Air and Surface Purification Using Heterogeneous Photocatalysis: Enhanced Indoor Sanitisation Through W18O49 and ZnO Catalyst Systems
Abstract
1. Introduction
2. Photocatalytic System Design
2.1. Catalyst Characterisation
2.2. Photocatalytic Mechanism
2.3. Safety Assessment Methodology
3. Effectiveness and Safety of Low Concentrations of Hydrogen Peroxide
4. Enhanced Photocatalytic Performance and Safety of W18O49/ZnO Systems
Low-Level H2O2 for Indoor Air Sanitisation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACGIH | American Conference of Governmental Industrial Hygienists |
| CDC | Centers for Disease Control and Prevention |
| HEPA | High-Efficiency Particulate Air |
| H2O2 | Hydrogen Peroxide |
| PM2.5 | Particulate Matter with diameter ≤ 2.5 μm |
| ROS | Reactive Oxygen Species |
| OSHA | Occupational Safety and Health Administration |
| TiO2 | Titanium Dioxide |
| UV | Ultraviolet |
| TWA | Time-Weighted Average |
| WHO | World Health Organization |
| ZnO | Zinc Oxide |
| W18O49 | Non-stoichiometric Tungsten Oxide (Sub-oxide of WO3 used as a visible-light photocatalyst) |
References
- Assessment of Exposure to Indoor Air Pollutants; Jantunen, M., Jaakkola, J.J.K., Krzyżanowski, M., Eds.; WHO Regional Publications; World Health Organization Regional office for Europe: Copenhagen, Denmark, 1997; ISBN 978-92-890-1342-0. [Google Scholar]
- Carrazana, E.; Ruiz-Gil, T.; Fujiyoshi, S.; Tanaka, D.; Noda, J.; Maruyama, F.; Jorquera, M.A. Potential Airborne Human Pathogens: A Relevant Inhabitant in Built Environments but Not Considered in Indoor Air Quality Standards. Sci. Total Environ. 2023, 901, 165879. [Google Scholar] [CrossRef]
- Liao, H.; Lyon, C.J.; Ying, B.; Hu, T. Climate Change, Its Impact on Emerging Infectious Diseases and New Technologies to Combat the Challenge. Emerg. Microbes Infect. 2024, 13, 2356143. [Google Scholar] [CrossRef]
- Mora, C.; McKenzie, T.; Gaw, I.M.; Dean, J.M.; von Hammerstein, H.; Knudson, T.A.; Setter, R.O.; Smith, C.Z.; Webster, K.M.; Patz, J.A.; et al. Over Half of Known Human Pathogenic Diseases Can Be Aggravated by Climate Change. Nat. Clim. Change 2022, 12, 869–875. [Google Scholar] [CrossRef]
- Bruce, N.; Perez-Padilla, R.; Albalak, R. The Health Effects of Indoor Air Pollution Exposure in Developing Countries; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- NIOSH. Immediately Dangerous to Life or Health (IDLH) Value Profile: Bromine Trifluoride (CAS® No. 7787-71-5); Niemeier, R.T., Ed.; Publication No. 2020-123; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH): Cincinnati, OH, USA, 2020. [Google Scholar] [CrossRef]
- Bai, L.; He, Z.; Ni, S.; Chen, W.; Li, N.; Sun, S. Investigation of PM2.5 Absorbed with Heavy Metal Elements, Source Apportionment and Their Health Impacts in Residential Houses in the North-East Region of China. Sustain. Cities Soc. 2019, 51, 101690. [Google Scholar] [CrossRef]
- Abhijith, K.V.; Kukadia, V.; Kumar, P. Investigation of Air Pollution Mitigation Measures, Ventilation, and Indoor Air Quality at Three Schools in London. Atmos. Environ. 2022, 289, 119303. [Google Scholar] [CrossRef]
- Kumar, P.; Hama, S.; Abbass, R.A.; Nogueira, T.; Brand, V.S.; Wu, H.-W.; Abulude, F.O.; Adelodun, A.A.; Anand, P.; de Fatima Andrade, M.; et al. In-Kitchen Aerosol Exposure in Twelve Cities across the Globe. Environ. Int. 2022, 162, 107155. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.; Kumar, P.; Morawska, L. Indoor Air Quality Monitoring and Source Apportionment Using Low-Cost Sensors. Environ. Res. Commun. 2024, 6, 012001. [Google Scholar] [CrossRef]
- Shammi, M.; Rahman, M.M.; Tareq, S.M. Distribution of Bioaerosols in Association With Particulate Matter: A Review on Emerging Public Health Threat in Asian Megacities. Front. Environ. Sci. 2021, 9, 698215. [Google Scholar] [CrossRef]
- Yan, S.; Liu, C.; Hou, L.; Wang, B.; Zhang, Y. A New Filterless Indoor Air Purifier for Particulate Matter and Bioaerosol Based on Heterogeneous Condensation. Environ. Res. 2023, 218, 115034. [Google Scholar] [CrossRef]
- Gizaw, Z.; Gebrehiwot, M.; Yenew, C. High Bacterial Load of Indoor Air in Hospital Wards: The Case of University of Gondar Teaching Hospital, Northwest Ethiopia. Multidiscip. Respir. Med. 2016, 11, 24. [Google Scholar] [CrossRef]
- Ito, K.; Zhang, S. Willingness to Pay for Clean Air: Evidence from Air Purifier Markets in China. J. Political Econ. 2020, 128, 1627–1672. [Google Scholar] [CrossRef]
- Wong, N.H.; Huang, B. Comparative Study of the Indoor Air Quality of Naturally Ventilated and Air-Conditioned Bedrooms of Residential Buildings in Singapore. Build. Environ. 2004, 39, 1115–1123. [Google Scholar] [CrossRef]
- Jung, S.; Ahn, Y.; Lee, Y.; Lee, J. Removal Characteristics and Distribution of Indoor Tobacco Smoke Particles Using a Room Air Cleaner. Korean J. Chem. Eng. 2013, 30, 351–356. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium Dioxide (TiO2)-Based Photocatalyst Materials Activity Enhancement for Contaminants of Emerging Concern (CECs) Degradation: In the Light of Modification Strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Escobedo, S.; de Lasa, H. Photocatalysis for Air Treatment Processes: Current Technologies and Future Applications for the Removal of Organic Pollutants and Viruses. Catalysts 2020, 10, 966. [Google Scholar] [CrossRef]
- He, F.; Jeon, W.; Choi, W. Photocatalytic Air Purification Mimicking the Self-Cleaning Process of the Atmosphere. Nat Commun 2021, 12, 2528. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on Heterogeneous Photocatalytic Disinfection of Waterborne, Airborne, and Foodborne Viruses: Can We Win against Pathogenic Viruses? J. Colloid Interface Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Guo, C.; Yin, S.; Yan, M.; Kobayashi, M.; Kakihana, M.; Sato, T. Morphology-Controlled Synthesis of W18O49 Nanostructures and Their Near-Infrared Absorption Properties. Inorg. Chem. 2012, 51, 4763–4771. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible Light Driven Type II Heterostructures and Their Enhanced Photocatalysis Properties: A Review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Aliannezhadi, M.; Doost Mohamadi, F.; Jamali, M.; Shariatmadar Tehrani, F. Innovative ZnO-W18O49 Nanocomposites and ZnWO4 Nanostructures for Water Treatment. Sci Rep 2025, 15, 9224. [Google Scholar] [CrossRef]
- Rawal, S.B.; Bera, S.; Lee, W.I. Visible-Light Photocatalytic Properties of W18O49/TiO2 and WO3/TiO2 Heterocomposites. Catal. Lett. 2012, 142, 1482–1488. [Google Scholar] [CrossRef]
- Navalón, S.; Ong, W.-J.; Duan, X. Sustainable Catalytic Processes Driven by Graphene-Based Materials. Processes 2020, 8, 672. [Google Scholar] [CrossRef]
- Rabiee, N.; Iravani, S.; Varma, R.S. Biowaste-Derived Carbon Dots: A Perspective on Biomedical Potentials. Molecules 2022, 27, 6186. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Wang, H.; Chen, R.; Chen, L.; Li, K.; Dong, F. Advances and Challenges of Photocatalytic Technology for Air Purification. Natl. Sci. Open 2022, 1, 20220025. [Google Scholar] [CrossRef]
- Chang, X.; Sun, S.; Dong, L.; Yin, Y. Efficient Synthesis of Ag/AgCl/W18O49 Nanorods and Their Antibacterial Activities. Mater. Lett. 2012, 83, 133–135. [Google Scholar] [CrossRef]
- Tao, C.; Sun, G.; Tang, X.; Gan, Y.; Liang, G.; Wang, J.; Huang, Y. Bactericidal Efficacy of a Low Concentration of Vaporized Hydrogen Peroxide with Validation in a BSL-3 Laboratory. J. Hosp. Infect. 2022, 127, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy, A.M.; Neetoo, H.; Al-Asmari, F. Antimicrobial Activity of Hydrogen Peroxide for Application in Food Safety and COVID-19 Mitigation: An Updated Review. J. Food Prot. 2024, 87, 100306. [Google Scholar] [CrossRef]
- Amaeze, N.J.; Shareef, M.U.; Henriquez, F.L.; Williams, C.L.; Mackay, W.G. Influence of Delivery System on the Efficacy of Low Concentrations of Hydrogen Peroxide in the Disinfection of Common Healthcare-Associated Infection Pathogens. J. Hosp. Infect. 2020, 106, 189–195. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ciric, L.; Ren, G.; Edirisinghe, M. Comparative Study of the Antimicrobial Effects of Tungsten Nanoparticles and Tungsten Nanocomposite Fibres on Hospital Acquired Bacterial and Viral Pathogens. Nanomaterials 2020, 10, 1017. [Google Scholar] [CrossRef]
- Yuju, S.; Xiujuan, T.; Dongsheng, S.; Zhiruo, Z.; Meizhen, W. A Review of Tungsten Trioxide (WO3)-Based Materials for Antibiotics Removal via Photocatalysis. Ecotoxicol. Environ. Saf. 2023, 259, 114988. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Havill, N.L.; Otter, J.A.; McDonald, L.C.; Adams, N.M.T.; Cooper, T.; Thompson, A.; Wiggs, L.; Killgore, G.; Tauman, A.; et al. Impact of Hydrogen Peroxide Vapor Room Decontamination on Clostridium Difficile Environmental Contamination and Transmission in a Healthcare Setting. Infect. Control Hosp. Epidemiol. 2008, 29, 723–729. [Google Scholar] [CrossRef]
- Urushidani, M.; Kawayoshi, A.; Kotaki, T.; Saeki, K.; Mori, Y.; Kameoka, M. Inactivation of SARS-CoV-2 and Influenza A Virus by Dry Fogging Hypochlorous Acid Solution and Hydrogen Peroxide Solution. PLoS ONE 2022, 17, e0261802. [Google Scholar] [CrossRef]
- HYDROGEN PEROXIDE|Occupational Safety and Health Administration. Available online: https://www.osha.gov/chemicaldata/630 (accessed on 15 June 2025).
- WHO Guidelines for Indoor Air Quality: Selected Pollutants; Adamkiewicz, G., World Health Organization, Eds.; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2010; ISBN 978-92-890-0213-4. [Google Scholar]
- ACGIH. TLV/BEI Guidelines. Available online: https://www.acgih.org/science/tlv-bei-guidelines/ (accessed on 15 June 2025).
- Ernstgård, L.; Sjögren, B.; Johanson, G. Acute Effects of Exposure to Vapors of Hydrogen Peroxide in Humans. Toxicol. Lett. 2012, 212, 222–227. [Google Scholar] [CrossRef]
- Hydrogen Peroxide: Incident Management. Available online: https://assets.publishing.service.gov.uk/media/6748a2d675bb645366b3a1a2/Inident-management-hydrogen-peroxide.pdf (accessed on 15 June 2025).
- Sanguinet, J.; Edmiston, C. Evaluation of Dry Hydrogen Peroxide in Reducing Microbial Bioburden in a Healthcare Facility. Am. J. Infect. Control 2021, 49, 985–990. [Google Scholar] [CrossRef]
- Graham, D.; Lee, C.; Jordan, B.J. Dry Hydrogen Peroxide: One Molecule for a One Health Approach—A Narrative Review. J. Public Health Emerg. 2023, 7, 20. [Google Scholar] [CrossRef]
- Wright, D.; Christie, J.; Lawrence, J.; Vaughn, K.L.; Walsh, T.F. Effectiveness of Dry Hydrogen Peroxide in Reducing Air and Surface Bioburden in a Multicenter Clinical Setting. Infect. Control Hosp. Epidemiol. 2024, 45, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Watt, B.E.; Proudfoot, A.T.; Vale, J.A. Hydrogen Peroxide Poisoning. Toxicol. Rev. 2004, 23, 51–57. [Google Scholar] [CrossRef]
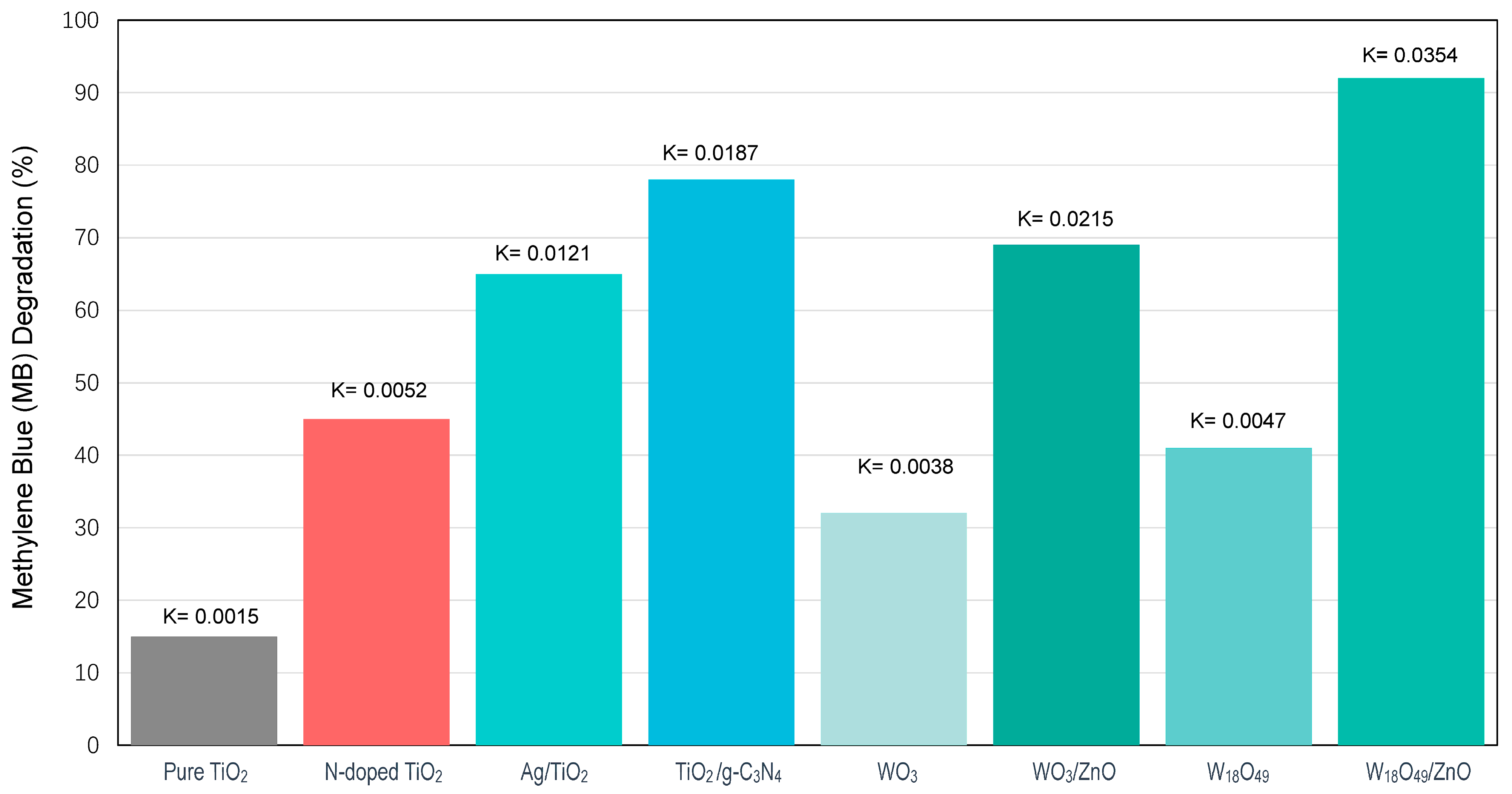
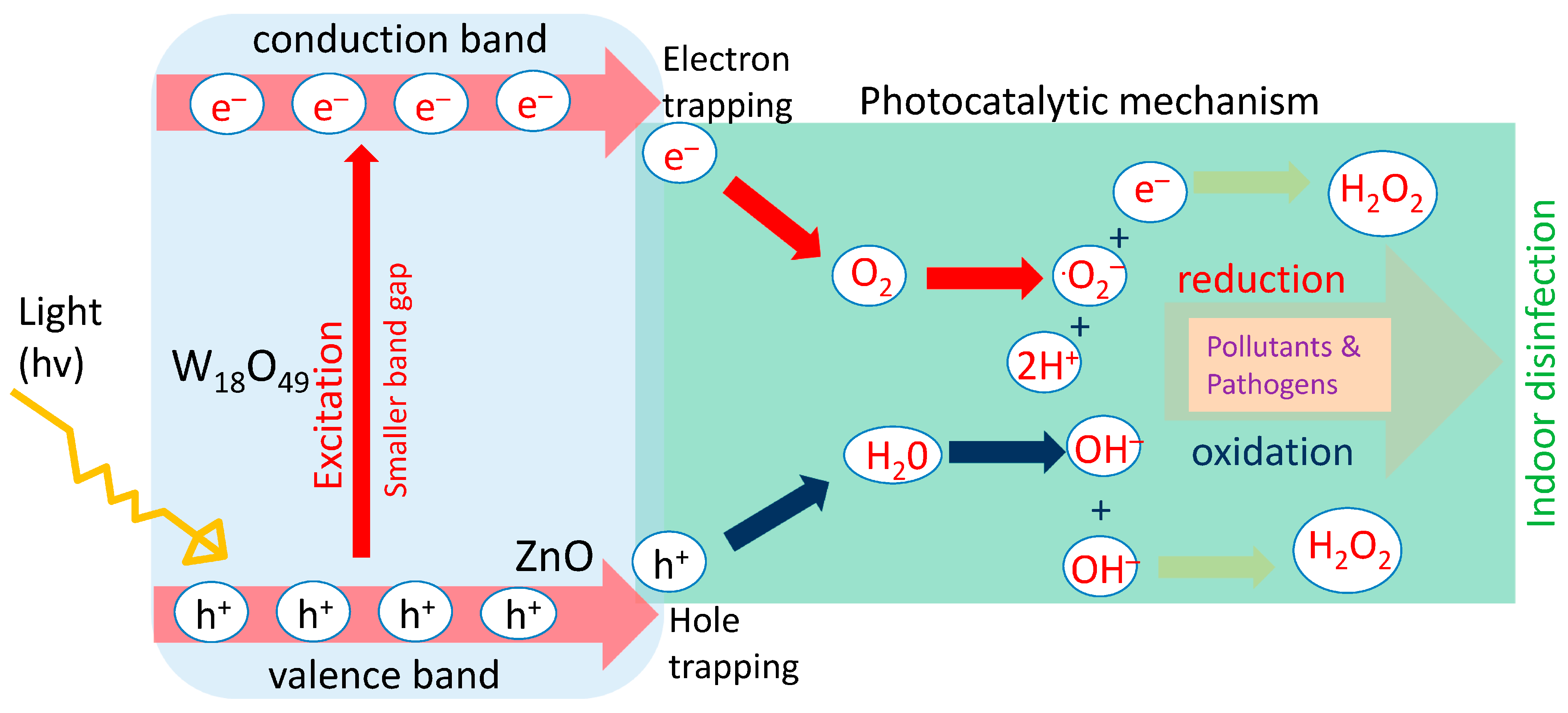
| Author (Year) | Focus Area | Key Finding | Implication | Exposure Duration | Light Condition |
|---|---|---|---|---|---|
| Sanguinet and Edmiston [43] | Air and Surface Disinfection | Dry H2O2 reduced microbial loads by >90% in clinical settings, including resistant organisms. | Supports effectiveness of 0.05 ppm H2O2 for continuous disinfection. | Continuous (multi-hour) | Not specified (ambient/room light assumed) |
| Wright et al. [45] | Surface Disinfection and Human Safety | Low-dose aerosol H2O2 significantly reduced S. aureus and C. difficile without health risks. | Demonstrates efficacy and safety of low-level H2O2. | Continuous (≥24 h/day for several weeks) | Not specified; ambient clinical lighting |
| Graham et al. [44] | Viral Inactivation | 98% reduction in SARS-CoV-2 titres within 2 h of H2O2 exposure. | Confirms antiviral potential of low-dose H2O2. | 2–4 h exposure per cycle | Not specified (ambient/room light assumed) |
| Rawal et al. [26] | Photocatalytic Performance (W18O49) | W18O49/TiO2 catalyst had >2× efficiency of WO3/TiO2 under ≥422 nm light. | W18O49 has superior visible-light activity for indoor use. | 2 h exposure | Visible-light (λ ≥ 422 nm) |
| Chang et al. [30] | ROS Generation and Bactericidal Activity | W18O49 hybrids generated abundant ROS under visible light. | Validates W18O49’s indoor bactericidal effectiveness. | 30 min irradiation + 24 h incubation | Full-spectrum light and Dark |
| Watt et al. [46] | Endogenous H2O2 Metabolism | Mitochondrial H2O2 is degraded by catalase and peroxidases. | Explains why low-dose H2O2 is safe in biological systems. | Minutes to hours (e.g., symptoms within minutes, pulmonary oedema up to 24–72 h post-exposure) | Not applicable (no light-based mechanism discussed) |
| Ernstgård et al. [41] | Inhalation Safety | H2O2 up to 0.5 ppm caused no lasting harm or inflammation. | Confirms indoor safety threshold for H2O2 exposure. | 30 min to 2 h | Visible light (λ ≥ 420 nm, 300 W Xe lamp) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez, P.; Paul, W.; Kumar, P. Air and Surface Purification Using Heterogeneous Photocatalysis: Enhanced Indoor Sanitisation Through W18O49 and ZnO Catalyst Systems. Atmosphere 2025, 16, 1108. https://doi.org/10.3390/atmos16091108
Fernandez P, Paul W, Kumar P. Air and Surface Purification Using Heterogeneous Photocatalysis: Enhanced Indoor Sanitisation Through W18O49 and ZnO Catalyst Systems. Atmosphere. 2025; 16(9):1108. https://doi.org/10.3390/atmos16091108
Chicago/Turabian StyleFernandez, Pablo, Wesley Paul, and Prashant Kumar. 2025. "Air and Surface Purification Using Heterogeneous Photocatalysis: Enhanced Indoor Sanitisation Through W18O49 and ZnO Catalyst Systems" Atmosphere 16, no. 9: 1108. https://doi.org/10.3390/atmos16091108
APA StyleFernandez, P., Paul, W., & Kumar, P. (2025). Air and Surface Purification Using Heterogeneous Photocatalysis: Enhanced Indoor Sanitisation Through W18O49 and ZnO Catalyst Systems. Atmosphere, 16(9), 1108. https://doi.org/10.3390/atmos16091108








