Remediation Effects of Potamogeton crispus on Nitrogen-Loaded Water Bodies and Its Greenhouse Gas Emission Mechanisms
Abstract
1. Introduction
2. Materials and Methods
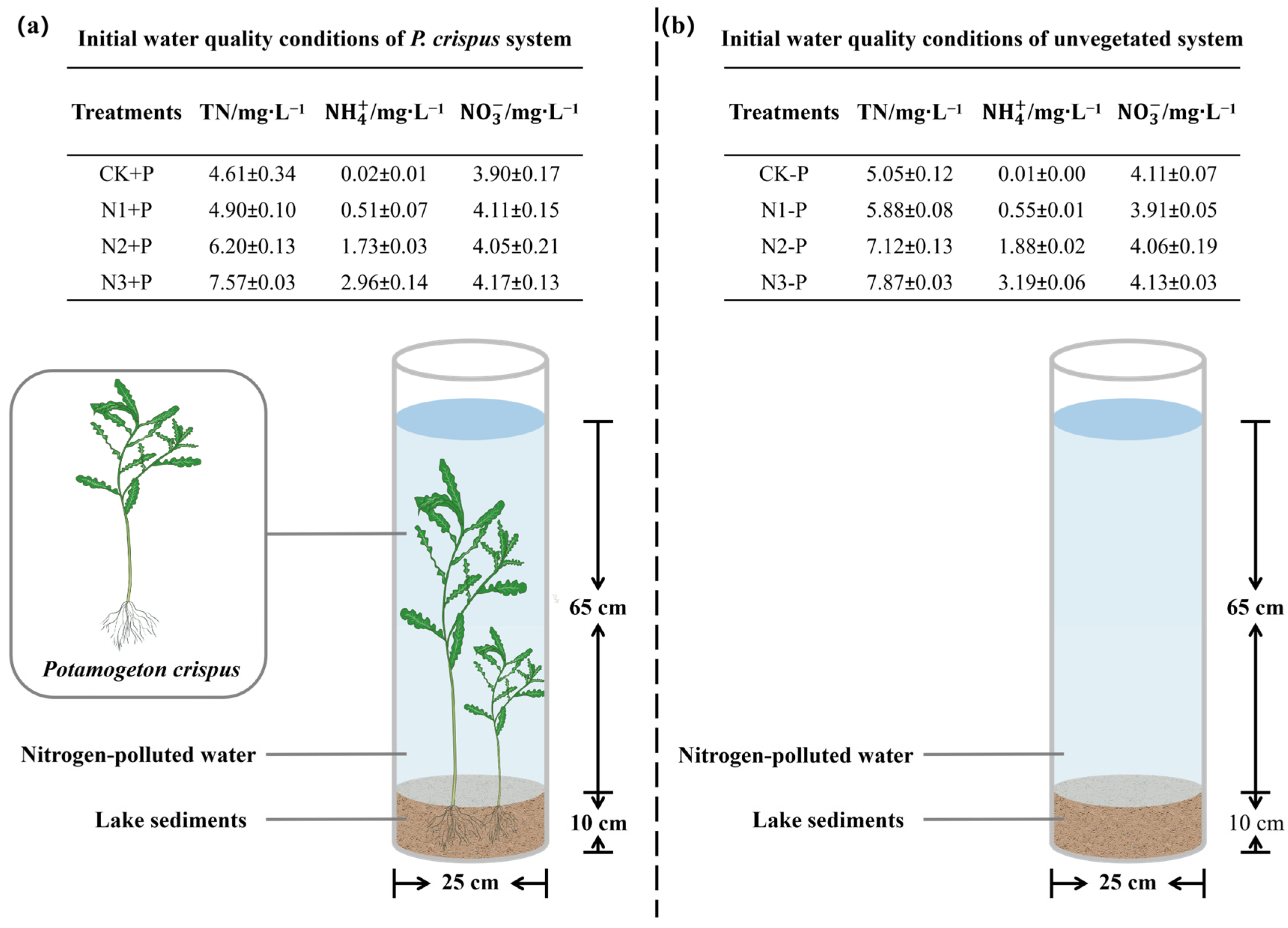
2.1. Expermental Site and Expermental Design
2.2. Sampling and Test
2.3. Data Analysis
3. Results and Discussion
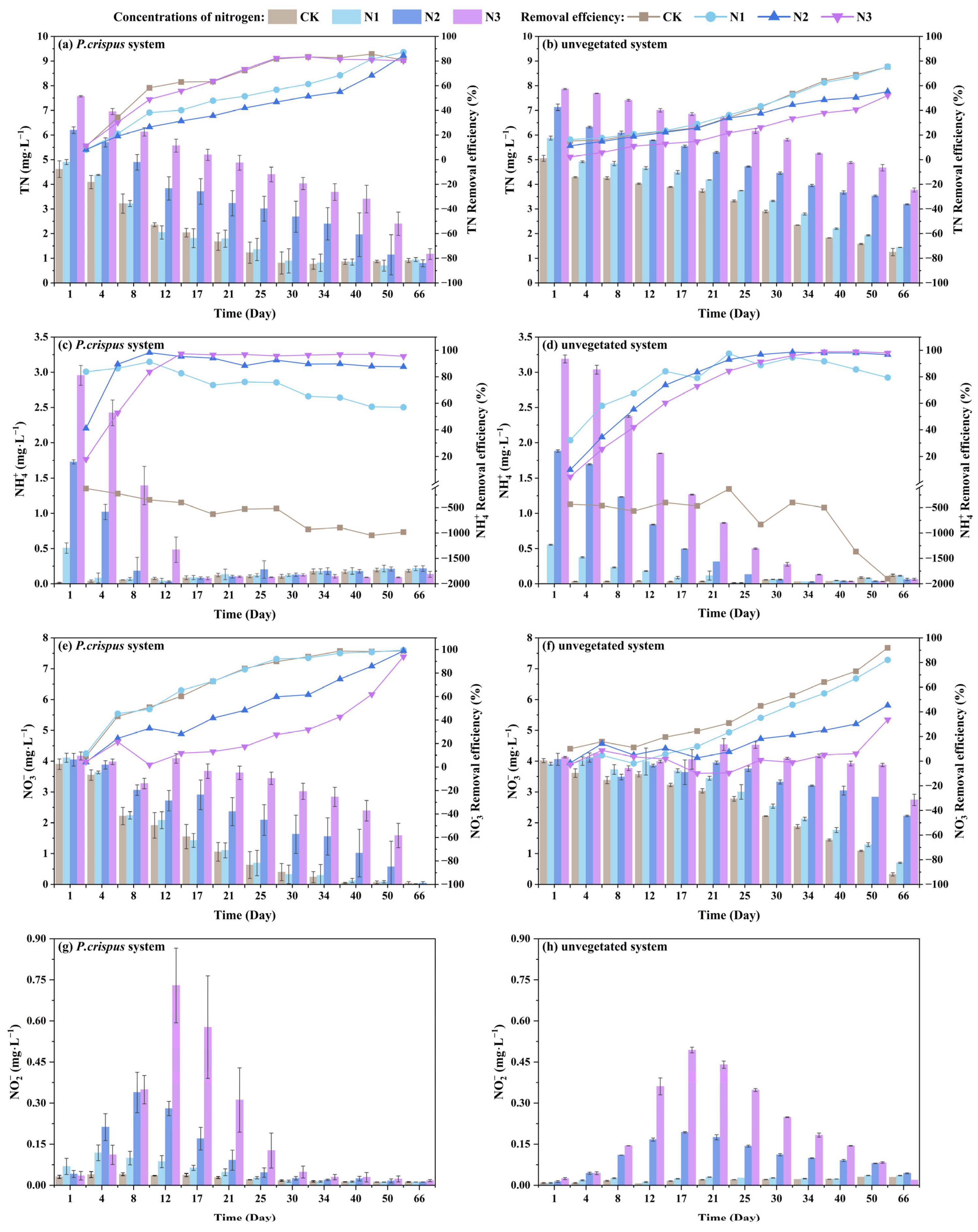
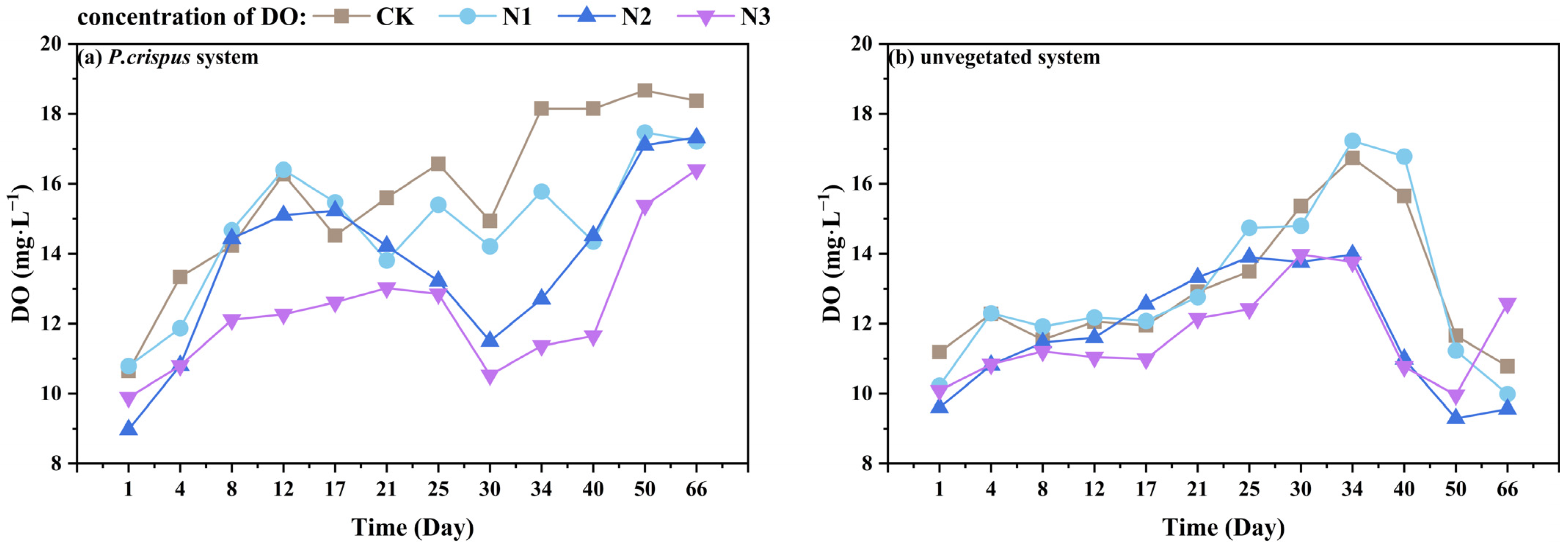
3.1. The Nitrogen Removal Efficiency of P. crispus in Water Body
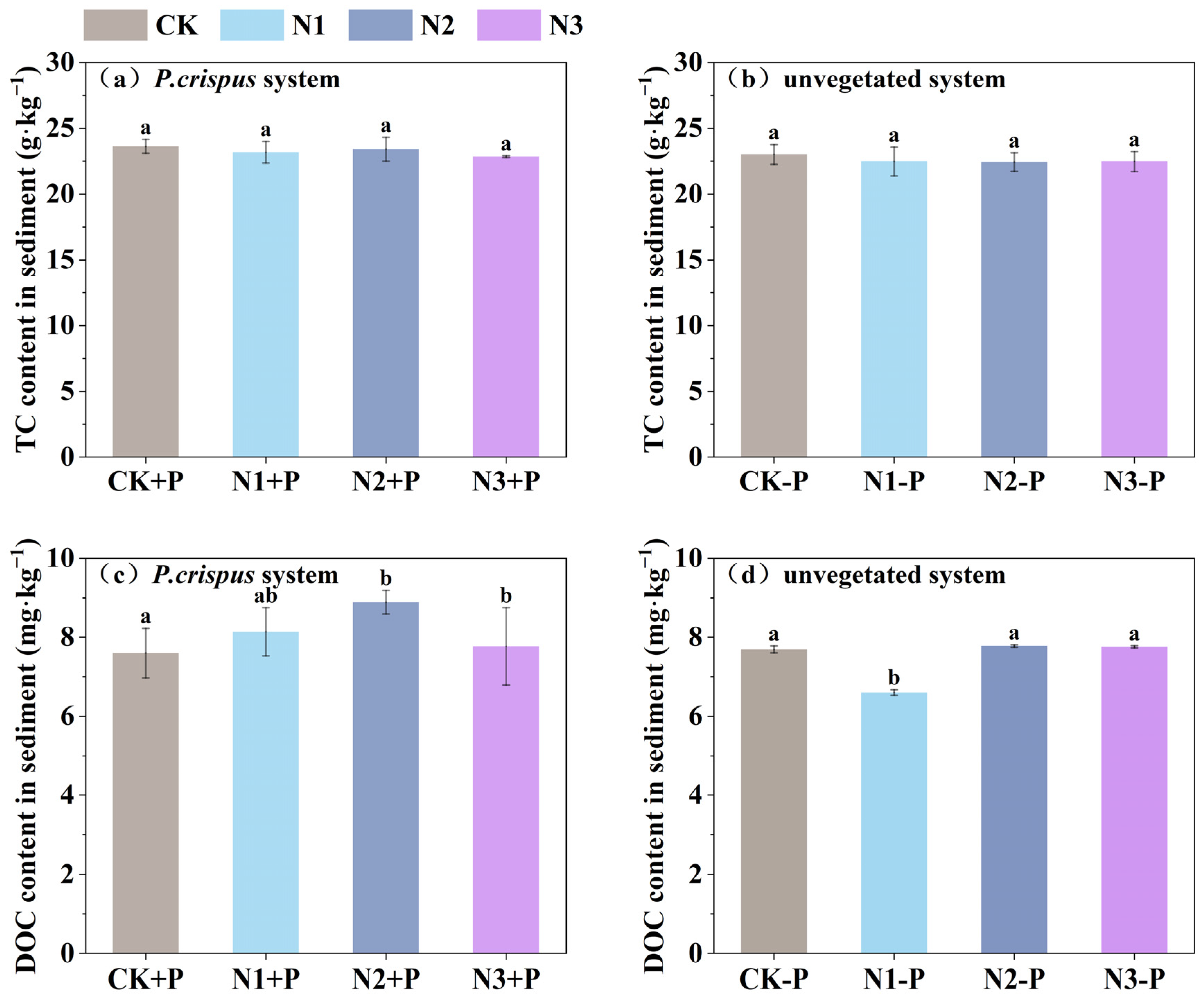
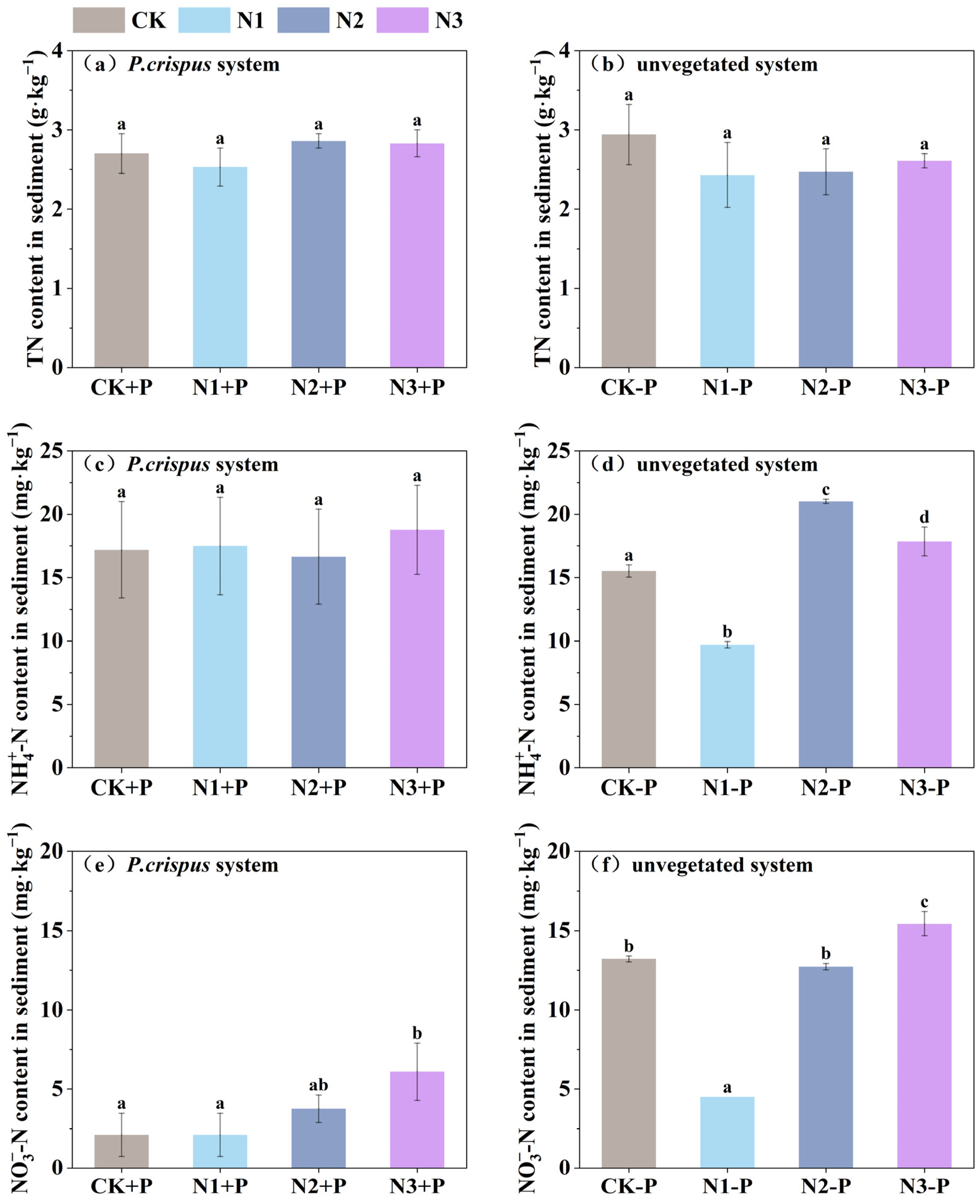
3.2. Variation Characteristics of Carbon and Nitrogen Fractions in Sediments
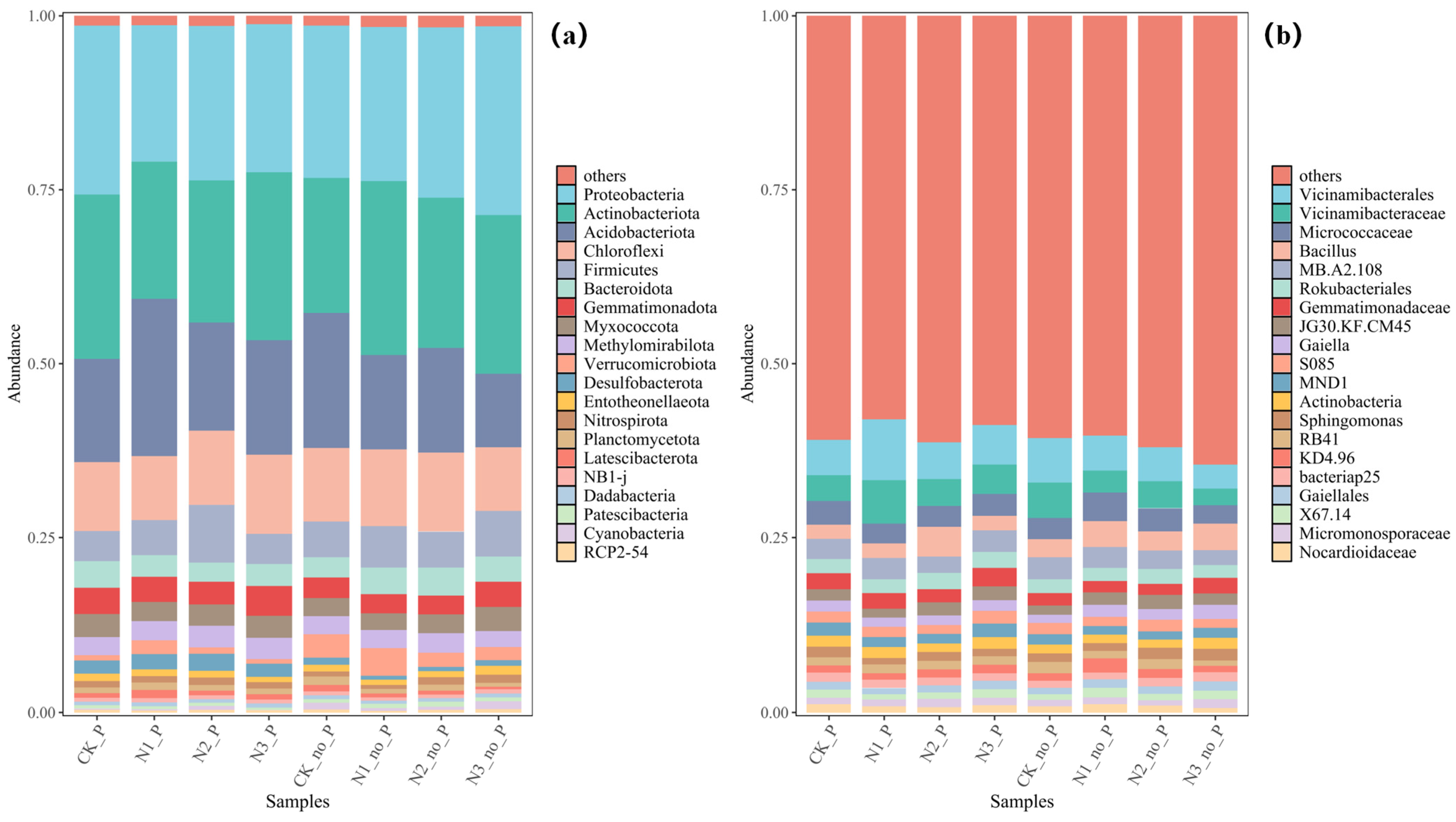
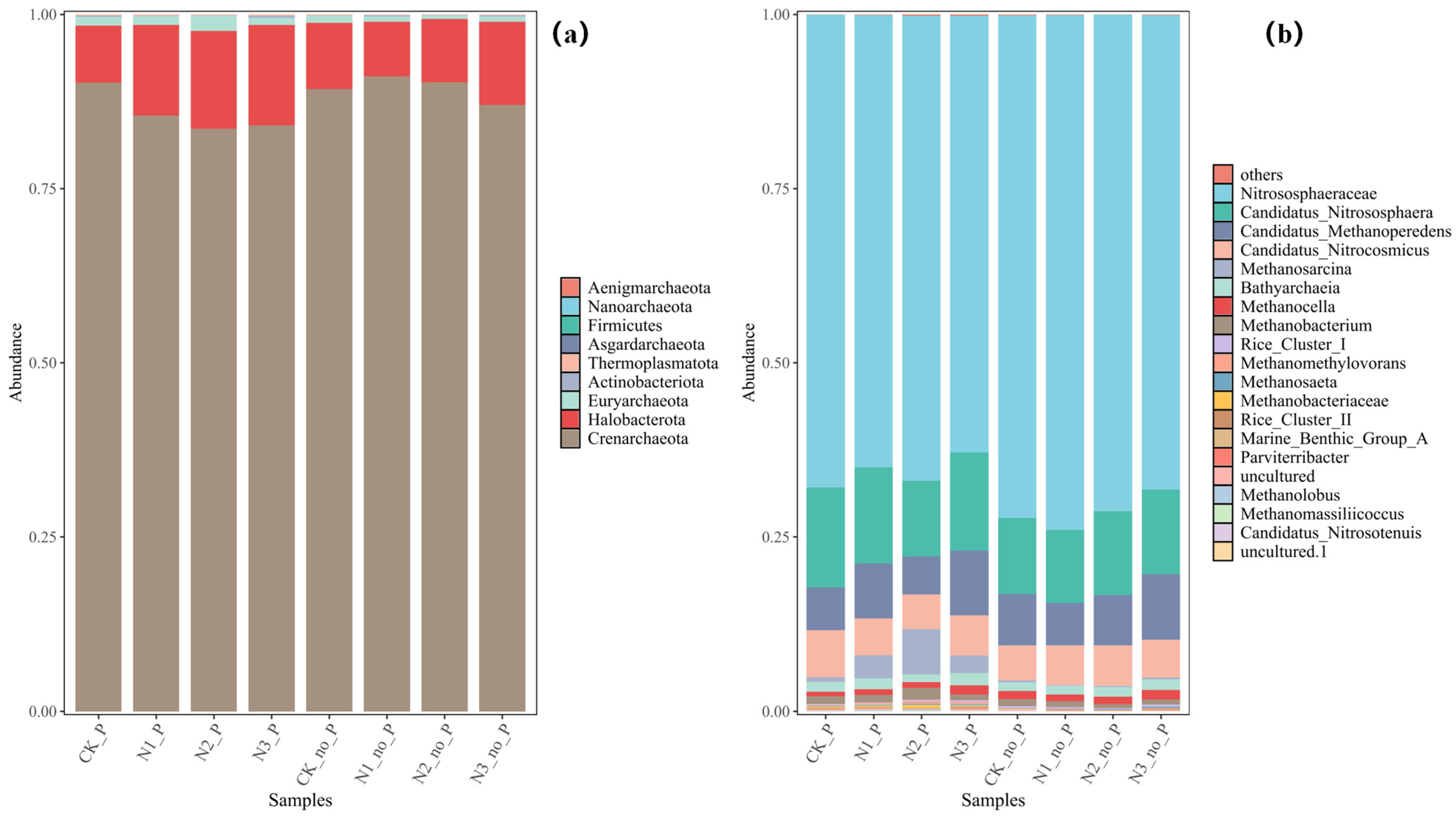
3.3. Responses of Microbial Community Structure to P. crispus and Nitrogen Loading
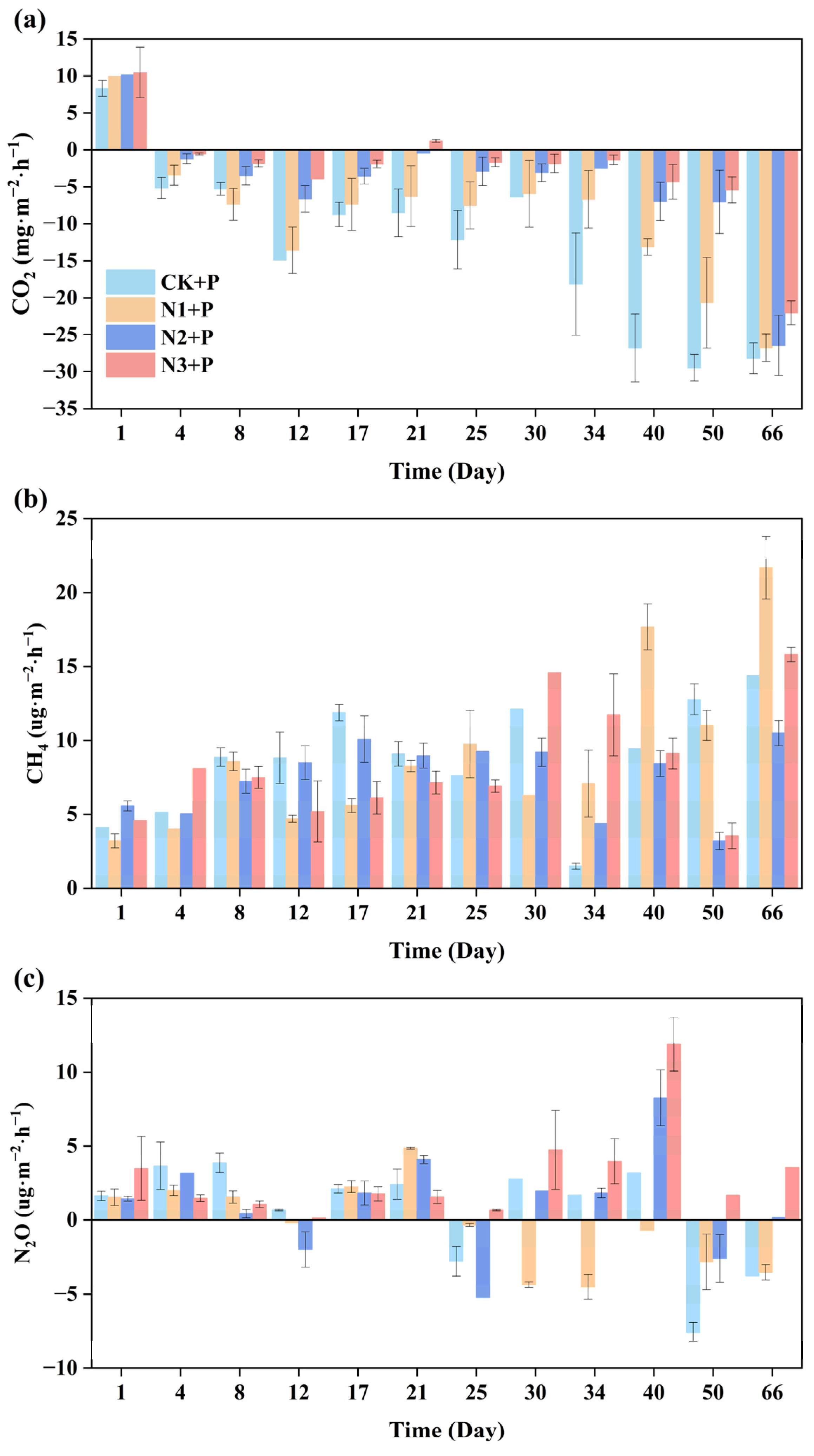
3.4. Greenhouse Gas Exchange Fluxes of P. crispus System Under Different Nitrogen-Loading Conditions
3.4.1. CO2 Exchange Fluxes
3.4.2. CH4 Exchange Fluxes
3.4.3. N2O Exchange Fluxes
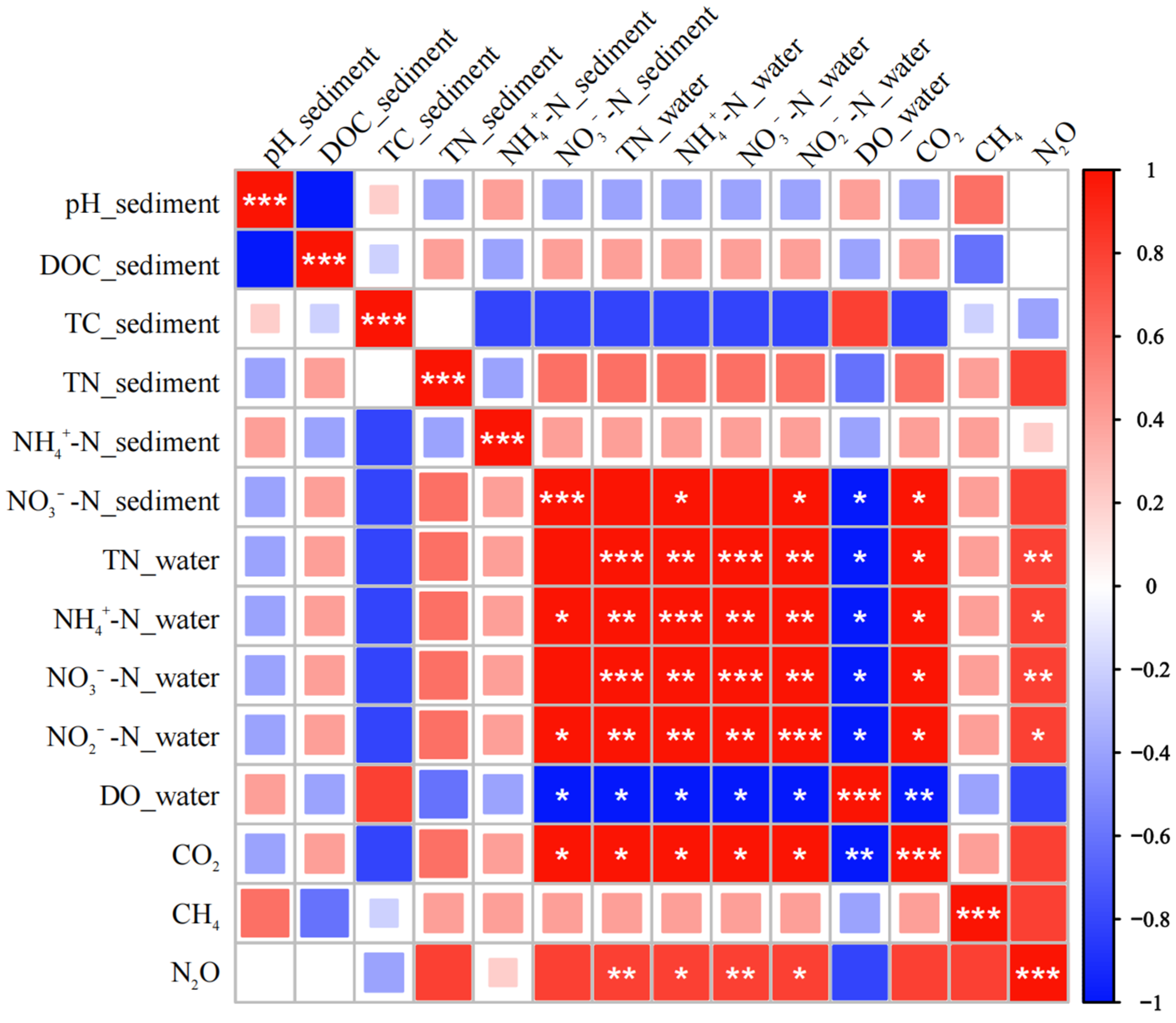
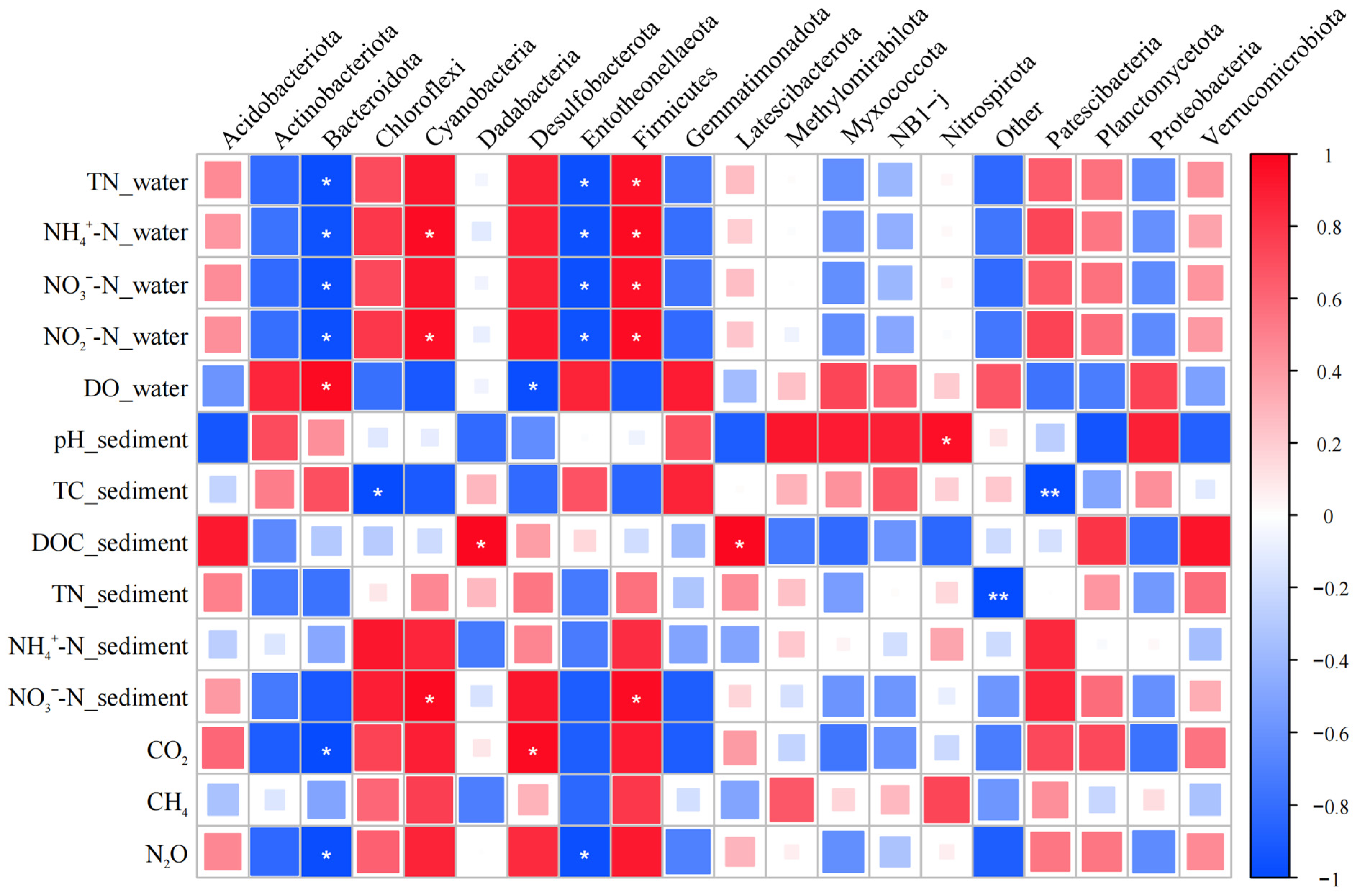
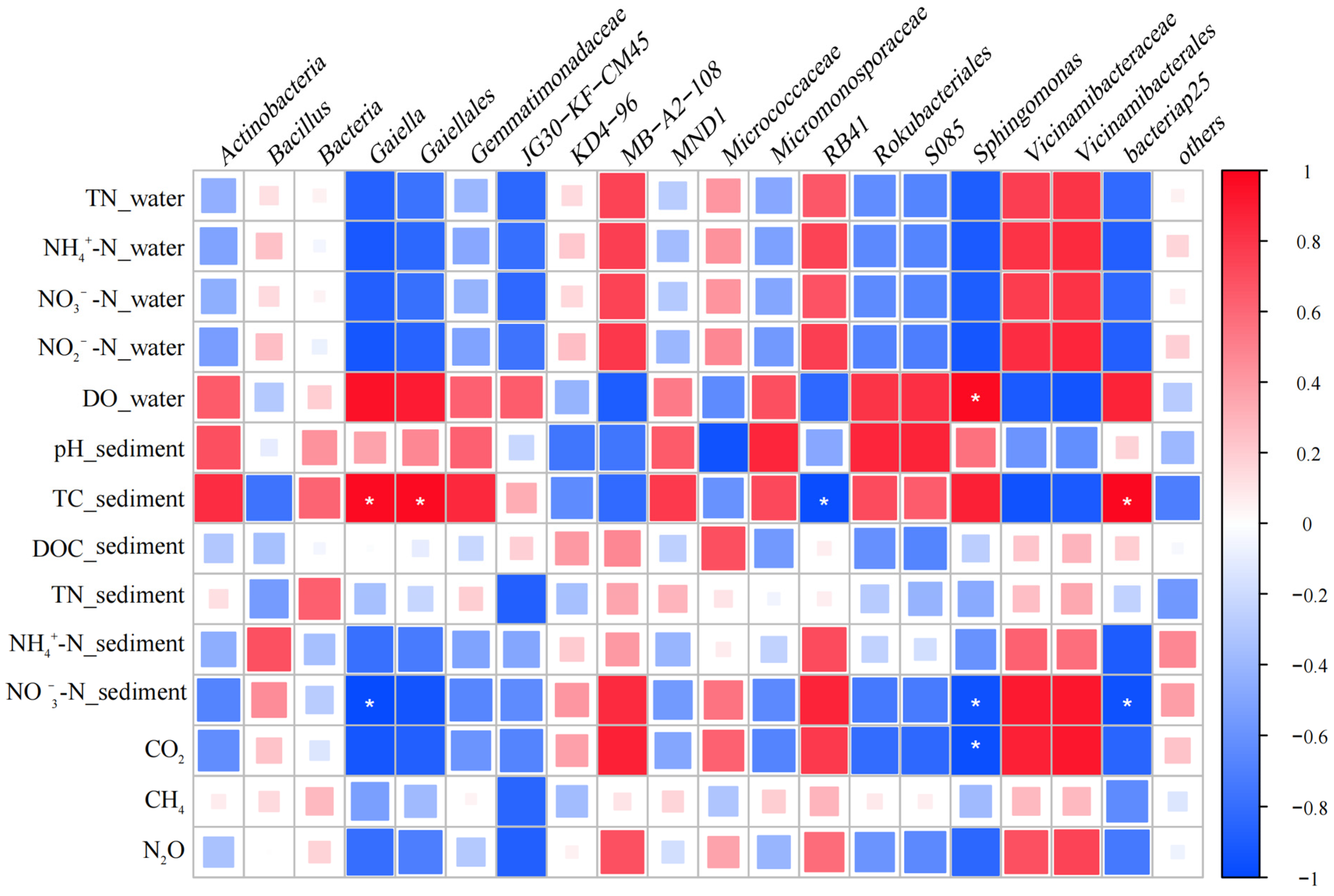
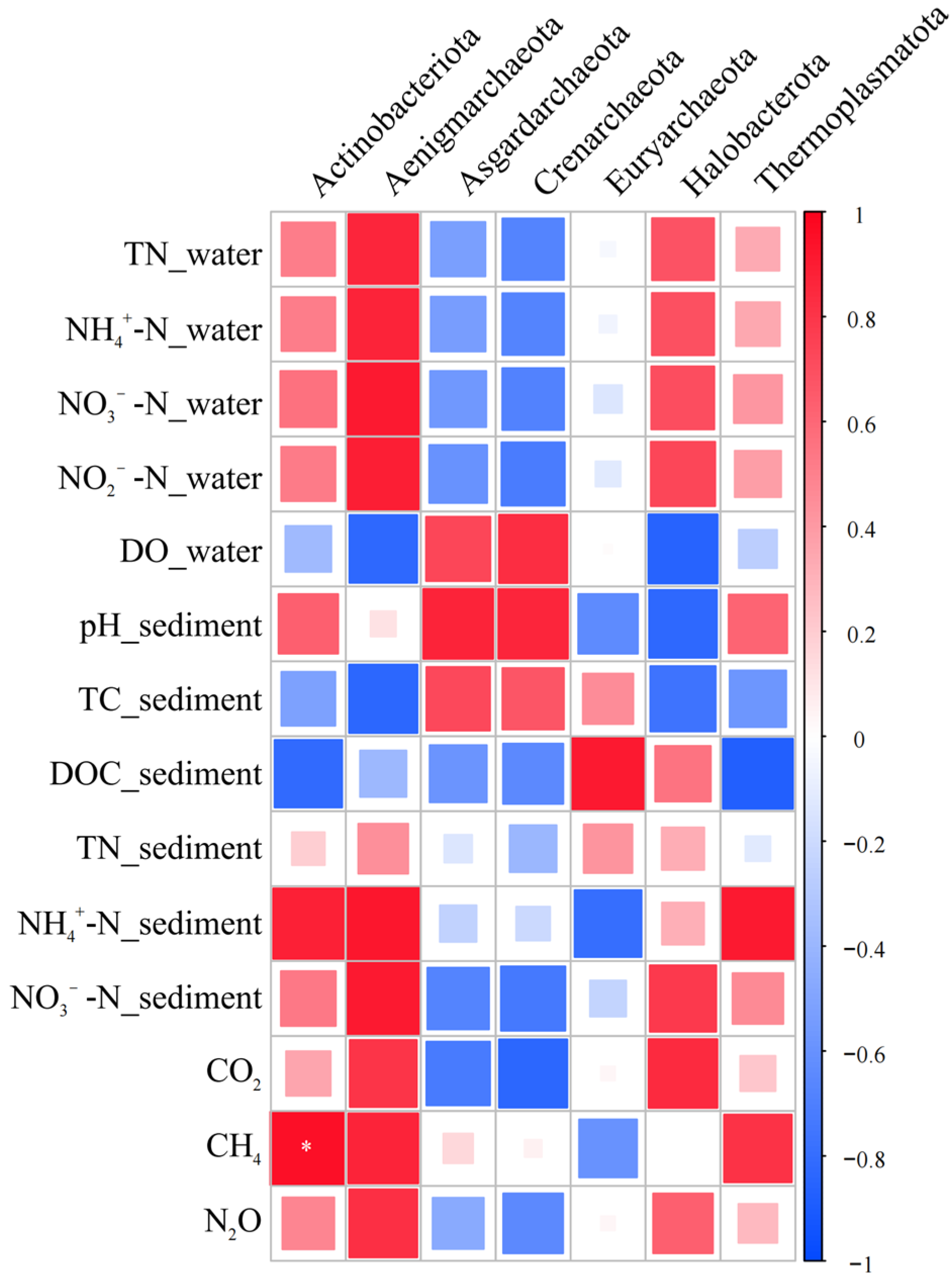
3.5. Effects of Environmental Factors and Microbial Communities on Greenhouse Gas Fluxes in P. crispus System
4. Conclusions
- P. crispus effectively removed NH4+-N, NO3−-N, and NO2−-N, and maintained a high denitrification capacity even under high-nitrogen loading. Under all nitrogen-loading conditions, TN removal rates exceeded 80%, while the removal efficiencies of NH4+-N and NO3−-N both exceeded 90%, with effective suppression of NO2−-N accumulation.
- Through root exudation and rhizosphere regulation, P. crispus contributed to the accumulation and transformation of carbon and nitrogen fractions in sediments, particularly promoting DOC enrichment and NO3−-N removal. However, high-nitrogen loading may disrupt microbial processes or weaken plant uptake capacity, thereby affecting sedimentary carbon–nitrogen balance.
- The dominant bacterial phyla were mainly Proteobacteria, Actinobacteriota, and Acidobacteriota, whose enrichment was promoted by the presence of P. crispus. In archaeal communities, Crenarchaeota was the predominant phylum, and its relative abundance was reduced by both P. crispus and increased nitrogen loading.
- The P. crispus system functioned overall as a carbon sink, demonstrating strong CO2 uptake capacity, although this carbon sink function weakened with increasing nitrogen load. CH4 emissions remained low but showed a slight increase under higher nitrogen loading. N2O flux was significantly influenced by nitrogen input, with negative fluxes observed under low-nitrogen conditions and substantial increases under high-nitrogen loading.
- Correlation analyses between greenhouse gas fluxes, environmental factors, and microbial communities in the P. crispus system identified several key microbial groups closely associated with CO2, CH4, and N2O emissions. These included bacterial taxa, such as Desulfobacterota, Bacteroidota, Entotheonellaeota, and Sphingomonas, and archaeal groups, including Actinobacteriota, Methanosaeta, Rice Cluster I, and Methanosarcina. The results indicated that CO2 and N2O fluxes were primarily regulated by microbial groups involved in carbon and nitrogen transformation, whereas CH4 emissions were mainly driven by methanogenic archaea and showed weaker direct correlations with environmental factors.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Jiang, W.; Wu, Z.; Peng, K.; Ling, Z.; Li, Z.; Wang, X. Assessing and Characterizing Carbon Storage in Wetlands of the Guangdong-Hong Kong-Macau Greater Bay Area, China, During 1995–2020. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2022, 15, 610–630. [Google Scholar] [CrossRef]
- Lal, R. Carbon Sequestration. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 815–830. [Google Scholar] [CrossRef]
- Roulet, N.T. Peatlands, Carbon Storage, Greenhouse Gases, and the Kyoto Protocol: Prospects and Significance for Canada. Wetlands 2000, 20, 605–615. [Google Scholar] [CrossRef]
- Fang, J.; Yu, G.; Liu, L.; Hu, S.; Chapin, F.S., III. Climate Change, Human Impacts, and Carbon Sequestration in China. Proc. Natl. Acad. Sci. USA 2018, 115, 4015–4020. [Google Scholar] [CrossRef]
- Harris, N.L.; Gibbs, D.A.; Baccini, A.; Birdsey, R.A.; de Bruin, S.; Farina, M.; Fatoyinbo, L.; Hansen, M.C.; Herold, M.; Houghton, R.A.; et al. Global Maps of Twenty-First Century Forest Carbon Fluxes. Nat. Clim. Change 2021, 11, 234–240. [Google Scholar] [CrossRef]
- IPCC. 2019 General Guidance and Reporting. In Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; Volume 1, Available online: https://www.ipcc.ch/ (accessed on 28 April 2025).
- Goodbred, S.L., Jr. Morphodynamic feedbacks in tidal estuaries under sea-level rise. Nat. Clim. Change 2019, 9, 907–910. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, J.; Zhang, P.; Wang, Q.; Wu, Y.; Ding, Y.; Wang, H.; Fu, C.; Sun, J. Review on strategies of close-to-natural wetland restoration and a brief case plan for a typical wetland in northern China. Chemosphere 2021, 285, 131534. [Google Scholar] [CrossRef]
- Chmura, G.L.; Anisfeld, S.C.; Cahoon, D.R.; Lynch, J.C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 2003, 17, 1111. [Google Scholar] [CrossRef]
- Duan, M.; Xu, W.; Yuan, F.; Meng, X.; Zhang, S.; Zhang, J. Analysis on the trend and nutritional status of water environment quality in Baiyangdian Lake in recent 5 years. Environ. Chem. 2022, 41, 1988–2000. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Wei, H.; Li, J.; Zhang, Y. Variation characteristics of nitrogen, phosphorus and heavy metals in waters from Baiyangdian Lake and the influencing factors. J. Water Resour. Water Eng. 2020, 31, 103–108. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, X.; Han, R.; Xu, X.; Wang, G.; Liu, X.; Bi, F.; Feng, D. Reproduction capacity of Potamogeton crispus fragments and its role in water purification and algae inhibition in eutrophic lakes. Sci. Total Environ. 2017, 580, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Li, Z. Biological processes and influencing factors of nitrite accumulation in aquatic systems: A review. J. Ecol. Rural. Environ. 2023, 39, 518–526. [Google Scholar]
- GB 3838-2002; Environmental Quality Standards for Surface Water. Ministry of Environmental Protection of China & General Administration of Quality Supervision, Inspection and Quarantine of China, China Environmental Science Press: Beijing, China, 2002.
- Liu, X.; Shi, B.; Meng, J.; Zhou, Y.; Ke, X.; Wang, T. Spatio-temporal variations in the characteristics of water eutrophication and sediment pollution in Baiyangdian Lake. Environ. Sci. 2020, 41, 2127–2136. [Google Scholar] [CrossRef]
- Ren, W.; Tian, Z.; Hu, X.; Liu, X.; Ning, G.; Wang, X.; Xie, J. Biomass of Potamogeton crispus and Dynamic Change of Water Quality on Different Growth Periods in Baiyangdian Lake. J. Agric. Univ. Hebei 2011, 34, 21–28. [Google Scholar]
- Jin, S.; Li, Y.; Ni, C.; Wang, B. Uptake by Potamogeton crispus of nitrogen and phosphorus from water and some affecting factors. Acta Ecol. Sin. 1994, 14, 169–173. [Google Scholar]
- Wang, H.; Li, X.; Liu, J.; Ao, H.; Liu, B. Study on the water purification performance of Potamogeton crispus in different habitats in winter. Environ. Pollut. Control 2012, 34, 40–44. [Google Scholar] [CrossRef]
- Luo, L.; Yu, J.; Zhu, L.; Gikas, P.; He, Y.; Xiao, Y.; Deng, S.; Zhang, Y.; Zhang, S.; Zhou, W.; et al. Nitrogen addition may promote soil organic carbon storage and CO2 emission but reduce dissolved organic carbon in Zoige peatland. J. Environ. Manag. 2022, 324, 116376. [Google Scholar] [CrossRef]
- Tao, B.; Liu, C.; Zhang, B.; Dong, J. Effects of inorganic and organic nitrogen additions on CO2 emissions in the coastal wetlands of the Yellow River Delta, China. Atmos. Environ. 2018, 185, 159–167. [Google Scholar] [CrossRef]
- Zhuang, S.; Ding, J.; Lin, W.; Zheng, Q.; Kou, X.; Li, Q.; Xu, C.; Mao, L.; Pan, Y.; Gao, Y.; et al. Effect of nitrogen loading on microbial nitrogen cycling and greenhouse gas emissions in paddy soil. Soil Biol. Biochem. 2024, 185, 108010. [Google Scholar] [CrossRef]
- Tubay, J.M.; Ito, H.; Uehara, T.; Kakishima, S.; Morita, S.; Togashi, T.; Tainaka, K.-I.; Niraula, M.P.; Casareto, B.E.; Suzuki, Y.; et al. The paradox of enrichment in phytoplankton by induced competitive interactions. Sci. Rep. 2013, 3, 2835. [Google Scholar] [CrossRef]
- Niu, C.; He, Z.X.; Ge, Y.; Chang, J.; Lu, Z.M. Effect of plant species richness on methane fluxes and associated microbial processes in wetland microcosms. Ecol. Eng. 2015, 84, 250–259. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, H.; Ye, C.; Chen, X.; Xie, B.; Huang, C.C.; Zhang, J.X.; Xu, M.N. Effects of plant species on soil microbial processes and CH4 emission from constructed wetlands. Environ. Pollut. 2013, 174, 273–278. [Google Scholar] [CrossRef] [PubMed]
- García-Lledó, A.; Vilar-Sanz, A.; Trias, R.; Hallin, S.; Bañeras, L. Genetic potential for N2O emissions from the sediment of a free water surface constructed wetland. Water Res. 2011, 45, 5621–5632. [Google Scholar] [CrossRef] [PubMed]
- Moseman-Valtierra, S.; Gonzalez, R.; Kroeger, K.D.; Tang, J.; Chao, W.C.; Crusius, J.; Bratton, J.; Green, A.; Shelton, J. Short-term nitrogen additions can shift a coastal wetland from a sink to a source of N2O. Atmos. Environ. 2011, 45, 4390–4397. [Google Scholar] [CrossRef]
- Feng, Y.; Song, Y.; Zhu, M.; Li, M.; Gong, C.; Luo, S.; Mei, W.; Feng, H.; Tan, W.; Song, C. Microbes drive more carbon dioxide and nitrous oxide emissions from wetland under long-term nitrogen enrichment. Water Res. 2025, 272, 122942. [Google Scholar] [CrossRef]

| Indices | SOC | TC | TN |
|---|---|---|---|
| Content | 13.65 ± 0.82 g∙kg−1 | 26.90 ± 8.18 g∙kg−1 | 2.64 ± 0.73 g∙kg−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lun, X.; Niu, J.; Zhang, L.; Wu, B.; Wang, X. Remediation Effects of Potamogeton crispus on Nitrogen-Loaded Water Bodies and Its Greenhouse Gas Emission Mechanisms. Atmosphere 2025, 16, 803. https://doi.org/10.3390/atmos16070803
Li X, Lun X, Niu J, Zhang L, Wu B, Wang X. Remediation Effects of Potamogeton crispus on Nitrogen-Loaded Water Bodies and Its Greenhouse Gas Emission Mechanisms. Atmosphere. 2025; 16(7):803. https://doi.org/10.3390/atmos16070803
Chicago/Turabian StyleLi, Xiaoyi, Xiaoxiu Lun, Jianzhi Niu, Lumin Zhang, Bo Wu, and Xinyue Wang. 2025. "Remediation Effects of Potamogeton crispus on Nitrogen-Loaded Water Bodies and Its Greenhouse Gas Emission Mechanisms" Atmosphere 16, no. 7: 803. https://doi.org/10.3390/atmos16070803
APA StyleLi, X., Lun, X., Niu, J., Zhang, L., Wu, B., & Wang, X. (2025). Remediation Effects of Potamogeton crispus on Nitrogen-Loaded Water Bodies and Its Greenhouse Gas Emission Mechanisms. Atmosphere, 16(7), 803. https://doi.org/10.3390/atmos16070803




