Abstract
The hydrolysis oxidation of 1,2-chlorobenzene (1,2-DCB) over Pd-Ti-Ni/ZSM-5(25) catalysts has been investigated as a safe and environmentally friendly method for the removal of chlorinated aromatic organic compounds. Experimental results demonstrate that hydrolysis oxidation technology can effectively suppress the formation of polychlorinated organic compounds. Among the catalysts studied, the 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) catalyst exhibited optimal hydrolysis oxidation performance, achieving complete conversion of 1,2-DCB at 425 °C. Notably, this technology significantly inhibits the formation of polychlorinated organic by-products during the catalytic degradation of 1,2-DCB. Although trace amounts of chlorobenzene were still detected, the overall reduction in hazardous by-products is remarkable. Characterization techniques, including X-Ray Diffraction (XRD), X-Ray Photoelectron Spectroscopy (XPS), Pyridine adsorption infrared Spectroscopy (pyridine IR) and Fourier transform infrared spectroscopy (FT-IR) analysis, revealed that the acidity and redox properties of the catalyst surface play a pivotal role in the hydrolysis oxidation process. The hydrolysis oxidation of chlorinated volatile organic compounds not only effectively reduces pollutant concentrations but also prevents the generation of more toxic by-products. This dual benefit not only protects the environment but also minimizes ecological risks, highlighting the potential of this technology for sustainable environmental remediation.
1. Introduction
Chlorinated volatile organic compounds (CVOCs) are organic compounds that contain chlorine atoms in their molecular structure and are volatile at room temperature and pressure. Their volatility enables them to spread rapidly in environmental media such as air. Common ones include chlorinated aliphatic hydrocarbons (e.g., methylene chloride, trichloroethane), chlorinated olefins (e.g., vinyl chloride), and chlorinated aromatic hydrocarbons (e.g., chlorobenzene). Chlorinated organic solvents are commonly used in dry cleaning agents, paint thinners, adhesives, etc., and will evaporate into the air when used; the combustion of chlorinated plastic products, chemicals, etc., as well as waste incineration processes, will generate a variety of CVOCs [1,2]. CVOCs can also be emitted during residential or industrial disinfection processes using bleach or similar agents [3,4].
Under sunlight, CVOCs undergo a series of complex photochemical reactions with nitrogen oxides. These reactions generate secondary pollutants such as ozone and peroxyacetyl nitrate (PAN), thereby forming photochemical smog [1,2,3,4,5]. Some CVOCs exhibit bioaccumulative properties in water. They can be transferred and magnified through the food chain, inflicting damage on the structure and function of aquatic ecosystems. This, in turn, impacts the survival, reproduction, growth, and development of marine organisms like fish and shellfish [1,6].
Given the widespread presence of CVOCs in the environment and their potential threats to human health (such as carcinogenicity and neurotoxicity) and ecological risks (such as photochemical smog formation and aquatic bioaccumulation), their efficient control has become an urgent issue in the field of environmental governance. Among various CVOCs treatment technologies, catalytic oxidation stands out due to its unique catalytic activation mechanism by lowering the activation energy of reactions. This technology significantly weakens the chemical stability of C-Cl bonds in CVOCs molecules, prompting directional transformation of chlorinated functional groups under mild conditions and thus greatly enhancing degradation efficiency. For typical chlorinated hydrocarbons (such as dichloromethane and trichloroethylene), catalytic oxidation can achieve a removal rate of over 90% within the low-temperature range of 200–400 °C [5,6], which is significantly lower than that of conventional thermal oxidation (800–1200 °C), effectively reducing energy consumption. At the same reaction residence time, the treatment capacity of this technology for chlorinated hydrocarbon pollutants is significantly higher than that of non-catalytic processes [7], making it particularly suitable for the deep purification of high-concentration CVOCs exhaust gases. However, catalytic oxidation technology has certain drawbacks. Chlorinated organic by-products have been detected during the catalytic oxidation of CVOCs [8,9], and these by-products sometimes have a greater environmental impact than the original pollutants.
In contrast to chlorinated hydrocarbons, chlorinated aromatic hydrocarbon molecules have a benzene ring structure, a highly stable conjugated system with a highly delocalized π-electron cloud, giving the entire molecule a high degree of stability. This conjugation effect causes the chemical bonds within the benzene ring system to exhibit a trend toward uniform distribution, rendering it difficult for them to undergo cleavage via conventional chemical reaction pathways. The catalytic degradation of chlorinated aromatic hydrocarbons is more challenging due to the difficulty of degradation and the complexity of the reaction mechanism. In addition, the distribution and formation mechanism of by-products such as polychlorinated biphenyls (PCBs), dioxins, and other non-persistent organic pollutants (POPs) in the catalytic oxidation of chlorinated aromatic hydrocarbons are complex [10] and are only partially understood. The emissions of CVOCs have given rise to numerous environmental and health problems. It has become extremely urgent to effectively control the emissions of CVOCs.
Van den Brink et al. found that during the catalytic oxidation of chlorobenzene (CB) over the Pt/γ-Al2O3 catalyst, a large number of polychlorinated organic by-products would be formed, such as 1,2,4-trichlorobenzene, 1,2,4,5-tetrachlorobenzene, pentachlorobenzene, and hexachlorobenzene. At the complete conversion temperature of CB, which is 440 °C, dichlorobenzene, trichlorobenzene, tetrachlorobenzene, pentachlorobenzene, and hexachlorobenzene were still detected [11,12,13]. Salvatore Scirè et al. investigated the catalytic oxidation of CB over Pd-based molecular sieve catalysts [14,15] and found that the yields of polychlorinated organic by-products were as high as 1.7% at 450 °C. In addition, Giraudon et al. found that polychlorinated organic by-products were formed during the catalytic oxidation of chlorobenzene over Pd-based catalysts [16], TiO2 and ZrO2 nanocatalysts [17]. In addition, polychlorinated organic by-products were detected in the catalytic oxidation of CB over Pd/molecular sieves [8], Pd/Fe [18], alumina-pillared clay supported with Pd or Pt [19], V2O5-based catalysts [20], and Ce-based composite-oxide catalysts [21,22]. Cai et al. detected organic by-products such as benzene, CB, and 1,2,4-trichlorobenzene during the catalytic oxidation of dichlorobenzene over Mn/Co composite oxides with different ratios [23]. Recently, Huang et al. investigated the effect of Ru/CeO2 catalysts with cerium oxide nanoparticles having different morphologies and crystalline surfaces on the oxidation activity of CB [24]. In this process, they also found the formation of 1,2-dichlorobenzene, 1,3-dichlorobenzene, and 1,4-dichlorobenzene. Weng et al. detected organic by-products of chlorinated alkanes and chlorinated aromatic hydrocarbons during the catalytic oxidation of CB over MnxCe1-xO2/H-ZSM5 catalysts [25,26]. In addition, Liu et al. evaluated the catalytic oxidation activity of CB over TiO2-loaded different noble metal catalysts. In addition, Liu et al. evaluated the catalytic oxidation activity of chlorobenzene over different noble metal catalysts supported on TiO2. They also detected polychlorinated organic by-products, and even polychlorinated biphenyls (PCBs). The concentration of PCBs was 0.0055 ng WHO-TEQ/Nm3 (the toxic concentration of pollutants calculated in accordance with the World Health Organization’s (WHO) Toxic Equivalent (TEQ) standard per normal cubic meter of air) [27].
Researchers have carried out a great deal of research work during the safe catalytic degradation process of CVOCs. Many research results indicate that water vapor plays a crucial role in inhibiting the formation of polychlorinated organic compounds [28]. During the catalytic oxidation of CVOCs, H2O can facilitate the removal of Cl species from the catalyst surface [29,30]. Finally, H2O can also interact with chlorine to produce HCl via the Deacon reaction, thus altering the HCl/Cl2 selectivity [31]. López-Fonseca et al. similarly found that input of water during the catalytic oxidation of trichloroethene over H-β and Pt/H-β catalysts significantly increased the selectivity of HCl and CO2 [32]. The catalytic oxidation of dichloromethane over 0.12%Pt-0.11% Pd/Al2O3-CeO2 catalyst with the addition of 1.5 vol.% H2O has the potential to improve the selectivity of HCl significantly. The yield of HCl increased from 37% to 68% at 500 °C [33]. H-type molecular sieve catalysts (HY, H-ZSM5, H-MOR) were used as potential materials for the catalytic oxidation of CVOCs to investigate their catalytic oxidation performance on trichloroethylene. The catalytic oxidation order of the catalysts under dry conditions was found to be H-MOR, H-ZSM5, HY. The order of activity of the individual molecular sieve catalysts did not change under humid conditions. Excess H2O led to an increase in the conversion of trichloroethylene over H-MOR and H-ZSM5 catalysts at lower temperatures, which implies that both O2 and H2O are involved in the catalytic oxidation reaction of trichloroethylene [34,35,36]. H2O is also capable of promoting the removal of Cl species from the catalyst surface during the catalytic oxidation of CB over VOx/TiO2, WOx/TiO2, and VOx-MoOx/TiO2 [29,37].
In addition, during the catalytic oxidation of 4,4,6-trichlorophenol over V2O5-WO3/TiO2 catalysts, H2O was able to affect the activity and product distribution due to competitive adsorption with 4,4,6-trichlorophenol [38]. Abdullah et al. analyzed the effect of water vapor on the catalytic oxidation performance of dichloromethane, trichloromethane, and trichloroethylene catalytic oxidation performance [39]. The conversion of dichloromethane and trichloromethane decreased from 99% to 86%, while the conversion of trichloroethylene decreased from 94% to 88% when the feed contained 0.9 vol.% of water vapor. H2O increased the CO2 yield although there was competitive adsorption with pollutants and inhibited the conversion of CVOCs. Wang et al. found that H2O had an inhibitory effect on the formation of organic by-products during the catalytic oxidation of p-dichlorobenzene [40]. He et al. explored the catalytic oxidation behavior of CB in the presence of water vapor over a typical manganese-based catalyst. The results showed that water vapor significantly promoted the deep oxidation of chlorobenzene and inhibited the formation of Cl2 and dichlorobenzene [41].
According to our previous studies, lowering the oxygen concentration and adding water vapor to the reaction atmosphere not only improves the catalytic activity but also reduces the formation of chlorinated organic by-products, mainly due to its ability to inhibit the formation of chlorinated palladium species during chlorination reactions [8]. In addition, the complete catalytic degradation of 1,2-dichlorobenzene (1,2-DCB) over Pd/ZSM-5(25) catalyst with water as the only oxidant (hydrolysis oxidation) was achieved; only benzene, chlorobenzene, and 1,3-dichlorobenzene were detected during the reaction [42].
This study delves into the feasibility of utilizing H2O as an alternative oxidant to oxygen in the catalytic decomposition of CVOCs. The overarching objective is to surmount the significant challenge of polychlorinated organic by-product generation during conventional catalytic oxidation, thereby enabling the safe and efficient conversion of CVOCs. A series of palladium-based bimetallic catalysts with different Ti loadings (0.5% Pd-x%Ti-(10-x)%Ni/ZSM-5(25), where x = 0, 1, 2, 8) will be meticulously designed and synthesized. Using 1,2-DCB as a model pollutant, a CVOC hydrolysis oxidation reaction system will be constructed to evaluate the catalytic performance. A comprehensive investigation of the catalysts will be conducted using a series of advanced characterization techniques to elucidate the structure-activity relationships and systematically analyze the intrinsic correlations between physicochemical properties (such as acid site distribution and redox capacity) and the hydrolysis oxidation activity toward 1,2-DCB. By closely monitoring the formation of polychlorinated organic pollutants during the reaction, the effectiveness of H2O as an oxidant in minimizing the generation of chlorinated by-products will be validated. In summary, through the synergistic approach of oxidant innovation and catalyst design, this study endeavors to overcome the limitations of traditional catalytic oxidation technologies, thereby paving the way for the industrial implementation of environmentally benign CVOC abatement strategies.
2. Materials and Methods
2.1. Catalyst Preparation
The loaded Pd-Ti-Ni-based catalysts were prepared for the equal volume impregnation method. The loadings of noble metals Pd, Ti, and Ni were 0.5%Pd-10% Ni, 0.5%Pd-1%Ti-9%Ni, 0.5%Pd-2%Ti-8%Ni, and 0.5%Pd-8%Ti-2%Ni, respectively, and the precious metal precursors Pd(NO3)2, TiCl3, and Ni(NO3)2·6H2O were pipetted and diluted to the corresponding impregnation volume of the carriers by ultrapure water. The solution of Pd(NO3)2, TiCl3, and Ni(NO3)2·6H2O was diluted with ultrapure water to the corresponding impregnation volume of the carriers, and then the H-ZSM5 carriers were added into the beaker, stirred thoroughly for 10 min and sonicated for 15 min to make the mixture homogeneous. The impregnated solid was dried at 80 °C for 12 h. After drying, the samples were roasted in air at 600 °C (with a heating rate of 5 °C·min−1) for 4 h. The baked samples were reduced in an H2 atmosphere at 450 °C for 2 h (with a heating rate of 3 °C·min−1).
2.2. Catalyst Characterization
The samples’ XRD spectra were obtained by testing on a PANalytical X’pert PRO powder diffractometer (PANalytical B.V., Almelo, The Netherlands). Cu Kα was used as the excitation light source, with λ of 0.154 nm, tube pressure of 40 kV, tube current of 40 mA, test range of 5–90°, and scan rate of 1°·min−1.
The elemental composition and chemical valence states of the sample surfaces were tested on a Thermo ESCALAB 250 X-ray photoelectron spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) using Al Kα as the excitation source for X-rays and the C1s binding energy peak as the internal standard (binding energy of 284.6 eV).
Fourier transform infrared spectroscopy (FT-IR) was used to analyze the surface of the catalyst material to detect changes in its surface hydroxyl groups. The FT-IR analysis of the catalyst material was performed on a TENSOR 27 infrared spectrometer from Bruker (Karlsruhe, Germany), which is equipped with a high-low vacuum system. An LN-MCT detector was used, and the measurement range was 400–4000 cm−1 with a resolution of 4 cm−1. The surface of the material was characterized by FT-IR. The fine powdered sample was placed in a diffuse reflection cell, and its surface was carefully flattened to ensure optimal measurement conditions.
Pyridine adsorption infrared spectroscopic (pyridine IR) studies were conducted on a Nicolet 6700 infrared spectrometer with a photoconductive (MCT) detector (Thermo Fisher Scientific, Waltham, USA). First, the samples were treated in situ under vacuum at 500 °C for 1 h and then cooled to room temperature. Pyridine vapor was introduced into the transmission cell and the physically adsorbed pyridine was withdrawn. Finally, the samples were heated to 150 °C and the spectra were recorded.
2.3. Evaluation of Catalytic Performance
A fixed-bed continuous flow CVOCs catalytic oxidation reaction device was used to evaluate the catalytic performance of the catalyst for 1,2-DCB, and the catalyst was pressed into 20~40 mesh particles of about 400 mg. The N2 carrier gas was passed through a double-layer CVOCs generator (the outer layer was a constant-temperature hydrothermal, and the temperature was controlled to be 55 °C), which brought out a high-concentration gas stream of 1,2-DCB. The N2 carrier gas passes through a double-layer H2O generator, which brings out a highly concentrated H2O gas stream, and then the above two gases are mixed with the other N2 to enter the fixed-bed reactor. The reaction gas comprised 500 ppm 1,2-DCB, 3.2 vol.% H2O, and N2. The total gas flow rate was 250 mL·min−1, the gas–air speed (GHSV) was 15,000 h−1, and the temperature range was between 200 and 600 °C. The gas stream from the reactor was passed through a six-way valve into an Agilent 6890 gas chromatograph, which was equipped with a DB-624 capillary column (30 m × 0.32 mm × 1.8 μm) and was used as an analytical tool for the determination of 1,2-DCB and organic by-products using an FID detector (Agilent Technologies, Santa Clara, USA). During the detection process, the column oven temperature was first held at 50 °C for 1 min, then ramped up to 140 °C at a rate of 10 °C per minute, and finally maintained for 5 min.
The concentrations of CO2, CO, and HCl at the outlet were analyzed online using an FT-IR spectrometer (MultiGas™2030, MKS Instruments, Inc., Andover, MA, USA). The generation of Cl2 was measured by bubbling the outlet gas stream into 0.0125 M NaOH solution for 20 min. The concentration of Cl− (produced from Cl2) was determined using an ultraviolet-visible spectrophotometer (TU-1810PC, Purkinje, Beijing, China) with N,N-diethyl-p-phenylenediamine (DPD) as the indicator.
3. Results and Discussion
3.1. 1,2-DCB Hydrolysis Oxidative Activity and Inorganic Product Analysis
The hydrolysis oxidation activity of 500 ppm 1,2-DCB was tested over catalysts of 0.5%Pd-10%Ni/ZSM-5(25), 0.5%Pd-1%Ti-9%Ni/ZSM-5(25), 0.5%Pd-2%Ti-8%Ni/ZSM-5(25), and 0.5%Pd-8%Ti-2%Ni/ZSM-5(25), with conversion curves shown in Figure 1. In the temperature range of 200–500 °C, the conversion of 1,2-DCB increased with temperature. Notably, 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) achieved complete conversion at 425 °C, exhibiting a lower reaction temperature window compared to the Ti-undoped 0.5%Pd-10%Ni/ZSM-5(25) (complete conversion at 450 °C). This feature is critical for industrial waste gas treatment: lower reaction temperatures reduce energy consumption and minimize the formation of by-products like NOx under high temperatures, avoiding additional atmospheric pressure from secondary pollution.
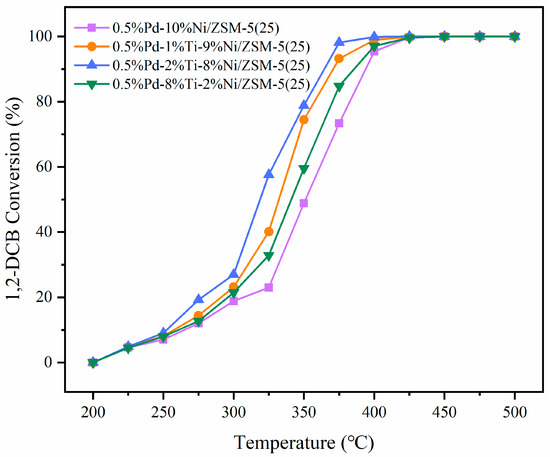
Figure 1.
1,2-DCB hydrolysis oxidation activity curve.
The characteristic activity data (T10, T50, T90), representing the temperatures required for 10%, 50%, and 90% conversion of 1,2-DCB during hydrolysis oxidation, are listed in Table 1, reflecting the catalysts’ capability to remove low-concentration pollutants. For instance, 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) shows a significantly lower T10 than other catalysts, indicating its superiority in treating low-concentration 1,2-DCB in the atmosphere. Since ambient VOC concentrations typically remain below ppm levels, this catalyst initiates catalytic reactions at lower temperatures, aligning with the demand for “low-temperature and high-efficiency governance” of atmospheric pollution.

Table 1.
Temperatures required when the conversion rate reaches 10%, 50% and 100%.
The CO, CO2, and HCl productions during the hydrolysis oxidation of 1,2-DCB are shown in Figure 2. With the increase in reaction temperature, the CO yield showed a continuous increase over 0.5%Pd-10%Ni/ZSM-5(25) catalyst, which reached 85.0% at 500 °C; while the CO yield increased over 0.5%Pd-1%Ti-9%Ni/ZSM-5(25), 0.5% Pd-2%Ti-8%Ni/ZSM-5(25), and 0.5%Pd 8%Ti-2%Ni/ZSM-5(25) catalysts catalyzed the first increase and then decrease in CO production, and the highest CO production was reached at 450 °C, which was 70.7%, 76.4% and 67.2%, respectively. As the second most significant greenhouse gas after CO2, carbon monoxide (CO) has an atmospheric lifetime of approximately 2 months. It indirectly promotes the accumulation of CO2 through participating in the methane oxidation reaction (CO + OH·→ CO2 + H·). The high CO yield of the 0.5% Pd-10% Ni/ZSM-5(25) catalyst at high temperatures may exacerbate the local atmospheric greenhouse effect, while Ti-doped catalysts may reduce such risks by decreasing the peak CO yield (e.g., the CO yield decreases after 450 °C). CO, together with NOx and VOCs, participates in photochemical reactions and serves as a key precursor for ozone (O3) formation. The decrease in CO yield of Ti-doped catalysts after 450 °C suggests that they can suppress the formation of O3 precursors under high-temperature conditions, which is of positive significance for atmospheric governance in areas with high incidences of photochemical smog in summer.
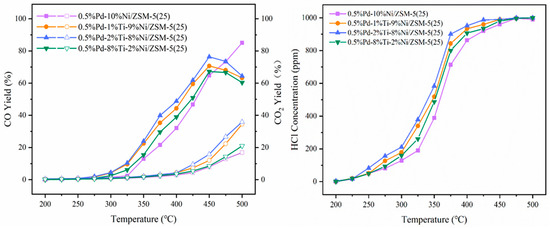
Figure 2.
Variation in CO (solid) and CO2 (hollow) yields and HCl concentration with temperature during hydrolysis oxidation of 1,2-DCB.
Although the CO2 yield was generally lower than that of CO (e.g., the CO2 yield of 0.5% Pd-2% Ti-8% Ni/ZSM-5(25) was 35.9% at 500 °C), its continuous increasing trend still warrants attention. As a major greenhouse gas, the CO2 selectivity (CO2/CO ratio) of the catalyst can serve as an indicator for evaluating the environmental friendliness of the catalytic system. The data showed that the order of CO2 yield for Ti-doped catalysts was consistent with the order of catalytic activity (0.5%Pd-2%Ti-8%Ni/ZSM-5(25) > 0.5%Pd-1%Ti-9%Ni/ZSM-5(25) > 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) > 0.5%Pd-10%Ni/ZSM-5(25)), indicating that more efficient degradation of 1,2-DCB might be accompanied by higher CO2 conversion efficiency. In the future, carbon capture technologies should be integrated to optimize industrial application scenarios.
In the reaction temperature range of 200–500 °C, the HCl concentration showed a positive correlation with temperature, exhibiting a continuous upward trend. The HCl concentrations on the catalysts of 0.5% Pd-1% Ti-9% Ni/ZSM-5(25), 0.5% Pd-2% Ti-8% Ni/ZSM-5(25), and 0.5% Pd-8% Ti-2% Ni/ZSM-5(25) reached 1000 ppm at 500 °C, 475 °C, and 500 °C, respectively, achieving chlorine equilibrium. In contrast, the HCl concentration of 0.5% Pd-10% Ni/ZSM-5(25) was only 991 ppm at 500 °C, failing to achieve chlorine equilibrium. The 0.5% Pd-2% Ti-8% Ni/ZSM-5(25) catalyst showed the most significant selectivity for HCl generation and required the lowest temperature to achieve chlorine equilibrium. HCl, being highly soluble in water, forms hydrochloric acid and serves as a potential contributor to atmospheric acid rain (pH < 5.6). In a humid atmospheric environment, HCl can react with NH3 to form NH4Cl particles, exacerbating PM2.5 pollution. Given that Ti-doped catalysts accelerate HCl generation, it is necessary to simultaneously consider post-treatment technologies for tail gases (such as alkali liquor absorption) to reduce acidic gas emissions.
3.2. Distribution of Chlorinated Organic By-Products
The production of organic by-products during the hydrolysis oxidation of 1,2-DCB on different catalysts is shown in Figure 3. Two organic by-products, benzene and CB, were detected during the hydrolysis oxidation of 1,2-DCB, but the production of benzene was much higher than that of CB. As can be seen from Figure 3, the main generation temperature of benzene was 325–450 °C, the reaction temperature was high, and the benzene concentration showed a trend of first increasing and then decreasing with the temperature. When the reaction temperature is 400 °C, the production amount of benzene reaches the maximum. The maximum concentrations of benzene generated on the catalysts of 0.5%Pd-10%Ni/ZSM-5(25), 0.5%Pd-1%Ti-9%Ni/ZSM-5(25), 0.5%Pd-2%Ti-8%Ni/ZSM-5(25), and 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) are 205 ppm, 178 ppm, 149 ppm, and 190 ppm, respectively. It can be seen that benzene generation was the least on the 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) catalyst. Benzene exhibits an OFP (Photochemical Ozone Formation Potential) value of approximately 0.22 g O3/g VOCs. Its reaction with hydroxyl radicals (C6H6 + OH· → C6H5O· + H2O) generates phenolic intermediates, which further contribute to the formation of secondary organic aerosols (SOA). The reduced benzene production by the 0.5% Pd-2% Ti-8% Ni/ZSM-5(25) catalyst at 400 °C suggests an inhibition of approximately 15–20% of local ozone precursors, particularly significant for mitigating ozone pollution in summer-prone regions.
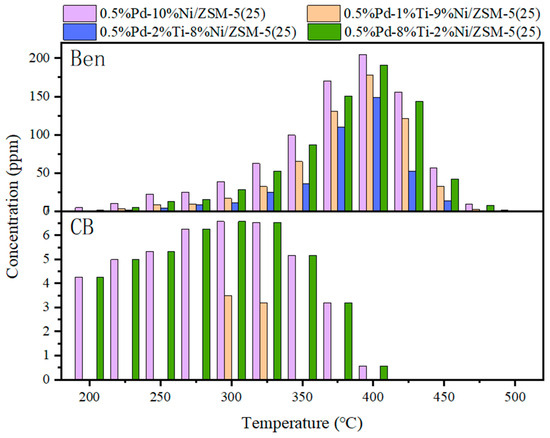
Figure 3.
Distribution of organic by-products (“Ben” is the abbreviation for benzene, and “CB” is the abbreviation for chlorobenzene) in the hydrolysis oxidation of 1,2-DCB.
Within the reaction temperature range of 200–500 °C, the maximum total production of CB is only approximately 6 ppm; additionally, chlorobenzene was not detected at 500 °C. The formation temperature is mainly within the range of 200–325 °C, and the required reaction temperature is relatively low. Only a small amount of CB is produced at 300 °C and 325 °C on the 0.5%Pd-1%Ti-9%Ni/ZSM-5(25) catalyst. Relatively more CB is produced on the 0.5%Pd-10%Ni/ZSM-5(25) and 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) catalysts. Compared with other catalysts, the 0.5%Pd-10%Ni/ZSM-5(25) catalyst has the largest production amount of CB during the hydrolysis oxidation process, which is approximately 6 ppm. When the reaction temperature is above 425 °C, no CB is detected. Moreover, it can be found that no CB is detected during the catalytic degradation of 1,2-DCB over the 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) catalyst.
The photolysis of CB can release Cl radicals (C6H5Cl + hν → C6H5· + Cl·), which participate in the O3 decomposition cycle (Cl + O3 → ClO + O2). Ti-doped catalysts suppress CB formation, reducing CB emissions by 80–100% and cutting off this potential risk pathway. The reaction products of CB with ·OH radicals include HCl and phenolic compounds, where HCl exacerbates acid rain and phenols can be further oxidized to form hygroscopic substances like nitrophenols, promoting PM2.5 formation. In industrial emission scenarios, using the 0.5% Pd-2% Ti-8% Ni/ZSM-5(25) catalyst avoids CB emissions, thereby reducing acidic gas and secondary particulate precursor loads by approximately 5–10%.
3.3. Catalyst XRD Analysis
The catalysts, which had been doped with diverse proportions of Ti, underwent XRD characterization, and the ensuing diffraction profiles are illustrated in Figure 4. The characteristic diffraction peaks of ZSM-5 were recognized. This revelation indicates that the infusion of different proportions of Ni and Ti metals did not undermine the framework of the ZSM-5 zeolite molecular sieves that served as the catalyst carrier. For 0.5%Pd-10%Ni/ZSM-5(25), 0.5%Pd-1%Ti-9%Ni/ZSM-5(25), and 0.5%Pd-2%Ti-8% Ni/ZSM-5(25), the diffraction peaks corresponding to Ni were discerned at 45.0°, 52.2°, and 77.1°. Nonetheless, these diffraction peaks manifested a progressive attenuation with a steady decrement in Ni loaded onto the catalyst. The Ni crystals were detected only at 2θ of 45.0° for 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) catalyst. For others, the Ti diffraction peaks detected at 35.2°, 37.1°, and 53.1° are weaker, and it is hypothesized that Ti has a relatively high dispersion [43].
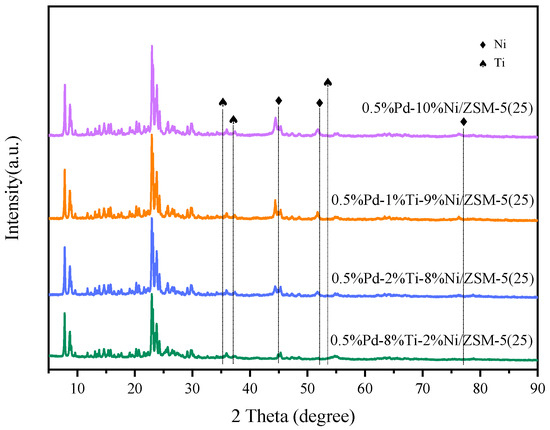
Figure 4.
XRD patterns of different catalysts.
3.4. Hydroxyl Group Changes on the Catalyst Surface
The hydroxyl groups on the surface of the catalysts were determined by FT-IR spectroscopy, and the plots are shown in Figure 5. ZSM-5 zeolite has a strong infrared absorption up to 1300 cm−1. As can be seen from the figure, the characteristic absorption peaks of ZSM-5 appeared, which indicates that the loaded Ti and Ni metals did not destroy the original structure of the carriers, and this is in agreement with the XRD analyses. The absorption peak of the spectrum at 1641 cm−1 corresponds to the hydroxyl bending vibration peak in H2O. In the high-frequency region, two inverted peaks at 3737 cm−1 and 3607 cm−1 were detected. The inverted peak at 3737 cm−1 is the terminal hydroxyl group bound to the silicon skeleton atom [44,45], while the inverted peak at 3607 cm−1 is due to the bridging hydroxyl group (Si-OH-Al) attributed to the Brønsted acid site. The intensity of the band at 3607 cm−1 is much more pronounced compared to the inverted peak at 3737 cm−1.
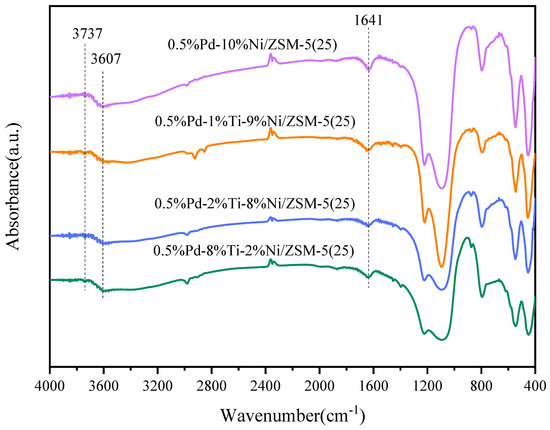
Figure 5.
FT-IR spectra of different catalyst surfaces.
3.5. The Surface Acidity and Basicity of the Catalyst
To further verify the acid-base nature of the catalyst surface, pyridine-IR tests were performed on the catalyst, and the results are shown in Figure 6. The IR peaks at 1450 cm−1 and 1540 cm−1 are attributed to Lewis and Brønsted acidic sites. In addition, the pyridine IR peak at 1490 cm−1 is the sum of Brønsted and Lewis acidic sites. The intensity of Lewis acid centers decreased in the following order: 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) > 0.5%Pd-1%Ti-9%Ni/ZSM-5(25) > 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) > 0.5%Pd-10%Ni/ZSM-5(25). This is directly proportional to the hydrolysis oxidation activity of the catalyst and inversely proportional to the number of organic by-products generated during the hydrolysis oxidation process. According to the literature, Lewis acidic sites contribute to the adsorption of CVOCs on the catalyst surface [46].
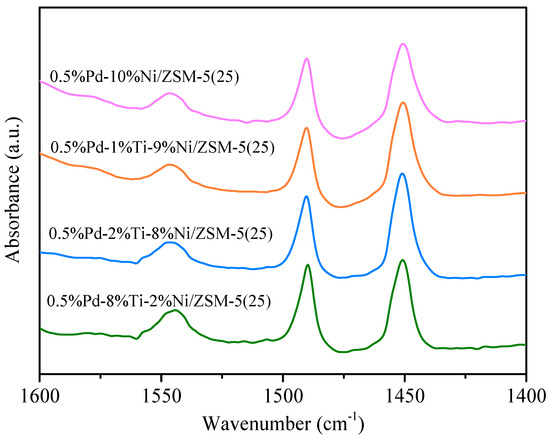
Figure 6.
Pyridine-IR profiles for different catalysts.
The strength of Brønsted acidic sites on the surface of 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) and 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) catalysts was greater than that of the other two catalysts. The stronger the acidity of Brønsted acidic sites, the more likely they are to donate protons and trigger the protonation of H2O, thus facilitating the decomposition reaction of H2O on the Brønsted acid sites. At the same time, the Brønsted acidic site also promotes the breaking of C–C bonds, which in turn accelerates the catalytic oxidation process [26,47,48].
3.6. Catalyst Surface Redox Properties
Further oxidation of carbon species is closely related to the redox capacity of the catalysts, therefore the redox capacity of each catalyst was tested by XPS as shown in Figure 7 and Figure 8. On the 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) catalyst, the Ni 2p1/2 binding energy was observed around 873.2 eV, which can be attributed to Ni2+ in NiO [49], and the binding energy at 855.6 eV corresponded to Ni2+ 2p3/2. With increasing Ni loading, the binding energy of Ni 2p gradually shifted to a lower position, in which the binding energy at Ni 2p1/2 corresponds to a larger degree of shift, and the binding energy at Ni 2p3/2 remains unchanged. Compared with the 0.5%Pd-1%Ti-9%Ni/ZSM-5(25) and 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) catalysts, the highest binding energy of Ni 2p was observed for the 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) catalyst. Therefore, the higher the Ti/Ni loading, the higher the Ni 2p binding energy, indicating that the higher the positive charge of Ni, the higher its redox capacity.
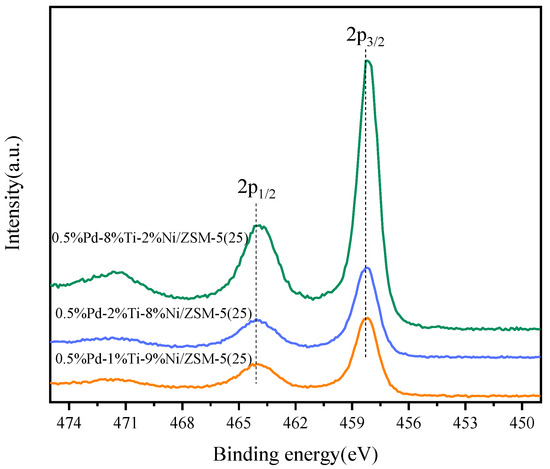
Figure 7.
Ni 2p XPS spectra for different catalysts.
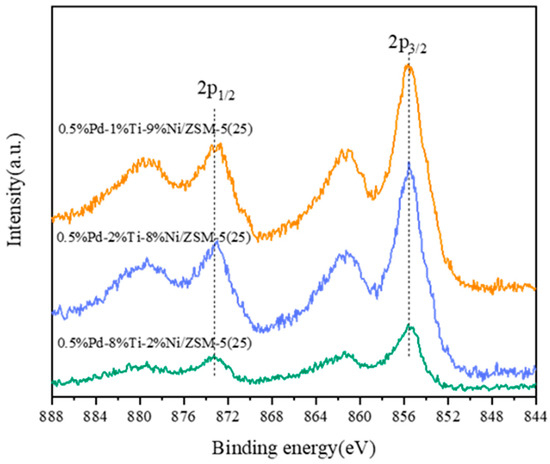
Figure 8.
Ti 2p XPS spectra for different catalysts.
The XPS spectra of different catalysts of Ti 2p are shown in Figure 8. Ti4+ 2p1/2 and Ti4+ 2p3/2 were observed at 464.1 and 458.3 eV, respectively [50]. Similarly, the binding energy of Ti 2p is gradually shifted to a lower position with increasing Ti loading. Compared with the 0.5%Pd-8%Ti-2%Ni/ZSM-5(25) catalyst, 0.5%Pd-1%Ti-9%Ni/ZSM-5(25) and 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) showed higher binding energies at Ti 2p1/2. Moreover, the Ti 2p binding energy is higher as the loading of Ti/Ni decreases. Therefore, encyclopedic consideration of the effect of the loading of Ti/Ni on the binding energies at Ni 2p and Ti 2p shows that the 0.5%Pd-2%Ti-8%Ni/ZSM-5(25) catalyst has the strongest redox ability. The CxHyOz species undergoes a redox reaction with the reactive hydroxyls decomposed on the active sites of the catalyst to generate CO2 and CO; therefore, the stronger the catalyst’s redox property is the ability to decompose CxHyOz species, the higher the reaction activity.
4. Conclusions
Through studying the effects of four catalysts with different dopings on the hydrolysis oxidation activity and product distribution of 1,2-DCB under the reaction condition of 3.2 vol.% H2O, it can be concluded that the 0.5% Pd-2%Ti-8% Ni/ZSM-5(25) catalyst exhibits the optimal catalytic efficiency. The complete conversion of 1,2-DCB achieved at 425 °C indicates effective suppression of atmospheric release of Cl radicals, which is of dual significance for controlling photochemical smog and acid rain precursors.
Analysis of the characterization data from various tests revealed that the acidity, active protons, and redox properties of the catalyst surface can affect the hydrolysis oxidation activity of the catalyst. Lewis acid on the catalyst surface can promote the active adsorption of 1,2-DCB, Bronsted acidic sites can promote the protonation of water molecules, and active protons can promote the further decomposition of adsorbed species. High-valent metals present on the catalyst surface are capable of facilitating the further oxidation of carbon species. As a consequence, the acidity, active protons, and redox characteristics of the catalyst surface all assume critically significant roles in the hydrolysis oxidation processes of CVOCs. In the future, it is advisable to combine the characteristics of atmospheric complex pollution to further explore the synergistic removal effect of the catalyst on multi-component VOCs, as well as its stability under actual atmospheric conditions such as humidity and particulate matter. This will provide technical support for the in-depth treatment of industrial source exhaust gases and the improvement of regional atmospheric quality.
Author Contributions
Formal analysis, Y.L. (Yingjie Li) and W.S.; Investigation, J.Z.; Writing—original draft, Y.L. (Yuqing Li), B.L. and N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Shandong Province (ZR2021QB133).
Data Availability Statement
All data supporting this study are included in the article.
Acknowledgments
Thank you to all the teachers for their assistance during the characterization of catalytic materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jia, H.; Xing, Y.; Zhang, L.; Zhang, W.; Wang, J.; Zhang, H.; Su, W. Progress of catalytic oxidation of typical chlorined volatile organic compounds (CVOCs): A review. Sci. Total Environ. 2023, 865, 161063. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Yang, L. Zeolite-based materials for the catalytic oxidation of VOCs: A mini review. Front. Chem. 2021, 10, 751581. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.G.; Bhattacharyya, N.; Blomdahl, D.; Tang, M.; Abue, P.; Novoselac, A.; Ruiz, L.H.; Misztal, P.K. Influence of application method on disinfectant byproduct formation during indoor bleach cleaning: A case study on phenol chlorination. ACS EST Air 2024, 1, 16–24. [Google Scholar] [CrossRef]
- Odabasi, M.; Elbir, T.; Dumanoglu, Y.; Sofuoglu, S.C. Halogenated volatile organic compounds in chlorine-bleach-containing household products and implications for their use. Atm. Environ. 2014, 92, 376–383. [Google Scholar] [CrossRef]
- Chen, G.; Cai, Y.; Zhang, H.; Hong, D.; Shao, S.; Tu, C.; Chen, Y.; Wang, F.; Chen, B.; Bai, Y.; et al. Pt and Mo Co-decorated MnO2 nanorods with superior resistance to H2O, sintering, and HCl for catalytic oxidation of chlorobenzene. Environ. Sci. Technol. 2021, 55, 14204–14214. [Google Scholar] [CrossRef]
- Wang, P.; Wang, L.; Zhao, Y.; Zhang, B.; Wang, D. Progress in degradation of volatile organic compounds by catalytic oxidation: A review based on the kinds of active components of catalysts. Water Air Soil Poll. 2024, 235, 7. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, S.; Bai, B.; Li, L.; Kang, Y.; Hu, X.; Liao, Z.; He, C. Biotemplate fabrication of hollow tubular CexSr1–xTiO3 with regulable surface acidity and oxygen mobility for efficient destruction of chlorobenzene: Intrinsic synergy effect and reaction mechanism. Environ. Sci. Technol. 2022, 56, 5796–5807. [Google Scholar] [CrossRef]
- Li, N.; Cheng, J.; Xing, X.; Sun, Y.; Hao, Z. Distribution and formation mechanisms of polychlorinated organic by-products upon the catalytic oxidation of 1,2-dichlorobenzene with palladium-loaded catalysts. J. Hazard. Mater. 2020, 393, 122412. [Google Scholar] [CrossRef]
- Dai, Q.; Shen, K.; Deng, W.; Cai, Y.; Yan, J.; Wu, J.; Guo, L.; Liu, R.; Wang, X.; Zhan, W. HCl-tolerant HxPO4/RuOx–CeO2 catalysts for extremely efficient catalytic elimination of chlorinated VOCs. Environ. Sci. Technol. 2021, 55, 4007–4016. [Google Scholar] [CrossRef]
- Xing, D.; Wang, S.; Fan, Y.; Zhang, H.; Wang, T.; Wang, M.; Wang, S.; Wang, J.; Pan, D.; Song, X. Formation of PCDD/Fs, PCBs and HCl during catalytic combustion of chlorobenzene over supported transition metal (Cr, V and Cu) oxide catalysts. J. Environ. Chem. Eng. 2023, 11, 109267. [Google Scholar] [CrossRef]
- Van den Brink, R.; Louw, R.; Mulder, P. Increased combustion rate of chlorobenzene on Pt/γ-Al2O3 in binary mixtures with hydrocarbons and with carbon monoxide. Appl. Catal. B Environ. 2000, 25, 229–237. [Google Scholar] [CrossRef]
- Van den Brink, R.W.; De Jong, V.; Louw, R.; Maggi, P.; Mulder, P. Hydrogen–deuterium isotope effects in the reactions of chlorobenzene and benzene on a Pt/γ-Al2O3 catalyst. Catal. Lett. 2001, 71, 15–20. [Google Scholar] [CrossRef]
- Brink, R.v.D.; Krzan, M.; Feijen-Jeurissen, M.; Louw, R.; Mulder, P. The role of the support and dispersion in the catalytic combustion of chlorobenzene on noble metal based catalysts. Appl. Catal. B Environ. 2000, 24, 255–264. [Google Scholar] [CrossRef]
- Scirè, S.; Minicò, S. The role of the support in the oxidative destruction of chlorobenzene on Pt/zeolite catalysts: An FT-IR investigation. Catal. Lett. 2003, 91, 199–205. [Google Scholar] [CrossRef]
- Scirè, S.; Minicò, S.; Crisafulli, C. Pt catalysts supported on H-type zeolites for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2003, 45, 117–125. [Google Scholar] [CrossRef]
- Giraudon, J.M.; Elhachimi, A.; Leclercq, G. Catalytic oxidation of chlorobenzene over Pd/perovskites. Appl. Catal. B Environ. 2008, 84, 251–261. [Google Scholar] [CrossRef]
- Giraudon, J.M.; Nguyen, T.; Leclercq, G.; Siffert, S.; Lamonier, J.F.; Aboukaïs, A.; Vantomme, A.; Su, B.L. Chlorobenzene total oxidation over palladium supported on ZrO2, TiO2 nanostructured supports. Catal. Today 2008, 137, 379–384. [Google Scholar] [CrossRef]
- Zhu, B.; Wei, Z.B.; Lim, T.; Thye, L.T. Catalytic reduction of chlorobenzenes with Pd/Fe nanoparticles: Reactive sites, catalyst stability, particle aging, and regeneration. Environ. Sci. Technol. 2007, 41, 7523–7529. [Google Scholar] [CrossRef]
- Aznárez, A.; Delaigle, R.; Eloy, P.; Gaigneaux, E.; Korili, S.; Gil, A. Catalysts based on pillared clays for the oxidation of chlorobenzene. Catal. Today 2015, 246, 15–27. [Google Scholar] [CrossRef]
- Cho, C.H.; Ihm, S.K. Development of new vanadium-based oxide catalysts for decomposition of chlorinated aromatic pollutants. Environ. Sci. Technol. 2002, 36, 1600–1606. [Google Scholar] [CrossRef]
- Yang, P.; Yang, S.; Shi, Z.; Meng, Z.; Zhou, R. Deep oxidation of chlorinated VOCs over CeO2-based transition metal mixed oxide catalysts. Appl. Catal. B Environ. 2015, 162, 227–235. [Google Scholar] [CrossRef]
- Yang, P.; Shi, Z.; Yang, S.; Zhou, R. High catalytic performances of CeO2–CrOx catalysts for chlorinated VOCs elimination. Chem. Eng. Sci. 2015, 126, 361–369. [Google Scholar] [CrossRef]
- Cai, T.; Huang, H.; Deng, W.; Dai, Q.; Liu, W.; Wang, X. Catalytic combustion of 1,2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts. Appl. Catal. B Environ. 2015, 166, 393–405. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.; Wang, X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2014, 158, 96–105. [Google Scholar] [CrossRef]
- Weng, X.; Sun, P.; Long, Y.; Meng, Q.; Wu, Z. Catalytic oxidation of chlorobenzene over MnxCe1–xO2/HZSM-5 catalysts: A study with practical implications. Environ. Sci. Technol. 2017, 51, 8057–8066. [Google Scholar] [CrossRef]
- Sun, P.; Wang, W.; Dai, X.; Weng, X.; Wu, Z. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1-xO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Zhu, T.; Ning, R. Catalytic oxidation of chlorobenzene over noble metals (Pd, Pt, Ru, Rh) and the distributions of polychlorinated by-products. J. Hazard. Mater. 2019, 363, 90–98. [Google Scholar] [CrossRef]
- Duan, X.; Zhao, T.; Niu, B.; Wei, Z.; Li, G.; Zhang, Z.; Cheng, J.; Hao, Z. Simultaneously constructing active sites and regulating Mn–O strength of Ru-substituted perovskite for efficient oxidation and hydrolysis oxidation of chlorobenzene. Adv. Sci. 2023, 10, 2205054. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Rivas, J.A.; Amiridis, M.D. Catalytic oxidation of 1,2-dichlorobenzene over supported transition metal oxides. J. Catal. 2000, 193, 264–272. [Google Scholar] [CrossRef]
- Wu, S.; Lv, X.; Hao, X.; Chen, J.; Jia, H. Enhancement of mineralization ability and water resistance of vanadium-based catalysts for catalytic oxidation of chlorobenzene by platinum loading. Environ. Sci. Technol. 2024, 58, 15836–15845. [Google Scholar] [CrossRef]
- González-Velasco, J.; Aranzabal, A.; López-Fonseca, R.; Ferret, R.; González-Marcos, J. Enhancement of the catalytic oxidation of hydrogen-lean chlorinated VOCs in the presence of hydrogen-supplying compounds. Appl. Catal. B Environ. 2000, 24, 33–43. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Gutiérrez-Ortiz, J.I.; Gutiérrez-Ortiz, M.A.; González-Velasco, J.R. Catalytic oxidation of aliphatic chlorinated volatile organic compounds over Pt/H-BETA zeolite catalyst under dry and humid conditions. Catal. Today 2005, 107, 200–207. [Google Scholar] [CrossRef]
- Pitkäaho, S.; Ojala, S.; Maunula, T.; Savimäki, A.; Kinnunen, T.; Keiski, R.L. Oxidation of dichloromethane and perchloroethylene as single compounds and in mixtures. Appl. Catal. B Environ. 2011, 102, 395–403. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Aranzabal, A.; Steltenpohl, P.; Gutiérrez-Ortiz, J.; González-Velasco, J. Performance of zeolites and product selectivity in the gas-phase oxidation of 1,2-dichloroethane. Catal. Today 2000, 62, 367–377. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Aranzabal, A.; Gutiérrez-Ortiz, J.; Álvarez-Uriarte, J.; González-Velasco, J. Comparative study of the oxidative decomposition of trichloroethylene over H-type zeolites under dry and humid conditions. Appl. Catal. B Environ. 2001, 30, 303–313. [Google Scholar] [CrossRef]
- López-Fonseca, R.; de Rivas, B.; Gutiérrez-Ortiz, J.; Aranzabal, A.; González-Velasco, J. Enhanced activity of zeolites by chemical dealumination for chlorinated VOC abatement. Appl. Catal. B Environ. 2003, 41, 31–42. [Google Scholar] [CrossRef]
- Bertinchamps, F.; Attianese, A.; Mestdagh, M.; Gaigneaux, E.M. Catalysts for chlorinated VOCs abatement: Multiple effects of water on the activity of VOx based catalysts for the combustion of chlorobenzene. Catal. Today 2006, 112, 165–168. [Google Scholar] [CrossRef]
- Lomnicki, S.; Lichtenberger, J.; Xu, Z.; Waters, M.; Kosman, J.; Amiridis, M.D. Catalytic oxidation of 2,4,6-trichlorophenol over vanadia/titania-based catalysts. Appl. Catal. B Environ. 2003, 46, 105–119. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Bakar, M.Z.A.; Bhatia, S. Combustion of chlorinated volatile organic compounds (VOCs) using bimetallic chromium-copper supported on modified H-ZSM-5 catalyst. J. Hazard. Mater. 2006, 129, 39–49. [Google Scholar] [CrossRef]
- Wang, P.; Ding, S.; Wu, S.; Fang, N.; Zhang, Q.; Chu, Y. Investigation of the by-product selectivity of industrialized support for the catalytic elimination of o-DCB over Pt-catalysts. Microporous Mesoporous Mater. 2025, 381, 113334. [Google Scholar] [CrossRef]
- Lv, X.; Wu, S.; Shao, S.; Yan, D.; Xu, W.; Jia, H.; He, H. Efficient catalytic elimination of chlorobenzene based on the water vapor-promoting effect within Mn-based catalysts: Activity enhancement and polychlorinated byproduct inhibition. Environ. Sci. Technol. 2024, 58, 3985–3996. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xing, X.; Cheng, J.; Zhang, Z.; Hao, Z. Influence of oxygen and water content on the formation of polychlorinated organic by-products from catalytic degradation of 1,2-dichlorobenzene over a Pd/ZSM-5 catalyst. J. Hazard. Mater. 2021, 403, 123952. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Sun, Z.; Yang, T.; Wang, J.; Tang, Q.; Huang, T.; Tang, C.; Gao, F.; Dong, L. Effect of different introduction methods of cerium and tin on the properties of titanium-based catalysts for the selective catalytic reduction of NO by NH3. J. Colloid. Interface Sci. 2022, 613, 320–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wen, Z.; Chai, C.; Li, N.; Zong, K.; Li, Z. Amine-free synthesis of high-silica ZSM-5 assisted with calcined silicalite-1 and ethanol with the investigation of mechanism. Microporous Mesoporous Mater. 2024, 375, 113160. [Google Scholar] [CrossRef]
- Badvi, K.; Javanbakht, V. Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean. Prod. 2021, 280, 124518. [Google Scholar] [CrossRef]
- Aristizábal, B.H.; de Correa, C.M.; Serykh, A.I.; Hetrick, C.E.; Amiridis, M.D. In situ FTIR study of the adsorption and reaction of ortho-dichlorobenzene over Pd-promoted Co-HMOR. Microporous Mesoporous Mater. 2008, 112, 432–440. [Google Scholar] [CrossRef]
- Aranzabal, A.; Romero-Sáez, M.; Elizundia, U.; González-Velasco, J.R.; González-Marcos, J.A. Deactivation of H-zeolites during catalytic oxidation of trichloroethylene. J. Catal. 2012, 296, 165–174. [Google Scholar] [CrossRef]
- González-Velasco, J.; López-Fonseca, R.; Aranzabal, A.; Gutiérrez-Ortiz, J.; Steltenpohl, P. Evaluation of H-type zeolites in the destructive oxidation of chlorinated volatile organic compounds. Appl. Catal. B Environ. 2000, 24, 233–242. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Q.; Su, G.; Pang, J.; Sun, B.; Meng, J.; Shi, B. Catalytic degradation of chlorinated volatile organic compounds (CVOCs) over Ce-Mn-Ti composite oxide catalysts. J. Environ. Sci. 2024, 138, 326–338. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Z.; Osatiashtiani, A.; Nabavi, S.A.; Clough, P.T. Ni-based bimetallic catalysts for hydrogen production via (sorption-enhanced) steam methane reforming. Chem. Eng. J. 2024, 486, 150170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).