Molecular Characterization of Organic Aerosol in Summer Suburban Shanghai Under High Humidity
Abstract
1. Introduction
2. Data Sources and Methods
2.1. Sample Collection
2.2. Sample Analysis
2.3. Data Processing
3. Results and Discussion
3.1. General Molecular Characterization and BrC Absorption
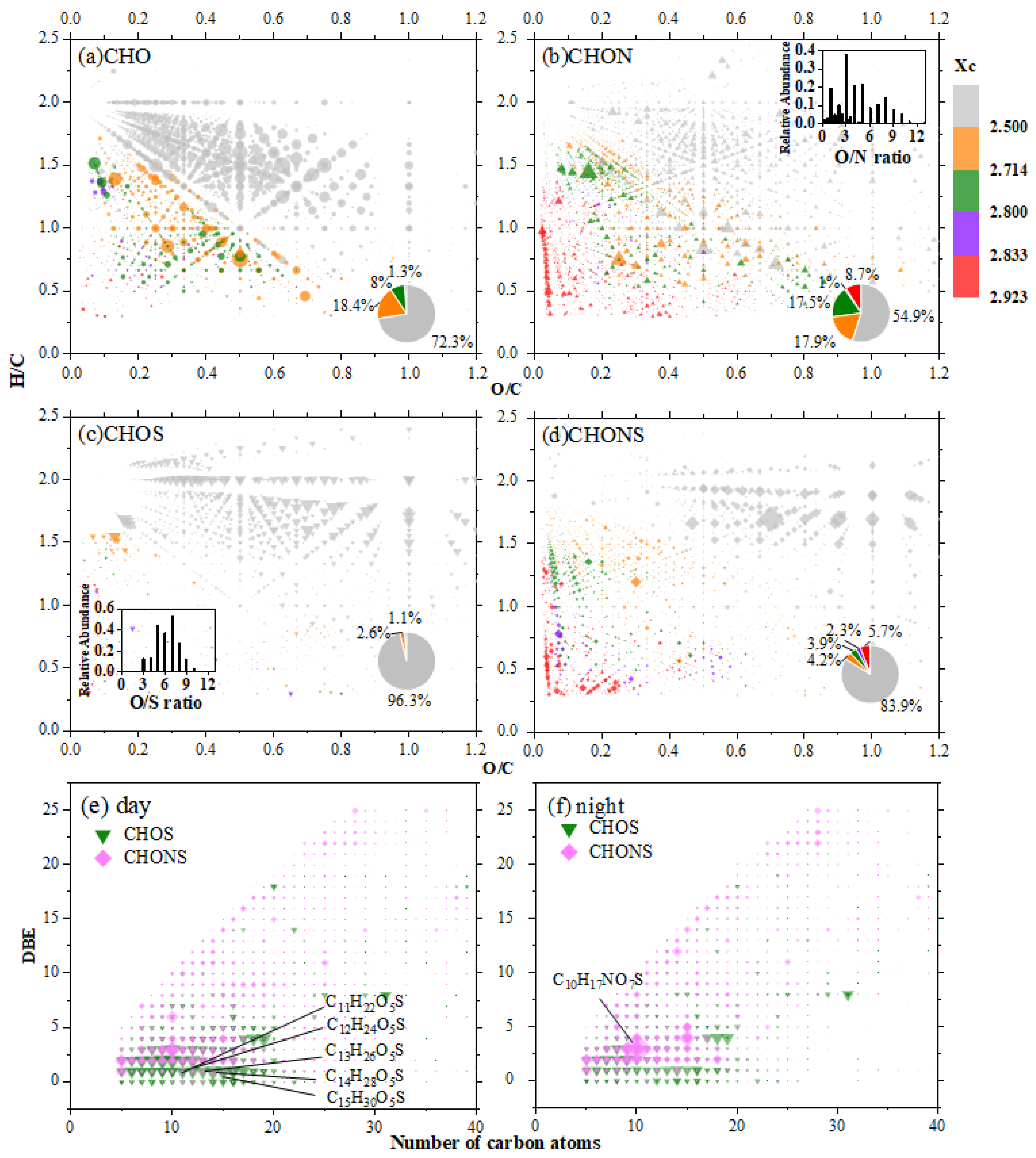
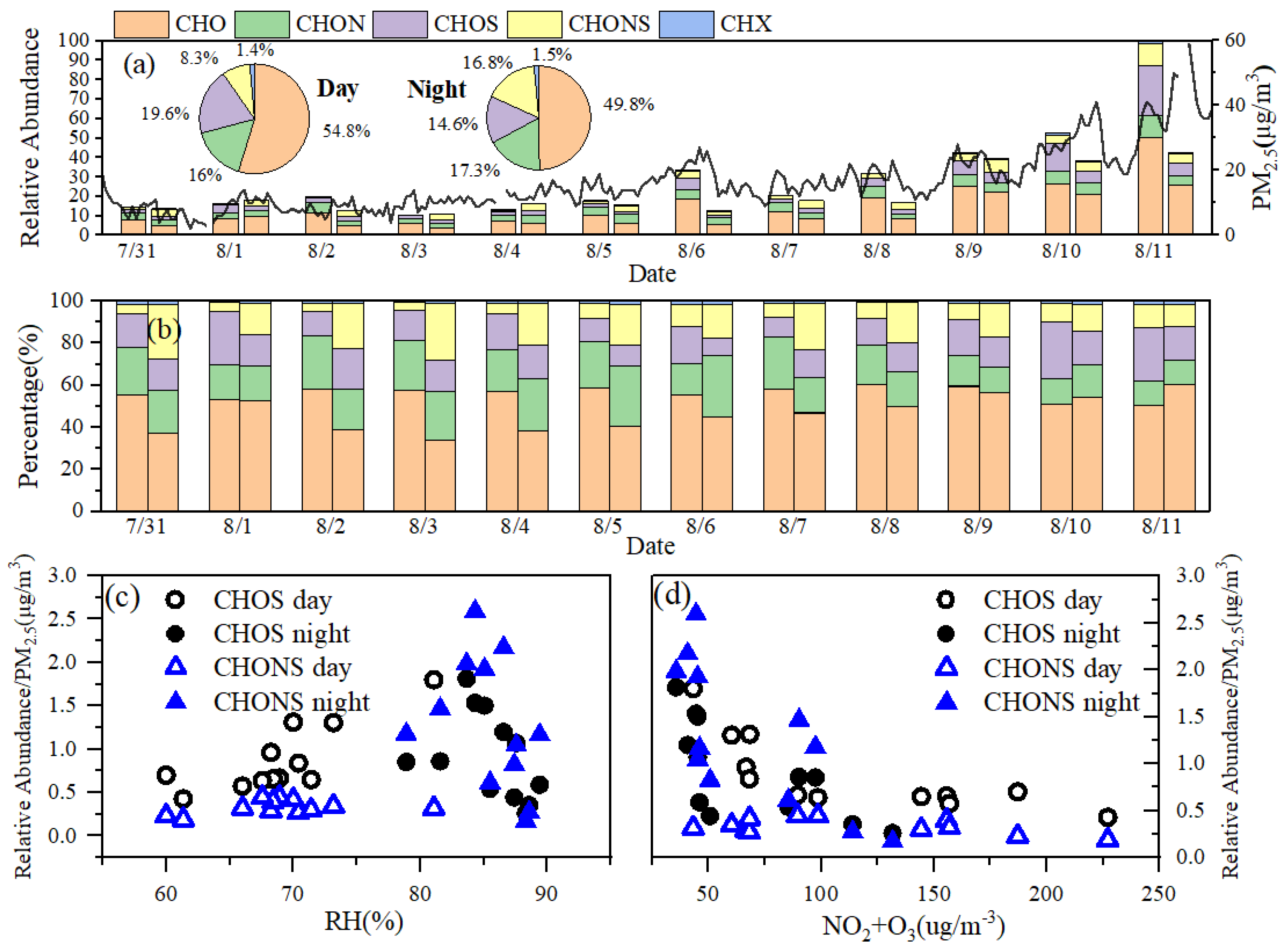
3.2. Diurnal Variations Characterization of CHO, CHON, CHOS, and CHONS Compounds
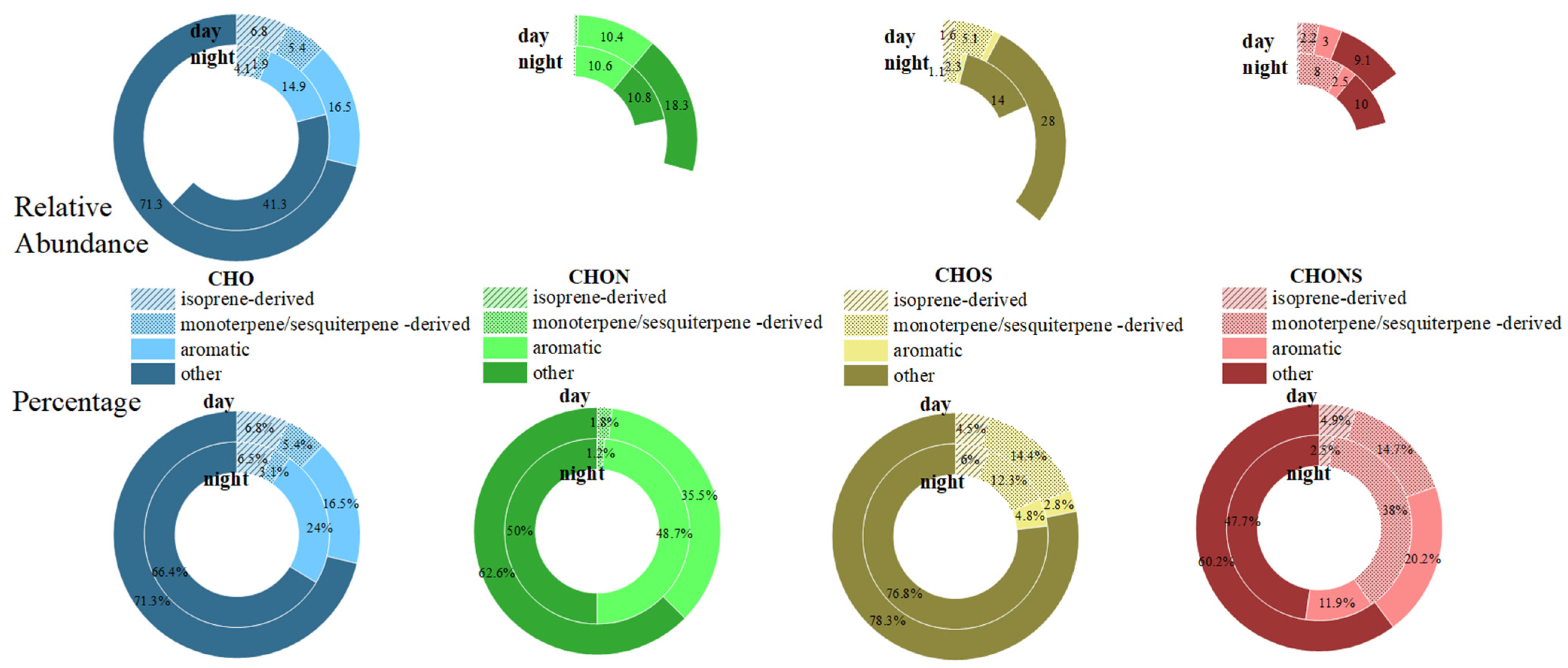
3.3. Contribution of Terpene Derivatives to SOA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, X.; Wei, S.; Zhu, M.; Song, J.; Peng, P. Comprehensive characterization of humic-like substances in smoke PM2.5 emitted from the combustion of biomass materials and fossil fuels. Atmos. Chem. Phys. 2016, 16, 13321–13340. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Jaoui, M.; Lewandowski, M.; Offenberg, J.H.; Lewis, C.W.; Bhave, P.V.; Edney, E.O. Estimates of the contributions of biogenic and anthropogenic hydrocarbons to secondary organic aerosol at a southeastern US location. Atmos. Environ. 2007, 41, 8288–8300. [Google Scholar] [CrossRef]
- Seinfeld, J. Black carbon and brown clouds. Nat. Geosci. 2008, 1, 15–16. [Google Scholar] [CrossRef]
- Mather, T.A.; Allen, A.G.; Oppenheimer, C.; Pyle, D.M.; McGonigle, A.J.S. Size-Resolved Characterisation of Soluble Ions in the Particles in the Tropospheric Plume of Masaya Volcano, Nicaragua: Origins and Plume Processing. J. Atmos. Chem. 2003, 46, 207–237. [Google Scholar] [CrossRef]
- Gentner, D.R.; Jathar, S.H.; Gordon, T.D.; Bahreini, R.; Day, D.A.; El Haddad, I.; Hayes, P.L.; Pieber, S.M.; Platt, S.M.; de Gouw, J.; et al. Review of Urban Secondary Organic Aerosol Formation from Gasoline and Diesel Motor Vehicle Emissions. Environ. Sci. Technol. 2017, 51, 1074–1093. [Google Scholar] [CrossRef]
- Hoffmann, T.; Huang, R.-J.; Kalberer, M. Atmospheric Analytical Chemistry. Anal. Chem. 2011, 83, 4649–4664. [Google Scholar] [CrossRef]
- Cheng, Y.; Mao, J.; Bai, Z.; Zhang, W.; Zhang, L.; Chen, H.; Wang, L.; Li, L.; Chen, J. The Significant Contribution of Polycyclic Aromatic Nitrogen Heterocycles to Light Absorption in the Winter North China Plain. Sustainability 2023, 15, 8568. [Google Scholar] [CrossRef]
- Nie, W.; Yan, C.; Huang, D.D.; Wang, Z.; Liu, Y.; Qiao, X.; Guo, Y.; Tian, L.; Zheng, P.; Xu, Z.; et al. Secondary organic aerosol formed by condensing anthropogenic vapours over China’s megacities. Nat. Geosci. 2022, 15, 255–261. [Google Scholar] [CrossRef]
- Hallquist, M.; Wenger, J.C.; Baltensperger, U.; Rudich, Y.; Simpson, D.; Claeys, M.; Dommen, J.; Donahue, N.M.; George, C.; Goldstein, A.H.; et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos. Chem. Phys. 2009, 9, 5155–5236. [Google Scholar] [CrossRef]
- Tolocka, M.P.; Turpin, B. Contribution of Organosulfur Compounds to Organic Aerosol Mass. Environ. Sci. Technol. 2012, 46, 7978–7983. [Google Scholar] [CrossRef]
- Li, H.; Duan, F.; Ma, T.; Ma, Y.; Xu, Y.; Wang, S.; Zhang, Q.; Jiang, J.; Zhu, L.; Li, F.; et al. Molecular Characterization of Organosulfur and Organonitrogen Compounds in Summer and Winter PM2.5 via UHPLC-Q-Orbitrap MS/MS. Environ. Sci. Technol. 2024, 58, 21692–21701. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, Y.; Ye, Q.; Ma, Y.J.; Wang, Y.C.; Yu, J.Z.; Duan, Y.S.; Li, C.X.; Xiao, H.W.; Li, Z.Y.; et al. Spatial and diurnal variations of aerosol organosulfates in summertime Shanghai, China: Potential influence of photochemical processes and anthropogenic sulfate pollution. Atmos. Chem. Phys. 2023, 23, 13433–13450. [Google Scholar] [CrossRef]
- Brüggemann, M.; Xu, R.; Tilgner, A.; Kwong, K.C.; Mutzel, A.; Poon, H.Y.; Otto, T.; Schaefer, T.; Poulain, L.; Chan, M.N.; et al. Organosulfates in Ambient Aerosol: State of Knowledge and Future Research Directions on Formation, Abundance, Fate, and Importance. Environ. Sci. Technol. 2020, 54, 3767–3782. [Google Scholar] [CrossRef] [PubMed]
- Surratt, J.; Gómez-González, Y.; Chan, A.; Vermeylen, R.; Shahgholi, M.; Kleindienst, T.; Edney, E.; Offenberg, J.; Lewandowski, M.; Jaoui, M.; et al. Organosulfate formation in biogenic secondary organic aerosol. J. Geophys. Res. Atmos. 2008, 112, 8345–8378. [Google Scholar] [CrossRef]
- Iinuma, Y.; Müller, C.; Berndt, T.; Böge, O.; Claeys, M.; Herrmann, H. Evidence for the Existence of Organosulfates from β-Pinene Ozonolysis in Ambient Secondary Organic Aerosol. Environ. Sci. Technol. 2007, 41, 6678–6683. [Google Scholar] [CrossRef]
- Cai, D.; Wang, X.; Chen, J.; Li, X. Molecular Characterization of Organosulfates in Highly Polluted Atmosphere Using Ultra-High-Resolution Mass Spectrometry. J. Geophys. Res. Atmos. 2020, 125, e2019JD032253. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Hu, W.; Zheng, J.; Niu, H.; Fang, X.; Xu, N.; Wu, Z.; Guo, S.; Wu, Y.; et al. Secondary Formation of Aerosols Under Typical High-Humidity Conditions in Wintertime Sichuan Basin, China: A Contrast to the North China Plain. J. Geophys. Res. Atmos. 2021, 126, e2021JD034560. [Google Scholar] [CrossRef]
- Gentner, D.R.; Isaacman, G.; Worton, D.R.; Chan, A.W.H.; Dallmann, T.R.; Davis, L.; Liu, S.; Day, D.A.; Russell, L.M.; Wilson, K.R.; et al. Elucidating secondary organic aerosol from diesel and gasoline vehicles through detailed characterization of organic carbon emissions. Proc. Natl. Acad. Sci. USA 2012, 109, 18318–18323. [Google Scholar] [CrossRef]
- Riva, M.; Da Silva Barbosa, T.; Lin, Y.H.; Stone, E.A.; Gold, A.; Surratt, J.D. Chemical characterization of organosulfates in secondary organic aerosol derived from the photooxidation of alkanes. Atmos. Chem. Phys. 2016, 16, 11001–11018. [Google Scholar] [CrossRef]
- Tao, S.; Lu, X.; Levac, N.; Bateman, A.P.; Nguyen, T.B.; Bones, D.L.; Nizkorodov, S.A.; Laskin, J.; Laskin, A.; Yang, X. Molecular Characterization of Organosulfates in Organic Aerosols from Shanghai and Los Angeles Urban Areas by Nanospray-Desorption Electrospray Ionization High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2014, 48, 10993–11001. [Google Scholar] [CrossRef]
- Kundu, S.; Quraishi, T.A.; Yu, G.; Suarez, C.; Keutsch, F.N.; Stone, E.A. Evidence and quantitation of aromatic organosulfates in ambient aerosols in Lahore, Pakistan. Atmos. Chem. Phys. 2013, 13, 4865–4875. [Google Scholar] [CrossRef]
- Chan, M.N.; Surratt, J.D.; Chan, A.W.H.; Schilling, K.; Offenberg, J.H.; Lewandowski, M.; Edney, E.O.; Kleindienst, T.E.; Jaoui, M.; Edgerton, E.S.; et al. Influence of aerosol acidity on the chemical composition of secondary organic aerosol from β-caryophyllene. Atmos. Chem. Phys. 2011, 11, 1735–1751. [Google Scholar] [CrossRef]
- Yang, N.; Xie, Q.; Zhang, X.; Zhong, S.; Hu, W.; Deng, J.; Wu, L.; Sheng, M.; Niu, M.; Liu, D.; et al. Unsaturated Fatty Acids Enhance Aqueous Atmospheric Oxidation Ability by Producing Oxygen—Containing Radicals in Fog Droplets. J. Geophys. Res. Atmos. 2023, 128, e2022JD038069. [Google Scholar] [CrossRef]
- Xie, Q.; Su, S.; Chen, S.; Xu, Y.; Cao, D.; Chen, J.; Ren, L.; Yue, S.; Zhao, W.; Sun, Y.; et al. Molecular characterization of firework-related urban aerosols using Fourier transform ion cyclotron resonance mass spectrometry. Atmos. Chem. Phys. 2020, 20, 6803–6820. [Google Scholar] [CrossRef]
- Kuang, B.Y.; Lin, P.; Hu, M.; Yu, J.Z. Aerosol size distribution characteristics of organosulfates in the Pearl River Delta region, China. Atmos. Environ. 2016, 130, 23–35. [Google Scholar] [CrossRef]
- Hettiyadura, A.P.S.; Al-Naiema, I.M.; Hughes, D.D.; Fang, T.; Stone, E.A. Organosulfates in Atlanta, Georgia: Anthropogenic influences on biogenic secondary organic aerosol formation. Atmos. Chem. Phys. 2019, 19, 3191–3206. [Google Scholar] [CrossRef]
- Zhang, M.; Cai, D.; Lin, J.; Liu, Z.; Li, M.; Wang, Y.; Chen, J. Molecular characterization of atmospheric organic aerosols in typical megacities in China. npj Clim. Atmos. Sci. 2024, 7, 230. [Google Scholar] [CrossRef]
- Lin, P.; Rincon, A.G.; Kalberer, M.; Yu, J.Z. Elemental Composition of HULIS in the Pearl River Delta Region, China: Results Inferred from Positive and Negative Electrospray High Resolution Mass Spectrometric Data. Environ. Sci. Technol. 2012, 46, 7454–7462. [Google Scholar] [CrossRef]
- Lin, P.; Yu, J.Z.; Engling, G.; Kalberer, M. Organosulfates in Humic-like Substance Fraction Isolated from Aerosols at Seven Locations in East Asia: A Study by Ultra-High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2012, 46, 13118–13127. [Google Scholar] [CrossRef]
- Mao, J.; Cheng, Y.; Bai, Z.; Zhang, W.; Zhang, L.; Chen, H.; Wang, L.; Li, L.; Chen, J. Molecular characterization of nitrogen-containing organic compounds in the winter North China Plain. Sci. Total Environ. 2022, 838, 156189. [Google Scholar] [CrossRef]
- Wang, X.; Hayeck, N.; Brüggemann, M.; Yao, L.; Chen, H.; Zhang, C.; Emmelin, C.; Chen, J.; George, C.; Wang, L. Chemical Characteristics of Organic Aerosols in Shanghai: A Study by Ultrahigh-Performance Liquid Chromatography Coupled with Orbitrap Mass Spectrometry. J. Geophys. Res. Atmos. 2017, 122, 11–703. [Google Scholar] [CrossRef]
- Xing, C.; Wan, Y.; Wang, Q.; Kong, S.; Huang, X.; Ge, X.; Xie, M.; Yu, H. Molecular Characterization of Brown Carbon Chromophores in Atmospherically Relevant Samples and Their Gas-Particle Distribution and Diurnal Variation in the Atmosphere. J. Geophys. Res. Atmos. 2023, 128, e2022JD038142. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Huang, R.-J.; Wang, M.; Ni, H.; Kampf, C.J.; Cheng, Y.; Bilde, M.; Glasius, M.; Hoffmann, T. Molecular Characterization and Source Identification of Atmospheric Particulate Organosulfates Using Ultrahigh Resolution Mass Spectrometry. Environ. Sci. Technol. 2019, 53, 6192–6202. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, R.J.; Brüggemann, M.; Zhang, Y.; Yang, L.; Ni, H.; Guo, J.; Wang, M.; Han, J.; Bilde, M.; et al. Urban organic aerosol composition in eastern China differs from north to south: Molecular insight from a liquid chromatography–mass spectrometry (Orbitrap) study. Atmos. Chem. Phys. 2021, 21, 9089–9104. [Google Scholar] [CrossRef]
- Campbell, J.R.; Battaglia, M., Jr.; Dingilian, K.; Cesler-Maloney, M.; St Clair, J.M.; Hanisco, T.F.; Robinson, E.; DeCarlo, P.; Simpson, W.; Nenes, A.; et al. Source and Chemistry of Hydroxymethanesulfonate (HMS) in Fairbanks, Alaska. Environ. Sci. Technol. 2022, 56, 7657–7667. [Google Scholar] [CrossRef]
- Cai, D.; Wang, X.; George, C.; Cheng, T.; Herrmann, H.; Li, X.; Chen, J. Formation of Secondary Nitroaromatic Compounds in Polluted Urban Environments. J. Geophys. Res. Atmos. 2022, 127, e2021JD036167. [Google Scholar] [CrossRef]
- Chan, M.N.; Choi, M.Y.; Ng, N.L.; Chan, C.K. Hygroscopicity of Water-Soluble Organic Compounds in Atmospheric Aerosols: Amino Acids and Biomass Burning Derived Organic Species. Environ. Sci. Technol. 2005, 39, 1555–1562. [Google Scholar] [CrossRef]
- Triesch, N.; van Pinxteren, M.; Salter, M.; Stolle, C.; Pereira, R.; Zieger, P.; Herrmann, H. Sea Spray Aerosol Chamber Study on Selective Transfer and Enrichment of Free and Combined Amino Acids. ACS Earth Space Chem. 2021, 5, 1564–1574. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.; Hatcher, P. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef]
- Lin, P.; Bluvshtein, N.; Rudich, Y.; Nizkorodov, S.A.; Laskin, J.; Laskin, A. Molecular Chemistry of Atmospheric Brown Carbon Inferred from a Nationwide Biomass Burning Event. Environ. Sci. Technol. 2017, 51, 11561–11570. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of Emissions from Air Pollution Sources. 5. C1−C32 Organic Compounds from Gasoline-Powered Motor Vehicles. Environ. Sci. Technol. 2002, 36, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, Y.; Zhao, Y.; Hu, H.; Yang, Y.; Wang, Y.; Yu, J.-Z.; Chen, T.; Cheng, Z.; Li, C.; et al. Biogenic and Anthropogenic Contributions to Atmospheric Organosulfates in a Typical Megacity in Eastern China. J. Geophys. Res. Atmos. 2023, 128, e2023JD038848. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, S.; Deng, J.; Fan, Y.; Zhang, Q.; Xie, Q.; Qi, Y.; Hu, W.; Wu, L.; Li, X.; et al. Impact of biogenic secondary organic aerosol (SOA) loading on the molecular composition of wintertime PM2.5 in urban Tianjin: An insight from Fourier transform ion cyclotron resonance mass spectrometry. Atmos. Chem. Phys. 2023, 23, 2061–2077. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wang, Y.; Yu, J.Z.; Shao, J.; Liu, P.; Zhu, W.; Cheng, Z.; Li, Z.; Yan, N.; et al. Organosulfates in atmospheric aerosols in Shanghai, China: Seasonal and interannual variability, origin, and formation mechanisms. Atmos. Chem. Phys. 2021, 21, 2959–2980. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Kuang, B.; Lin, P.; Liang, Y.; Huang, C.; Yu, J.Z. Abundance of organosulfates derived from biogenic volatile organic compounds: Seasonal and spatial contrasts at four sites in China. Sci. Total Environ. 2022, 806, 151275. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Mao, J.; Cai, D.; Zhang, Z.; Nong, H.; Li, L.; Chen, J. Molecular Characterization of Organic Aerosol in Summer Suburban Shanghai Under High Humidity. Atmosphere 2025, 16, 659. https://doi.org/10.3390/atmos16060659
Tang X, Mao J, Cai D, Zhang Z, Nong H, Li L, Chen J. Molecular Characterization of Organic Aerosol in Summer Suburban Shanghai Under High Humidity. Atmosphere. 2025; 16(6):659. https://doi.org/10.3390/atmos16060659
Chicago/Turabian StyleTang, Xiancheng, Junfang Mao, Dongmei Cai, Zhiwei Zhang, Haixin Nong, Ling Li, and Jianmin Chen. 2025. "Molecular Characterization of Organic Aerosol in Summer Suburban Shanghai Under High Humidity" Atmosphere 16, no. 6: 659. https://doi.org/10.3390/atmos16060659
APA StyleTang, X., Mao, J., Cai, D., Zhang, Z., Nong, H., Li, L., & Chen, J. (2025). Molecular Characterization of Organic Aerosol in Summer Suburban Shanghai Under High Humidity. Atmosphere, 16(6), 659. https://doi.org/10.3390/atmos16060659






