In Silico Characterization of Molecular Interactions of Aviation-Derived Pollutants with Human Proteins: Implications for Occupational and Public Health
Abstract
1. Introduction
2. Methods
2.1. Identification of Target Proteins and Aviation-Derived Air Pollutants
| Protein Name | Protein Structure | PDB ID | Normal Function in Cells | Key Health Effects |
|---|---|---|---|---|
| Estrogen receptor (ER) α ligand-binding domain | 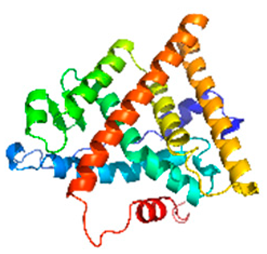 | 3ERT | Nuclear hormone receptor; ligand-activated transcription factor for estrogen-responsive genes. | Endocrine disruption linked to breast cancer and fertility disorders in exposed populations [17]. |
| Androgen receptor (AR) ligand-binding domain | 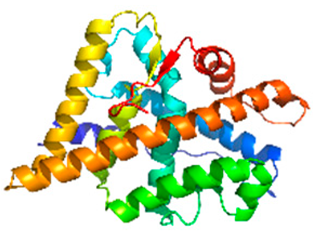 | 2AM9 | Ligand-activated transcription factor controlling male sexual differentiation and reproductive function. | Endocrine disruption affects pubertal development and spermatogenesis [18]. |
| Thyroid hormone receptor β ligand-binding domain | 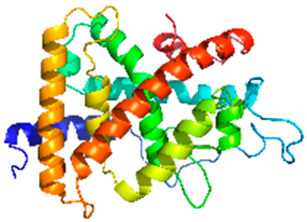 | 1N46 | Nuclear receptor for T3; regulates basal metabolism and development via gene transcription. | Exposure to air pollutants is associated with increased risk of thyroid cancer and hypothyroidism [19]. |
| Vitamin D3 receptor ligand-binding domain | 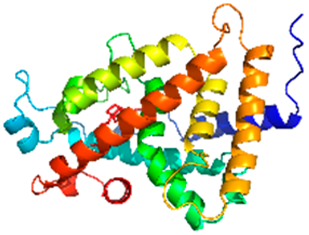 | 1IE9 | Ligand-activated transcription factor controlling calcium/phosphate homeostasis. | Vitamin D deficiency exacerbates asthma and chronic obstructive pulmonary disorder (COPD) in chronically exposed cohorts [20]. |
| Acetylcholinesterase |  | 4M0E | Hydrolyzes acetylcholine to terminate neurotransmission at cholinergic synapses. | Structural alterations observed in different types of tumors from the brain, lung, breast, renal, and colon cancers [21]. |
| Human serum albumin (HSA) |  | 1H9Z | Main plasma carrier for fatty acids, hormones, and drugs; maintains oncotic pressure. | Forms adducts with pollutants like PAHs and is used as a biomarker for pollutant exposure [22]. |
| Hemoglobin (α-chain) |  | 2DN1 | Transports O2 from lungs to tissues and CO2 back to lungs. | Exposure to air pollutants has been associated with reduced hemoglobin levels and anemia [23]. |
| Cytochrome P450 1A1 (CYP1A1) |  | 4I8V | Monooxygenase metabolizing xenobiotics (PAHs; drugs) and endogenous lipids. | Impacts pulmonary inflammation due to PAH-induced activity of CYP1A1 [24]. |
2.2. Molecular Docking Simulations
2.3. Determination of Protein–Pollutant Interactions
3. Results and Discussion
3.1. Selection of Pollutants and Proteins for Molecular Docking Analysis
3.2. Binding Affinities of Pollutant–Protein Complexes
3.3. Structural Interactions of High-Affinity Protein–Pollutant Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bendtsen, K.M.; Bengtsen, E.; Saber, A.T.; Vogel, U. A review of health effects associated with exposure to jet engine emissions in and around airports. Environ. Health 2021, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Brook, R.D.; Li, Y.; Rajagopalan, S.; Kim, J.B. Air Pollution, Built Environment, and Early Cardiovascular Disease. Circ. Res. 2023, 132, 1707–1724. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.C.; Pastorinho, M.R.; Masjedi, M.R.; Urrutia-Pereira, M.; Arrais, M.; Nunes, E.; To, T.; Ferreira, A.J.; Robalo-Cordeiro, C.; Borreg, C.; et al. Issue 1–‘Update on adverse respiratory effects of outdoor air pollution’ Part 2: Outdoor air pollution and respiratory diseases: Perspectives from Angola, Brazil, Canada, Iran, Mozambique and Portugal. Pulmonology 2022, 28, 376–395. [Google Scholar] [CrossRef] [PubMed]
- Dimakakou, E.; Johnston, H.J.; Streftaris, G.; Cherrie, J.W. Exposure to Environmental and Occupational Particulate Air Pollution as a Potential Contributor to Neurodegeneration and Diabetes: A Systematic Review of Epidemiological Research. Int. J. Environ. Res. Public Health 2018, 15, 1704. [Google Scholar] [CrossRef] [PubMed]
- How Air Pollution Affects Our Health. 2024. Available online: https://www.eea.europa.eu/en/topics/in-depth/air-pollution/eow-it-affects-our-health (accessed on 31 March 2025).
- Hudda, N.; Durant, L.W.; Fruin, S.A.; Durant, J.L. Impacts of Aviation Emissions on Near-Airport Residential Air Quality. Environ. Sci. Technol. 2020, 54, 8580–8588. [Google Scholar] [CrossRef]
- Nagar, N.; Saxena, H.; Pathak, A.; Mishra, A.; Poluri, K.M. A review on structural mechanisms of protein-persistent organic pollutant (POP) interactions. Chemosphere 2023, 332, 138877. [Google Scholar] [CrossRef]
- Låg, M.; Øvrevik, J.; Refsnes, M.; Holme, J.A. Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases. Respir. Res. 2020, 21, 299. [Google Scholar] [CrossRef]
- Plunk, E.C.; Richards, S.M. Endocrine-Disrupting Air Pollutants and Their Effects on the Hypothalamus-Pituitary-Gonadal Axis. Int. J. Mol. Sci. 2020, 21, 9191. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zeng, G.; Shao, B.; Chen, M.; Li, Z.; Jiang, Y.; Liu, Y.; Zhang, Y.; Zhong, H. Application of molecular docking for the degradation of organic pollutants in the environmental remediation: A review. Chemosphere 2018, 203, 139–150. [Google Scholar] [CrossRef]
- Montero-Pérez, Y.; Pájaro-Castro, N.; Coronado-Posada, N.; Ahumedo-Monterrosa, M.; Olivero-Verbel, J. Human Target Proteins for Benzo(a)pyrene and Acetaminophen (And Its Metabolites): Insights from Inverse Molecular Docking and Molecular Dynamics Simulations. Sci. Pharm. 2024, 92, 55. [Google Scholar] [CrossRef]
- Chushak, Y.G.; Chapleau, R.R.; Frey, J.S.; Mauzy, C.A.; Gearhart, J.M. Identifying potential protein targets for toluene using a molecular similarity search, in silico docking and in vitro validation. Toxicol. Res. 2015, 4, 519–526. [Google Scholar] [CrossRef]
- Liu, L.; Cui, H.; Huang, Y.; Yan, H.; Zhou, Y.; Wan, Y. Molecular docking and in vitro evaluations reveal the role of human cytochrome P450 3A4 in the cross-coupling metabolism of phenolic xenobiotics. Environ. Res. 2023, 220, 115256. [Google Scholar] [CrossRef]
- Vincent-Hall, T.D.; Bergeron, J.G.; Eftim, S.E.; Lindahl, A.J.; Weinberger, K.R.; Haver, C.E.; Snow, S.J. Health effects of occupational exposure to jet fuels used in the military: A systematic review of the epidemiologic literature. Environ. Int. 2025, 196, 109278. [Google Scholar] [CrossRef]
- Mokalled, T.; Gérard, J.A.; Abboud, M.; Trocquet, C.; Nasreddine, R.; Person, V.; le Calvé, S. VOC tracers from aircraft activities at Beirut Rafic Hariri International Airport. Atmos. Pollut. Res. 2019, 10, 537–551. [Google Scholar] [CrossRef]
- Souza, T.L.; da Luz, J.Z.; Barreto Ldos, S.; de Oliveira Ribeiro, C.A.; Neto, F.F. Structure-based modeling to assess binding and endocrine disrupting potential of polycyclic aromatic hydrocarbons in Danio rerio. Chem.-Biol. Interact. 2024, 398, 111109. [Google Scholar] [CrossRef]
- Gearhart-Serna, L.M.; Davis, J.B.; Jolly, M.K.; Jayasundara, N.; Sauer, S.J.; Di Giulio, R.T.; Devi, G.R. A polycyclic aromatic hydrocarbon-enriched environmental chemical mixture enhances AhR, antiapoptotic signaling and a proliferative phenotype in breast cancer cells. Carcinogenesis 2020, 41, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D. Overview of air pollution and endocrine disorders. Int. J. Gen. Med. 2018, 11, 191–207. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, K.; Qian, T.; Li, X.; Yi, W.; Pan, R.; Huang, Y.; Ji, Y.; Su, H. Association between ambient air pollution and thyroid hormones levels: A systematic review and meta-analysis. Sci. Total Environ. 2023, 904, 166780. [Google Scholar] [CrossRef]
- Janssens, W.; Decramer, M.; Mathieu, C.; Korf, H. Vitamin D and chronic obstructive pulmonary disease: Hype or reality? Lancet Respir. Med. 2013, 1, 804–812. [Google Scholar] [CrossRef]
- Xi, H.-J.; Wu, R.-P.; Liu, J.-J.; Zhang, L.-J.; Li, Z.-S. Role of acetylcholinesterase in lung cancer. Thorac. Cancer 2015, 6, 390–398. [Google Scholar] [CrossRef]
- Smith, J.W.; O’mEally, R.N.; Ng, D.K.; Chen, J.-G.; Kensler, T.W.; Cole, R.N.; Groopman, J.D. Biomonitoring of ambient outdoor air pollutant exposure in humans using targeted serum albumin adductomics. Chem. Res. Toxicol. 2021, 34, 1183–1196. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, H.-J. Association of ambient air pollution with hemoglobin levels and anemia in the general population of Korean adults. BMC Public Health 2024, 24, 988. [Google Scholar] [CrossRef]
- Arlt, V.M.; Krais, A.M.; Godschalk, R.W.; Riffo-Vasquez, Y.; Mrizova, I.; Roufosse, C.A.; Corbin, C.; Shi, Q.; Frei, E.; Stiborova, M.; et al. Pulmonary Inflammation Impacts on CYP1A1-Mediated Respiratory Tract DNA Damage Induced by the Carcinogenic Air Pollutant Benzo[a]pyrene. Toxicol. Sci. 2015, 146, 213–225. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, Version 3.0; Schrödinger LLC: Palo Alto, CA, USA, 2024.
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- UCSF ChimeraX: Tools for Structure Building and Analysis-Meng-2023-Protein Science-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/pro.4792 (accessed on 30 May 2025).
- Lai, C.-H.; Chuang, K.-Y.; Chang, J.-W. Characteristics of nano-/ultrafine particle-bound PAHs in ambient air at an international airport. Environ. Sci. Pollut. Res. 2013, 20, 1772–1780. [Google Scholar] [CrossRef]
- Anderson, B.E.; Chen, G.; Blake, D.R. Hydrocarbon emissions from a modern commercial airliner. Atmos. Environ. 2006, 40, 3601–3612. [Google Scholar] [CrossRef]
- Schürmann, G.; Schäfer, K.; Jahn, C.; Hoffmann, H.; Bauerfeind, M.; Fleuti, E.; Rappenglück, B. The impact of NOx, CO and VOC emissions on the air quality of Zurich airport. Atmos. Environ. 2007, 41, 103–118. [Google Scholar] [CrossRef]
- Shimada, T.; Fujii-Kuriyama, Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and1B1. Cancer Sci. 2004, 95, 1–6. [Google Scholar] [CrossRef]
- Rostamnezhad, F.; Hossein Fatemi, M. Comprehensive investigation of binding of some polycyclic aromatic hydrocarbons with bovine serum albumin: Spectroscopic and molecular docking studies. Bioorganic Chem. 2022, 120, 105656. [Google Scholar] [CrossRef]
- Harrison, V.; Mackenzie Ross, S.J. An emerging concern: Toxic fumes in airplane cabins. Cortex 2016, 74, 297–302. [Google Scholar] [CrossRef]
- Guo, Y.; Li, N. Network toxicology and molecular docking to investigative the non-acetylcholinesterase mechanisms and targets of cardiotoxicity injury induced by organophosphorus pesticides. Medicine 2024, 103, e39963. [Google Scholar] [CrossRef]
- Olsson, A.; Guha, N.; Bouaoun, L.; Kromhout, H.; Peters, S.; Siemiatycki, J.; Ho, V.; Gustavsson, P.; Boffetta, P.; Vermeulen, R.; et al. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Lung Cancer Risk: Results from a Pooled Analysis of Case–Control Studies (SYNERGY). Cancer Epidemiol. Biomark. Prev. 2022, 31, 1433–1441. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Arcaro, K.; Spink, D.C. Understanding the human health effects of chemical mixtures. Environ. Health Perspect. 2002, 110, 25–42. [Google Scholar] [CrossRef]
- Andersen, M.H.G.; Saber, A.T.; Frederiksen, M.; Clausen, P.A.; Sejbaek, C.S.; Hemmingsen, C.H.; Ebbehøj, N.E.; Aimonen, K.; Koivisto, J.; Loft, S. Occupational Exposure and Markers of Genetic Damage, Systemic Inflammation and Lung Function: A Danish Cross-Sectional Study among Air Force Personnel. Sci. Rep. 2021, 11, 17998. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.E.; Branham, H.-S.; Hudgins, C.H.; Plant, J.V.; Ballenthin, J.O.; Miller, T.M.; Viggiano, A.A.; Blake, D.R.; Boudries, H.; Canagaratna, M. Experiment to Characterize Aircraft Volatile Aerosol and Trace-Species Emissions (EXCAVATE). NASA/TM-2005-213783, 1 August 2005. Available online: https://ntrs.nasa.gov/citations/20050214696 (accessed on 30 March 2025).
- Campo, L.; Buratti, M.; Fustinoni, S.; Cirla, P.E.; Martinotti, I.; Longhi, O.; Cavallo, D.; Foà, V. Evaluation of Exposure to PAHs in Asphalt Workers by Environmental and Biological Monitoring. Ann. N. Y. Acad. Sci. 2006, 1076, 405–420. [Google Scholar] [CrossRef]
- Fushimi, A.; Saitoh, K.; Fujitani, Y.; Takegawa, N. Identification of Jet Lubrication Oil as a Major Component of Aircraft Exhaust Nanoparticles. Atmos. Chem. Phys. 2019, 19, 6389–6399. [Google Scholar] [CrossRef]
- Shi, Q.; Guo, W.; Shen, Q.; Han, J.; Lei, L.; Chen, L.; Yang, L.; Feng, C.; Zhou, B. In Vitro Biolayer Interferometry Analysis of Acetylcholinesterase as a Potential Target of Aryl-Organophosphorus Flame-Retardants. J. Hazard. Mater. 2021, 409, 124999. [Google Scholar] [CrossRef]
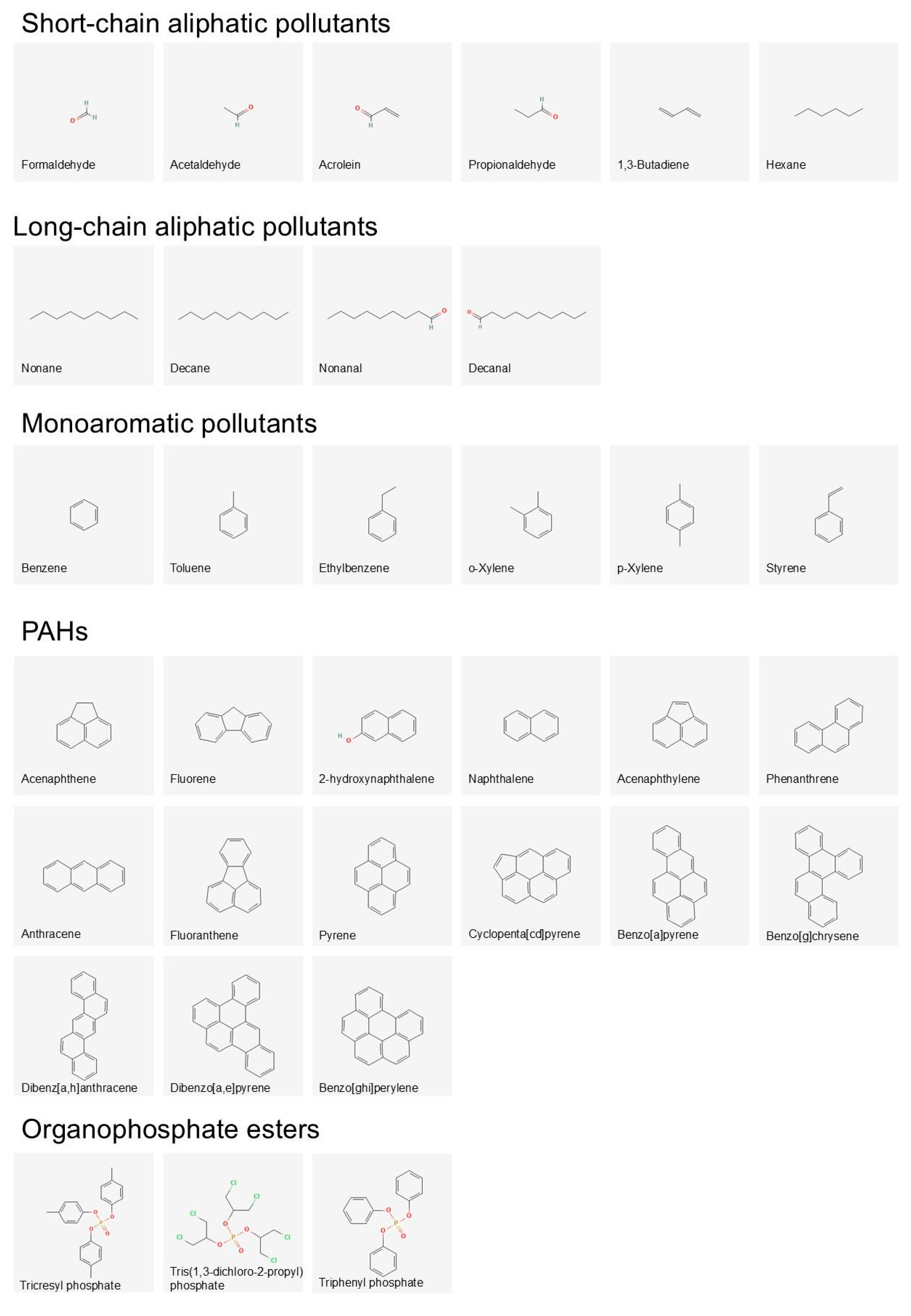
| Pollutant Category | Chemical Pollutant | Estrogen Receptor | Androgen Receptor | Thyroid Receptor | Nuclear Receptor Vit D | Acetylcholinesterase | Human Serum Albumin | Hemoglobin Alpha | Cytochrome P450 1A1 | Average Affinity Across Proteins | Average Affinity for Pollutant Category |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Light chain aliphatic VOCs | Formaldehyde | −1.8 | −1.7 | −1.9 | −2.3 | −2.0 | −2.0 | −1.7 | −1.7 | −1.9 | −3.1 |
| Acetaldehyde | −2.6 | −2.6 | −2.6 | −3.0 | −3.0 | −2.6 | −2.3 | −2.5 | −2.6 | ||
| Acrolein | −2.8 | −3.3 | −3.2 | −3.2 | −3.1 | −3.3 | −2.8 | −3.3 | −3.1 | ||
| Propionaldehyde | −2.9 | −3.2 | −3.3 | −2.8 | −3.0 | −3.4 | −2.9 | −3.4 | −3.1 | ||
| 1,3-Butadiene | −3.1 | −3.6 | −3.3 | −2.9 | −3.3 | −3.7 | −3.2 | −4.0 | −3.4 | ||
| Hexane | −3.8 | −4.0 | −4.2 | −3.4 | −4.1 | −4.5 | −4.1 | −5.0 | −4.1 | ||
| Heavy chain aliphatic VOCs | Nonane | −4.4 | −3.8 | −4.8 | −3.8 | −5.0 | −5.6 | −4.5 | −5.9 | −4.7 | −4.7 |
| Decane | −4.1 | −3.9 | −3.5 | −3.9 | −5.1 | −5.7 | −4.7 | −6.2 | −4.7 | ||
| Nonanal | −4.3 | −4.0 | −4.6 | −3.8 | −5.0 | −5.4 | −3.7 | −5.9 | −4.6 | ||
| Decanal | −4.3 | −4.2 | −4.1 | −4.1 | −5.0 | −5.7 | −5.3 | −6.2 | −4.9 | ||
| Monoaromatic VOCs | Benzene | −4.2 | −5.0 | −4.5 | −3.7 | −4.9 | −5.2 | −4.4 | −5.6 | −4.7 | −5.5 |
| Toluene | −5.0 | −5.0 | −5.2 | −4.3 | −5.5 | −6.1 | −5.3 | −6.4 | −5.3 | ||
| Ethylbenzene | −5.0 | −5.0 | −5.2 | −4.5 | −5.7 | −6.5 | −5.5 | −6.8 | −5.5 | ||
| o-Xylene | −5.5 | −5.3 | −5.7 | −4.6 | −6.1 | −6.8 | −5.8 | −7.1 | −5.9 | ||
| p-Xylene | −5.3 | −5.3 | −5.8 | −4.7 | −5.9 | −6.8 | −5.5 | −7.1 | −5.8 | ||
| Styrene | −4.9 | −5.3 | −5.5 | −4.5 | −5.7 | −6.5 | −5.6 | −6.8 | −5.6 | ||
| PAHs - two (top) to five (bottom) aromatic rings | Naphthalene | −6.3 | −5.9 | −6.4 | −5.2 | −7.2 | −8.3 | −7.0 | −8.5 | −6.9 | −9.0 |
| 2-hydroxynaphthalene | −6.2 | −6.1 | −5.6 | −5.3 | −7.0 | −8.3 | −5.7 | −8.8 | −6.6 | ||
| Acenaphthene | −6.9 | −7.4 | −7.3 | −5.7 | −8.4 | −9.6 | −8.3 | −9.9 | −7.9 | ||
| Fluorene | −7.5 | −7.6 | −6.6 | −5.8 | −8.9 | −10.0 | −7.8 | −10.5 | −8.1 | ||
| Acenaphthylene | −6.9 | −7.4 | −8.0 | −5.8 | −8.4 | −9.8 | −8.3 | −9.9 | −8.1 | ||
| Phenanthrene | −7.9 | −8.2 | −8.2 | −6.1 | −9.6 | −10.7 | −8.3 | −11.2 | −8.8 | ||
| Anthracene | −7.6 | −6.7 | −8.1 | −6.1 | −9.4 | −10.7 | −7.9 | −11.2 | −8.5 | ||
| Fluoranthene | −8.4 | −9.0 | −8.0 | −6.8 | −10.2 | −12.0 | −8.6 | −12.6 | −9.5 | ||
| Pyrene | −8.2 | −7.6 | −6.9 | −6.7 | −10.5 | −11.8 | −10.4 | −12.3 | −9.3 | ||
| Cyclopenta-cd-pyrene | −8.4 | −8.3 | −8.2 | −7.1 | −11.2 | −13.2 | −7.6 | −13.9 | −9.7 | ||
| Benzo-a-pyrene | −8.6 | −9.5 | −8.0 | −7.1 | −12.2 | −13.3 | −7.3 | −15.3 | −10.2 | ||
| Benzo-g-chrysene | −9.3 | −7.8 | −8.2 | −7.7 | −10.9 | −14.5 | −8.1 | −16.3 | −10.3 | ||
| Dibenz-ah-anthracene | −8.8 | −8.3 | −8.3 | −7.8 | −12.6 | −14.0 | −8.0 | −16.3 | −10.5 | ||
| Dibenzo-ae-pyrene | −10.1 | −8.6 | −9.4 | −8.0 | −12.2 | −15.0 | −8.4 | −17.6 | −11.1 | ||
| Benzo-ghi-perylene | −8.9 | −8.1 | −8.2 | −7.4 | −10.8 | −14.6 | −7.4 | −16.1 | −10.2 | ||
| Organophosphate esters | TCP | −7.5 | −7.4 | −7.1 | −6.6 | −8.9 | −9.8 | −6.5 | −9.5 | −7.9 | −6.7 |
| TDCIPP | −4.6 | −4.3 | −5.0 | −4.5 | −5.4 | −5.4 | −4.0 | −6.6 | −5.0 | ||
| TPHP | −6.9 | −6.5 | −6.5 | −6.5 | −7.3 | −8.7 | −6.3 | −9.5 | −7.3 | ||
| Average affinity of protein for all pollutants | -6.0 | −5.9 | −5.9 | −5.2 | −7.2 | −8.2 | −6.0 | −8.8 | |||
| Protein | Ligand | Interaction | Residues |
|---|---|---|---|
| Estrogen receptor | Benzo-a-pyrene | Hydrophobic interactions | Ala350, Leu354, Trp383, Leu525, Leu536 |
| Pi-stacking | Trp383 | ||
| Dibenzo-ae-pyrene | Hydrophobic interactions | Thr347, Leu354, Trp383, Leu525, Leu536 | |
| Pi-stacking | Trp383 | ||
| Tricresyl phosphate | Hydrophobic interactions | Leu354, Trp383, Leu525, Tyr526, Lys529, Val533, Leu536 | |
| Pi-stacking | Trp383 | ||
| Androgen receptor | Benzo-a-pyrene | Hydrophobic interactions | Leu707, Gln711, Met745, Val746, Leu873 |
| Pi-stacking | Phe764 | ||
| Dibenzo-ae-pyrene | Hydrophobic interactions | Ile816, Val818, Lys912, Ile914, Tyr915 | |
| Tricresyl phosphate | Hydrogen bonds | Trp751 | |
| Hydrophobic interactions | Glu681, Pro682, Val684, Ala748, Arg752, Pro801, Phe804, Leu805 | ||
| Pi-cation interactions | Arg752 | ||
| Thyroid receptor | Benzo-a-pyrene | Hydrophobic interactions | Ile303, Lys306, Ala436, Phe459 |
| Dibenzo-ae-pyrene | Hydrophobic interactions | Ile303, Lys306, Ala436, Phe459 | |
| Pi-cation interactions | Arg383 | ||
| Tricresyl phosphate | Hydrogen bonds | Lys411, His412, Phe417, Trp418 | |
| Salt bridges | Lys211 | ||
| Hydrophobic interactions | Ile407, Lys411, Trp418 | ||
| Pi-cation interactions | Lys211 | ||
| Nuclear receptor Vit D | Benzo-a-pyrene | Hydrophobic interactions | Gln364, Arg368, Leu378, Tyr380 |
| Dibenzo-ae-pyrene | Hydrophobic interactions | Gln152, Phe153, Tyr236, Thr415 | |
| Tricresyl phosphate | Hydrogen bonds | Arg343, Asn394 | |
| Hydrophobic interactions | Pro344, Arg391, Asn394, Glu395 | ||
| Acetylcholinesterase | Benzo-a-pyrene | Hydrophobic interactions | Tyr72, Trp286, Val294, Phe338, Tyr341 |
| Pi-stacking | Trp286, Tyr341 | ||
| Dibenzo-ae-pyrene | Hydrophobic interactions | Tyr72, Trp286, Leu289, Phe338, Tyr341 | |
| Pi-stacking | Trp286 | ||
| Tricresyl phosphate | Hydrophobic interactions | Trp286, Leu289, Glu292, Tyr337, Phe338, Tyr341 | |
| Pi-stacking | Tyr341 | ||
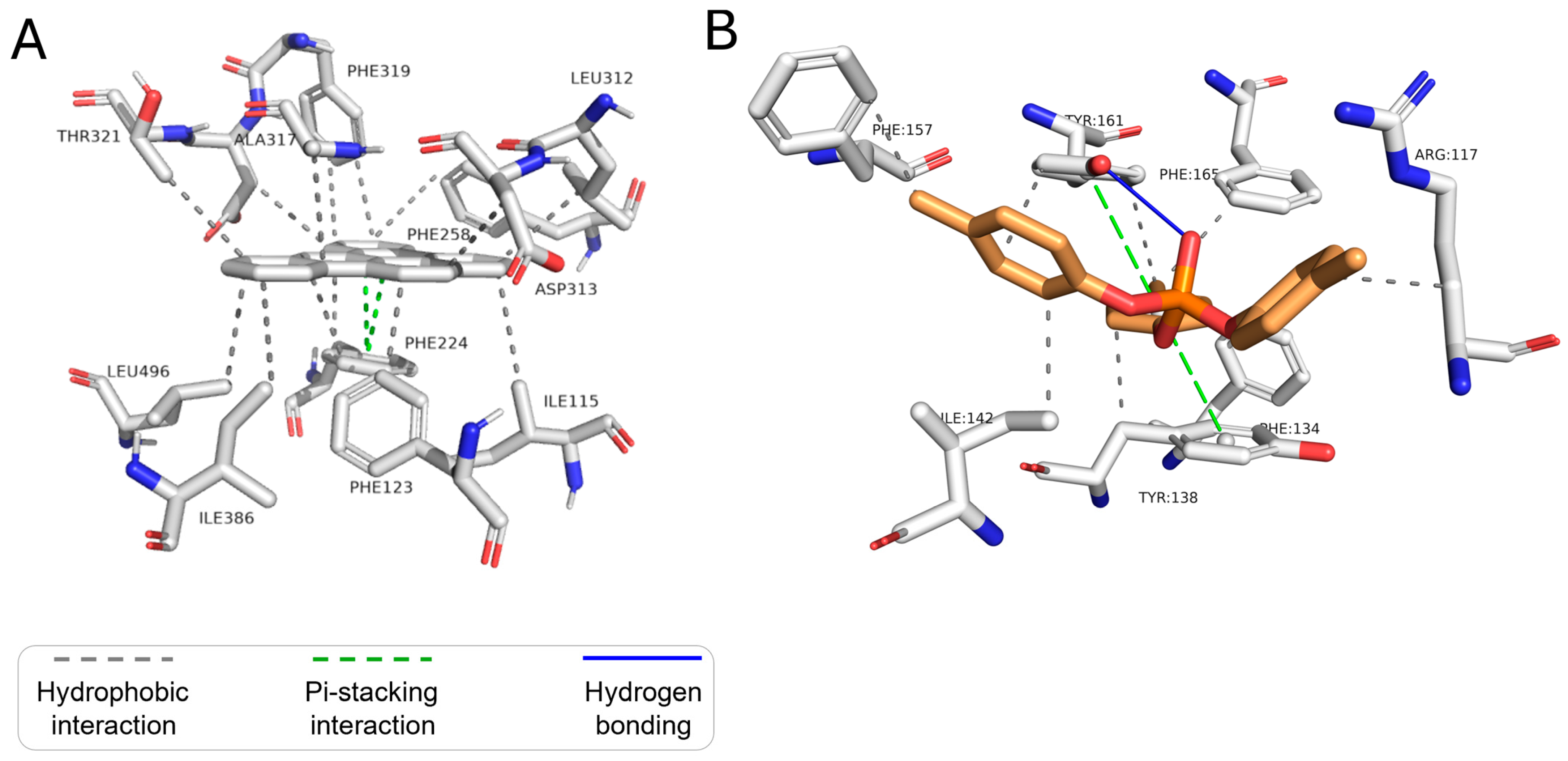
| Human Serum Albumin (HSA) | Benzo-a-pyrene | Hydrophobic interactions | Arg117, Phe134, Tyr138, Ile142, Ala158, Tyr161, Phe165 |
| Pi-stacking | Tyr138, Tyr161 | ||
| Dibenzo-ae-pyrene | Hydrophobic interactions | Tyr138, Ile142, Leu154, Tyr161 | |
| Pi-stacking | Tyr161 | ||
| Tricresyl phosphate | Hydrogen bonds | Tyr161 | |
| Hydrophobic interactions | Arg117, Phe134, Tyr138, Ile142, Phe157, Tyr161, Phe165 | ||
| Pi-stacking | Tyr138, Tyr161 | ||
| Hemoglobin alpha | Benzo-a-pyrene | Hydrophobic interactions | Glu23, Glu27, Glu30, Val55 |
| Dibenzo-ae-pyrene | Hydrophobic interactions | Glu23, Ala26, Glu27, Glu30, Val55, Lys56 | |
| Tricresyl phosphate | Hydrophobic interactions | Glu23, Ala26, Val55, Lys56 | |
| Cytochrome P450 1A1 (CYP 1A1) | Benzo-a-pyrene | Hydrophobic interactions | Phe123, Phe224, Phe258, Ala317, Phe319, Asp320, Thr321, Ile386, Leu496 |
| Pi-stacking | Phe224 | ||
| Dibenzo-ae-pyrene | Hydrophobic interactions | Ile115, Phe123, Phe224, Phe258, Leu312, Asp313, Ala317, Phe319, Asp320, Thr321, Ile386, Leu496 | |
| Pi-stacking | Phe224 | ||
| Tricresyl phosphate | Hydrophobic interactions | Ile115, Phe224, Leu254, Phe258, Leu312, Ala317, Phe319, Asp320, Ile386, Leu496 | |
| Pi-stacking | Phe123, Phe224 | ||
| Hydrogen bonds | Ala317 |
| Individual Pollutant Binding Affinity | Multi-Pollutant Binding Affinity | |||||
|---|---|---|---|---|---|---|
| Protein | VOC (p-xylene) | PAH (Benzo-a-pyrene) | OPE (Tricresyl phosphate) | VOC-PAH | PAH-OPE | VOC-OPE |
| Androgen receptor | −5.4 | −10.5 | −7.4 | −16 | −13 | −9.9 |
| Human serum albumin | −5.9 | −12.2 | −9 | −19.3 | −18.5 | −14.6 |
| Acetylcholinesterase | −6.7 | −13.3 | −10 | −18 | −17.6 | −12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, C.; Nazarenko, Y. In Silico Characterization of Molecular Interactions of Aviation-Derived Pollutants with Human Proteins: Implications for Occupational and Public Health. Atmosphere 2025, 16, 919. https://doi.org/10.3390/atmos16080919
Narayanan C, Nazarenko Y. In Silico Characterization of Molecular Interactions of Aviation-Derived Pollutants with Human Proteins: Implications for Occupational and Public Health. Atmosphere. 2025; 16(8):919. https://doi.org/10.3390/atmos16080919
Chicago/Turabian StyleNarayanan, Chitra, and Yevgen Nazarenko. 2025. "In Silico Characterization of Molecular Interactions of Aviation-Derived Pollutants with Human Proteins: Implications for Occupational and Public Health" Atmosphere 16, no. 8: 919. https://doi.org/10.3390/atmos16080919
APA StyleNarayanan, C., & Nazarenko, Y. (2025). In Silico Characterization of Molecular Interactions of Aviation-Derived Pollutants with Human Proteins: Implications for Occupational and Public Health. Atmosphere, 16(8), 919. https://doi.org/10.3390/atmos16080919







