Long-Term Caragana korshinskii Restoration Enhances SOC Stability but Reduces Sequestration Efficiency over 40 Years in Degraded Loess Soils
Abstract
1. Introduction
2. Material and Method
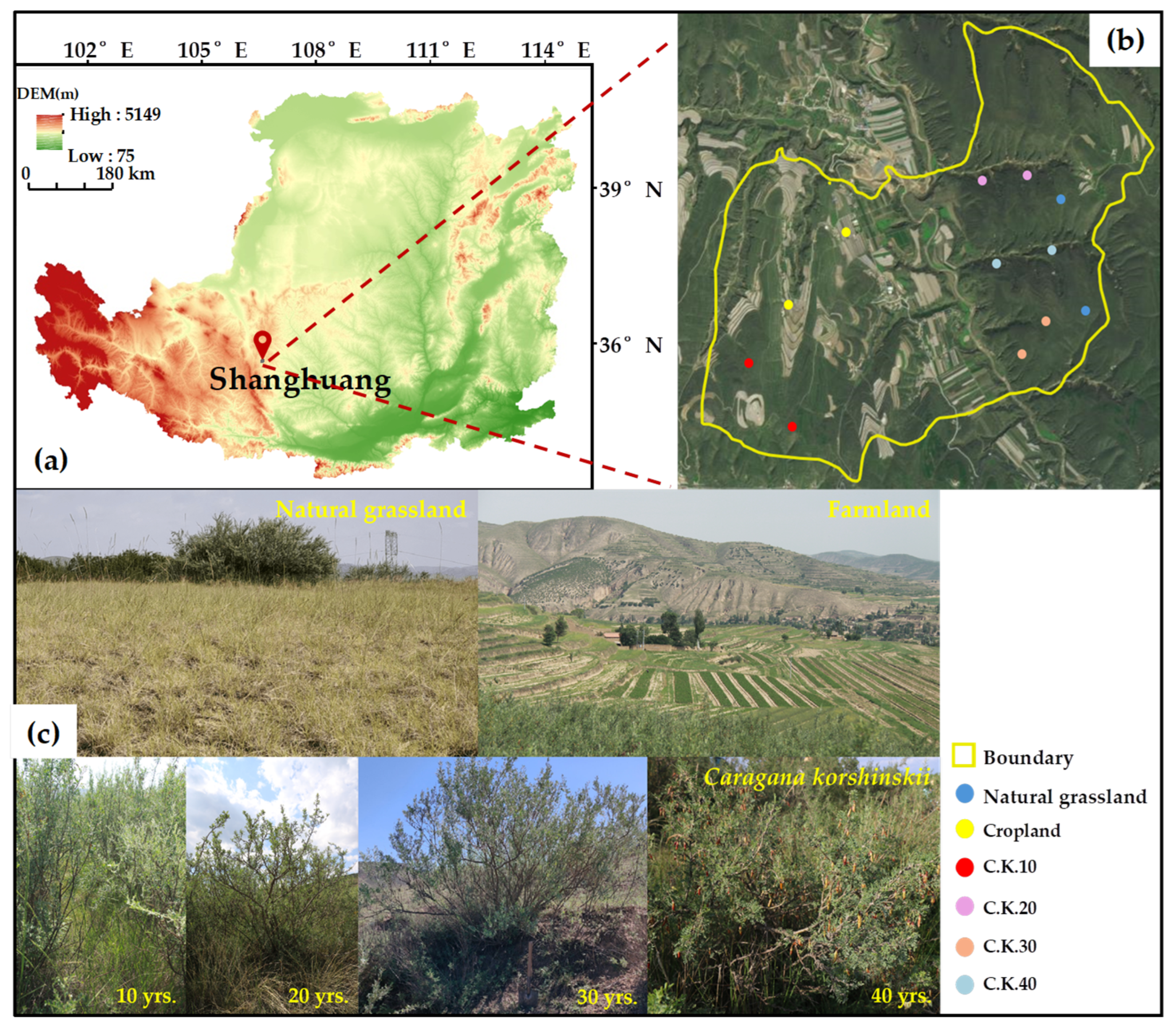
2.1. Study Area
2.2. C. korshinskii Plantations and Management
2.3. Soil Sampling
2.4. Measurement of Soil Properties and Soil Organic C Fractions
2.5. Statistical Analyses
3. Results
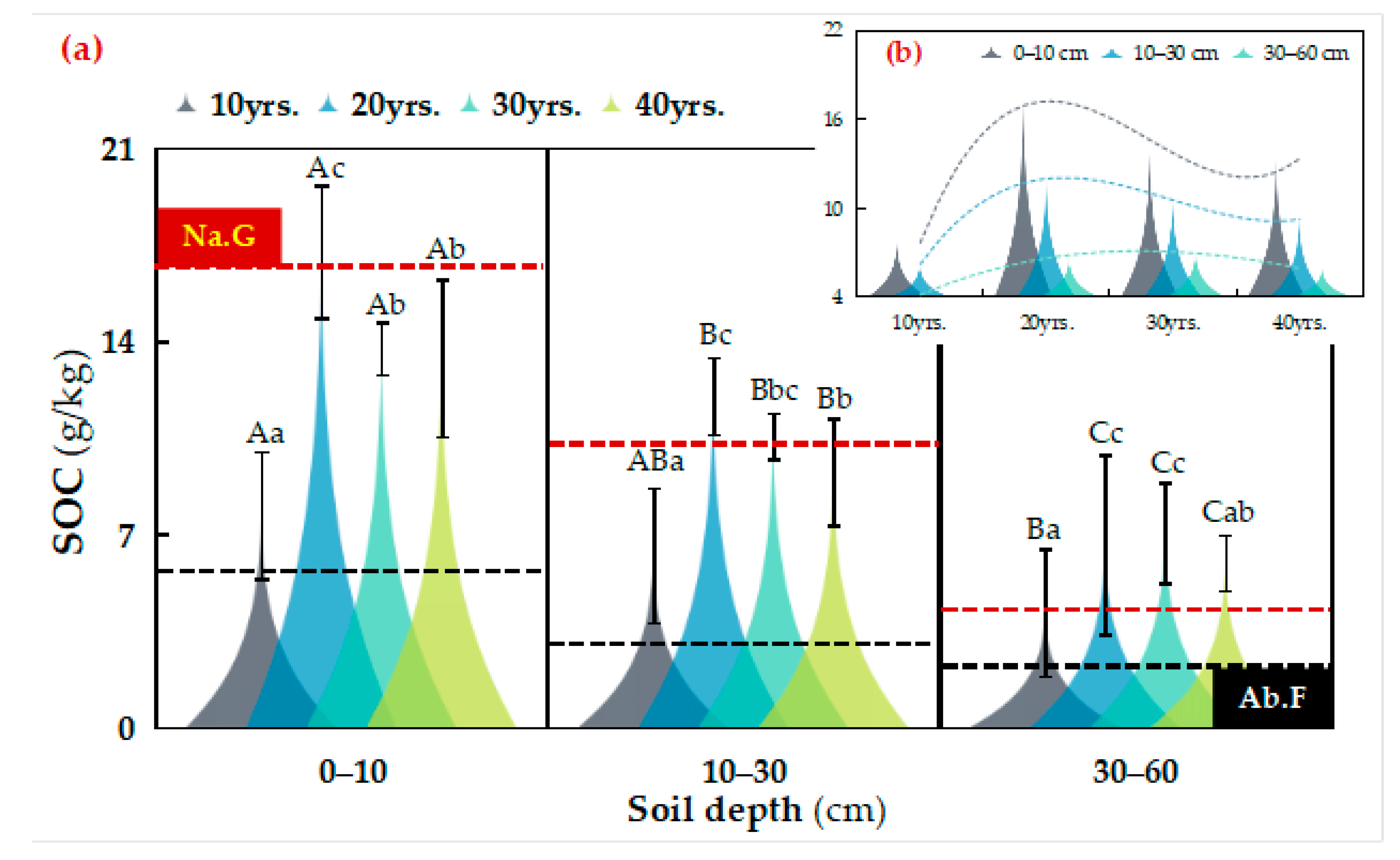
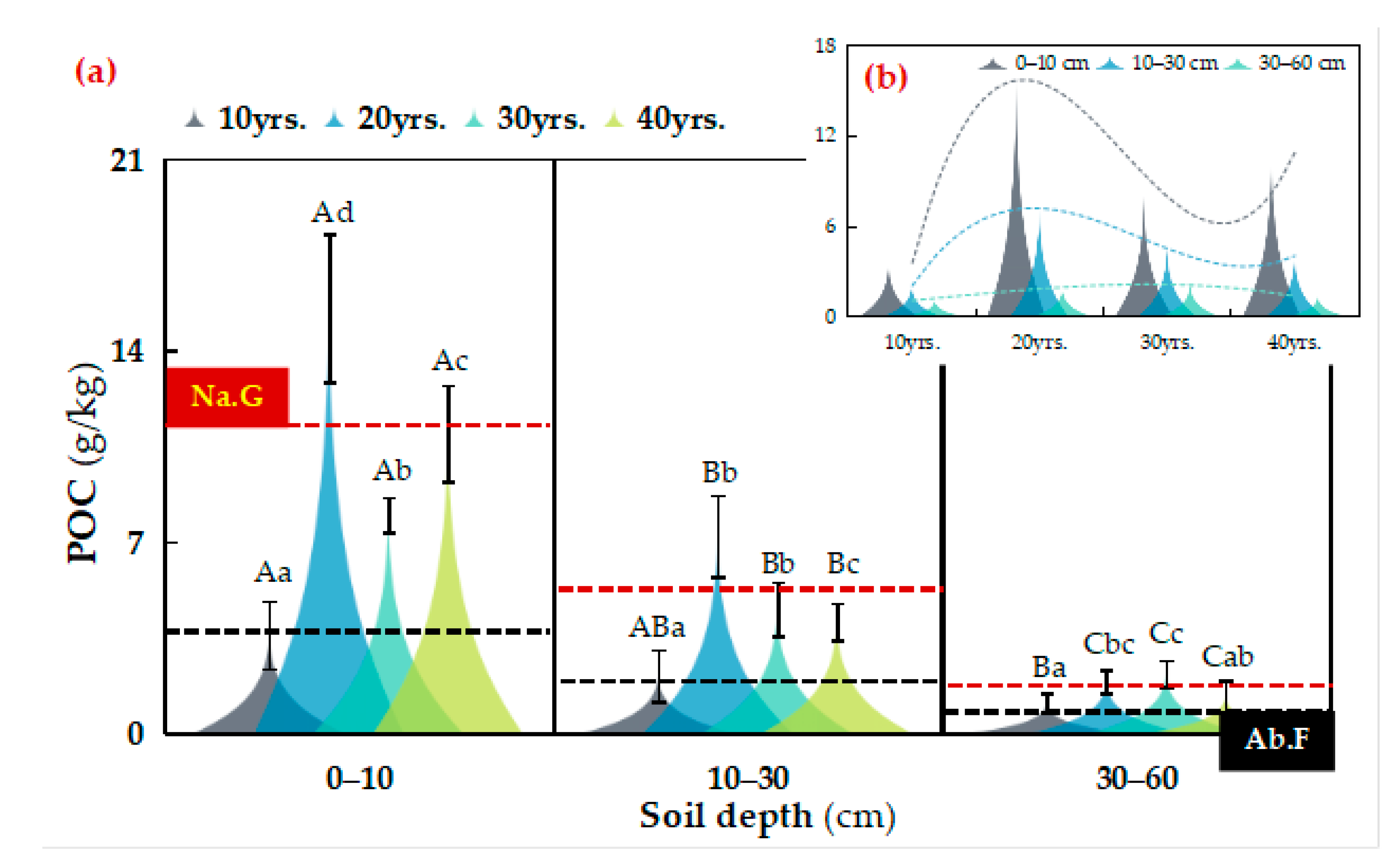
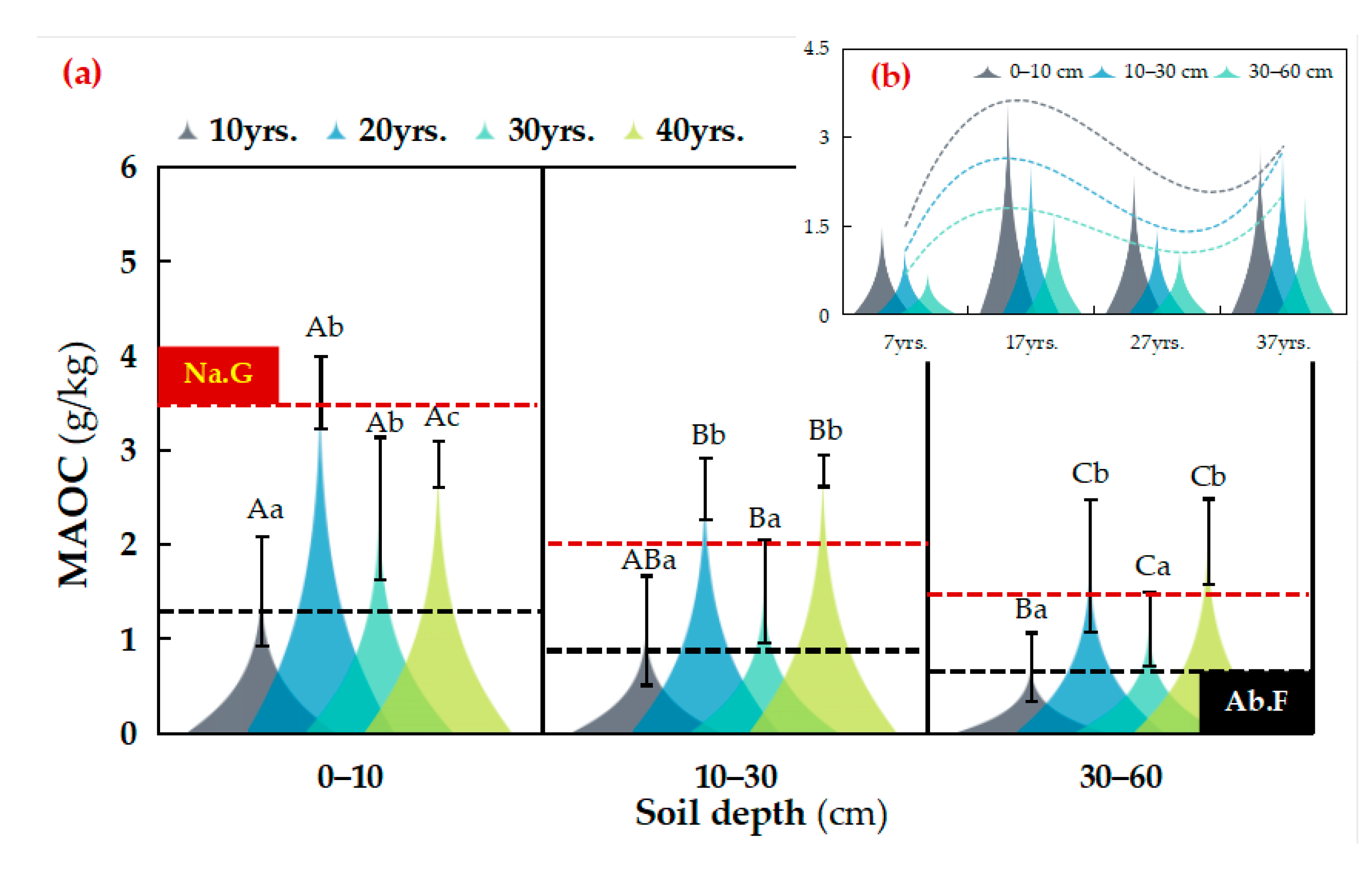
3.1. Distribution of SOC Fractions in Different Vegetation Restoration Chronosequence Years
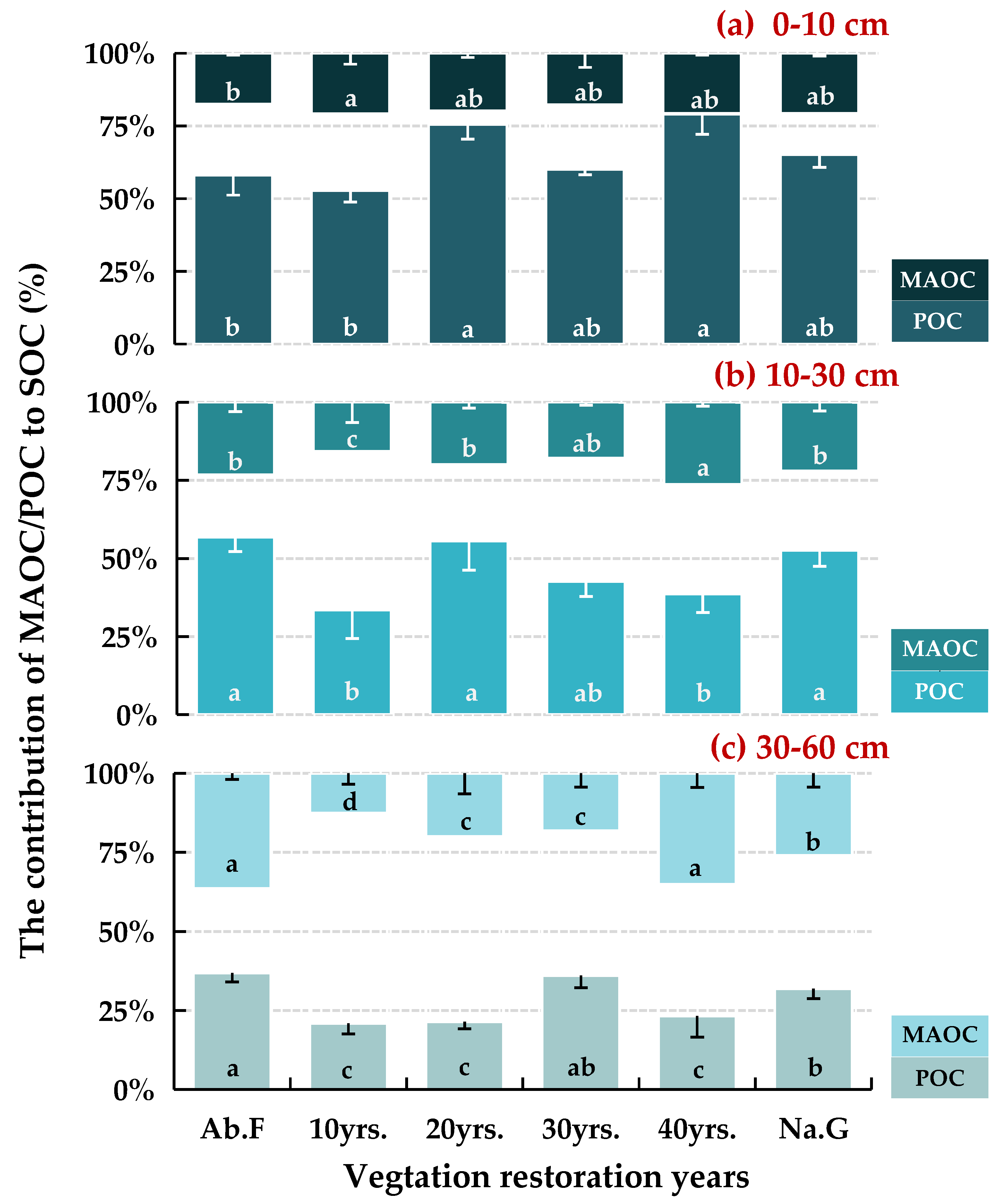
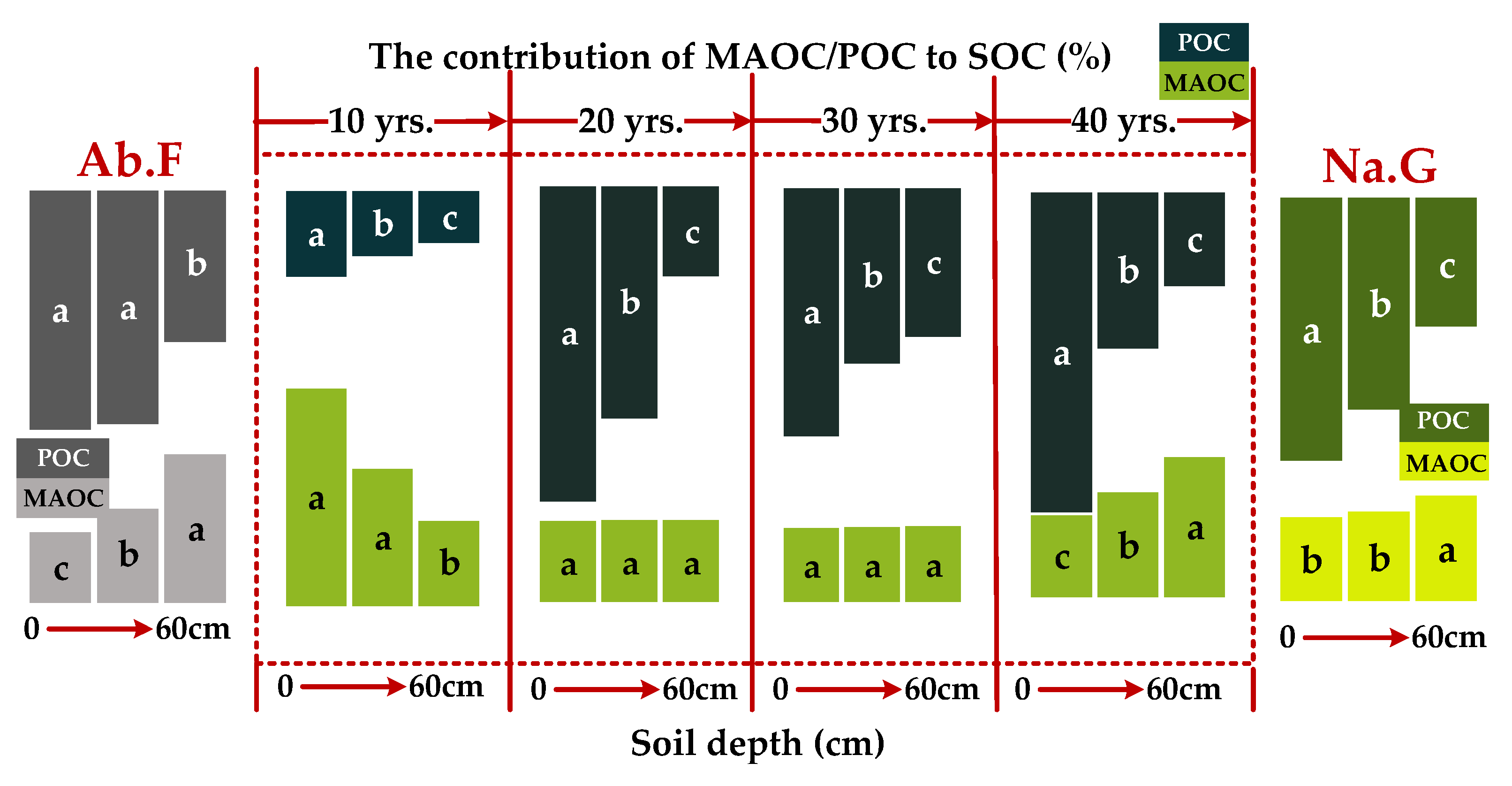
3.2. The Correlation Between POC, MAOC, and SOC in Different Restoration Chronosequence Periods
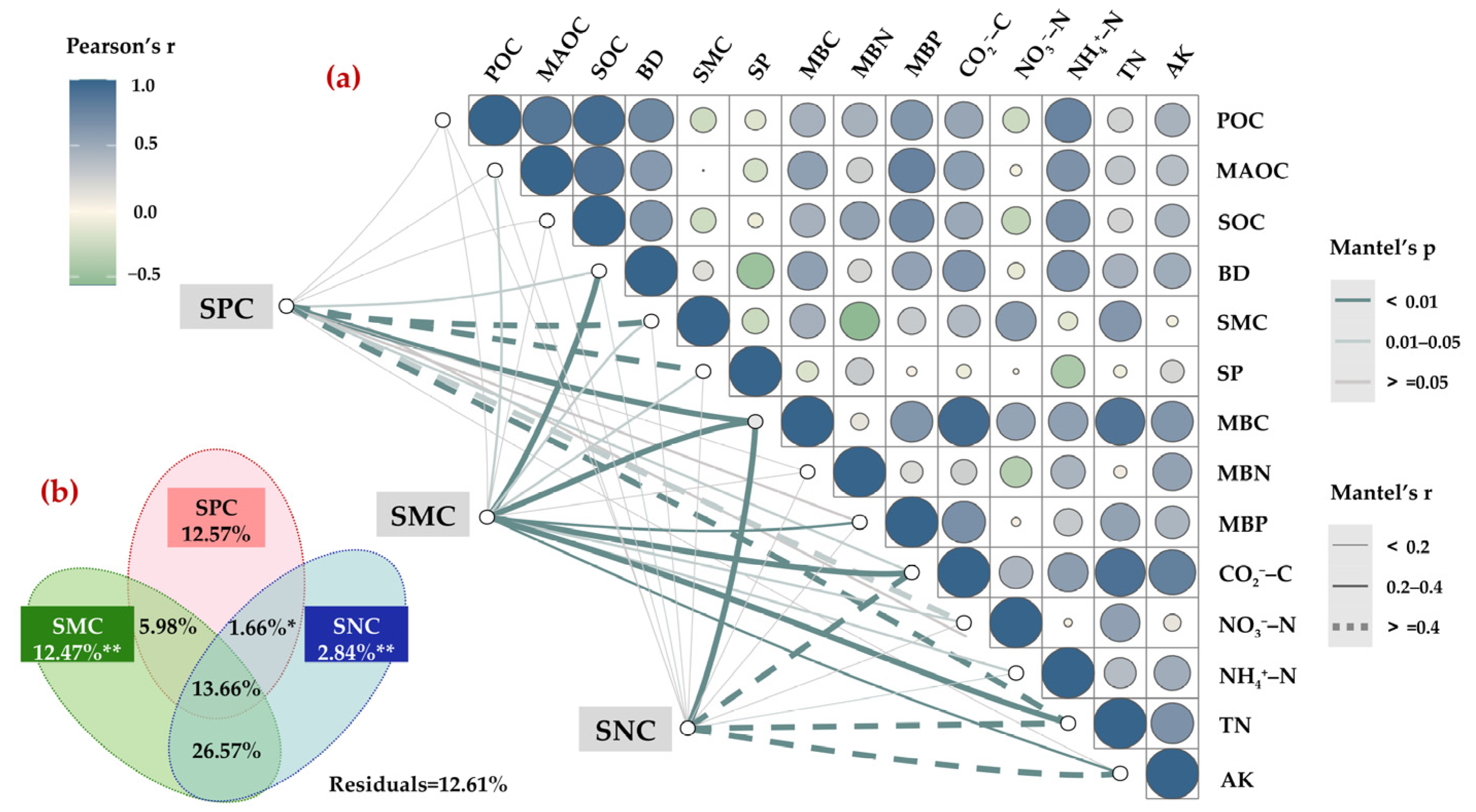
3.3. Influencing Factors of SOC Fractions
4. Discussion
4.1. Influence of C. korshinskii Chronosequence Stage on SOC Sequestration
4.2. Dominance of MAOC Contribution to SOC Stability
4.3. MAOC Accumulation Depends on Depth Stabilization Mechanisms
4.4. Microbial Drivers Mediate Soil C Pool Stabilization in Managed Restoration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, K.; Owens, P.R.; Libohova, Z.; Miller, D.M.; Wills, S.A.; Nemecek, J. Assessing soil organic carbon stock of Wisconsin, USA and its fate under future land use and climate change. Sci. Total Environ. 2019, 667, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.; Crawford, J.W.; Field, D.J.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Xu, L.; Yu, G.; He, N.; Wang, Q.; Gao, Y.; Ding, W.; Li, S.; Niu, S.; Ge, J. Carbon storage in China’s terrestrial ecosystems: A synthesis. Sci. Rep. 2018, 8, 2806. [Google Scholar] [CrossRef]
- Amelung, W.; Bossio, D.; de Vries, W.; Kögel-Knabner, I.; Amundson, R.; Bol, R.; Collins, C.; Lal, R.; Leifeld, J.; Minasny, B. Towards a global-scale soil climate mitigation strategy. Nat. Commun. 2020, 2020, 5427. [Google Scholar] [CrossRef]
- Zhang, X.P.; Lin, P.F.; Chen, H.; Yan, R.; Zhang, J.; Yu, Y.; Liu, E.; Yang, Y.; Zhao, W.; Lv, D.; et al. Understanding land use/cover change impacts on runoff and sediment load at flood events on the Loess Plateau, China. Hydrol. Process. 2018, 32, 576–589. [Google Scholar] [CrossRef]
- Shi, P.; Li, P.; Li, Z.; Sun, J.; Wang, D.; Min, Z. Effects of grass vegetation coverage and position on runoff and sediment yields on the slope of Loess Plateau, China. Agric. Water Manag. 2022, 259, 107231. [Google Scholar] [CrossRef]
- Gu, C.; Mu, X.; Gao, P.; Zhao, G.; Sun, W.; Tatarko, J.; Tan, X. Influence of vegetation restoration on soil physical properties in the Loess Plateau, China. J. Soils Sediments 2019, 2019, 716–728. [Google Scholar] [CrossRef]
- Wang, A.; Zha, T.; Zhang, Z. Variations in soil organic carbon storage and stability with vegetation restoration stages on the Loess Plateau of China. Catena 2023, 228, 107142. [Google Scholar] [CrossRef]
- Li, Q.; Jia, W.; Wu, J.; Wang, L.; Huang, F.; Cheng, X. Changes in soil organic carbon stability in association with microbial carbon use efficiency following 40 years of afforestation. Catena 2023, 228, 107179. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Gao, X.; Hu, Q.; Song, J.; Ma, N.; Song, X.; Siddique, K.; Wu, P.; Zhao, X. Divergent responses of deep SOC sequestration to large-scale revegetation on China’s Loess Plateau. Agric. Ecosyst. Environ. 2023, 349, 108433. [Google Scholar] [CrossRef]
- Zhao, R.; He, M.; Yue, P.; Huang, L.; Liu, F. Linking soil organic carbon stock to microbial stoichiometry, carbon sequestration and microenvironment under long-term forest conversion. J. Environ. Manag. 2022, 301, 113940. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Bai, J.; Wang, J.; Zhou, Y.; Kang, T.; Wang, J.; Hu, C.; Guo, H.; Chen, P.; Xie, P.; et al. Carbon storage patterns of Caragana korshinskii in areas of reduced environmental moisture on the Loess Plateau, China. Sci. Rep. 2016, 6, 28883. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, W.; Wang, G.; Yang, S.; Pereira, P. Effects of long-term afforestation and natural grassland recovery on soil properties and quality in Loess Plateau. Sci. Total Environ. 2021, 770, 144833. [Google Scholar] [CrossRef]
- Bao, J.T.; Wang, J.; Li, X.R.; Zhang, Z.; Su, J. Age-related changes in photosynthesis and water relations of revegetated Caragana korshinskii in the Tengger desert, Northern China. Trees 2015, 29, 1749–1760. [Google Scholar] [CrossRef]
- Xu, H.; Qu, Q.; Lu, B.; Zhang, Y.; Liu, G.; Xue, S. Variation in soil organic carbon stability and driving factors after vegetation restoration in different vegetation zones on the Loess Plateau, China. Soil Tillage Res. 2020, 204, 104727. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, B.; Wu, X.; Wang, Z.; Liu, L.; Yang, H. Revegetation promotes soil mineral-associated organic carbon sequestration and soil carbon stability in the Tengger Desert, northern China. Soil Biol. Biochem. 2023, 185, 109155. [Google Scholar] [CrossRef]
- Cunningham, S.C.; Metzeling, K.J.; Nally, R.M.; Thomson, J.R.; Cavagnaro, T.R. Changes in soil carbon of pastures after afforestation with mixed species: Sampling, heterogeneity and surrogates. Agric. Ecosyst. Environ. 2012, 158, 58–65. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Yao, Y.; Qin, F.; Guo, Y.; Gao, Y.; Zhang, M. Spatial distribution of soil organic carbon and its influencing factors at different soil depths in a semiarid region of China. Environ. Earth Sci. 2017, 76, 654. [Google Scholar] [CrossRef]
- Albaladejo, J.; Ortiz, R.; Garcia-Franco, N.; Navarro, A.; Almagro, M.; Pintado, J.; Martínez-Mena, M. Land use and climate change impacts on soil organic carbon stocks in semi-arid Spain. J. Soils Sediments 2013, 13, 265–277. [Google Scholar] [CrossRef]
- Zhou, L.; Li, S.; Liu, B.; Wu, P.; Heal, K.; Ma, X. Tissue-specific carbon concentration, carbon stock, and distribution in Cunninghamia lanceolata (Lamb.) Hook plantations at various developmental stages in subtropical China. Ann. For. Sci. 2019, 76, 70. [Google Scholar] [CrossRef]
- Villarino, S.H.; Pinto, P.; Jackson, R.B.; Piñeiro, G. Plant rhizodeposition: A key factor for soil organic matter formation in stable fractions. Sci. Adv. 2021, 7, eabd3176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, L.; Knighton, J.; Evaristo, J.; Wassen, M. Contrasting adaptive strategies by Caragana korshinskii and Salix psammophila in a semiarid revegetated ecosystem. Agric. For. Meteorol. 2021, 300, 168–1923. [Google Scholar] [CrossRef]
- Su, Z.; Zhong, Y.; Zhu, X.; Wu, Y.; Shen, Z.; Shangguan, Z. Vegetation restoration altered the soil organic carbon composition and favoured its stability in a Robinia pseudoacacia plantation. Sci. Total Environ. 2023, 899, 165665. [Google Scholar] [CrossRef]
- Fu, B.J.; Zhao, W.; Chen, L.; Zhang, J.; Lv, H.; Gulinck, J. Assessment of soil erosion at large watershed scale using RUSLE and GIS: A case study in the Loess Plateau of China. Land Degrad. Dev. 2005, 16, 73–85. [Google Scholar] [CrossRef]
- Jian, S.; Zhao, C.; Fang, S.; Yu, K. Effects of different vegetation restoration on soil water storage and water balance in the Chinese Loess Plateau. Agric. For. Meteorol. 2015, 206, 85–96. [Google Scholar] [CrossRef]
- Liao, J.; Yang, X.; Dou, Y.; Wang, B.; Xue, Z.; Sun, H.; Yang, Y.; An, S. Divergent contribution of particulate and mineral-associated organic matter to soil carbon in grassland. J. Environ. Manag. 2023, 344, 0301–4797. [Google Scholar] [CrossRef]
- Shi, J.; Song, M.; Yang, L.; Zhao, F.; Wu, J.; Li, J.; Yu, Z.; Li, A.; Shangguan, Z.; Deng, L. Recalcitrant organic carbon plays a key role in soil carbon sequestration along a long-term vegetation succession on the Loess Plateau. Catena 2023, 233, 107528. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, J.; Li, G.; Ma, Z.; Zhang, X.; Zhang, Q.; Yang, Z.; Huang, G. Caragana korshinskii of different planting years altered the soil water, carbon, and nitrogen storage in the different layers within level ditch on slope in a semi-arid area. Catena 2024, 242, 108126. [Google Scholar] [CrossRef]
- Cotrufo, M.; Ranalli, M.; Haddix, J.; Six, J.; Lugato, E. Soil carbon storage informed by particulate and mineral-associated organic matter. Nat. Geosci. 2019, 12, 989–994. [Google Scholar] [CrossRef]
- Lavallee, J.; Soong, J.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yang, F.; Song, X.; Yang, S.; Zhang, G. Edaphic regulation of soil organic carbon fractions in the mattic layer across the Qinghai-Tibetan Plateau. Sci. Total Environ. 2024, 943, 173814. [Google Scholar] [CrossRef] [PubMed]
- Villarino, S.H.; Studdert, G.A.; Baldassini, P.; Cendoya, M.; Giuffoli, L.; Mastrángelo, M.; Piñeiro, G. Deforestation impacts on soil organic carbon stocks in the Semiarid Chaco Region, Argentina. Sci. Total Environ. 2017, 575, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Raphael, A.; Rossel, V.; Wang, G.; Xiao, L.; Wang, M.; Zhang, S.; Luo, Z. Particulate and mineral-associated organic carbon turnover revealed by modelling their long-term dynamics. Soil Biol. Biochem. 2022, 173, 108780. [Google Scholar] [CrossRef]
- Amorim, H.; Luis, C.; Ivan, H.; Zinn, Y. C:N ratios of bulk soils and particle-size fractions: Global trends and major drivers. Geoderma 2022, 425, 116026. [Google Scholar] [CrossRef]
- Rocci, K.; Lavallee, J.; Stewart, C.; Cotrufo, M. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 793, 148569. [Google Scholar] [CrossRef]
- Feng, W.; Plante, A.; Aufdenkampe, A.; Six, J. Soil organic matter stability in organo-mineral complexes as a function of increasing C loading. Soil Biol. Biochem. 2014, 69, 398–405. [Google Scholar] [CrossRef]
- Ramesh, T.; Nanthi, S.; Kirkham, M.; Wijesekara, H.; Manjaiah, K.M.; Ch, S.; Sasidharan, S.; Rinklebe, J.; Yong, S.; Choudbury, B.; et al. Freeman II, Soil organic carbon dynamics, Impact of land use changes and management practices, A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Liu, J.; Zhao, Z.; Yan, Y.; Ali, A.; Ahmed, Z.; He, D.; Yu, M.; Hang, J.; Perven, M.; Nazir, T.; et al. Effect of alfalfa habitat change on dispersal behavior of Harmonia axyridis Pallas and Hippodamia variegata Goeze (Coleoptera, Coccinellidae). J. Asia-Pac. Entomol. 2021, 24, 997–1003. [Google Scholar] [CrossRef]
- An, S.S.; Huang, Y.M.; Liu, M.Y. Soil organic carbon density and land restorations example of southern mountain area of Ningxia province, northwest China. Commun. Soil Sci. Plant Anal. 2020, 4, 181–189. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar]
- Peng, X.; Huang, Y.; Duan, X.; Yamg, H.; Liu, J. Particulate and mineral-associated organic carbon fractions reveal the roles of soil aggregates under different land-use types in a karst faulted basin of China. Catena 2023, 220, 106721. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis: Part 3 Chemical Methods; American Society of Agronomy: Madison, WI, USA, 1996; Volume 5, pp. 1085–1121. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorous. In Methods of Soil Analysis: Part 2, Chemical and Microbial Properties; Agronomy Monograph; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Society of America: Madison, WI, USA, 1982; Volume 9, pp. 403–430. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Nicoloso, R.; Rice, C.; Amado, T.; Costa, C.; Akley, E. Carbon saturation and translocation in a no-till soil under organic amendments. Agric. Ecosyst. Environ. 2018, 264, 73–84. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, P.; Li, P.; Li, Z.; Min, Z.; Sun, J.; Cui, L.; Niu, H.; Zu, P.; Cao, M. Effects of vegetation restoration on soil organic carbon in the Loess Plateau: A meta-analysis. Land Degrad. Dev. 2023, 34, 2088–2097. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.; Nierop, K.; Simpson, M. Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Pan, Y. Particulate organic carbon is more sensitive to nitrogen addition than mineral-associated organic carbon, A meta-analysis. Soil Tillage Res. 2022, 216, 106405. [Google Scholar] [CrossRef]
- Don, A.; Rebmann, C.; Kolle, O.; Scherer-lorenzen, M.; Schulze, E. Impact of afforestation-associated management changes on the carbon balance of grassland. Glob. Chang. Biol. 2009, 15, 1990–2002. [Google Scholar] [CrossRef]
- Chai, Q.; Ma, Z.; An, Q.; Wu, G.; Chang, X.; Zheng, J.; Wang, G. Does artificial Caragana korshinskii plantation increase soil carbon continuously in a water-limited landscape on the Loess Plateau? Land Degrad. Dev. 2019, 30, 1691–1698. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Yao, S.; Ye, Y.; Zhang, B. Responses of soil mineral-associated and particulate organic carbon to carbon input: A meta-analysis. Sci. Total Environ. 2022, 829, 0048–9697. [Google Scholar] [CrossRef]
- Li, Z.; Duan, X.; Guo, X.; Gao, W.; Li, Y.; Zhou, P.; Zhu, Q.; O’Donnell, A.G.; Dai, K.; Wu, J. Microbial metabolic capacity regulates the accrual of mineral-associated organic carbon in subtropical paddy soils. Soil Biol. Biochem. 2024, 195, 109457. [Google Scholar] [CrossRef]
- Lu, F.; Hu, H.; Sun, W.; Zhu, J.; Liu, G.; Zhou, W.; Zhang, Q.; Shi, P.; Liu, X.; Wu, X.; et al. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Proc. Natl. Acad. Sci. USA 2018, 115, 4039–4044. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Han, P.; Zhang, P.; Ding, F.; Ma, C. The impact of afforestation on soil organic carbon sequestration on the Qinghai Plateau, China. PLoS ONE 2015, 10, e0116591. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, K.; Li, J.; Li, Q.; Na, W.; Gao, Y.; Gao, Z. Response of soil water in deep dry soil layers to monthly precipitation, plant species, and surface mulch in a semi-arid hilly loess region of China. Agric. Water Manag. 2024, 291, 108612. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, C.; Wang, Y.; Zhu, Y.; Wei, X.; Shao, M. Traditional dry soil layer index method overestimates soil desiccation severity following conversion of cropland into forest and grassland on China’s Loess Plateau. Agric. Ecosyst. Environ. 2020, 291, 106794. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P. Chapter One: Mineral-Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 1–140. [Google Scholar]
- Poeplau, C.; Don, A.; Six, J.; Kaiser, M.; Benbi, D.; Chenu, C.; Cotrufo, M.; Derrien, D.; Gioacchini, P.; Grand, S.; et al. Rolf Nieder, Isolating organic carbon fractions with varying turnover rates in temperate agricultural soils—A comprehensive method comparison. Soil Biol. Biochem. 2018, 125, 10–26. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Chang. Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef]
- Isabelle, B.D. Reviews and syntheses: The mechanisms underlying carbon storage in soil. Biogeosciences 2020, 17, 5223–5242. [Google Scholar]
- Feng, Y.; Sui, X.; Tang, J.; Liu, R.; Ling, X.; Liang, W.; Wei, X. Responses of belowground fine root biomass and morphology in Robinia pseudoacacia L. plantations to aboveground environmental factors. Glob. Ecol. Conserv. 2024, 50, e02863. [Google Scholar] [CrossRef]
- Jia, Y.; Li, T.; Shao, M.; Hao, J.; Wang, Y.; Jia, X.; Zeng, C.; Fu, X.; Liu, B.; Gan, M. Disentangling the formation and evolvement mechanism of plants-induced dried soil layers on China’s Loess Plateau. Agric. For. Meteorol. 2019, 269–270, 57–70. [Google Scholar] [CrossRef]
- Han, L.; Nan, G.; He, X.; Wang, J.; Zhao, J.; Zhang, X. Soil moisture and soil organic carbon coupled effects in apple orchards on the Loess Plateau, China. Sci. Rep. 2024, 14, 12281. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Rich, R.L.; Hobbie, S.E.; Montgomery, R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 2018, 562, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, C.; Frey, S.; Grandy, A. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef] [PubMed]
- Jilling, A.; Keiluweit, M.; Gutknecht, J.; Grandy, A.S. Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol. Biochem. 2021, 158, 108265. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Z.; Wang, S.; Wang, A.; Huang, S.; Qu, T.; Chen, Q.; Li, X.; Wang, R.; Liu, Z.; Dong, Z. Long-Term Caragana korshinskii Restoration Enhances SOC Stability but Reduces Sequestration Efficiency over 40 Years in Degraded Loess Soils. Atmosphere 2025, 16, 662. https://doi.org/10.3390/atmos16060662
Xue Z, Wang S, Wang A, Huang S, Qu T, Chen Q, Li X, Wang R, Liu Z, Dong Z. Long-Term Caragana korshinskii Restoration Enhances SOC Stability but Reduces Sequestration Efficiency over 40 Years in Degraded Loess Soils. Atmosphere. 2025; 16(6):662. https://doi.org/10.3390/atmos16060662
Chicago/Turabian StyleXue, Zhijing, Shuangying Wang, Anqi Wang, Shengwei Huang, Tingting Qu, Qin Chen, Xiaoyun Li, Rui Wang, Zhengyao Liu, and Zhibao Dong. 2025. "Long-Term Caragana korshinskii Restoration Enhances SOC Stability but Reduces Sequestration Efficiency over 40 Years in Degraded Loess Soils" Atmosphere 16, no. 6: 662. https://doi.org/10.3390/atmos16060662
APA StyleXue, Z., Wang, S., Wang, A., Huang, S., Qu, T., Chen, Q., Li, X., Wang, R., Liu, Z., & Dong, Z. (2025). Long-Term Caragana korshinskii Restoration Enhances SOC Stability but Reduces Sequestration Efficiency over 40 Years in Degraded Loess Soils. Atmosphere, 16(6), 662. https://doi.org/10.3390/atmos16060662







