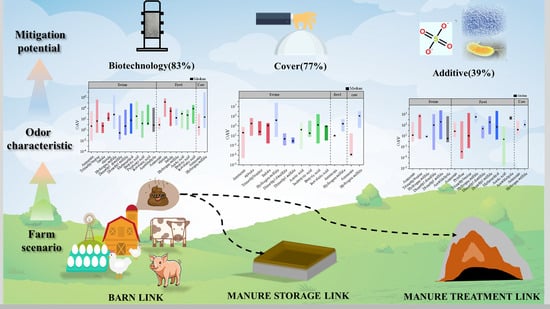
The Characteristics of Key Odorants from Livestock Farms and Their Mitigation Potential: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature and Search
2.2. Study Selection Criteria
2.3. Data Collection
2.4. Calculation of Odor Characteristics and Odor Activity Value (OAV) in Farm
2.5. Meta-Analysis
2.6. Gas Classification and Definition of Categories
2.7. Relative Importance Analysis
3. Results and Discussion
3.1. Odor Emission and OAV Characteristics in Livestock Farms
3.2. Odor Emission and OAV Characteristics of Different Breeds and Links
3.3. Factors Driving Changes in OAV During Manure Storage and Treatment Link
3.4. Mitigation of Different Odor Control Technologies
3.5. Different Odor Control Technologies for Different Breeds
3.6. Limitations and Future Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/ (accessed on 20 January 2023).
- Hu, Y.; Cheng, H.; Tao, S. Environmental and Human Health Challenges of Industrial Livestock and Poultry Farming in China and Their Mitigation. Environ. Int. 2017, 107, 111–130. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Letter on Printing and Distributing the Report on the Analysis of National Odor Pollution Complaints in 2018–2020. Available online: http://www.mee.gov.cn/xxgk2018/xxgk/sthjbsh/202108/t20210802_853623.html/ (accessed on 8 September 2021).
- Blanes-Vidal, V.; Suh, H.; Nadimi, E.S.; Løfstrøm, P.; Ellermann, T.; Andersen, H.V.; Schwartz, J. Residential Exposure to Outdoor Air Pollution from Livestock Operations and Perceived Annoyance among Citizens. Environ. Int. 2012, 40, 44–50. [Google Scholar] [CrossRef]
- Piccardo, M.T.; Geretto, M.; Pulliero, A.; Izzotti, A. Odor Emissions: A Public Health Concern for Health Risk Perception. Environ. Res. 2022, 204, 112121. [Google Scholar] [CrossRef]
- Settimo, G.; Avino, P. State-of-Art of the Legislation on Odour Emissions with a Focus on the Italian Studies. Environ. Pollut. 2024, 348, 123525. [Google Scholar] [CrossRef]
- Simões, M.; Janssen, N.; Heederik, D.J.J.; Smit, L.A.M.; Vermeulen, R.; Huss, A. Residential Proximity to Livestock Animals and Mortality from Respiratory Diseases in The Netherlands: A Prospective Census-Based Cohort Study. Environ. Int. 2022, 161, 107140. [Google Scholar] [CrossRef]
- Liao, X.; Wu, Y.; Wang, Y.; Blue, T.; Wang, J.; Mi, J. Research Progress on Comprehensive Treatments of Farm Odor. China Poult. 2019, 41, 1–8. [Google Scholar] [CrossRef]
- Akdeniz, N.; Jacobson, L.D.; Hetchler, B.P.; Bereznicki, S.D.; Heber, A.J.; Koziel, J.A.; Cai, L.; Zhang, S.; Parker, D.B. Odor and Odorous Chemical Emissions from Animal Buildings: Part 2. Odor Emissions. Trans. ASABE 2012, 55, 2335–2345. [Google Scholar] [CrossRef]
- Jo, S.-H.; Kim, K.-H.; Jeon, B.-H.; Lee, M.-H.; Kim, Y.-H.; Kim, B.-W.; Cho, S.-B.; Hwang, O.-H.; Bhattacharya, S.S. Odor Characterization from Barns and Slurry Treatment Facilities at a Commercial Swine Facility in South Korea. Atmos. Environ. 2015, 119, 339–347. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, P.; Zhao, L.; Meng, H.; Cheng, H. Component Analysis of Volatile Organic Compounds and Determination of Key Odor in Pig Manure Aerobic Fermentation Process. Chin. Soc. Agric. Eng. 2016, 32, 205–210. [Google Scholar]
- Cao, T.; Zheng, Y.; Dong, H. Control of Odor Emissions from Livestock Farms: A Review. Environ. Res. 2023, 225, 115545. [Google Scholar] [CrossRef]
- Konkol, D.; Popiela, E.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Moustakas, K.; Opaliński, S.; Korczyński, M.; Witek-Krowiak, A.; Chojnacka, K. Recent Innovations in Various Methods of Harmful Gases Conversion and Its Mechanism in Poultry Farms. Environ. Res. 2022, 214, 113825. [Google Scholar] [CrossRef]
- Adamu, G.; Hassim, H.A.; Kumar, P.; Sazili, A.Q.; Mohd Zainudin, M.H. Controlling Odour Emissions in Poultry Production through Dietary Interventions: Prospects and Challenges. World’s Poult. Sci. J. 2024, 80, 1101–1122. [Google Scholar] [CrossRef]
- Blanes-Vidal, V.; Hansen, M.N.; Adamsen, A.P.S.; Feilberg, A.; Petersen, S.O.; Jensen, B.B. Characterization of Odor Released during Handling of Swine Slurry: Part II. Effect of Production Type, Storage and Physicochemical Characteristics of the Slurry. Atmos. Environ. 2009, 43, 3006–3014. [Google Scholar] [CrossRef]
- Cong, Q.; Wang, Y.; Zhang, Y.; Yin, F.; Zhang, W.; Cao, T.; Dong, H. Effects of Self-Produced Lactic Fermentation (SPLF) on GHG and VSC Emissions during Swine Slurry Storage. Environ. Res. 2023, 231, 116240. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, Y.S.; Roh, Y.M.; Kim, C.N. Effect of Ventilation Rate on Gradient of Aerial Contaminants in the Confinement Pig Building. Environ. Res. 2007, 103, 352–357. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Bai, Z.; Chadwick, D.; Misselbrook, T.; Sommer, S.G.; Qin, W.; Ma, L. Mitigation of Ammonia, Nitrous Oxide and Methane Emissions during Solid Waste Composting with Different Additives: A Meta-Analysis. J. Clean. Prod. 2019, 235, 626–635. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, R.; Li, L.; Zheng, G.; Wang, J.; Wang, G.; Bao, Z.; Yin, Z.; Li, G.; Yuan, J. A Global Meta-Analysis of Greenhouse Gas Emissions and Carbon and Nitrogen Losses during Livestock Manure Composting: Influencing Factors and Mitigation Strategies. Sci. Total Environ. 2023, 885, 163900. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Han, M.-F.; Jia, T.-P.; Hu, X.-R.; Zhu, H.-Q.; Tong, Z.; Lin, Y.-T.; Wang, C.; Liu, D.-Z.; Peng, Y.-Z.; et al. Emissions, Measurement, and Control of Odor in Livestock Farms: A Review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef]
- Cui, Y.; Tian, Z.; Deng, D.; Lu, H.; Liu, Z.; Wang, G.; Ma, X. Effects of Citrus Extract on Intestinal Antioxidant Indices, Digestive Enzyme Activities, Ammonia Nitrogen Contents and Fecal Nitrogen, Phosphorus and Odor Contents of Finishing Pigs. Chin. J. Anim. Nutr. 2021, 33, 2585–2594. [Google Scholar]
- Clanton, C.; Schmidt, D.R.; Jacobson, L.D.; Nicolai, R.E.; Goodrich, P.R.; Janni, K.A. Swine Manure Storage Covers for Odor control. Appl. Eng. Agric. 1999, 15, 567–572. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Li, P.; Jenks, W.S. Mitigation of Air Pollutants by UV-A Photocatalysis in Livestock and Poultry Farming: A Mini-Review. Catalysts 2022, 12, 782. [Google Scholar] [CrossRef]
- Conti, C.; Guarino, M.; Bacenetti, J. Measurements Techniques and Models to Assess Odor Annoyance: A Review. Environ. Int. 2020, 134, 105261. [Google Scholar] [CrossRef]
- Hayes, J.E.; Barczak, R.J.; “Mel” Suffet, I.; Stuetz, R.M. The Use of Gas Chromatography Combined with Chemical and Sensory Analysis to Evaluate Nuisance Odours in the Air and Water Environment. Environ. Int. 2023, 180, 108214. [Google Scholar] [CrossRef]
- Zhai, Z.; Wang, G.; Li, W.; Meng, J.; Lu, F. Advances and Perspectives on Accurate Identification Technology for Key Odour Substances in Odor Pollution. Sci. Technol. Rev. 2022, 40, 35–41. [Google Scholar]
- Blazy, V.; de Guardia, A.; Benoist, J.C.; Daumoin, M.; Guiziou, F.; Lemasle, M.; Wolbert, D.; Barrington, S. Correlation of Chemical Composition and Odor Concentration for Emissions from Pig Slaughterhouse Sludge Composting and Storage. Chem. Eng. J. 2015, 276, 398–409. [Google Scholar] [CrossRef]
- Rincón, C.A.; De Guardia, A.; Couvert, A.; Le Roux, S.; Soutrel, I.; Daumoin, M.; Benoist, J.C. Chemical and Odor Characterization of Gas Emissions Released during Composting of Solid Wastes and Digestates. J. Environ. Manag. 2019, 233, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shao, L.; Kang, X.; Zhang, H.; Lü, F.; He, P. A Critical Review on Odor Measurement and Prediction. J. Environ. Manag. 2023, 336, 117651. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Li, S.; Zhang, X.; Zhu, F.; Li, P.; Sheng, R.; Chen, X.; Zhang, L.-M.; He, J.-Z.; Wei, W.; et al. Fertilizer Nitrogen Use Efficiency and Fates in Maize Cropping Systems across China: Field 15N Tracer Studies. Soil Tillage Res. 2020, 197, 104498. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Zhang, L.; Zhang, X.; Cao, Y.; Xiao, R.; Bai, Z.; Ma, L. Meta-Analysis Addressing the Potential of Antibiotic Resistance Gene Elimination through Aerobic Composting. Waste Manag. 2024, 182, 197–206. [Google Scholar] [CrossRef]
- Abdellah, Y.A.Y.; Shi, Z.-J.; Luo, Y.-S.; Hou, W.-T.; Yang, X.; Wang, R.-L. Effects of Different Additives and Aerobic Composting Factors on Heavy Metal Bioavailability Reduction and Compost Parameters: A Meta-Analysis. Environ. Pollut. 2022, 307, 119549. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.; Gurevitch, J.; Curtis, P. The Meta-Analysis of Response Ratios in Experimental Ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Lin, S.; Li, J.; Yan, X.; Pei, L.; Shang, X. Maternal Pesticide Exposure and Risk of Preterm Birth: A Systematic Review and Meta-Analysis. Environ. Int. 2023, 178, 108043. [Google Scholar] [CrossRef]
- Gao, G. Biotrickling Filter Degradation of Ammonia and Hydrogen Sulfide in Farm Odor; Taiyuan University of Technology: Shanxi, China, 2013. [Google Scholar]
- Nagata, Y.; Takeuchi, N. Measurement of Odor Threshold by Triangle Odor Bag Method. Odor Meas. Rev. 2003, 118, 118–127. [Google Scholar]
- Wan, X.; Li, J.; Xie, L.; Wei, Z.; Wu, J.; Tong, Y.W.; Wang, X.; He, Y.; Zhang, J. Machine Learning Framework for Intelligent Prediction of Compost Maturity towards Automation of Food Waste Composting System. Bioresour. Technol. 2022, 365, 128107. [Google Scholar] [CrossRef]
- Wang, W.; Du, W.; Han, Y.; Cao, B.; Li, W.; Tong, Y.; Dai, S.; Liu, B. Ammonia Emission Characteristics and Construction of an Emission Reduction System for Livestock and Poultry Farming in China. J. Agro-Environ. Sci. 2021, 40, 2305–2316. [Google Scholar]
- Wang, X.; Bai, Z.; Yao, Y.; Gao, B.; Chadwick, D.; Chen, Q.; Hu, C.; Ma, L. Composting with Negative Pressure Aeration for the Mitigation of Ammonia Emissions and Global Warming Potential. J. Clean. Prod. 2018, 195, 448–457. [Google Scholar] [CrossRef]
- Santawee, N.; Treesubsuntorn, C.; Thiravetyan, P. Using Modified Coir Pith–Glucose Syrup Beads Inoculated with Bacillus Thuringiensis as a Packing Material in Trimethylamine (Fishy Odor) Biofilter. Atmos. Pollut. Res. 2019, 10, 1312–1319. [Google Scholar] [CrossRef]
- Sintermann, J.; Schallhart, S.; Kajos, M.; Jocher, M.; Bracher, A.; Münger, A.; Johnson, D.; Neftel, A.; Ruuskanen, T. Trimethylamine Emissions in Animal Husbandry. Biogeosciences 2014, 11, 5073–5085. [Google Scholar] [CrossRef]
- Ma, Q.; Meng, N.; Li, Y.; Wang, J. Occurrence, Impacts, and Microbial Transformation of 3-Methylindole (Skatole): A Critical Review. J. Hazard. Mater. 2021, 416, 126181. [Google Scholar] [CrossRef]
- Turan, N.G.; Akdemir, A.; Ergun, O.N. Removal of Volatile Organic Compounds by Natural Materials during Composting of Poultry Litter. Bioresour. Technol. 2009, 100, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Li, C.; How, B.S.; Chin, B.L.F.; et al. A State-of-the-Art Review on Capture and Separation of Hazardous Hydrogen Sulfide (H2S): Recent Advances, Challenges and Outlook. Environ. Pollut. 2022, 314, 120219. [Google Scholar] [CrossRef]
- OSHA. Hydrogen Sulfide. Available online: https://www.osha.gov/hydrogen-sulfide/hazards (accessed on 4 September 2021).
- Arogo, J.; Zhang, R.H.; Riskowski, G.L.; Day, D.L. Hydrogen Sulfide Production from Stored Liquid Swine Manure: A Laboratory Study. Trans. ASAE Am. Soc. Agric. Eng. 2000, 43, 1241–1245. [Google Scholar] [CrossRef]
- Gautam, D.P.; Rahman, S.; Fortuna, A.-M.; Borhan, M.S.; Saini-Eidukat, B.; Bezbaruah, A.N. Characterization of Zinc Oxide Nanoparticle (nZnO) Alginate Beads in Reducing Gaseous Emission from Swine Manure. Environ. Technol. 2017, 38, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cao, Z.; Jing, B.; Chen, W.; Wen, X.; Han, M.; Wang, Y.; Liao, X.; Wu, Y.; Chen, T. The Production of Methyl Mercaptan Is the Main Odor Source of Chicken Manure Treated with a Vertical Aerobic Fermenter. Environ. Res. 2024, 260, 119634. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, F.; Cheng, J.; Xu, Z.; Zang, B.; Li, G.; Xie, X.; Luo, W. Emission of Volatile Sulphur Compounds during Swine Manure Composting: Source Identification, Odour Mitigation and Assessment. Waste Manag. 2022, 153, 129–137. [Google Scholar] [CrossRef]
- Jiao, L.; Li, Y.; Wei, H.; Chen, G. A Review on the Mechanism of Adding Iron and Iron Compounds to Control Volatile Sulfur Compounds during Anaerobic Digestion of Sludge. China Environ. Sci. 2023, 43, 3454–3463. [Google Scholar] [CrossRef]
- Zang, B.; Li, S.; Michel, F.C.; Li, G.; Zhang, D.; Li, Y. Control of Dimethyl Sulfide and Dimethyl Disulfide Odors during Pig Manure Composting Using Nitrogen Amendment. Bioresour. Technol. 2017, 224, 419–427. [Google Scholar] [CrossRef]
- Li, B.; Ho, S.S.H.; Li, X.; Guo, L.; Chen, A.; Hu, L.; Yang, Y.; Chen, D.; Lin, A.; Fang, X. A Comprehensive Review on Anthropogenic Volatile Organic Compounds (VOCs) Emission Estimates in China: Comparison and Outlook. Environ. Int. 2021, 156, 106710. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Kumar Awasthi, S.; Ren, X.; Liu, X.; Zhang, Z. Influence of Fine Coal Gasification Slag on Greenhouse Gases Emission and Volatile Fatty Acids during Pig Manure Composting. Bioresour. Technol. 2020, 316, 123915. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, X.; Cheng, S.; Lei, Y.; Sun, W.; Wang, K.; Li, Z. A Review of Volatile Fatty Acids Production from Organic Wastes: Intensification Techniques and Separation Methods. J. Environ. Manag. 2024, 360, 121062. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Zeng, M.; Xu, X. Research Progress of Fishy Odor in Aquatic Products: From Substance Identification, Formation Mechanism, to Elimination Pathway. Food Res. Int. 2024, 178, 113914. [Google Scholar] [CrossRef] [PubMed]
- Zgarbová, E.; Vrzal, R. Skatole: A Thin Red Line between Its Benefits and Toxicity. Biochimie 2023, 208, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Ma, R.; Wang, G.; Yuan, J. Effects of Reflux of Mature Compost on Sulfur Odor Emission during Pig Manure Composting. J. Agro-Environ. Sci. 2021, 40, 2456–2464. [Google Scholar]
- Parker, D.B.; Cai, L.; Kim, K.-H.; Hales, K.E.; Spiehs, M.J.; Woodbury, B.L.; Atkin, A.L.; Nickerson, K.W.; Patefield, K.D. Reducing Odorous VOC Emissions from Swine Manure Using Soybean Peroxidase and Peroxides. Bioresour. Technol. 2012, 124, 95–104. [Google Scholar] [CrossRef]
- Fornós, M.; Sanz Fernández, S.; Jiménez-Moreno, E.; Carrión, D.; Gasa, J.; Rodríguez-Estévez, V. The Feeding Behaviour Habits of Growing-Finishing Pigs and Its Effects on Growth Performance and Carcass Quality: A Review. Animals 2022, 12, 1128. [Google Scholar] [CrossRef]
- Simeneh, G. Review on the Effect of Feed and Feeding on Chicken Performance. Anim. Husb. Dairy Vet. Sci. 2019, 3. [Google Scholar] [CrossRef][Green Version]
- Smith, J. Feeding and Nutritional Management of Beef Cattle. Available online: https://www.msdvetmanual.com/management-and-nutrition/nutrition-beef-cattle/feeding-and-nutritional-management-of-beef-cattle (accessed on 1 September 2024).[Green Version]
- Wu, M.; Fang, G.; Li, R.; Wu, H.; Yang, X.; Liu, Z.; Zhou, H. Solve Systematically Resource Utilization of Livestock and Poultry Manure. China Anim. Ind. 2022, 7, 27–31. [Google Scholar][Green Version]
- Zhu, Z.; Dong, H.; Wei, S.; Ma, J.; Pengying, X. Impact of Changes in Livestock Manure Management on Greenhouse Gas Emissions in China. J. Agro-Environ. Sci. 2020, 39, 743–748. [Google Scholar][Green Version]
- Cao, J.; Wu, Y. Evaluation of the Material Composition of Chicken Manure and Its Treatment Technology. Guangdong Feed. 2017, 26, 19–23. [Google Scholar][Green Version]
- Estrada, J.M.; Hernández, S.; Muñoz, R.; Revah, S. A Comparative Study of Fungal and Bacterial Biofiltration Treating a VOC Mixture. J. Hazard. Mater. 2013, 250, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption Materials for Volatile Organic Compounds (VOCs) and the Key Factors for VOCs Adsorption Process: A Review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Hoff, S.J.; Bundy, D.S.; Nelson, M.A.; Zelle, B.C.; Jacobson, L.D.; Heber, A.J.; Ni, J.; Zhang, Y.; Koziel, J.A.; Beasley, D.B. Emissions of Ammonia, Hydrogen Sulfide, and Odor before, during, and after Slurry Removal from a Deep-Pit Swine Finisher. J. Air Waste Manag. Assoc. 2006, 56, 581–590. [Google Scholar] [CrossRef]
- Paszek, D.; Jacobson, L.; Johnson, V.; Nicolai, R. Design and Management of an Oil Sprinkling System to Control Dust, Odor, and Gases in and from a Curtain-Sided Pig Finishing Barn. In Proceedings of the 2001 ASAE Annual Meeting, St. Joseph, MI, USA, 29 July–1 August 2001; p. 1. [Google Scholar]
- Rathnayake, D.; Mun, H.-S.; Dilawar, M.; Chung, I.-B.; Park, K.-W.; Lee, S.-R.; Yang, C.-J. Effect of Air Heat Pump Cooling System as a Greener Energy Source on the Air Quality, Housing Environment and Growth Performance in Pig House. Atmosphere 2021, 12, 1474. [Google Scholar] [CrossRef]
- Yao, Q.; Torrents, A.; Li, H.; Buser, M.; Downey, P.; Hapeman, C. Using a Vegetative Environmental Buffer to Reduce the Concentrations of Volatile Organic Compounds in Poultry House Atmospheric Emissions. J. Agric. Food Chem. 2018, 66, 8231–8236. [Google Scholar] [CrossRef]
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.S.; Chen, B.; Li, P.; Banik, C. Mitigation of Odor and Gaseous Emissions from Swine Barn with UV-A and UV-C Photocatalysis. Atmosphere 2021, 12, 585. [Google Scholar] [CrossRef]
- Lebrero, R.; Bouchy, L.; Stuetz, R.; Muñoz, R. Odor Assessment and Management in Wastewater Treatment Plants: A Review. Crit. Rev. Environ. Sci. Technol. 2011, 41, 915–950. [Google Scholar] [CrossRef]
- Eskicioglu, C.; Galvagno, G.; Cimon, C. Approaches and Processes for Ammonia Removal from Side-Streams of Municipal Effluent Treatment Plants. Bioresour. Technol. 2018, 268, 797–810. [Google Scholar] [CrossRef]
- Pokorna, D.; Zabranska, J. Sulfur-Oxidizing Bacteria in Environmental Technology. Biotechnol. Adv. 2015, 33, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.; Brunsch, R.; Pazsiczki, I. Greenhouse Gas Emissions from Covered Slurry Compared with Uncovered during Storage. Agric. Ecosyst. Environ. 2006, 112, 129–134. [Google Scholar] [CrossRef]
- Prado, J.; Chieppe, J.; Raymundo, A.; Fangueiro, D. Bio-Acidification and Enhanced Crusting as an Alternative to Sulphuric Acid Addition to Slurry to Mitigate Ammonia and Greenhouse Gases Emissions during Short Term Storage. J. Clean. Prod. 2020, 263, 121443. [Google Scholar] [CrossRef]
- Pineda, P.A.L.; Demeestere, K.; Toledo, M.; Van Langenhove, H.; Walgraeve, C. Enhanced Removal of Hydrophobic Volatile Organic Compounds in Biofilters and Biotrickling Filters: A Review on the Use of Surfactants and the Addition of Hydrophilic Compounds. Chemosphere 2021, 279, 130757. [Google Scholar] [CrossRef] [PubMed]
- Sunesson, A.L.; Gullberg, J.; Blomquist, G. Airborne chemical compounds on dairy farms. J. Environ. Monit. 2001, 3, 210–216. [Google Scholar] [CrossRef]
- Jacobson, L.D.; Schmidt, D.R.; Lake, J.K.; Johnson, V.J. Ammonia, Hydrogen Sulfide, Odor, and Pm10 Emissions from Deep-Bedded Hoop and Curtain-Sided Pig Finishing Barns in Minnesota. In Air Pollution from Agricultural Operations III; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003; pp. 283–291. [Google Scholar]
- Blunden, J.; Aneja, V.P.; Lonneman, W.A. Characterization of non-methane volatile organic compounds at swine facilities in eastern North Carolina. Atmos. Environ. 2005, 39, 6707–6718. [Google Scholar] [CrossRef]
- Nie, E.; Zheng, G.; Ma, C. Characterization of odorous pollution and health risk assessment of volatile organic compound emissions in swine facilities. Atmos. Environ. 2020, 223, 117233. [Google Scholar] [CrossRef]
- Rumsey, I.C.; Aneja, V.P.; Lonneman, W.A. Characterizing reduced sulfur compounds emissions from a swine concentrated animal feeding operation. Atmos. Environ. 2014, 94, 458–466. [Google Scholar] [CrossRef]
- Uzal Seyfi, S. Daily and seasonal variation of air pollutants and some climatic parameters in freestall and loose dairy cattle houses in Konya, Turkey. J. Food Agric. Environ. 2012, 10, 992–1000. [Google Scholar]
- Huang, D.; Guo, H. Diurnal and seasonal variations of odor and gas emissions from a naturally ventilated free-stall dairy barn on the Canadian prairies. J. Air Waste Manag. Assoc. 2017, 67, 1092–1105. [Google Scholar] [CrossRef]
- Sun, G.; Guo, H.; Peterson, J.; Predicala, B.; Laguë, C. Diurnal Odor, Ammonia, Hydrogen Sulfide, and Carbon Dioxide Emission Profiles of Confined Swine Grower/Finisher Rooms. J. Air Waste Manag. Assoc. (1995) 2008, 58, 1434–1448. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Sakamoto, Y.; Yamashiro, T.; Yasui, S.; Iwasaki, M.; Ihara, I.; Tsuji, O.; Umetsu, K. Effects of handling parameters on hydrogen sulfide emission from stored dairy manure. J. Environ. Manag. 2015, 154, 110–116. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Liu, S.; Diehl, C.A.; Lim, T.-T.; Bogan, B.W.; Chen, L.; Chai, L.; Wang, K.; Heber, A.J. Emission factors and characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter at two high-rise layer hen houses. Atmos. Environ. 2017, 154, 260–273. [Google Scholar] [CrossRef]
- Kafle, G.; Chen, L. Emissions of Odor, Ammonia, Hydrogen Sulfide, and Volatile Organic Compounds from Shallow-Pit Pig Nursery Rooms. J. Biosyst. Eng. 2014, 39, 76–86. [Google Scholar] [CrossRef]
- Yasmeen, R.; Ali, Z.; Tyrrel, S.; Nasir, Z.A. Estimation of particulate matter and gaseous concentrations using low-cost sensors from broiler houses. Environ. Monit. Assess. 2019, 191, 470. [Google Scholar] [CrossRef] [PubMed]
- Chénard, L.; Lemay, S.; Laguë, C. Hydrogen sulfide assessment in shallow-pit swine housing and outside manure storage. J. Agric. Saf. Health 2003, 9, 285–302. [Google Scholar] [CrossRef]
- Blunden, J.; Aneja, V.P.; Westerman, P.W. Measurement and analysis of ammonia and hydrogen sulfide emissions from a mechanically ventilated swine confinement building in North Carolina. Atmos. Environ. 2008, 42, 3315–3331. [Google Scholar] [CrossRef]
- Maasikmets, M.; Teinemaa, E.; Kaasik, A.; Kimmel, V. Measurement and analysis of ammonia, hydrogen sulphide and odour emissions from the cattle farming in Estonia. Biosyst. Eng. 2015, 139, 48–59. [Google Scholar] [CrossRef]
- Shi, Z.; Xi, L.; Zhao, X. Measurement of Ammonia and Hydrogen Sulfide Emission from Three Typical Dairy Barns and Estimation of Total Ammonia Emission for the Chinese Dairy Industry. Animals 2023, 13, 2301. [Google Scholar] [CrossRef]
- Alvarado, A.C.; Predicala, B.Z. Occupational Exposure Risk for Swine Workers in Confined Housing Facilities. J. Agric. Saf. Health 2019, 25, 37–50. [Google Scholar] [CrossRef]
- Rahman, S.; Newman, D.E. Odor, Ammonia, and Hydrogen Sulfide Concentration and Emissions from Two Farrowing-Gestation Swine Operations in North Dakota. Appl. Eng. Agric. 2012, 28, 107–115. [Google Scholar] [CrossRef]
- Trabue, S.; Scoggin, K.; Tyndall, J.; Sauer, T.; Hernandez-Ramirez, G.; Pfeiffer, R.; Hatfield, J. Odorous compounds sources and transport from a swine deep-pit finishing operation: A case study. J. Environ. Manag. 2019, 233, 12–23. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, H.S.; Kim, C.N. Sulfuric odorous compounds emitted from pig-feeding operations. Atmos. Environ. 2007, 41, 4811–4818. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Heber, A.; Diehl, C.; Lim, T.; Duggirala, R.; Haymore, B. Summertime concentrations and emissions of hydrogen sulfide at a mechanically ventilated swine finishing building. Trans. Am. Soc. Agric. Eng. 2002, 45, 193–199. [Google Scholar] [CrossRef][Green Version]
- Park, J.; Kang, T.; Heo, Y.; Lee, K.; Kim, K.; Lee, K.; Yoon, C. Evaluation of Short-Term Exposure Levels on Ammonia and Hydrogen Sulfide During Manure-Handling Processes at Livestock Farms. Saf. Health Work. 2020, 11, 109–117. [Google Scholar] [CrossRef]
- Lin, H.; Liu, W.; Gan, J.; Wang, Y.; Hu, B. Simulation of Hydrogen Sulfide Emission from Deep-Pit Manure Storage During Agitation. Trans. ASABE 2018, 61, 1951–1967. [Google Scholar] [CrossRef]
- Chung, Y.; Huang, C.; Tseng, C.-P. Reduction of H2S/NH3 production from pig feces by controlling environmental conditions. J. Environ. Sci. Health Part. A-Toxic Hazard. Subst. Environ. Eng. 1996, 31, 139–155. [Google Scholar]
- Vtoryi, V.F.; Vtoryi, S.; Gordeev, V. Hydrogen sulfide emissions from cattle manure: Experimental study. Agron. Res. 2020, 18, 1090–1098. [Google Scholar]
- Saludes, R.B.; Iwabuchi, K.; Miyatake, F.; Abe, Y.; Honda, Y. Characterization of dairy cattle manure/wallboard paper compost mixture. Bioresour. Technol. 2008, 99, 7285–7290. [Google Scholar] [CrossRef]
- Avidov, R.; Sudharsan Varma, V.; Saadi, I.; Hanan, A.; Yoselevich, I.; Lublin, A.; Chen, Y.; Laor, Y. Physical and chemical indicators of transformations of poultry carcass parts and broiler litter during short term thermophilic composting. Waste Manag. 2021, 119, 202–214. [Google Scholar] [CrossRef]
- Turan, N.G.; Akdemir, A.; Ergun, O.N. Emission of Volatile Organic Compounds during Composting of Poultry Litter. Water Air Soil Pollut. 2007, 184, 177–182. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, W.; Tang, W.; Zhou, X.; Zhu, X.; Xiao, H. Effects of oxygenated water on the air quality in pigsty and the pig growth. J. Zhejiang Agric. Sci. 2022, 63, 1400–1404. [Google Scholar] [CrossRef]
- Arriaga, H.; Salcedo, G.; Martínez-Suller, L.; Calsamiglia, S.; Merino, P. Effect of dietary crude protein modification on ammonia and nitrous oxide concentration on a tie-stall dairy barn floor. J. Dairy Sci. 2010, 93, 3158–3165. [Google Scholar] [CrossRef]
- Cheng, Z.; Fan, Y.; Zhang, H.; Liao, G.; Liao, Q.; Su, Z.; Wang, G. The effect of Yucca extract and Bacillus subtilis on odor emissions in broiler houses. Feed Res. 2012, 25–27. [Google Scholar] [CrossRef]
- Pepple, L. Impacts of Feeding Dried Distillers Grains with Solubles on Aerial Emissions When Fed to Swine. 2011. Available online: https://porkcheckoff.org/research/impacts-of-feeding-ddgs-to-swine-aerial-emissions-and-potential-management-strategies/ (accessed on 4 September 2021).
- Dai, R.; Cao, G.; Song, F.; Huang, P.; Jinh, S.; Deng, H.; Zhou, S.; Xu, D.; Zheng, H.; Chen, C. The effect of plant deodorants on broiler performance and odor compounds in feces. J. Livest. Ecol. 2007, 55–58. [Google Scholar]
- Dai, R.; Zhou, X.; Ding, Y.; Ding, Y.; Chen, C.; Peng, X.; Luo, Y. Regulation of Chinese herbal deodorant on odor emissions and nitrogen and phosphorus discharge in chicken manure. Chin. J. Vet. Med. 2008, 30–32. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Zhang, X.; Sun, B. Experimental observation on the effect of Chinese herbal additives on odor in broiler chicken houses. Mod. Anim. Husb. Vet. Med. 2012, 38–41. [Google Scholar]
- Wu-Haan, W.; Powers, W.J.; Angel, C.R.; Hale, C.E.; Applegate, T.J. Effect of an Acidifying Diet Combined with Zeolite and Slight Protein Reduction on Air Emissions from Laying Hens of Different Ages. Poult. Sci. 2007, 86, 182–190. [Google Scholar] [CrossRef]
- Parker, D. Effectiveness of a Manure Scraper System for Odor Control in Tunnel-Ventilated Swine Finisher Barns. Trans. ASABE (Am. Soc. Agric. Biol. Eng.) 2011, 54, 315–324. [Google Scholar] [CrossRef]
- Hansen, M.J.; Jonassen, K.E.N.; Løkke, M.M.; Adamsen, A.P.S.; Feilberg, A. Multivariate prediction of odor from pig production based on in-situ measurement of odorants. Atmos. Environ. 2016, 135, 50–58. [Google Scholar] [CrossRef]
- Wi, J.; Lee, S.; Kim, E.; Lee, M.; Koziel, J.A.; Ahn, H. Effects of Treated Manure Conditions on Ammonia and Hydrogen Sulfide Emissions from a Swine Finishing Barn Equipped with Semicontinuous Pit Recharge System in Summer. Atmosphere 2020, 11, 713. [Google Scholar] [CrossRef]
- Choi, Y.; Ha, D.-M.; Lee, S.; Kim, D.-H. Seasonal atmospheric characteristics in a swine finishing barn equipped with a continuous pit recirculation system using aerobically treated manure. Anim. Biosci. 2022, 35, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Predicala, B.Z.; Cortus, E.L.; Lemay, S.P.; Laguë, C. Effectiveness of a Manure Scraper System for Reducing Concentrations of Hydrogen Sulfide and Ammonia in a Swine Grower-Finisher Room. Trans. ASABE 2007, 50, 999–1006. [Google Scholar] [CrossRef]
- Wi, J.; Lee, S.; Kim, E.; Lee, M.; Koziel, J.A.; Ahn, H. Evaluation of Semi-Continuous Pit Manure Recharge System Performance on Mitigation of Ammonia and Hydrogen Sulfide Emissions from a Swine Finishing Barn. Atmosphere 2019, 10, 170. [Google Scholar] [CrossRef]
- Spiehs, M.J.; Brown-Brandl, T.M.; Parker, D.B.; Miller, D.N.; Jaderborg, J.P.; DiCostanzo, A.; Berry, E.D.; Wells, J.E. Use of Wood-Based Materials in Beef Bedded Manure Packs: 1. Effect on Ammonia, Total Reduced Sulfide, and Greenhouse Gas Concentrations. J. Environ. Qual. 2014, 43, 1187–1194. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Q.; Huang, A. Evaluation of a plant material-based air purifier for removing H2S, NH3 and swine manure odour. Environ. Technol. 2012, 33, 2751–2756. [Google Scholar] [CrossRef]
- Heber, A.; Ni, J.-Q.; Lim, T.; Diehl, C.; Sutton, A.; Duggirala, R.; Haymore, B.; Kelly, D.; Adamchuk, V. Effect of a manure additive on ammonia emission from swine finishing buildings. Trans. ASAE 2000, 43, 1895–1902. [Google Scholar] [CrossRef]
- Ni, J.Q.; Heber, A.J.; Diehl, C.A.; Lim, T.T. Ammonia, hydrogen sulphide and carbon dioxide release from pig manure in under-floor deep pits. J. Agric. Eng. Res. 2000, 77, 53–66. [Google Scholar] [CrossRef]
- Kafle, G.K.; Chen, L.; Neibling, H.; Brian He, B. Field evaluation of wood bark-based down-flow biofilters for mitigation of odor, ammonia, and hydrogen sulfide emissions from confined swine nursery barns. J. Environ. Manag. 2015, 147, 164–174. [Google Scholar] [CrossRef]
- Chen, L.; Hoff, S.; Cai, L.; Koziel, J.; Zelle, B. Evaluation of Wood Chip-Based Biofilters to Reduce Odor, Hydrogen Sulfide, and Ammonia from Swine Barn Ventilation Air. J. Air Waste Manag. Assoc. 2012, 59, 520–530. [Google Scholar] [CrossRef]
- Melse, R.W.; Hol, J.M.G. Biofiltration of exhaust air from animal houses: Evaluation of removal efficiencies and practical experiences with biobeds at three field sites. Biosyst. Eng. 2017, 159, 59–69. [Google Scholar] [CrossRef]
- Shelford, T.J.; Gooch, C.A.; Lansing, S.A. Performance and Economic Results for Two Full-scale Biotrickling Filters to Remove H2S from Dairy Manure-derived Biogas. Appl. Eng. Agric. 2019, 35, 283–291. [Google Scholar] [CrossRef]
- Lim, T.-T.; Jin, Y.; Ni, J.-Q.; Heber, A.J. Field evaluation of biofilters in reducing aerial pollutant emissions from a commercial pig finishing building. Biosyst. Eng. 2012, 112, 192–201. [Google Scholar] [CrossRef]
- Wang, Y.; Cho, J.H.; Chen, Y.J.; Yoo, J.S.; Huang, Y.; Kim, H.J.; Kim, I.H. The effect of probiotic BioPlus 2B® on growth performance, dry matter and nitrogen digestibility and slurry noxious gas emission in growing pigs. Livest. Sci. 2009, 120, 35–42. [Google Scholar] [CrossRef]
- Cho, J.H.; Chen, Y.J.; Min, B.J.; Yoo, J.S.; Wang, Y.; Kim, I.H. Effects of reducing dietary crude protein on growth performance, odor gas emission from manure and blood urea nitrogen and IGF--1 concentrations of serum in nursery pigs. Anim. Sci. J. 2008, 79, 453–459. [Google Scholar] [CrossRef]
- Wang, Y.; Cho, J.H.; Chen, Y.J.; Yoo, J.S.; Kim, H.J.; Huang, Y.; Shin, S.; Zhou, T.X.; Kim, I.H.J.J.o.A.; Sciences, F. Effect of dietary soyabean hulls and metal-amino acid chelated mineral supplementation on growth performance, nutrient digestibility and noxious gas emission in growing pigs. J. Anim. Feed. Sci. 2008, 17, 171–181. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Lee, K.Y.; Kim, I.H. Influence of protected organic acid blends and diets with different nutrient densities on growth performance, nutrient digestibility and faecal noxious gas emission in growing pigs. Vet. Med. 2018, 59, 491–497. [Google Scholar] [CrossRef]
- Yoo, J.S.; Cho, J.H.; Chen, Y.G.; Kim, H.J.; Wang, Q.; Hyun, Y.; Ko, T.G.; Park, C.S.; Kim, I.-S. The Effects of Environment-Friendly Diets on the Growth Performance, Nutrient Digestibility, Fecal Excretion, Nitrogen Excretion and Emission Gases in Manure for Growing Pigs. J. Anim. Sci. Technol. 2007, 49, 491–500. [Google Scholar] [CrossRef][Green Version]
- Yin, J.; Kim, H.S.; Kim, Y.M.; Kim, I.H. Effects of dietary fermented red ginseng marc and red ginseng extract on growth performance, nutrient digestibility, blood profile, fecal microbial, and noxious gas emission in weanling pigs. J. Appl. Anim. Res. 2018, 46, 1084–1089. [Google Scholar] [CrossRef]
- Kwak, W.G.; Park, I.H.; Yun, W.; Lee, J.H.; Lee, C.H.; Oh, S.Y.; Oh, H.J.; Liu, S.; Kim, Y.H.; Park, J.C.; et al. Effects of various additives to enhance growth performance, blood profiles, and reduce malodour emissions in growing pigs. S. Afr. J. Anim. Sci. 2017, 47, 535–541. [Google Scholar] [CrossRef]
- Yan, L.; Wang, J.P.; Kim, H.J.; Meng, Q.W.; Ao, X.; Hong, S.M.; Kim, I.H. Influence of essential oil supplementation and diets with different nutrient densities on growth performance, nutrient digestibility, blood characteristics, meat quality and fecal noxious gas content in grower–finisher pigs. Livest. Sci. 2010, 128, 115–122. [Google Scholar] [CrossRef]
- Kumar, S.; Seok, W.; Ha, S.; Jin, S.; Kim, I.H. Supplemental effect of coated refined fish oil on the performance of finishing pigs fed diets containing soybean meal as a partial alternative to barley or wheat feed ingredient. Can. J. Anim. Sci. 2022, 102, 332–341. [Google Scholar] [CrossRef]
- Lan, R.X.; Lee, S.I.; Kim, I.H. Effects of multistrain probiotics on growth performance, nutrient digestibility, blood profiles, faecal microbial shedding, faecal score and noxious gas emission in weaning pigs. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1130–1138. [Google Scholar] [CrossRef]
- Park, C.; Sun, S. Effect of Dietary Supplementation of Enzyme and Microorganism on Growth Performance, Carcass Quality, Intestinal Microflora and Feces Odor in Broiler Chickens. Korean J. Poult. Sci. 2020, 47, 275–283. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Tran, H.; Yun, H.-M.; Kim, I.-S. Influence of a cocktail of protease and xylanase in different energy density of corn and soybean meal-based diet on growth performance, nutrient digestibility, carcass quality, and gas emission in broilers. Can. J. Anim. Sci. 2017, 98, 271–278. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.H. Effects of lactulose supplementation on performance, blood profiles, excreta microbial shedding ofLactobacillusandEscherichia coli, relative organ weight and excreta noxious gas contents in broilers. J. Anim. Physiol. Anim. Nutr. 2013, 98, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Kim, Y.-I.; Kwak, W.S. Effects of Dietary Addition of Bentonite on Manure Gas Emission, Health, Production, and Meat Characteristics of Hanwoo (Bos taurus coreanae) Steers. Asian-Australas. J. Anim. Sci. 2010, 23, 1594–1600. [Google Scholar] [CrossRef]
- Saksrithai, K.; King, A.J. Lactobacillus and dietary sunflower meal supplementation in layer diets: Effects on specific serum content and hydrogen sulfide concentration in layer manure. Res. Vet. Sci. 2019, 122, 64–71. [Google Scholar] [CrossRef]
- Dilawar, M.A.; Saturno, J.F.L.; Mun, H.-S.; Kim, D.-H.; Jeong, M.-G.; Yang, C.-J. Influence of Two Plant Extracts on Broiler Performance, Oxidative Stability of Meat and Odorous Gas Emissions from Excreta. Ann. Anim. Sci. 2019, 19, 1099–1113. [Google Scholar] [CrossRef]
- Lan, R.; Li, T.; Kim, I. Effects of xylanase supplementation on growth performance, nutrient digestibility, blood parameters, fecal microbiota, fecal score and fecal noxious gas emission of weaning pigs fed corn--soybean meal--based diet. Anim. Sci. J. 2017, 88, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Trabue, S.; Kerr, B.; Scoggin, K. Odor and Odorous Compound Emissions from Manure of Swine Fed Standard and Dried Distillers Grains with Soluble Supplemented Diets. J. Environ. Qual. 2016, 45, 915–923. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Heber, A.J.; Sutton, A.L.; Kelly, D.T.; Patterson, J.A.; Kim, S.-T. Effect of swine manure dilution on ammonia, hydrogen sulfide, carbon dioxide, and sulfur dioxide releases. Sci. Total Environ. 2010, 408, 5917–5923. [Google Scholar] [CrossRef]
- Hile, M.L.; Fabian-Wheeler, E.E.; Murphy, D.J.; Meinen, R.J.; Hill, D.E.; Elliott, H.A.; Bryant, R.B. Gypsum Bedding Impact on Hydrogen Sulfide Release from Dairy Manure Storages. Trans. ASABE 2018, 61, 937–941. [Google Scholar] [CrossRef]
- Fuchs, A.; Dalby, F.R.; Liu, D.; Kai, P.; Feilberg, A. Improved effect of manure acidification technology for gas emission mitigation by substituting sulfuric acid with acetic acid. Clean. Eng. Technol. 2021, 4, 100263. [Google Scholar] [CrossRef]
- Meiirkhanuly, Z.; Koziel, J.A.; Chen, B.; Białowiec, A.; Lee, M.; Wi, J.; Banik, C.; Brown, R.C.; Bakshi, S. Mitigation of Gaseous Emissions from Swine Manure with the Surficial Application of Biochars. Atmosphere 2020, 11, 1179. [Google Scholar] [CrossRef]
- Choi, E.; Kim, J.; Choi, I.; Ahn, H.; Dong, J.I.; Kim, H. Microbial Additives in Controlling Odors from Stored Swine Slurry. Water Air Soil Pollut. 2015, 226, 104. [Google Scholar] [CrossRef]
- Duong, C.M.; Wang, H.; Lim, T.-T. Effectiveness of Manure Pit Additive in Reducing Emissions and Solids. Appl. Eng. Agric. 2021, 37, 309–317. [Google Scholar] [CrossRef]
- Moreno, L.; Predicala, B.; Nemati, M. Laboratory, semi-pilot and room scale study of nitrite and molybdate mediated control of H2S emission from swine manure. Bioresour. Technol. 2010, 101, 2141–2151. [Google Scholar] [CrossRef]
- Awume, B.; Tajallipour, M.; Nemati, M.; Predicala, B. Application of ZnO Nanoparticles in Control of H2S Emission from Low-Temperature Gases and Swine Manure Gas. Water Air Soil Pollut. 2017, 228, 147. [Google Scholar] [CrossRef]
- Chen, L.; Hile, M.L.; Fabian, E.E.; Xu, Z.; Bruns, M.A.; Brown, V. Iron Oxide to Mitigate Hydrogen Sulfide Gas Release from Gypsum-Bedded Dairy Manure Storages. Trans. ASABE 2018, 61, 1101–1112. [Google Scholar] [CrossRef]
- Berg, W. Reducing Ammonia Emissions by Combining Covering and Acidifying Liquid Manure. Available online: https://xueshu.baidu.com/ndscholar/browse/detail?paperid=1c5a44e6d9af0775f3d59789ec7eb8ee (accessed on 1 September 2024).
- Madhavaraj, L.; Lim, H.-D.; Kim, K.-M.; Kim, D.-H.; Han, G.H. Influence of Sargassum horneri Mitigating Odorous Gas Emissions from Swine Manure Storage Facilities. Sustainability 2020, 12, 7587. [Google Scholar] [CrossRef]
- Blanes-Vidal, V.; Hansen, M.N.; Sousa, P. Reduction of Odor and Odorant Emissions from Slurry Stores by Means of Straw Covers. J. Environ. Qual. 2009, 38, 1518–1527. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Lee, M.; O’Brien, S.C.; Li, P.; Brown, R.C. Mitigation of Acute Hydrogen Sulfide and Ammonia Emissions from Swine Manure during Three-Hour Agitation Using Pelletized Biochar. Atmosphere 2021, 12, 825. [Google Scholar] [CrossRef]
- Prandini, J.M.; da Silva, M.L.B.; Mezzari, M.P.; Pirolli, M.; Michelon, W.; Soares, H.M. Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour. Technol. 2016, 202, 67–75. [Google Scholar] [CrossRef]
- Ding, L.; Lin, H.; Hetchler, B.; Wang, Y.; Wei, W.; Hu, B. Electrochemical mitigation of hydrogen sulfide in deep-pit swine manure storage. Sci. Total Environ. 2021, 777, 146048. [Google Scholar] [CrossRef]
- Buelna, G.; Turgeon, N.; Dubé, R. Organic Bed Biofiltration: A new Technology for Simultaneously Deodorization of Liquid and Gaseous Effluents on Pig Farms. Ing. Investig. Y Tecnol. 2007, 8, 1–9. [Google Scholar] [CrossRef]
- Akdeniz, N.; Janni, K.A.; Hetchler, B. Mitigation of multiple air emissions from swine buildings using corn cob biofilters. Trans. ASABE 2014, 2, 902–909. [Google Scholar]
- Li, Y.; Sun, B.; Chen, J.; Peng, X.; Bai, Z.; Zhuang, X. Effects of Nano-membrane on Aerobic Composting Process and Odor Emission of Livestock Manure. Environ. Sci. 2021, 42, 5554–5562. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Zheng, C.; Zhu, Y.; Liang, Y.; Chen, F.; Jie, S.; Pi, J. Design of Deodorization Device for Livestock and Poultry Manure Composting and Analysis of Deodorizing Effect. J. Domest. Anim. Ecol. 2021, 42, 75–78. [Google Scholar]
- Yi, C.; Liu, Y.; Long, J.; Deng, J. Screening of Dominant Strains of Pig Manure Fermented by Three Starter Agents and Deodorization Experimental Research. J. Hunan Ecol. Sci. 2022, 9, 85–95. [Google Scholar]
- Bao, W. Microbial Removal of Ammonia from Composting Gases By Immobilized Ammonia-Oxidizing Bacteria. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2009. [Google Scholar]
- Gu, W.; Sun, W.; Lu, Y.; Li, X.; Xu, P.; Xie, K.; Sun, L.; Wu, H. Effect of Thiobacillus thioparus 1904 and sulphur addition on odour emission during aerobic composting. Bioresour. Technol. 2018, 249, 254–260. [Google Scholar] [CrossRef]
- Kuroda, K.; Tanaka, A.; Furuhashi, K.; Fukuju, N. Evaluation of ammonia emission reducing effect by adding waste cooking oil in pilot-scale composting of dairy cattle manure. Anim. Biosci. 2023, 36, 1612–1618. [Google Scholar] [CrossRef]
- Chen, W.; Li, G.; Ma, R.; Liu, Y.; Yuan, J. Effect of Fe2O3 on the emission of sulfur-containing odor during chicken manure composting. J. Agro-Environ. Sci. 2021, 40, 2465–2471. [Google Scholar]
- Yan, Z.; Yang, F.; Gao, X.; Chen, J.; Li, S.; Li, G.; Luo, W. Effect of sulfur-containing additives on methane and odor emissions during pig manure composting. J. Agro-Environ. Sci. 2021, 40, 2448–2455. [Google Scholar]
- Liu, Y.; Ma, R.; Li, D.; Qi, C.; Han, L.; Chen, M.; Fu, F.; Yuan, J.; Li, G. Effects of calcium magnesium phosphate fertilizer, biochar and spent mushroom substrate on compost maturity and gaseous emissions during pig manure composting. J. Environ. Manag. 2020, 267, 110649. [Google Scholar] [CrossRef]
- Shen, Y.; Meng, H.; Zhang, P.; Zhao, L.; Zhou, H.; Hou, Y. Generation law and influencing factors of volatile organic compounds during pig manure composting. Chin. Soc. Agric. Eng. 2017, 33, 211–216. [Google Scholar]
- Zang, B.; Li, S.; Michel, F.; Li, G.; Luo, Y.; Zhang, D.; Li, Y. Effects of mix ratio, moisture content and aeration rate on sulfur odor emissions during pig manure composting. Waste Manag. 2016, 56, 498–505. [Google Scholar] [CrossRef]
- Ma, R.; Li, D.; Qi, C.; Li, G.; Wang, G.; Liu, Y.; Sun, S.; Yuan, J. Effects of C/N ratio on maturity and odor emissions during chicken manure composting. Chin. Soc. Agric. Eng. 2020, 36, 194–202. [Google Scholar]
- Ma, R.; Liu, Y.; Wang, J.; Li, D.; Qi, C.; Li, G.; Yuan, J. Effects of oxygen levels on maturity, humification, and odor emissions during chicken manure composting. J. Clean. Prod. 2022, 369, 133326. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, B.; Wang, Y.; Xiao, J.; Wang, X. Composting process and odor emission varied in windrow and trough composting system under different air humidity conditions. Bioresour. Technol. 2020, 297, 122482. [Google Scholar] [CrossRef]
- Lu, R.; Wang, D.; Xiang, Q.; Chen, G.; Liao, Z. Effects of biofilter media and operating parameters on biofiltration of odor from chicken manure composting. Chin. Soc. Agric. Eng. 2008, 241–245. [Google Scholar]
- Shang, B.; Zhou, T.; Dong, H.; Tao, X.; Li, L.; Liu, Y. Pilot scale test on removal effect of odor from pig manure and carcass composting by biofiltration. Chin. Soc. Agric. Eng. 2017, 33, 226–232. [Google Scholar]
- Su, Q.; Dai, D.; Liao, Y.; Han, H.; Wu, J.; Ren, Z. Synthetic microbial consortia to enhance the biodegradation of compost odor by biotrickling filter. Bioresour. Technol. 2023, 387, 129698. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Zhang, R.; Zhang, L.; Wang, H.; Zhang, X.; Bai, Z.; Ma, L.; Wang, X. The Characteristics of Key Odorants from Livestock Farms and Their Mitigation Potential: A Meta-Analysis. Atmosphere 2025, 16, 1097. https://doi.org/10.3390/atmos16091097
Ren Y, Zhang R, Zhang L, Wang H, Zhang X, Bai Z, Ma L, Wang X. The Characteristics of Key Odorants from Livestock Farms and Their Mitigation Potential: A Meta-Analysis. Atmosphere. 2025; 16(9):1097. https://doi.org/10.3390/atmos16091097
Chicago/Turabian StyleRen, Yazhan, Ruifang Zhang, Lu Zhang, Hongge Wang, Xinyuan Zhang, Zhaohai Bai, Lin Ma, and Xuan Wang. 2025. "The Characteristics of Key Odorants from Livestock Farms and Their Mitigation Potential: A Meta-Analysis" Atmosphere 16, no. 9: 1097. https://doi.org/10.3390/atmos16091097
APA StyleRen, Y., Zhang, R., Zhang, L., Wang, H., Zhang, X., Bai, Z., Ma, L., & Wang, X. (2025). The Characteristics of Key Odorants from Livestock Farms and Their Mitigation Potential: A Meta-Analysis. Atmosphere, 16(9), 1097. https://doi.org/10.3390/atmos16091097