Numerical Study of Moisture Transfer and Methane Emission in Earthen Final Covers: Effects of Ambient Conditions
Abstract
1. Introduction
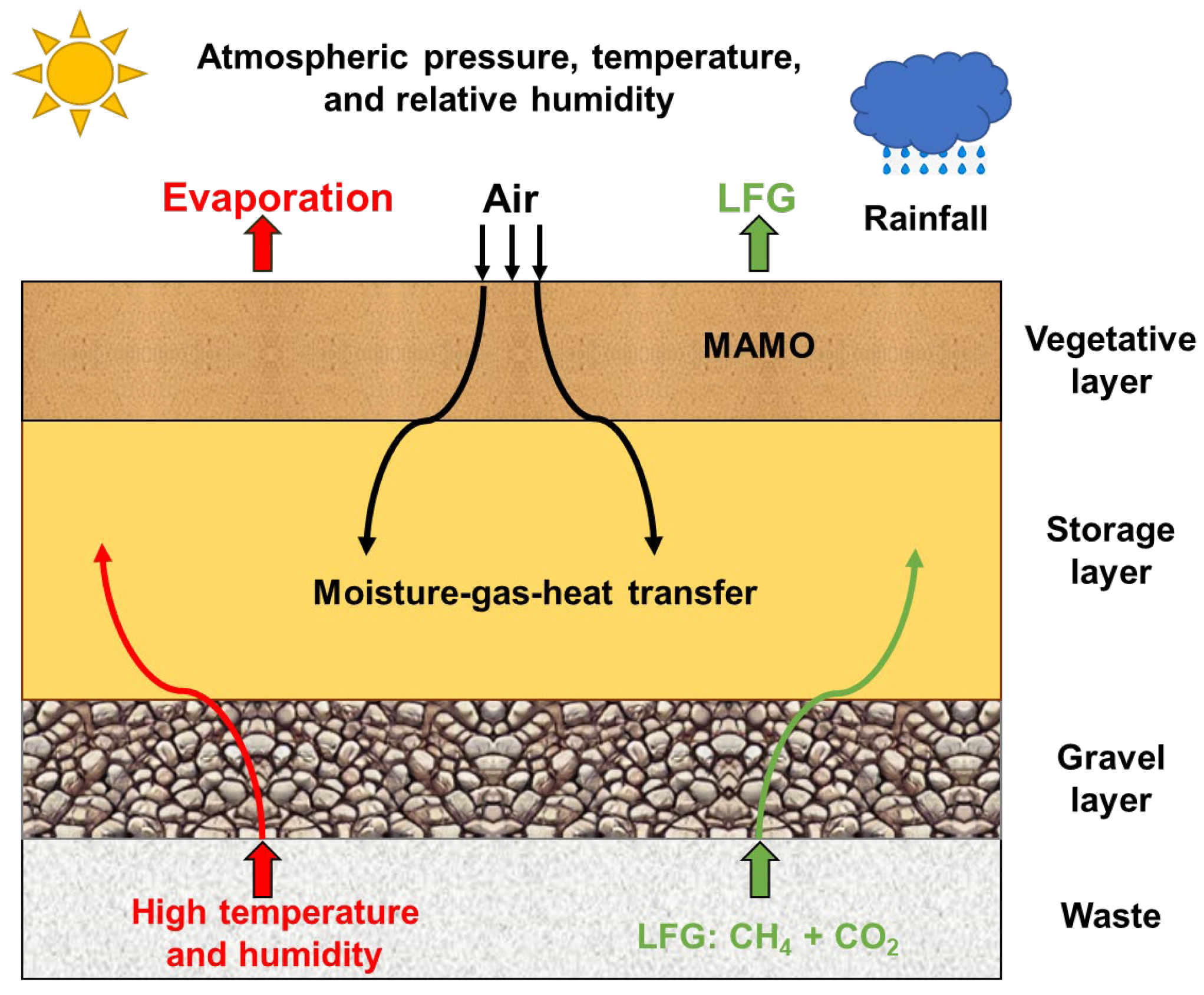
2. Methods
2.1. Governing Equation for Moisture Transfer
2.2. Governing Equation for Multicomponent Gas Transfer
2.3. Governing Equation for Heat Transfer
2.4. Microbial Aerobic CH4 Oxidation
2.5. Model Implementation
3. Results and Discussion
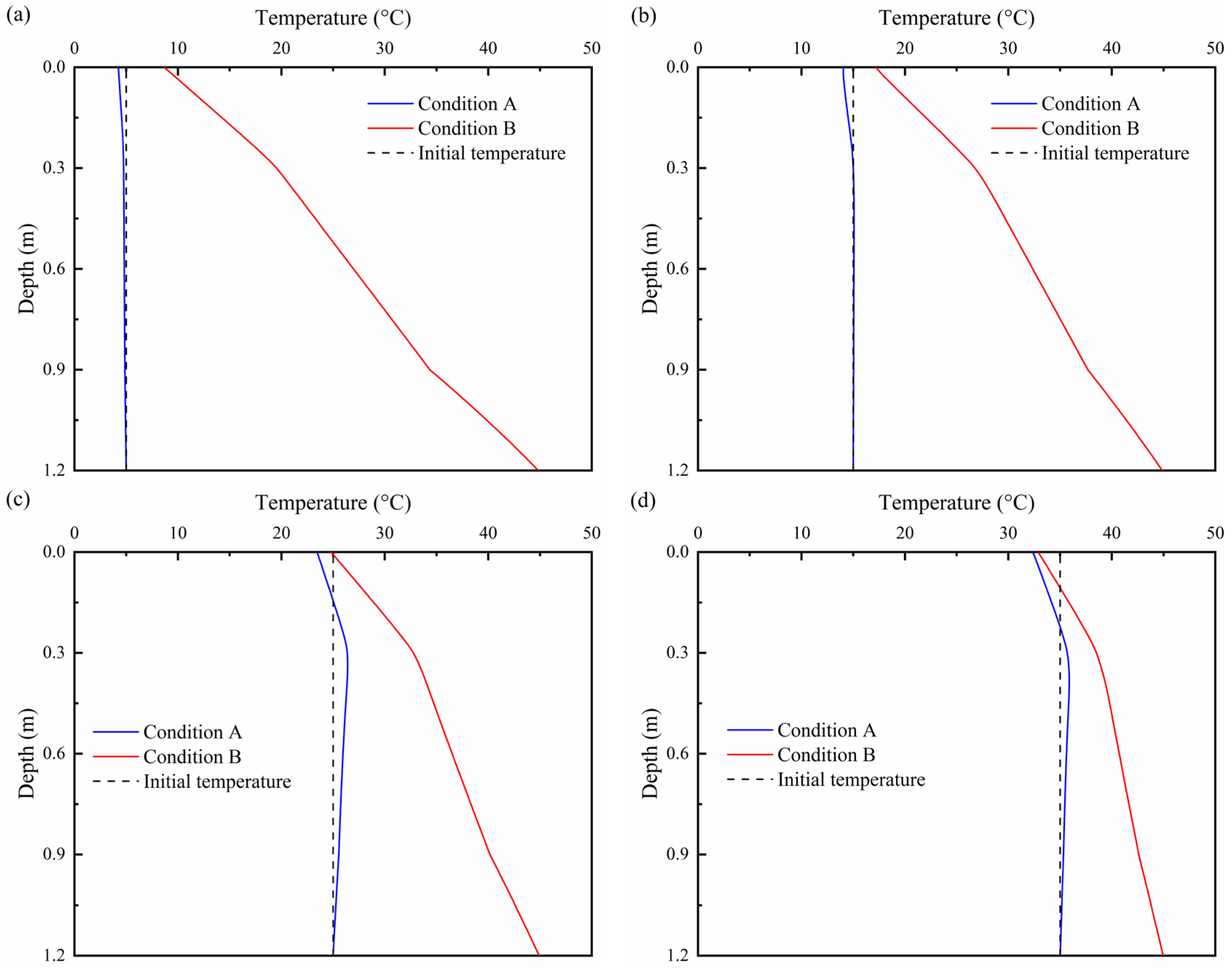
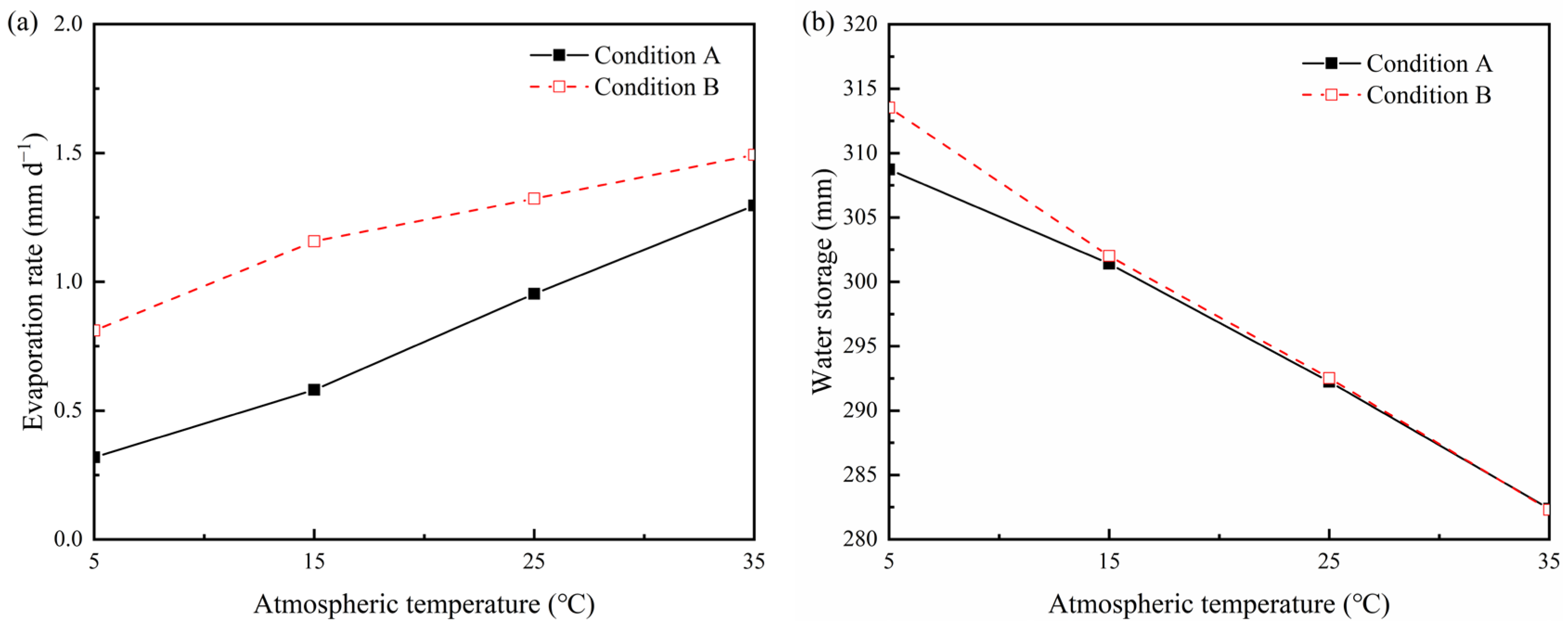
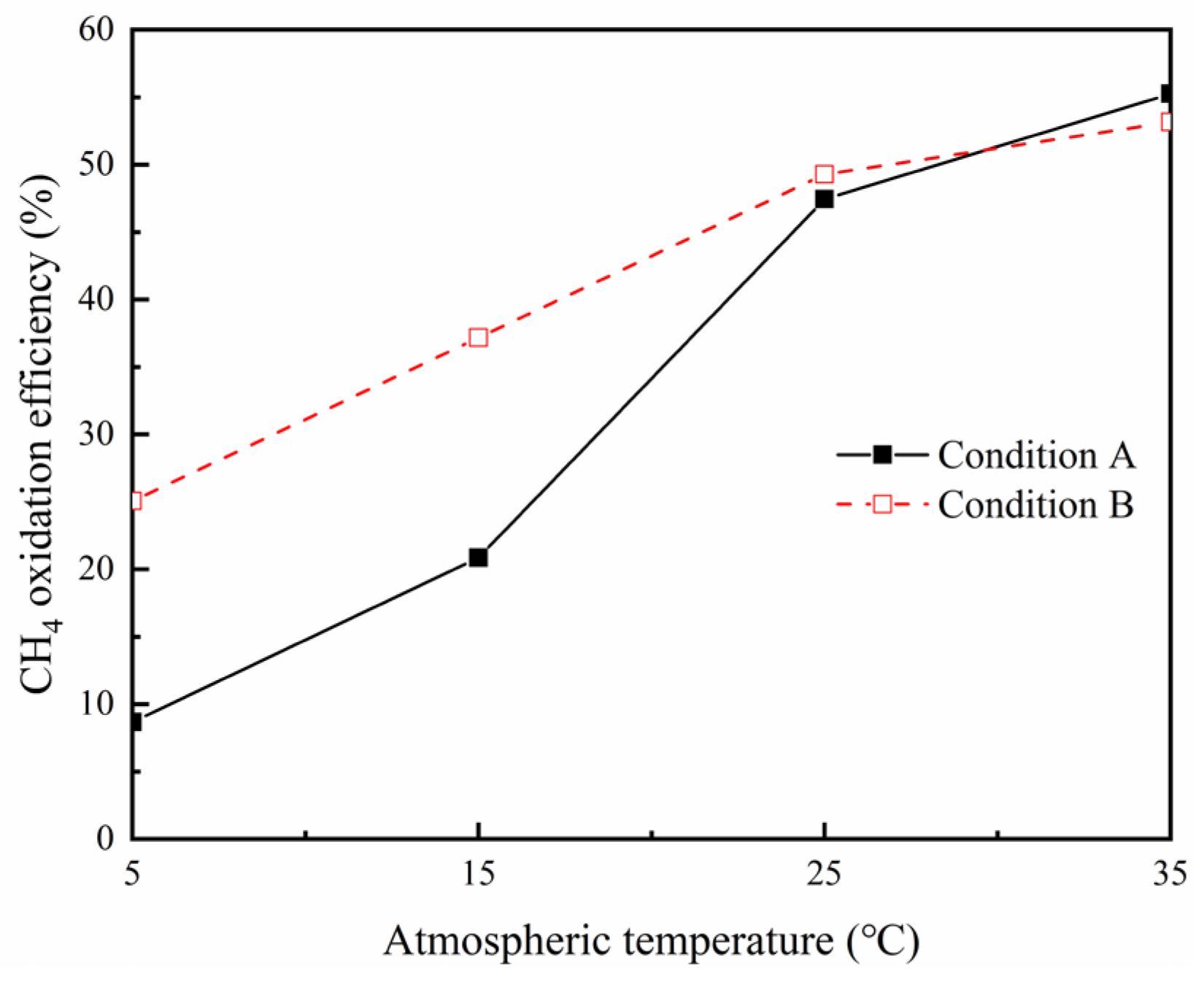
3.1. Effects of Ambient Temperature
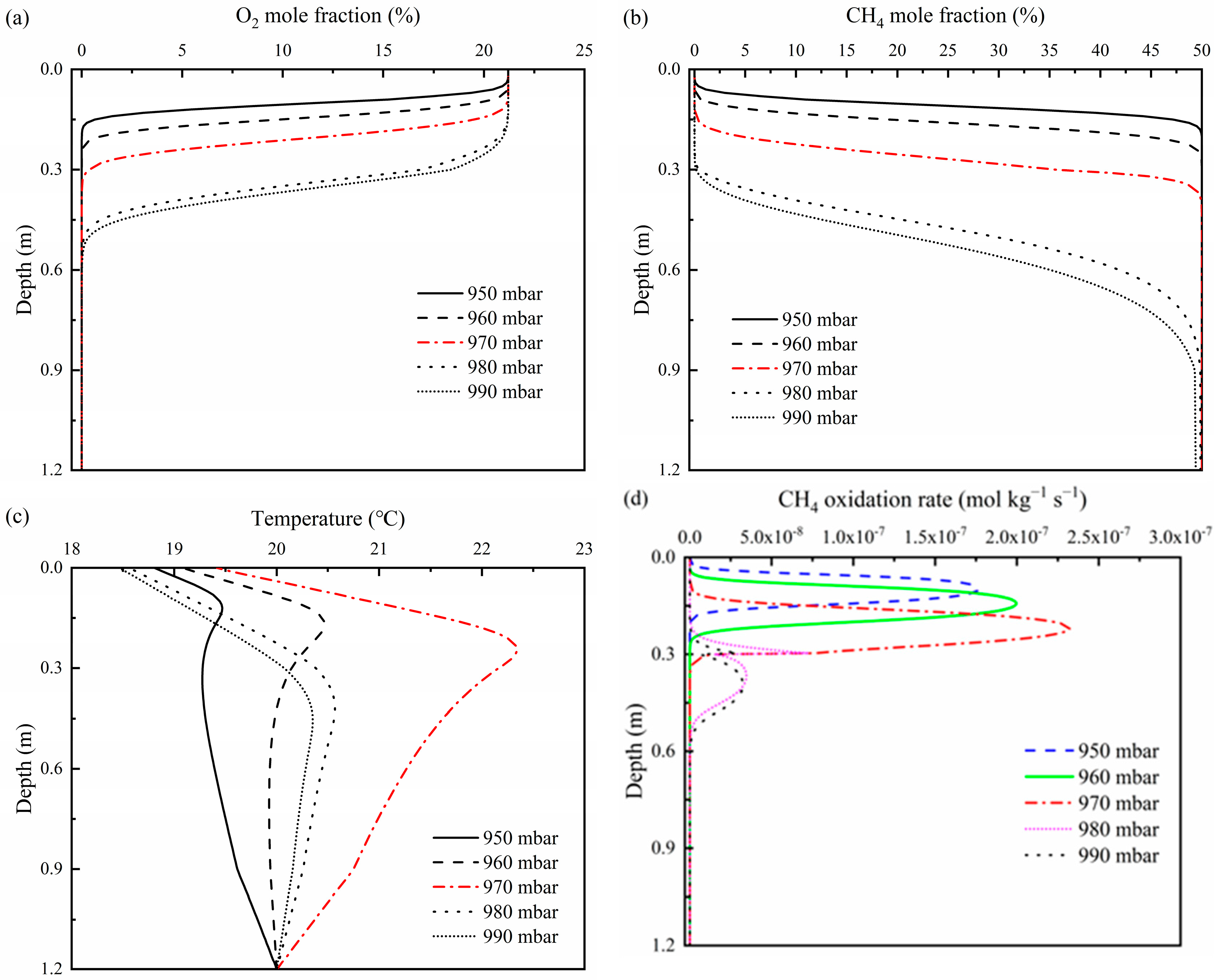
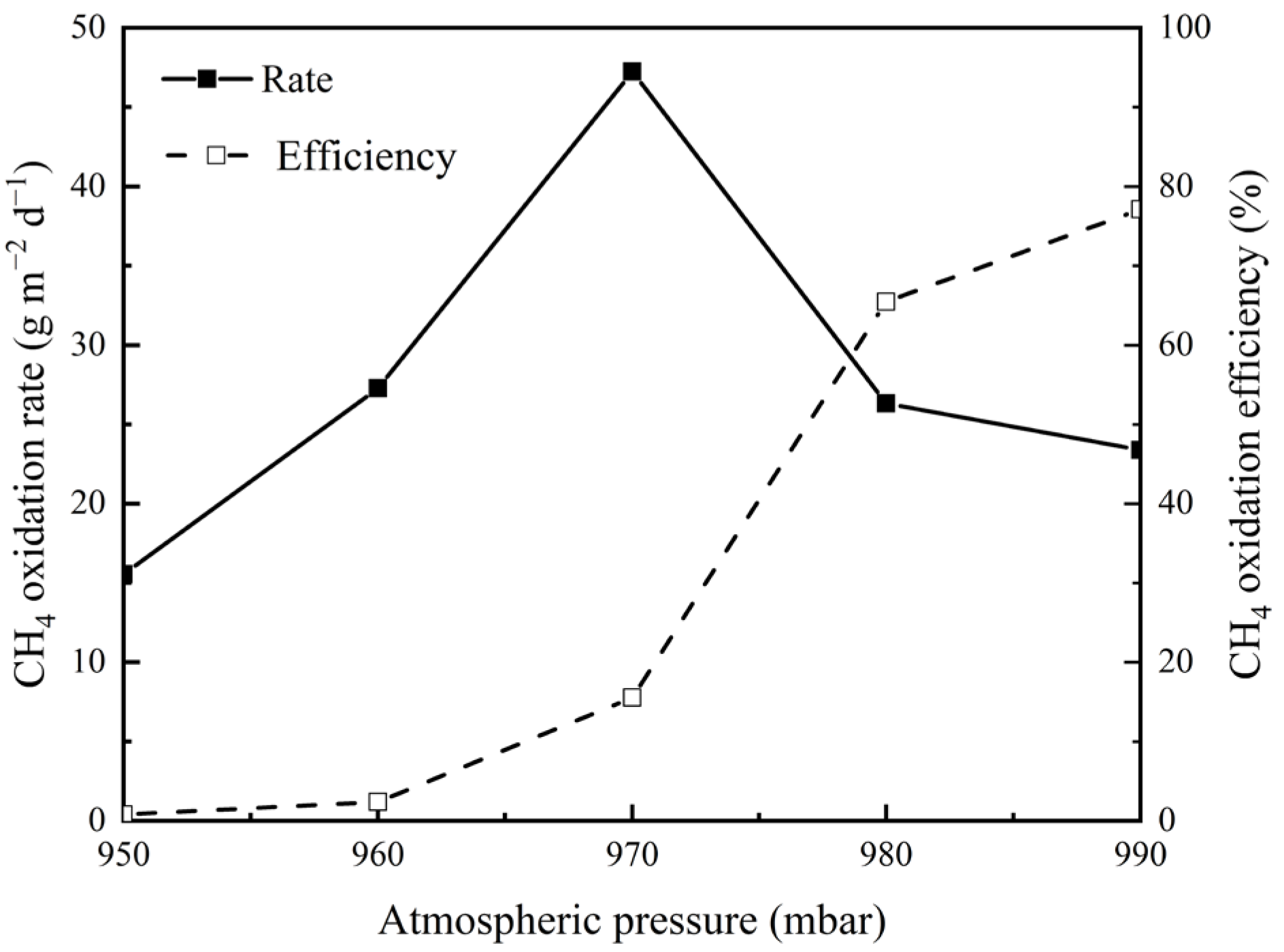
3.2. Effects of Atmospheric Pressure
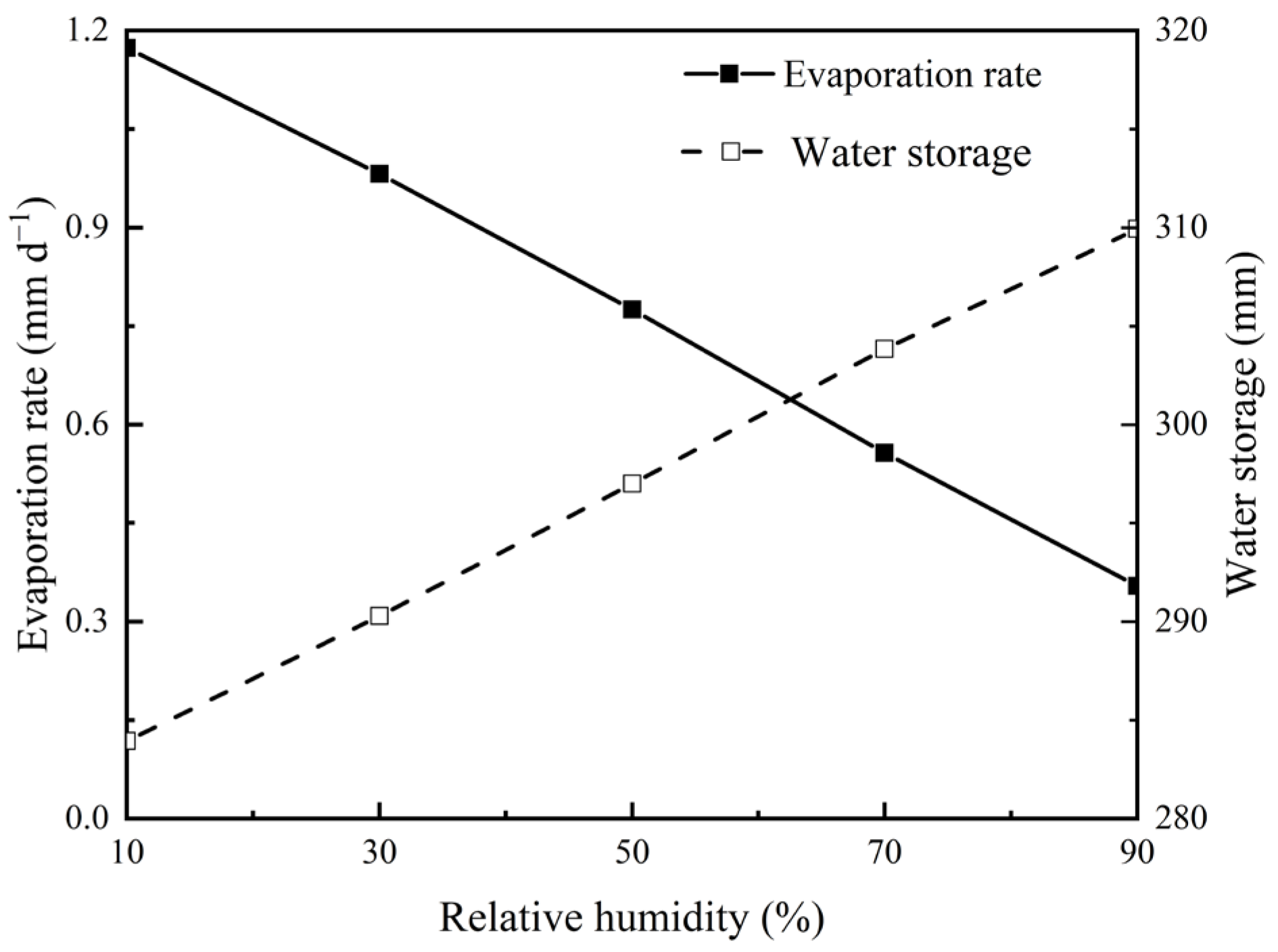
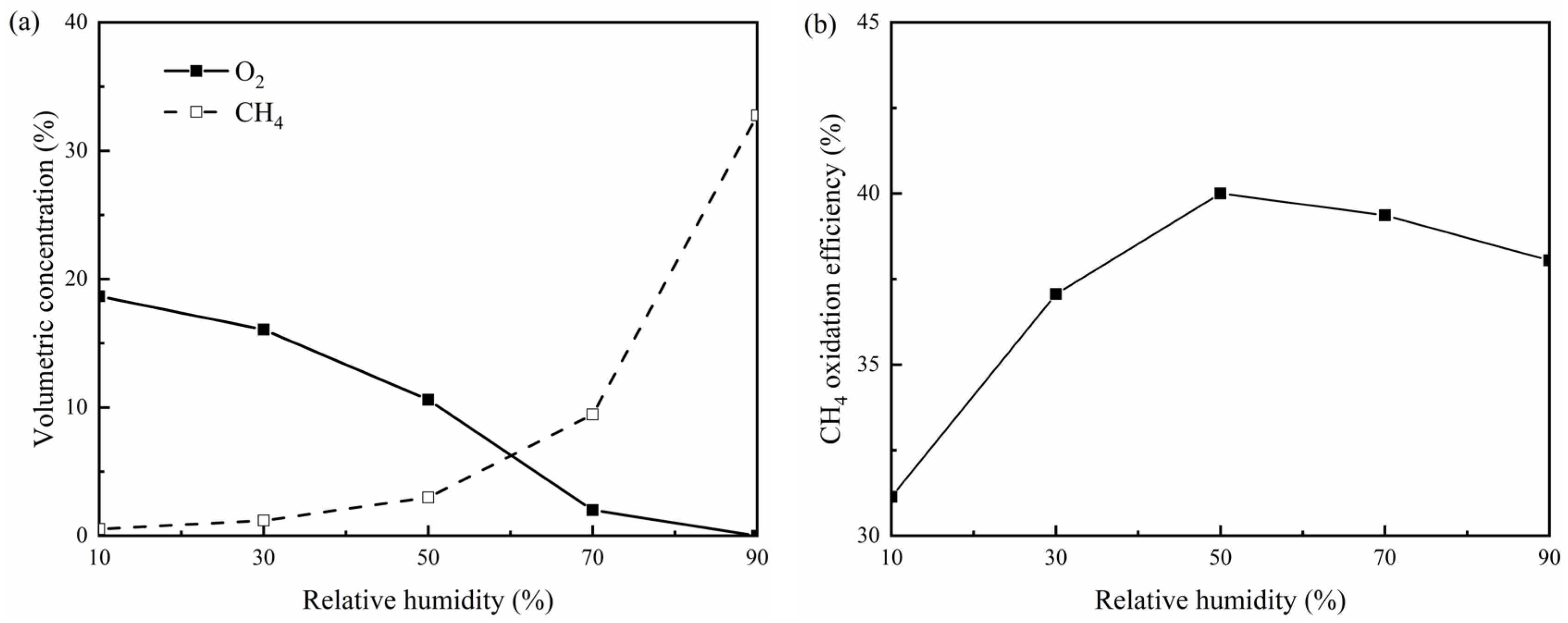
3.3. Effects of Atmospheric Relative Humidity
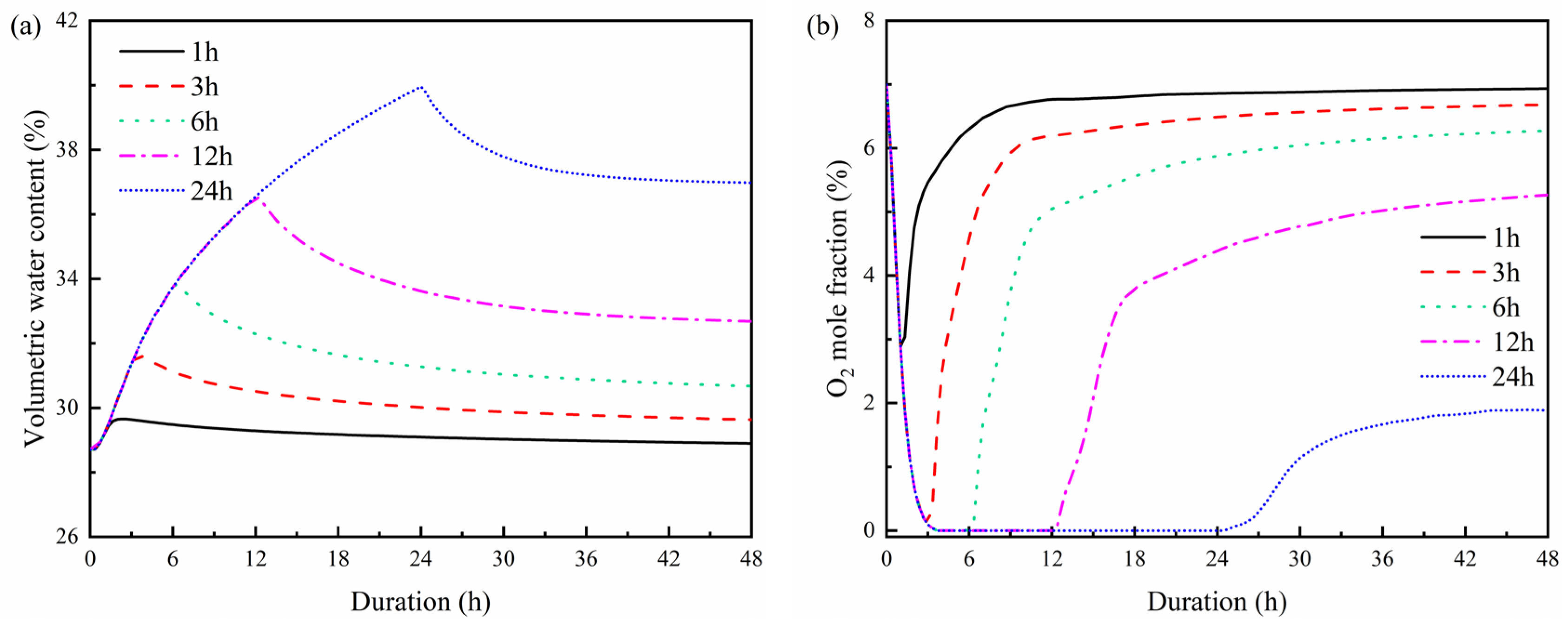
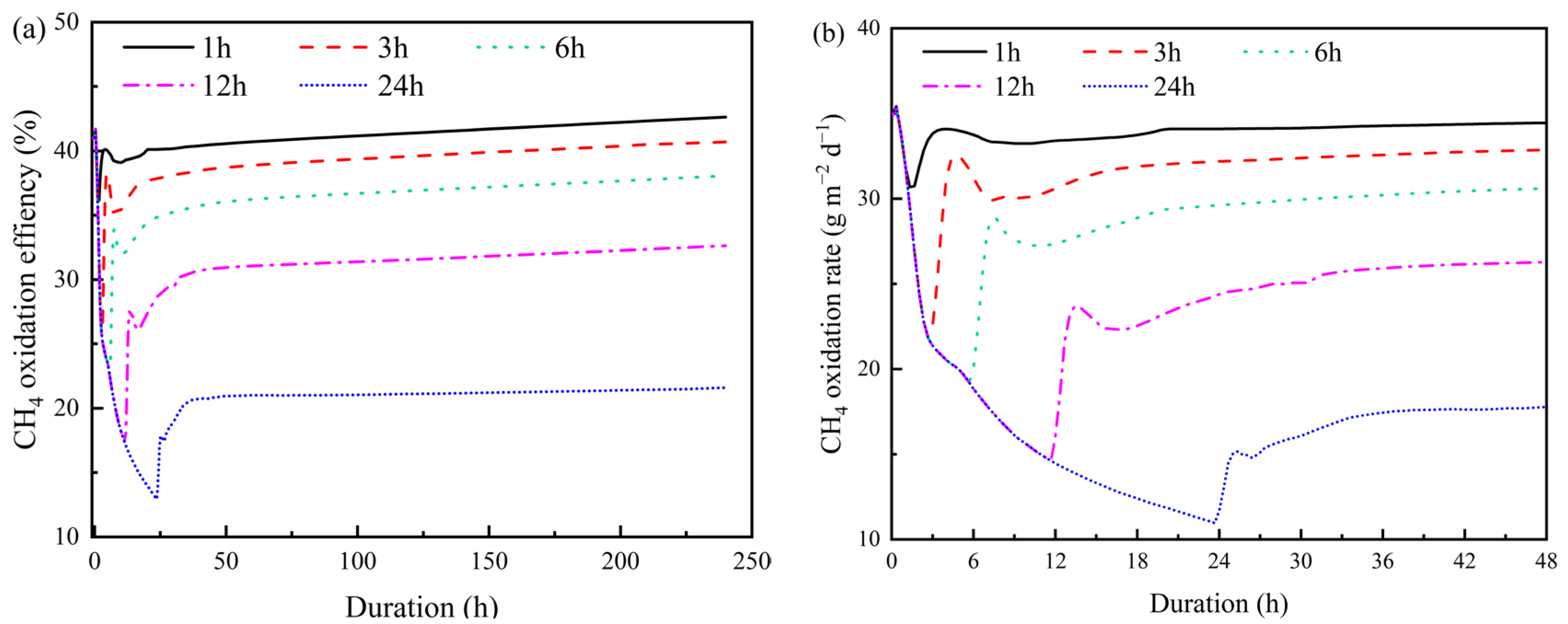
3.4. Effects of Rainfall
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balasus, N.; Jacob, D.J.; Maxemin, G.; Jenks, C.; Nesser, H.; Maasakkers, J.D.; Cusworth, D.H.; Scarpelli, T.R.; Varon, D.J.; Wang, X. Satellite Monitoring of Annual US Landfill Methane Emissions and Trends. Environ. Res. Lett. 2025, 20, 024007. [Google Scholar] [CrossRef]
- Feng, S.-J.; Bai, Z.-B.; Zheng, Q.-T.; Lu, S.-F.; Zhang, X.-L. A Finite-Volume Numerical Model for Temporal and Spatial Variability of Methane Oxidation in Landfill Covers. Comput. Geotech. 2020, 122, 103510. [Google Scholar] [CrossRef]
- Chen, C.; Zhan, L.-T.; Xie, H.-J.; Chen, Y.-M. Bayesian Updating for Improving Long-Term Performance Prediction of Composite Liners in Landfills with Temporally Variable Leachate Head. Comput. Geotech. 2025, 187, 107511. [Google Scholar] [CrossRef]
- Ng, C.W.W.; Guo, H.; Ni, J.; Zhang, Q.; Chen, R.; Zhang, Y. Effects of Plant-Biochar Interaction on the Performance of a Landfill Cover System: Field Monitoring and Numerical Modelling. Can. Geotech. J. 2023, 60, 1663–1680. [Google Scholar] [CrossRef]
- Zuo, X.; Chen, Y.; Wang, L.; Xie, H.; Shen, S. Multicomponent Landfill Gas Transport in Soil Cover: Column Tests and Numerical Modelling. Environ. Geotech. 2023, 10, 3–18. [Google Scholar] [CrossRef]
- Abichou, T.; Mahieu, K.; Chanton, J.; Romdhane, M.; Mansouri, I. Scaling Methane Oxidation: From Laboratory Incubation Experiments to Landfill Cover Field Conditions. Waste Manag. 2011, 31, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Q.; Fei, S.; Thomas, H.R.; Xu, W.; Chen, Y. A Two-Dimensional Semi-Analytical Model for Tracer Gas Injection and Transport in a Landfill System. Comput. Geotech. 2022, 151, 104995. [Google Scholar] [CrossRef]
- Scheutz, C.; Kjeldsen, P.; Bogner, J.E.; De Visscher, A.; Gebert, J.; Hilger, H.A.; Huber-Humer, M.; Spokas, K. Microbial Methane Oxidation Processes and Technologies for Mitigation of Landfill Gas Emissions. Waste Manag. Res. 2009, 27, 409–455. [Google Scholar] [CrossRef]
- Bian, R.; Chen, J.; Li, W.; Sun, Y.; Chai, X.; Wang, H.; Wang, Y.; Zhao, J. Numerical Modeling of Methane Oxidation and Emission from Landfill Cover Soil Coupling Water-Heat-Gas Transfer: Effects of Meteorological Factors. Process Saf. Environ. Prot. 2021, 146, 647–655. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, X.; Fei, S.; Xie, H.; Qiu, Z.; Sun, Z. A Semi-Analytical Model for Methane Transport and Oxidation in a Soil Cover. Comput. Geotech. 2021, 134, 104117. [Google Scholar] [CrossRef]
- Xie, H.; Zuo, X.; Yan, H.; Peng, Y.; Gu, X.; Chen, Y.; Chen, Y. A Steady-State Analytical Model for Coupled Methane Transport and Oxidation in Vegetated Landfill Cover Soil. Comput. Geotech. 2022, 152, 105006. [Google Scholar] [CrossRef]
- Gebert, J.; Groengroeft, A. Passive Landfill Gas Emission—Influence of Atmospheric Pressure and Implications for the Operation of Methane-Oxidising Biofilters. Waste Manag. 2006, 26, 245–251. [Google Scholar] [CrossRef]
- Czepiel, P.; Shorter, J.; Mosher, B.; Allwine, E.; McManus, J.; Harriss, R.; Kolb, C.; Lamb, B. The Influence of Atmospheric Pressure on Landfill Methane Emissions. Waste Manag. 2003, 23, 593–598. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, T.; Zhang, L.; Ruan, J.; Zhu, X. Measurement and Modeling of the Evaporation Rate of Loess under High Temperature. Int. J. Heat Mass Transf. 2023, 215, 124486. [Google Scholar] [CrossRef]
- Zhan, L.; Wu, T.; Feng, S.; Li, G.; He, H.; Lan, J.; Chen, Y. Full-Scale Experimental Study of Methane Emission in a Loess-Gravel Capillary Barrier Cover under Different Seasons. Waste Manag. 2020, 107, 54–65. [Google Scholar] [CrossRef]
- Spokas, K.A.; Bogner, J.E. Limits and Dynamics of Methane Oxidation in Landfill Cover Soils. Waste Manag. 2011, 31, 823–832. [Google Scholar] [CrossRef]
- Chen, Y.M.; Xu, W.J.; Ling, D.S.; Zhan, L.T.; Gao, W. A Degradation-Consolidation Model for the Stabilization Behavior of Landfilled Municipal Solid Waste. Comput. Geotech. 2020, 118, 103341. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Xu, W.; Zhan, L.; Ling, D.; Ke, H.; Hu, J.; Li, J. A Thermo-Hydro-Mechanical-Biochemical Coupled Model for Landfilled Municipal Solid Waste. Comput. Geotech. 2021, 134, 104090. [Google Scholar] [CrossRef]
- Jafari, N.H.; Stark, T.D.; Thalhamer, T. Spatial and Temporal Characteristics of Elevated Temperatures in Municipal Solid Waste Landfills. Waste Manag. 2017, 59, 286–301. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Zhan, L.; Chen, Y. Laboratory and Numerical Study on an Enhanced Evaporation Process in a Loess Soil Column Subjected to Heating. J. Zhejiang Univ. Sci. A 2016, 17, 553–564. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, X.; Tang, S.; Mohammad, A.; Singh, D.N.; Xie, H.; Chen, Y.; Zuo, X.; Sun, Z. Gas Transport in Landfill Cover System: A Critical Appraisal. J. Environ. Manag. 2022, 321, 116020. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Q.; Wu, J.; Chen, Y. Analytical Model for Methane Migration through Fractured Unsaturated Landfill Cover Soil. Eng. Geol. 2019, 255, 69–79. [Google Scholar] [CrossRef]
- Guan, C.; Xie, H.; Qiu, Z.; Chen, Y.; Chen, P. One-Dimensional Coupled Model for Landfill Gas and Water Transport in Layered Unsaturated Soil Cover Systems. J. Zhejiang Univ. Sci. A 2016, 17, 667–676. [Google Scholar] [CrossRef]
- Wu, T.; Zhan, L.; Feng, S.; Chen, P. Numerical Analysis of Moisture and Gas Transport in Earthen Final Covers Considering Effects of Vapor and Temperature Gradient. Soils Found. 2023, 63, 101262. [Google Scholar] [CrossRef]
- Philip, J.R.; De Vries, D.A. Moisture Movement in Porous Materials under Temperature Gradients. Eos Trans. Am. Geophys. Union 1957, 38, 222–232. [Google Scholar] [CrossRef]
- Saito, H.; Šimůnek, J.; Mohanty, B.P. Numerical Analysis of Coupled Water, Vapor, and Heat Transport in the Vadose Zone. Vadose Zone J. 2006, 5, 784–800. [Google Scholar] [CrossRef]
- Parker, J. Multiphase Flow and Transport in Porous Media. Rev. Geophys. 1989, 27, 311–328. [Google Scholar] [CrossRef]
- Thomas, H.R.; Ferguson, W.J. A Fully Coupled Heat and Mass Transfer Model Incorporating Contaminant Gas Transfer in an Unsaturated Porous Medium. Comput. Geotech. 1999, 24, 65–87. [Google Scholar] [CrossRef]
- De Visscher, A.; Van Cleemput, O. Simulation Model for Gas Diffusion and Methane Oxidation in Landfill Cover Soils. Waste Manag. 2003, 23, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Vereecken, H.; Weynants, M.; Javaux, M.; Pachepsky, Y.; Schaap, M.; Genuchten, M.T. van Using Pedotransfer Functions to Estimate the van Genuchten–Mualem Soil Hydraulic Properties: A Review. Vadose Zone J. 2010, 9, 795–820. [Google Scholar]
- Chen, Y.; Guo, R.; Li, Y.-C.; Liu, H.; Zhan, T.L. A Degradation Model for High Kitchen Waste Content Municipal Solid Waste. Waste Manag. 2016, 58, 376–385. [Google Scholar] [CrossRef]
- Al-Heetimi, O.T.; Van De Ven, C.J.C.; Van Geel, P.J.; Rayhani, M.T. Impact of Temperature on the Performance of Compost-Based Landfill Biocovers. J. Environ. Manag. 2023, 344, 118780. [Google Scholar] [CrossRef]
- Xu, L.; Lin, X.; Amen, J.; Welding, K.; McDermitt, D. Impact of Changes in Barometric Pressure on Landfill Methane Emission. Glob. Biogeochem. Cycles 2014, 28, 679–695. [Google Scholar] [CrossRef]
- Niemczyk, M.; Berenjkar, P.; Wilkinson, N.; Lozecznik, S.; Sparling, R.; Yuan, Q. Enhancement of CH4 Oxidation Potential in Bio-Based Landfill Cover Materials. Process Saf. Environ. Prot. 2021, 146, 943–951. [Google Scholar] [CrossRef]
- Kissas, K.; Ibrom, A.; Kjeldsen, P.; Scheutz, C. Methane Emission Dynamics from a Danish Landfill: The Effect of Changes in Barometric Pressure. Waste Manag. 2022, 138, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Kang, J.-Y.; Lee, N.-H. Estimation of Methane Emission Flux at Landfill Surface Using Laser Methane Detector: Influence of Gauge Pressure. Waste Manag. Res. 2016, 34, 784–792. [Google Scholar] [CrossRef]
- Rees-White, T.; Mønster, J.; Beaven, R.; Scheutz, C. Measuring Methane Emissions from a UK Landfill Using the Tracer Dispersion Method and the Influence of Operational and Environmental Factors. Waste Manag. 2019, 87, 870–882. [Google Scholar] [CrossRef]
- Kondo, J.; Saigusa, N.; Sato, T. A Parameterization of Evaporation from Bare Soil Surfaces. J. Appl. Meteorol. Climatol. 1990, 29, 385–389. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, D.; Wang, Y.; Meng, X.; Chen, W.; Yang, Y. Temporal and Spatial Variations of Urban Climate and Derivation of an Urban Climate Map for Xi’an, China. Sustain. Cities Soc. 2020, 52, 101850. [Google Scholar] [CrossRef]
- Bittelli, M.; Ventura, F.; Campbell, G.S.; Snyder, R.L.; Gallegati, F.; Pisa, P.R. Coupling of Heat, Water Vapor, and Liquid Water Fluxes to Compute Evaporation in Bare Soils. J. Hydrol. 2008, 362, 191–205. [Google Scholar] [CrossRef]
- Tollenaar, R.N.; Van Paassen, L.A.; Jommi, C. Observations on the Desiccation and Cracking of Clay Layers. Eng. Geol. 2017, 230, 23–31. [Google Scholar] [CrossRef]
- Pu, H.-F.; Wen, X.-J.; Min, M.; Chen, J.; Qiu, J.-W. Analytical Solution for Coupled Water–Gas Transport in Landfill Cover. Acta Geotech. 2023, 18, 4219–4231. [Google Scholar] [CrossRef]
- Maanoja, S.T.; Rintala, J.A. Methane Oxidation Potential of Boreal Landfill Cover Materials: The Governing Factors and Enhancement by Nutrient Manipulation. Waste Manag. 2015, 46, 399–407. [Google Scholar] [CrossRef]
- Zhan, L.; Qiu, Q.; Xu, W.; Chen, Y. Field Measurement of Gas Permeability of Compacted Loess Used as an Earthen Final Cover for a Municipal Solid Waste Landfill. J. Zhejiang Univ. Sci. A 2016, 17, 541–552. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids; McGraw Hill Book, Co.: New York, NY, USA, 1987. [Google Scholar]
- Garg, A.; Achari, G. A Comprehensive Numerical Model Simulating Gas, Heat, and Moisture Transport in Sanitary Landfills and Methane Oxidation in Final Covers. Environ. Model. Assess. 2010, 15, 397–410. [Google Scholar] [CrossRef]
- Mauder, M.; Foken, T.; Cuxart, J. Surface-Energy-Balance Closure over Land: A Review. Bound.-Layer Meteorol. 2020, 177, 395–426. [Google Scholar] [CrossRef]
- Zhou, K.; Mao, J.; Li, Y.; Hua, Z. Comparative Study on Thermal Performance of Horizontal Ground Source Heat Pump Systems with Dirichlet and Robin Boundary Conditions on Ground Surface. Energy Convers. Manag. 2020, 225, 113469. [Google Scholar] [CrossRef]
- Kämpf, M.; Holfelder, T.; Montenegro, H. Identification and Parameterization of Flow Processes in Artificial Capillary Barriers. Water Resour. Res. 2003, 39, 2002WR001860. [Google Scholar] [CrossRef]
- Zhan, L.; Li, G.; Jiao, W.; Wu, T.; Lan, J.; Chen, Y. Field Measurements of Water Storage Capacity in a Loess–Gravel Capillary Barrier Cover Using Rainfall Simulation Tests. Can. Geotech. J. 2017, 54, 1523–1536. [Google Scholar] [CrossRef]
- Feng, S.; Ng, C.W.W.; Leung, A.K.; Liu, H.W. Numerical Modelling of Methane Oxidation Efficiency and Coupled Water-Gas-Heat Reactive Transfer in a Sloping Landfill Cover. Waste Manag. 2017, 68, 355–368. [Google Scholar] [CrossRef]
- Zhan, T.L.T.; Yang, Y.B.; Chen, R.; Ng, C.W.W.; Chen, Y.M. Influence of Clod Size and Water Content on Gas Permeability of a Compacted Loess. Can. Geotech. J. 2014, 51, 1468–1474. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, L.; Chen, Y.; Shi, W. Methane Oxidation Capacity of Landfill Cover Loess and Its Impact Factors. China Environ. Sci. 2015, 35, 484–492. [Google Scholar] [CrossRef]
- De Visscher, A.; Thomas, D.; Boeckx, P.; Van Cleemput, O. Methane Oxidation in Simulated Landfill Cover Soil Environments. Environ. Sci. Technol. 1999, 33, 1854–1859. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Lu, J. Experimental Study on Coefficient of Thermal Conductivity and Specific Volume Heat of Loess. Yantu Lixue (Rock Soil Mech.) 2007, 28, 655–658. [Google Scholar] [CrossRef]
- Nastev, M. Modeling Landfill Gas Generation and Migration in Sanitary Landfills and Geological Formations. Ph.D. Thesis, University of Calgary, Calgary, AB, Canada, 1998. [Google Scholar]
- Bowers, S.A.; Hanks, R.J. Specific Heat Capacity of Soils and Minerals as Determined with a Radiation Calorimeter. Soil Sci. 1962, 94, 392–396. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Feng, S.; Chen, C.; Chen, G.; Zhang, Z. Numerical Study of Moisture Transfer and Methane Emission in Earthen Final Covers: Effects of Ambient Conditions. Atmosphere 2025, 16, 1058. https://doi.org/10.3390/atmos16091058
Wu T, Feng S, Chen C, Chen G, Zhang Z. Numerical Study of Moisture Transfer and Methane Emission in Earthen Final Covers: Effects of Ambient Conditions. Atmosphere. 2025; 16(9):1058. https://doi.org/10.3390/atmos16091058
Chicago/Turabian StyleWu, Tao, Song Feng, Cheng Chen, Guannian Chen, and Zhangjing Zhang. 2025. "Numerical Study of Moisture Transfer and Methane Emission in Earthen Final Covers: Effects of Ambient Conditions" Atmosphere 16, no. 9: 1058. https://doi.org/10.3390/atmos16091058
APA StyleWu, T., Feng, S., Chen, C., Chen, G., & Zhang, Z. (2025). Numerical Study of Moisture Transfer and Methane Emission in Earthen Final Covers: Effects of Ambient Conditions. Atmosphere, 16(9), 1058. https://doi.org/10.3390/atmos16091058






