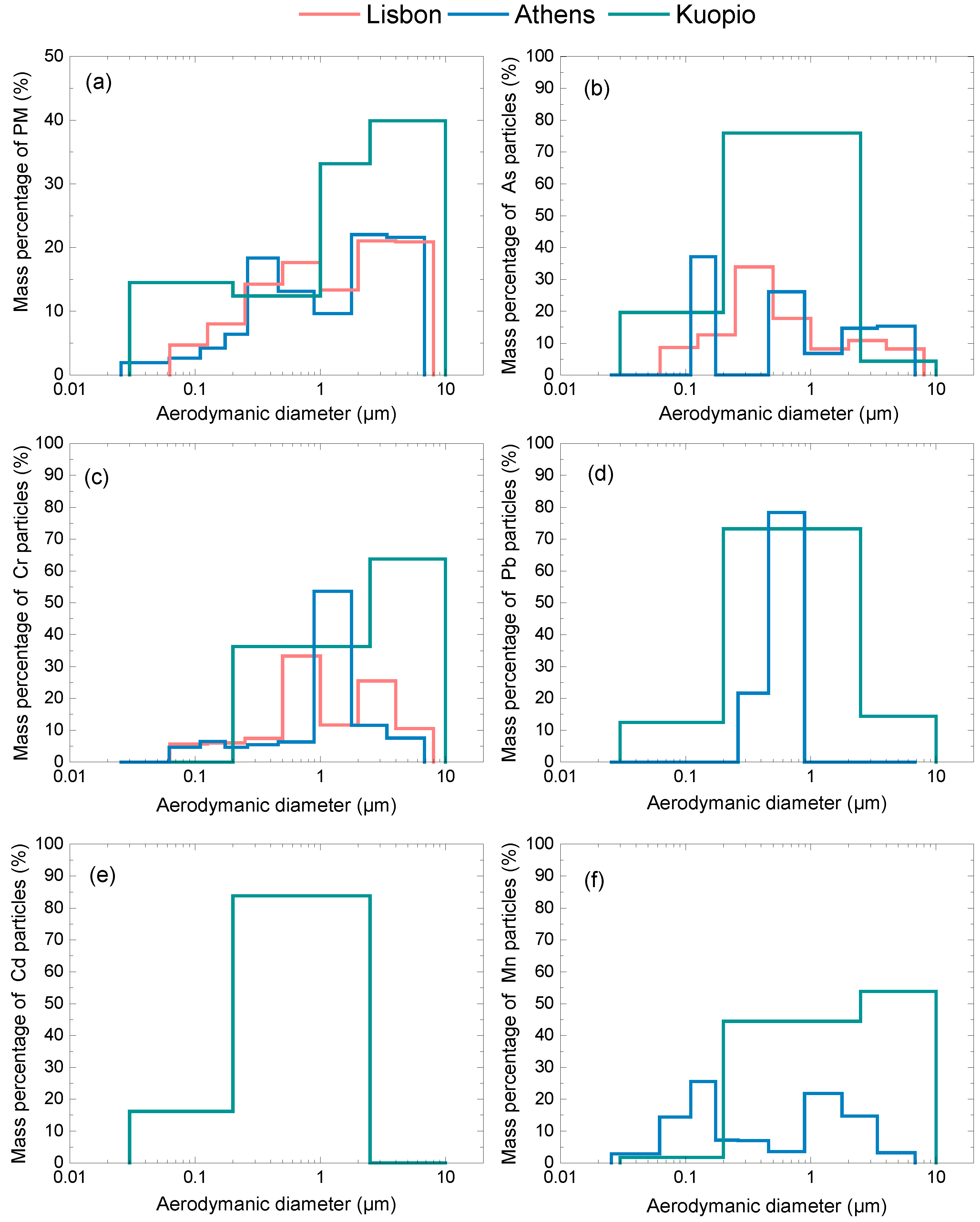
3.1. Particulate Matter Concentration and Size Distribution Data
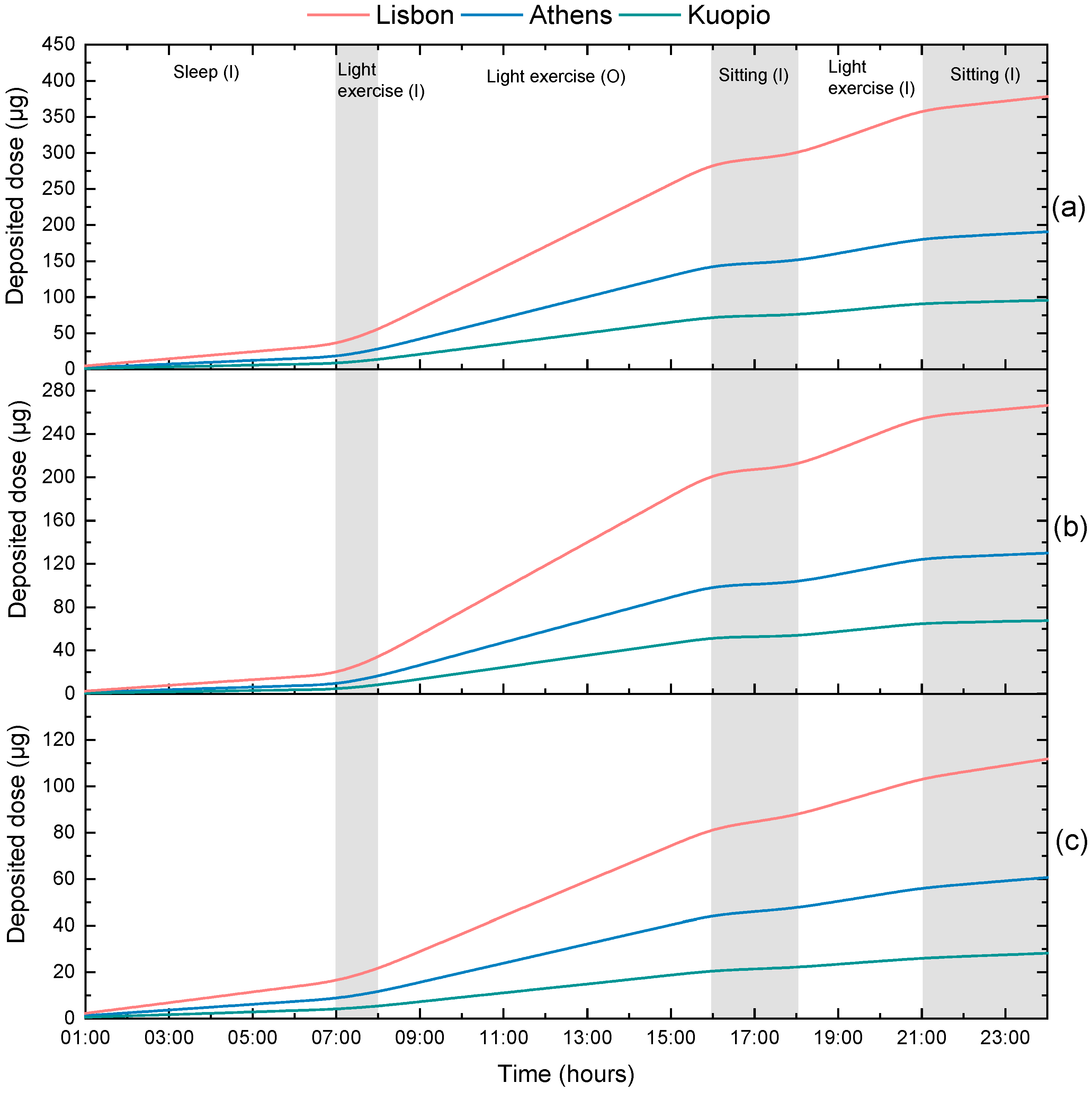
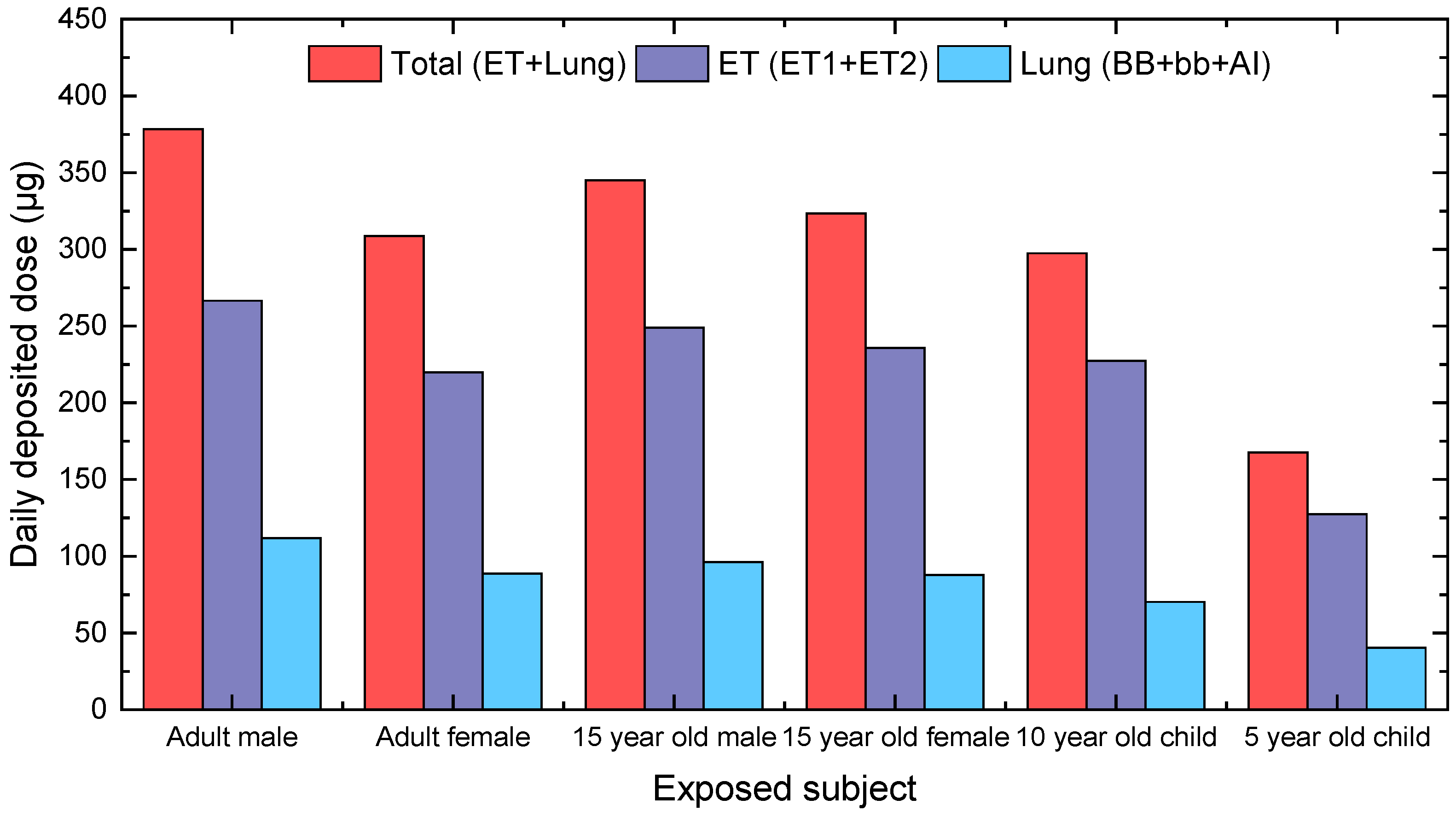
3.2. Calculation of Personal Deposited Dose and Internal Dose of Airborne Particles
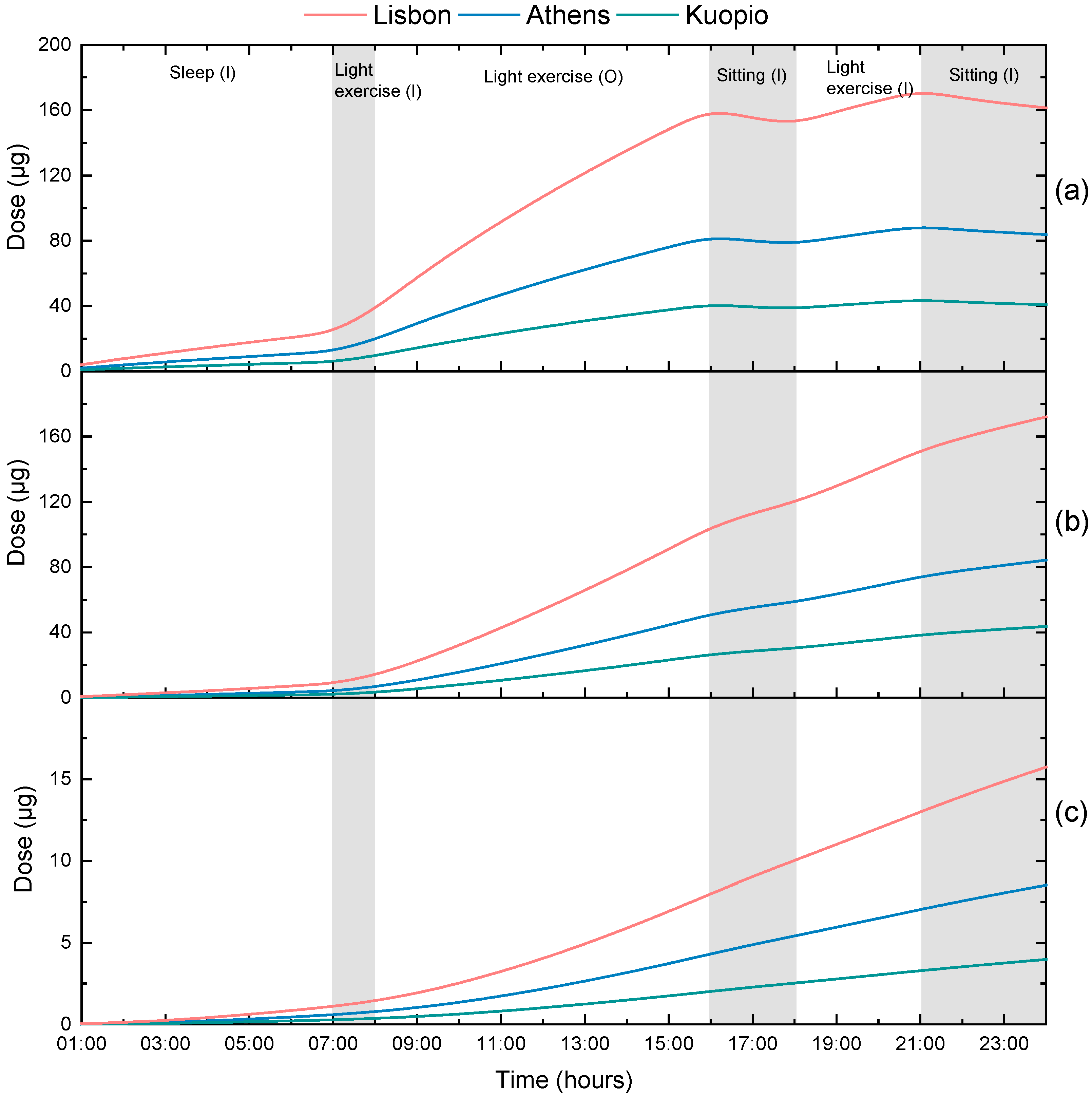
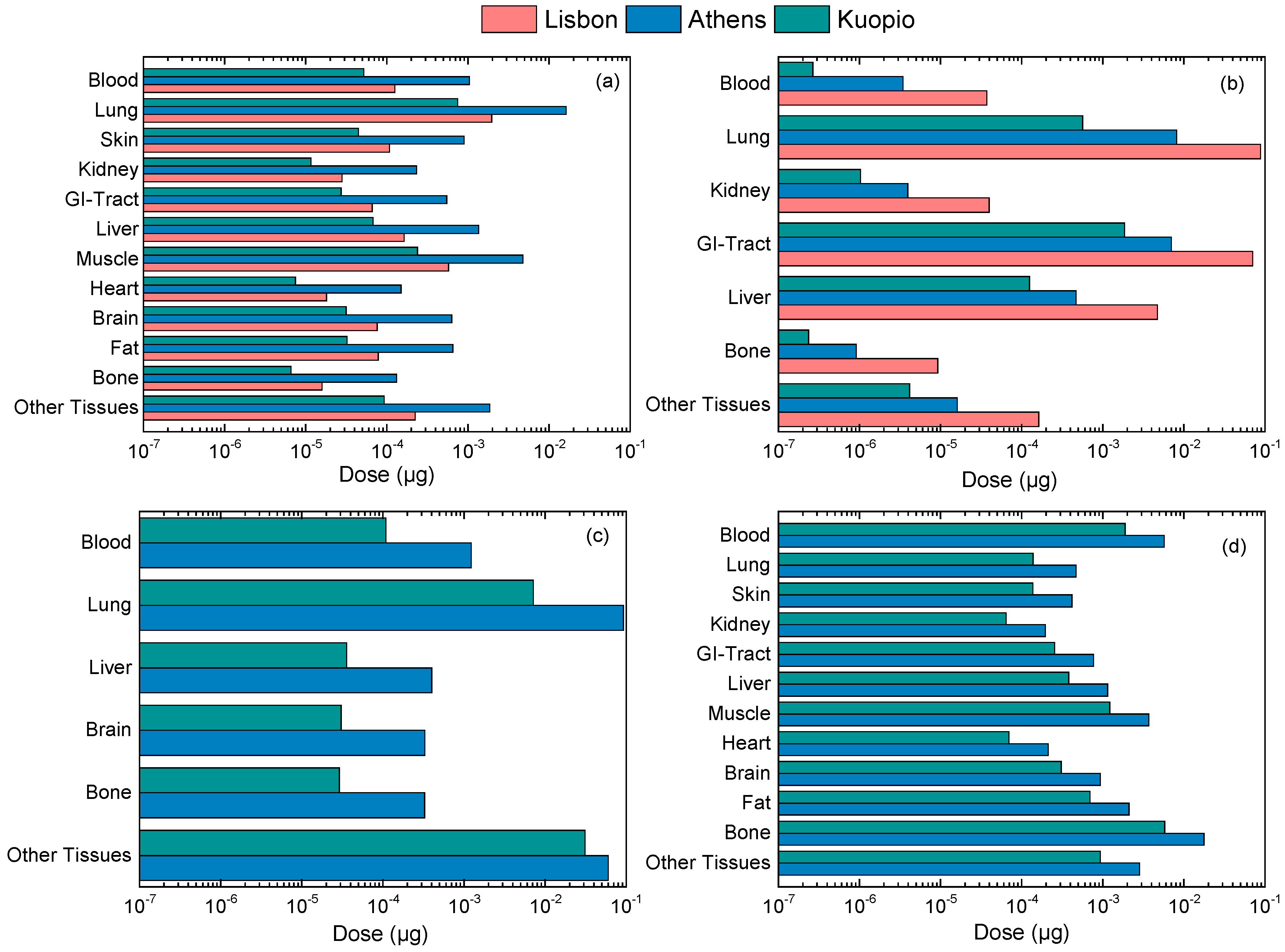
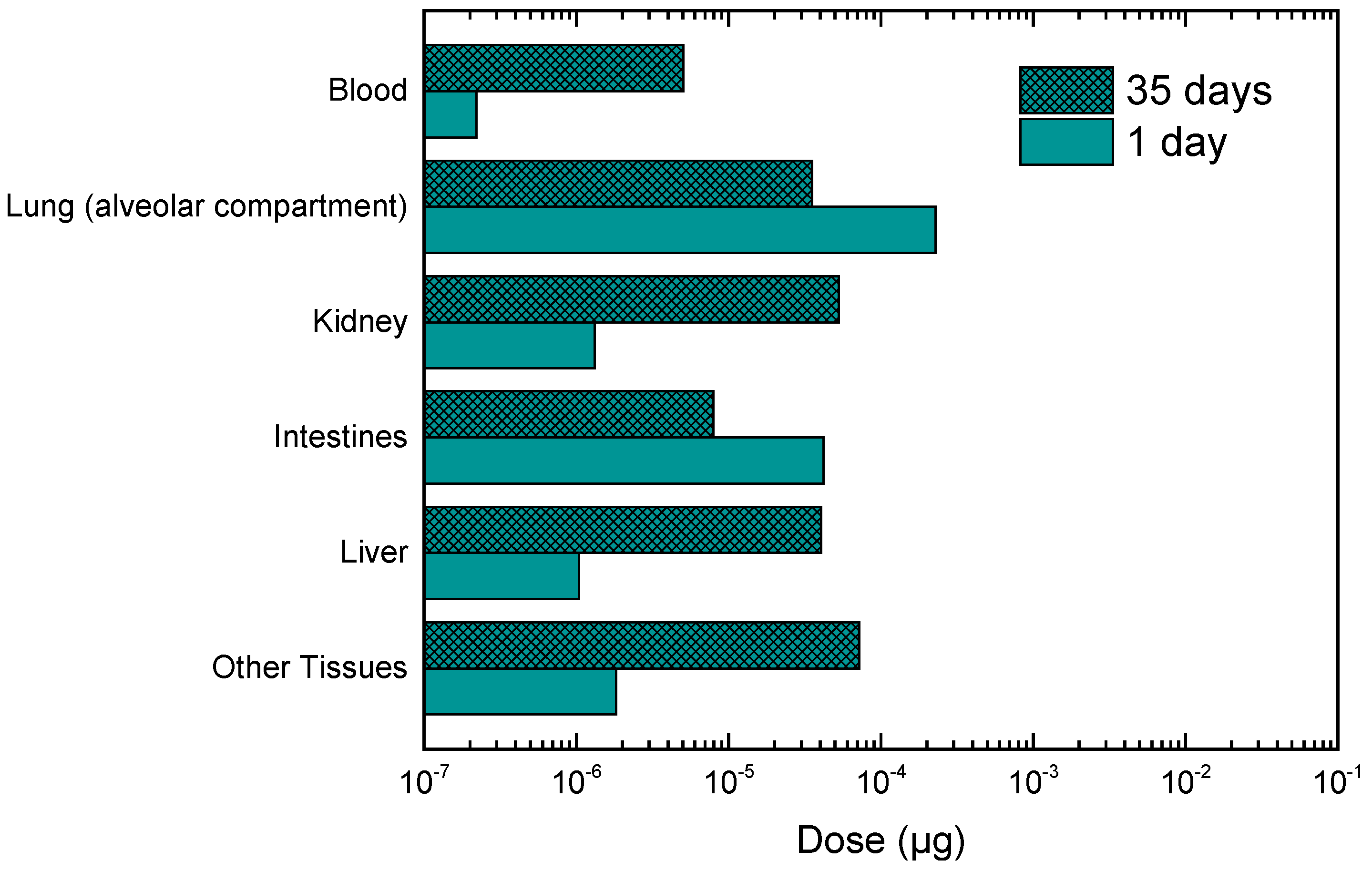
3.3. Calculation of Personal Deposited Dose and Internal Dose of Particle-Bound Metals
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alvarez, F.F.; Rodriguez, M.T.; Espinosa, A.J.F.; Daban, A.G. Physical speciation of arsenic, mercury, lead, cadmium and nickel in inhalable atmospheric particles. Anal. Chim. Acta 2004, 524, 33–40. [Google Scholar] [CrossRef]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Samet, J.M.; Dominici, F.; Curriero, F.C.; Coursac, I.; Zeger, S.L. Fine particulate air pollution and mortality in 20 U.S. Cities, 1987–1994. N. Engl. J. Med. 2000, 343, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Gu, S.; Chen, J.; Wu, X.; Yu, J. Assessment of health and economic effects by PM2.5 pollution in Beijing: A combined exposure–response and computable general equilibrium analysis. Environ. Technol. 2016, 37, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- WHO. Ambient (Outdoor) Air Quality and Health. 2016. Available online: http://www.who.int/mediacentre/factsheets/fs313/en/ (accessed on 3 June 2018).
- US EPA. Air Quality Criteria for Particulate Matter. EPA Report No. EPA/600/P-95/001Af; 1996. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=2832 (accessed on 3 June 2018).
- Kelly, F.J.; Fussell, J.C. Air pollution and public health: Emerging hazards and improved understanding of risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.A. An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Pope, C.A. Acute respiratory effects of particulate air-pollution. Annu. Rev. Public Health 1994, 15, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A. Respiratory hospital admission associated with PM10 pollution in Utah, Salt Lake, and Cache Valleys. Arch. Environ. Health 1991, 46, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Bates, D.V.; Raizenne, M.E. Health effects of particulate air pollution: Time for reassessment? Environ. Health Perspect. 1995, 103, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., III; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Adar, S.D.; Filigrana, P.A.; Clements, N.; Peel, J.L. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Curr. Environ. Health Rep. 2014, 1, 258–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buonanno, G.; Stabile, L.; Morawska, L. Personal exposure to ultrafine particles: The influence of time-activity patterns. Sci. Total Environ. 2014, 468–469, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US Counties, 1999–2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Kroll, A.; Gietl, J.K.; Wiesmüller, G.A.; Günsel, A.; Wohlleben, W.; Schnekenburger, J.; Klemm, O. In vitro toxicology of ambient particulate matter: Correlation of cellular effects with particle size and components. Environ. Toxicol. 2013, 28, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Kloog, I.; Ridgway, B.; Koutrakis, P.; Coull, B.A.; Schwartz, J.D. Long- and short-term exposure to PM2.5 and mortality: Using novel exposure models. Epidemiology 2013, 24, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Samek, L. Overal human mortality and morbidity due to exposure to air pollution. IJOMEH 2016, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, 69–74. [Google Scholar]
- IARC. Outdoor Air Pollution a Leading Environmental Cause of Cancer Deaths. 2013. Available online: https://www.iarc.fr/en/media-centre/iarcnews/pdf/pr221_E.pdf (accessed on 3 June 2018).
- IARC. Outdoor Air Pollution. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2016; Volume 109, Available online: http://monographs.iarc.fr/ENG/Monographs/vol109/index.php (accessed 3 June 2018).
- Behera, S.N.; Betha, R.; Huang, X.; Balasubramanian, R. Characterization and estimation of human airway deposition of size-resolved particulate-bound trace elements during a recent haze episode in Southeast Asia. Environ. Sci. Pollut. Res. 2015, 22, 4265–4280. [Google Scholar] [CrossRef] [PubMed]
- Costa, X.; Dreher, X. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environ. Health Perspect. 1997, 105, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Molinelli, A.R.; Madden, M.C.; McGee, J.K.; Stonehuerner, J.G.; Ghio, A.J. Effect of metal removal on the toxicity of airborne particulate matter from the Utah valley. Inhal. Toxicol. 2002, 14, 1069–1086. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaio, P.D.; Magli, E.; Caliendo, G.; Corvino, A.; Fiorino, F.; Frecentese, F.; Saccone, I.; Santagada, V.; Severino, B.; Onorati, G.; et al. Heavy Metals Size Distribution in PM10 and Environmental-Sanitary Risk Analysisin Acerra (Italy). Atmosphere 2018, 9, 58. [Google Scholar] [CrossRef]
- Ruzer, L.S.; Harley, N.H. Aerosols Handbook: Measurement, Dosimetry and Health Effects, 2nd ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2013; p. 666. ISBN 9781439855102. [Google Scholar]
- ATSDR. Toxicological Profile for Lead; Agency for Toxic Substance and Disease Registry, Division of Toxicology and Environmental Medicine/Applied Toxicology Branch: Atlanta, Georgia, 2007. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 3 June 2018).
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Ambient Air Pollution by As, Cd and Ni Compounds-Position Paper; Office for Official Publications of the European Communities: Luxembourg, 2001; Available online: http://ec.europa.eu/environment/air/pdf/pp_as_cd_ni.pdf (accessed on 3 June 2018).
- IPCS. Cadmium. In Environmental Health Criteria 134; WHO: Geneva, Switzerland, 1992; Available online: http://www.inchem.org/documents/ehc/ehc/ehc134.htm (accessed on 3 June 2018).
- WHO. Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications, Regional Office for Europe: Copenhagen, Denmark, 2000; Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/74732/E71922.pdf (accessed on 13 June 2018).
- ATSDR. Toxicological Profile for Manganese; Agency for Toxic Substance and Disease Registry, Division of Toxicology and Environmental Medicine/Applied Toxicology Branch: Atlanta, Georgia, 2012. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp151.pdf (accessed on 3 June 2018).
- Bi, X.; Sheng, G.; Peng, P.; Chen, Y.; Fu, J. Size distribution of n-alkanes and polycyclic aromatic hydrocarbons (PAHs) in urban and rural atmospheres of Guangzhou, China. Atmos. Environ. 2005, 39, 477–487. [Google Scholar] [CrossRef]
- Hussain, M.; Madl, P.; Khan, A. Lung deposition predictions of airborne particles and the emergence of contemporary diseases Part-I. Health 2011, 2, 51–59. [Google Scholar]
- Oberdorster, G. Effects and Fate of Inhaled Ultrafine Particles. In Nanotechnologyand the Environment; ACS Symposium Series, Chapter 7; American Chemical Society Publications: Washington, DC, USA, 2004; Volume 890, pp. 37–59. [Google Scholar]
- Zwozdziak, A.; Gini, M.I.; Samek, L.; Rogula-Kozlowska, W.; Sowka, I.; Eleftheriadis, K. Implications of the aerosol size distribution modal structure of trace and major elements on human exposure, inhaled dose and relevance to the PM2.5 and PM10 metrics in a European pollution hotspot urban area. J. Aerosol Sci. 2017, 103, 38–52. [Google Scholar] [CrossRef]
- Chan, Y.C.; Vowles, P.D.; McTainsh, G.H.; Sompson, R.W.; Cohen, D.D.; Bailey, G.M.; McOrist, G.D. Characterization and source identification of PM10 aerosol samples collected with a high volume cascade impactor in Brisbane (Australia). Sci. Total Environ. 2000, 262, 5–19. [Google Scholar] [CrossRef]
- Griffin, D.W.; Garrison, V.H.; Herman, J.R.; Shinn, E.A. African desert dust in the Caribbean atmosphere: Microbiology and public health. Aerobiologia 2001, 17, 203–213. [Google Scholar] [CrossRef]
- Harrison, R.M.; Yin, J. Particulate matter in the atmosphere: Which particle properties are important for its effects on health? Sci. Total Environ. 2000, 249, 85–101. [Google Scholar] [CrossRef]
- Hetland, R.B.; Cassee, F.R.; Refsnes, M.; Schwarze, P.E.; Lag, M.; Boere, A.J.F.; Dybing, E. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicol. Vitr. 2004, 18, 203–212. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology: Properties, Behavior and Measurement of Airborne Particles, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1999; p. 504. ISBN 978-0-471-19410-1. [Google Scholar]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.A.; Volckens, J. Development of a Sampler to Estimate Regional Deposition of Aerosol in the Human Respiratory Tract. Ann. Occup. Hyg. 2013, 57, 1138–1147. [Google Scholar] [PubMed] [Green Version]
- Aleksandropoulou, V.; Lazaridis, M. Development and application of a model (ExDoM) for calculating the respiratory tract dose and retention of particles under variable exposure conditions. Air Qual. Atmos. Health 2013, 6, 13–26. [Google Scholar] [CrossRef]
- Chalvatzaki, E.; Lazaridism, M. Development and application of a dosimetry model (ExDoM2) for calculating internal dose of specific particle bound metals in the human body. Inhal. Toxicol. 2015, 27, 308–320. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Human Respiratory Tract Model for Radiological Protection. ICRP Publication 66. Ann. ICRP 1994, 24. Available online: http://www.icrp.org/publication.asp?id=icrp%20publication%2066 (accessed on 3 June 2018).
- ICRP. Occupational Intakes of Radionuclides: Part 1. ICRP Publication 130. Ann. ICRP 2015, 44. Available online: http://www.icrp.org/publication.asp?id=ICRP%20Publication%20130 (accessed on 3 June 2018).
- Mitsakou, C.; Helmis, C.; Housiadas, C. Eulerianmodeling of lung deposition with sectional representation of aerosol dynamics. J. Aerosol Sci. 2005, 36, 75–94. [Google Scholar] [CrossRef]
- RIVM. Multiple Path Particle Dosimetry Model (MPPD v 1.0): A Model for Human and Rat Airway Particle Dosimetry; RIVA Report 650010030; RIVM: Bilthoven, The Netherlands, 2002.
- Salma, I.; Balashazy, I.; Winkler-Heil, R.; Hofmann, W.; Zaray, G. Effect of particle mass size distribution on the deposition of aerosols in the human respiratory tract. J. Aerosol Sci. 2002, 33, 119–132. [Google Scholar] [CrossRef]
- Manousakas, M.; Diapouli, E.; Papaefthymiou, H.; Kantarelou, V.; Zarkadas, C.; Kalogridis, A.C.; Karydas, A.G.; Eleftheriadis, K. XRF characterization and source apportionment of PM10 samples collected in a coastal city. X-Ray Spectrom. 2017, 47, 190–200. [Google Scholar] [CrossRef]
- Sippula, O.; Rintala, H.; Happo, M. Characterization of chemical and microbial species from size-segregated indoor and outdoor particulate samples. Aerosol Air Qual. Res. 2013, 13, 1212–1230. [Google Scholar] [CrossRef]
- Almeida, S.M.; Pio, C.A.; Freitas, M.C.; Reis, M.A.; Trancoso, M.A. Approaching PM2.5 and PM2.5–10 source apportionment by mass balance analysis, principal component analysis and particle size distribution. Sci. Total Environ. 2006, 368, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.M.; Freitas, M.C.; Reis, M.; Pinheiro, T.; Felix, P.M.; Pio, C.A. Fifteen years of nuclear techniques application to suspended particulate matter studies. J. Radioanal. Nucl. Chem. 2013, 297, 347–356. [Google Scholar] [CrossRef]
- ICRP. Dose Coefficients for Intake of Radionuclides by Workers. ICRP Publication 68. Ann. ICRP 1994, 24. Available online: http://www.icrp.org/publication.asp?id=ICRP%20Publication%2068 (accessed on 3 June 2018).
- ICRP. Occupational Intakes of Radionuclides: Part 3. ICRP Publication 137. Ann. ICRP 2017, 46. Available online: http://www.icrp.org/publication.asp?id=ICRP%20Publication%20137 (accessed on 3 June 2018).
- Chou, W.C.; Chio, C.O.; Liao, C.M. Assessing airborne PM-bound arsenic exposure risk in semiconductor manufacturing facilities. J. Hazard. Mater. 2009, 167, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.M.; Lin, T.L.; Chen, S.C. A Weibull-PBPK model for assessing risk of arsenic related children skin lesions. Sci. Total Environ. 2008, 392, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C.; Chou, W.C.; Chen, W.Y.; Liao, C.M. Assessing the cancer risk associated with arsenic-contaminated seafood. J. Hazard. Mater. 2010, 181, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.M.; Shen, H.H.; Chen, C.L.; Hsu, L.I.; Lin, T.L.; Chen, S.C.; Chen, C.J. Risk assessment of arsenic-induced internal cancer at long-term low dose exposure. J. Hazard. Mater. 2009, 165, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Plowman, S.A.; Smith, D.L. Exercise Physiology for Health, Fitness and Performance, 3rd ed.; Baltimore: Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; p. 750. ISBN 978-0-7817-7976-0. [Google Scholar]
- ICRP. Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values. ICRP Publication 89. Ann. ICRP 2003, 32. Available online: http://www.icrp.org/publication.asp?id=icrp%20publication%2089 (accessed on 3 June 2018).
- Lenz, T.L. Pharmacokinetic drug interactions with physical activity. Pharmacy review. Am. J. Lifestyle Med. 2010, 4, 226–229. [Google Scholar] [CrossRef]
- O’Flaherty, E.J.; Kerger, B.D.; Hays, S.M.; Paustenbach, D.J. A physiologically based model for the ingestion of chromium (III) and Chromium (VI) by humans. Toxicol. Sci. 2001, 60, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Pitz, M.; Cyrys, J.; Karg, E.; Wiedensohler, A.; Wichmann, H.E.; Heinrich, J. Variability of Apparent Particle Density of an Urban Aerosol. Environ. Sci. Technol. 2003, 37, 4336–4342. [Google Scholar] [CrossRef] [PubMed]
- DeCarlo, P.F.; Slowik, J.G.; Worsnop, D.R.; Davidovits, P.; Jimenez, J.L. Particle morphology and density characterization by combined mobility and aerodynamic diameter measurements. Part 1: Theory. Aerosol Sci. Technol. 2004, 38, 1185–1205. [Google Scholar] [CrossRef]
- Geller, M.; Biswas, S.; Sioutas, C. Determination of Particle Effective Density in Urban Environments with a Differential Mobility Analyzer and Aerosol Particle Mass Analyzer. Aerosol Sci. Technol. 2006, 40, 709–723. [Google Scholar] [CrossRef] [Green Version]
- Malloy, Q.G.J.; Nakao, S.; Qi, L.; Austin, R.; Stothers, C.; Hagino, H.; Cocker, D.R., III. Real-time aerosol density determination utilizing a modified scanning mobility particle size aerosol particle mass analyzer system. Aerosol Sci. Technol. 2009, 43, 673–678. [Google Scholar] [CrossRef]
- Morawska, L.; Salthammer, T. Indoor Environment: Airborne Particles and Settled Dust; Wiley-VCH: Weinheim, Germany, 2003; p. 450. [Google Scholar]
- Aleksandropoulou, V. Development of an Integrated Decision Support Tool for Estimation of Human Exposure to Airborne Particulate Matter and their Effect in Human Health. Ph.D. Thesis, Technical University of Crete, Chania, Greece, 2010. (In Greek). Available online: http://dias.library.tuc.gr/view/16655 (accessed on 3 June 2018).
- Lazaridis, M.; Aleksandropoulou, V.; Smolík, J.; Hansen, J.E.; Glytsos, T.; Kalogerakis, N.; Dahlin, E. Physico-chemical characterization of indoor/ outdoor particulate matter in two residential houses in Oslo, Norway: Measurements overview and physical properties—URBAN-AEROSOL Project. Indoor Air 2006, 16, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Zereini, F.; Alt, F.; Messerschmidt, J.; Wiseman, C.; Feldmann, I.; von Bohlen, A.; Müller, J.; Liebl, K.; Püttmann, W. Concentration and distribution of heavy metals in urban airborne particulate matter in Frankfurt am Main, Germany. Environ. Sci. Technol. 2005, 39, 2983–2989. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W.; Kozielska, B.; Klejnowski, K. Hazardous compounds in urban PM in the central part of upper Silesia (Poland) in Winter. Arch. Environ. Prot. 2013, 39, 53–65. [Google Scholar] [CrossRef]
- Widziewicz, K.; Rogula-kozlowska, W. Urban environment as a factor modulating metals deposition in the respiratory track and associated cancer risk. Atmos. Pollut. Res. 2017. [Google Scholar] [CrossRef]
- Canepari, S.; Perrino, C.; Olivieri, F.; Astolfi, M.L. Characterisation of the traffic sources of PM through size-segregated sampling, sequential leaching and ICP analysis. Atmos. Environ. 2008, 42, 8161–8175. [Google Scholar] [CrossRef]
- Eleftheriadis, K.; Oschenkuhn, K.; Lymperopoulou, T.; Karanasiou, A.; Razos, P.; Ochsenkuhn-Petropoulou, M. Influence of local andregional sources on the observed spatial and temporal variability of, size resolved atmospheric aerosol mass concentrations and water-solublespecies in the Athens metropolitan area. Atmos. Environ. 2014, 97, 252–261. [Google Scholar] [CrossRef]
- Grigoratos, T.; Martini, G. Brake wear particle emissions: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 2491–2504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahlin, P.; Berkowicz, R.; Palmgren, F. Characterisation of traffic-generated particulate matter in Copenhagen. Atmos Environ. 2006, 40, 2151–2159. [Google Scholar] [CrossRef]
- Garg, B.D.; Cadle, S.H.; Mulawa, P.A. Brake wear particulate matter emissions. Environ. Sci. Technol. 2000, 34, 4463–4469. [Google Scholar] [CrossRef]
- Iijima, A.; Sato, K.; Yano, K.; Kato, M.; Tago, H.; Kato, M.; Kimura, H.; Furuta, N. Particle size and composition distribution analysis of automotive brake abrasion dusts for the evaluation of antimony sources of airborne particulate matter. Atmos. Environ. 2007, 4, 4908–4919. [Google Scholar] [CrossRef]
- Davis, E.B. Trends in Environmental Research; Nova Science Publishers, Inc.: New York, NY, USA, 2006; ISBN 1-59454-485-9. [Google Scholar]
- Chalvatzaki, E.; Aleksandropoulou, V.; Glytsos, T.; Lazaridis, M. The effect of the dust emissions from open storage piles to particle ambient concentration and human exposure. Waste Manag. 2012, 32, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Chalvatzaki, E.; Kopanakis, I.; Lazaridis, M. Size distribution and metal composition of airborne particles in a waste management facility. J. Mater. Cycles Waste Manag. 2017, 20, 323–335. [Google Scholar] [CrossRef]
- Voutilainen, A.; Kaipio, J.P.; Pekkanen, J.; Timonen, K.L.; Ruuskanen, J. Theoretical analysis of the influence of aerosol size distribution and physical activity on particle deposition pattern in human lungs. J. Work Environ. Health 2004, 30, 73–79. [Google Scholar]
- Ferguson, M.D.; Migliaccio, C.; Ward, T. Comparison of how ambient PMc and PM2.5 influence the inflammatory potential. Inhal. Toxicol. 2013, 25, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Mundandhara, S.; Devlin, R.B.; Madden, M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol. Appl. Pharmacol. 2005, 207, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.W. Principles and Methods of Toxicology, 5th ed.; Informa Healthcare: New York, NY, USA, 2007; p. 748. ISBN 9781420005424. [Google Scholar]
- Kelly, J.T.; Avise, J.; Cai, C.; Kaduwela, A.P. Simulating particle size distributions over California and impactor on lung deposition fraction. Aerosol Sci. Technol. 2011, 45, 148–162. [Google Scholar] [CrossRef]
- Alexandropoulou, V.; Mitsakou, C.; Housiadas, C.; Lazaridis, M. Particulate Matter exposure and dose relationships derived from realistic exposure scenarios. Indoor Built Environ. 2008, 17, 237–246. [Google Scholar] [CrossRef]
- Neuberger, M.; Schimekc, M.G.; Horak, F., Jr.; Moshammerb, H.; Kundib, M.; Frischerd, T.; Gomisceka, B.; Puxbauma, H.; Hauck, H.; AUPHEP-Team. Acute effects of particulate matter on respiratory diseases, symptoms and functions: Epidemiological results of the Austrian Project on Health Effects of Particulate Matter (AUPHEP). Atmos. Environ. 2004, 38, 3971–3981. [Google Scholar] [CrossRef]
- Qiu, H.; Yu, I.T.; Tian, L.; Wang, X.; Tse, L.A.; Tam, W.; Wong, T.W. Effects of coarse particulate matter on emergency hospital admissions for respiratory diseases: A time-series analysis in Hong Kong. Environ. Health Perspect. 2012, 120, 572–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Health Aspects of Air Pollution with Particulate Matter, Ozone and Nitrogen Dioxide. In Report on a WHO Working Group; WHO: Bonn, Germany, 2003; Available online: http://www.euro.who.int/__data/assets/pdf_file/0005/112199/E79097.pdf (accessed on 3 June 2018).
- Daniels, M.J.; Dominici, F.; Samet, J.M.; Zeger, S.L. Estimating particulate matter-mortality dose-response curves and threshold levels: An analysis of daily time-series for the 20 largest US cities. Am. J. Epidemiol. 2000, 152, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Bennett, W.D.; Zeman, K.L.; Kim, C. Variability of fine particle deposition in healthy adults: Effect of age and gender. Am. J. Respir. Crit. Care Med. 1996, 153, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Oravisjärvi, K.; Pietikäinen, M.; Ruuskanen, J.; Rautio, A.; Voutilainen, A.; Keiski, R.L. Effects of Physical Activity on the Deposition of Traffic-Related Particles into the Human Lungs in Silico. Sci. Total Environ. 2011, 409, 4511–4518. [Google Scholar] [CrossRef] [PubMed]
- Kjellstrom, T.; Nordberg, G.F. A kinetic model of cadmium metabolism in the human being. Environ. Res. 1978, 16, 248–269. [Google Scholar] [CrossRef]
- Mammi-Galani, E.; Chalvatzaki, E.; Lazaridis, M. Personal exposure and dose of inhaled ambient particulate matter bound metals in five European cities. Aerosol Air Qual. Res. 2016, 16, 1452–1463. [Google Scholar] [CrossRef]
- Saha, J.C.; Dikshit, A.K.; Bandyopadhyay, M.; Saha, K.C. A review of arsenic poisoning and its effects on human health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 281–313. [Google Scholar] [CrossRef]
- Falk, H.; Caldwell, G.G.; Ishak, K.G.; Thomas, L.B.; Popper, H. Arsenic-Related Hepatic Angiosarcoma. Am. J. Ind. Med. 1981, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Medical Management Guidelines for Arsenic Trioxide; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2014. Available online: https://www.atsdr.cdc.gov/MMG/MMG.asp?id=1200&tid=279 (accessed on 3 June 2018).
- WHO. Manganese and Its Compounds. Concise International Chemical Assessment Document 12. 1999. Available online: http://whqlibdoc.who.int/publications/1999/924153012X.pdf (accessed on 3 June 2018).
- O’Flaherty, E.J. Physiologically based models for bone seeking elements: IV Kinetics of lead disposition in humans. Toxicol. Appl. Pharmacol. 1993, 118, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Maheshwari, M.; Morisawa, S. Dietary and inhalation intake of lead and estimation of blood lead levels in adults and children in Kanpur, India. Risk Anal. 2005, 25, 1573–1588. [Google Scholar] [CrossRef] [PubMed]
- IPCS. Inorganic Lead. World Health Organization, International Programme on Chemical Safety. Environmental Health Criteria 165. Geneva. 1995. Available online: http://www.inchem.org/documents/ehc/ehc/ehc165.htm (accessed on 3 June 2018).
- Rabinowitz, M.B. Toxicokinetics of bone lead. Environ. Health Perspect. 1991, 91, 33–37. [Google Scholar] [CrossRef] [PubMed]
- WHO. Lead Poisoning and Health. 2016. Available online: http://www.who.int/mediacentre/factsheets/fs379/en/ (accessed on 3 June 2018).
- Sasso, A.F.; Isukapalli, S.S.; Georgopoulos, P.G. A generalized physiologically-based toxicokinetic modelling system for chemical mixtures containing metals. Theor. Biol. Med. Modell. 2010, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cadmium and Cadmium Compounds. World Health Organization, International Agency for Research on Cancer. 2007. Available online: http://www.inchem.org/documents/iarc/vol58/mono58-2.html (accessed on 3 June 2018).

| Sleep/Rest | Light Activity | |
|---|---|---|
| Cardiac output (L/h) | 390 | 632 |
| (%CO) | ||
| Skin | 5 | 12.5 |
| Kidney | 19 | 8.6 |
| GI tract | 19 | 8.4 |
| Liver | 6.5 (hepatic artery) + 19 (portal vein) | 2.9 (hepatic artery) + 8.4 (portal vein) |
| Muscle | 17 | 40 |
| Heart | 4 | 4 |
| Brain | 12 | 7.4 |
| Fat | 5 | 12.5 |
| Bronchial tissues | 2.5 | 2.5 |
| Bone | 1.5 | 0.9 |
| Other tissues |
| Activity | Time | Environment |
|---|---|---|
| Sleep | 12 p.m.–7 a.m. | Indoors |
| Light exercise | 7 a.m.–8 a.m. | Indoors |
| Work (light exercise) | 8 a.m.–4 p.m. | Outdoors |
| Sitting awake (rest) | 4 p.m.–6 p.m. | Indoors |
| Light exercise | 6 p.m.–9 p.m. | Indoors |
| Sitting awake (rest) | 9 p.m.–12 p.m. | Indoors |
| City | PMx | As | Cd | Cr | Mn | Pb |
|---|---|---|---|---|---|---|
| Athens (PM6.8) | 20 | 2.9 | - | 2.4 | 15.0 | 6.0 |
| Kuopio (PM10) | 10 | 0.2 | 0.1 | 0.3 | 4.2 | 1.6 |
| Lisbon (PM8) | 37 | 0.5 | - | 23.8 | - | - |
| City | As | Pb | Cr | Mn |
|---|---|---|---|---|
| Athens | 3.5 | 4.1 | 2.5 | 18 |
| Kuopio | 0.17 | 1.5 | 0.5 | 5.7 |
| Lisbon | 0.42 | - | 25 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalvatzaki, E.; Chatoutsidou, S.E.; Mammi-Galani, E.; Almeida, S.M.; Gini, M.I.; Eleftheriadis, K.; Diapouli, E.; Lazaridis, M. Estimation of the Personal Deposited Dose of Particulate Matter and Particle-Bound Metals Using Data from Selected European Cities. Atmosphere 2018, 9, 248. https://doi.org/10.3390/atmos9070248
Chalvatzaki E, Chatoutsidou SE, Mammi-Galani E, Almeida SM, Gini MI, Eleftheriadis K, Diapouli E, Lazaridis M. Estimation of the Personal Deposited Dose of Particulate Matter and Particle-Bound Metals Using Data from Selected European Cities. Atmosphere. 2018; 9(7):248. https://doi.org/10.3390/atmos9070248
Chicago/Turabian StyleChalvatzaki, Eleftheria, Sofia Eirini Chatoutsidou, Eleni Mammi-Galani, Susana Marta Almeida, Maria I. Gini, Konstantinos Eleftheriadis, Evangelia Diapouli, and Mihalis Lazaridis. 2018. "Estimation of the Personal Deposited Dose of Particulate Matter and Particle-Bound Metals Using Data from Selected European Cities" Atmosphere 9, no. 7: 248. https://doi.org/10.3390/atmos9070248
APA StyleChalvatzaki, E., Chatoutsidou, S. E., Mammi-Galani, E., Almeida, S. M., Gini, M. I., Eleftheriadis, K., Diapouli, E., & Lazaridis, M. (2018). Estimation of the Personal Deposited Dose of Particulate Matter and Particle-Bound Metals Using Data from Selected European Cities. Atmosphere, 9(7), 248. https://doi.org/10.3390/atmos9070248









