Analysis of VOC Emission Characteristics in the Petrochemical Industry and Study on Fenceline Monitoring Techniques
Abstract
1. Introduction
1.1. Characteristics of VOC Emissions in Industrial Complexes and the Petroleum Refining and Petrochemical Industries
1.2. VOC Fenceline Monitoring Regulations and Rule 1180 in the United States
1.3. VOC Monitoring and Regulatory Standards in Canada
1.4. Regulation of VOCs and the Current Status of the Clean Air Conservation Act in South Korea
2. Materials and Methods
2.1. Study Subjects and Measurement Facilities
2.2. Measurement Techniques
2.2.1. Passive Sampling
2.2.2. Active Sampling
2.2.3. Sampling Dates
2.3. Analytes
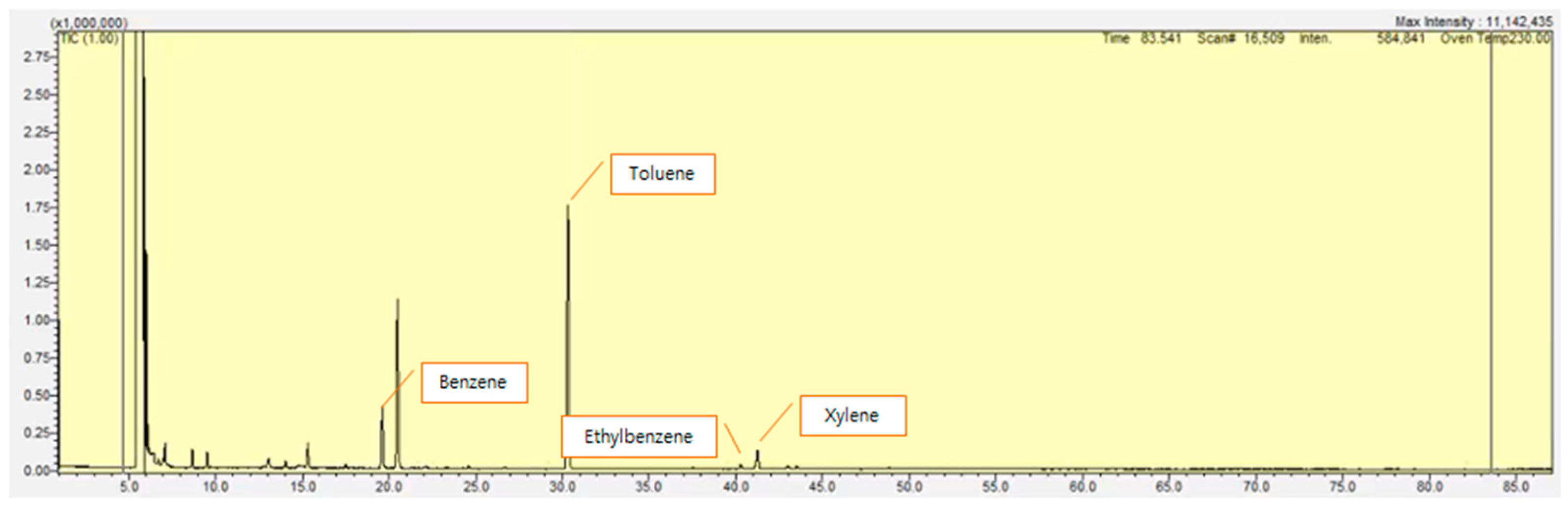
3. Results
3.1. Study Facility A
- (1)
- LD/LDD Process
- (2)
- ADL PE Process
- (3)
- EVA Process
- (4)
- PP4 Process
- (5)
- CFU/ARO#2 Process
- (6)
- PTA
- (7)
- TANK YARD
- (8)
- SM #1, #2
- (9)
- NCC Process
- (10)
- BTX Process
- (11)
- General Discussion and Observations
3.2. Study Facility B
- (1)
- Plant 1 CDU, VDU, HCR, HDS, DCU Processes
- (2)
- Plant 2 CDU, HDS Processes
- (3)
- BTX Process
- (4)
- WWT Process (Wastewater Treatment)
- (5)
- FCC RDS Process
- (6)
- CDU, BTX
- (7)
- Crude Oil/Product TANK FARM
- (8)
- Plant 3 CDU, VDU, HCR, HDS, DCU Processes
- (9)
- Warehouse
- (10)
- General Discussion and Conclusions
3.3. Comparison of Active and Passive Methods and Community Notification Levels
4. Discussion
4.1. Comparison of Passive and Active Measurement Results
4.2. Relationship Between VOC Concentrations and Meteorological Conditions (Wind Speed, Wind Direction)
4.3. Analysis of Major VOC Sources and Process Impact
4.4. Comparison with International Regulations and Applicability in Korea
4.5. Ozone Formation Potential and Regulatory Implications
4.6. Limitations of the Study and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.-W. A Study on the Measurement and Investigation of Air Quality in a University Library. J. Korean Libr. Inf. Sci. Soc. 2019, 50, 635–658. [Google Scholar]
- Kim, K.S.; Lee, H.S.; Kong, S.Y.; Ku, H.J. A Study on Public Opinion Poll and Policy on Indoor Air Pollution. 2001. Available online: https://www.osti.gov/etdeweb/biblio/20334334 (accessed on 31 March 2025).
- Chung, Y.H.; Kim, S.C.; Shin, S.K.; Kang, I.G.; Lee, J.I.; Lee, W.S.; Lee, J.B. A Study on the Characteristic Trace Organic Pollutants in the Industrial Wastewater. Anal. Sci. Technol. 1998, 11, 62–72. [Google Scholar]
- Daves, G. Refinery fence-line monitoring to impact petrochemical operators. Oil Gas J. 2017, 115, 68–73. [Google Scholar]
- DeWees, J.M. Refinery fenceline monitoring & method 325A/B. In National Air Toxics Monitoring and Data Analysis Workshop; EPA: Boston, MA, USA, 2015; Volume 28. [Google Scholar]
- Eisele, A.P.; Mukerjee, S.; Smith, L.A.; Thoma, E.D.; Whitaker, D.A.; Oliver, K.D.; Alston, L.; Cousett, T.A.; Miller, M.C.; Smith, D.M.; et al. Volatile organic compounds at two oil and natural gas production well pads in Colorado and Texas using passive samplers. J. Air Waste Manag. Assoc. 2016, 66, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Stutz, J.; Colosimo, S.F.; Cooperdock, S.; Pikelnaya, O.; Polidori, A. UV-DOAS Measurements of BTEX in Petrochemical Facilities: Performance, Quality Assessment, and Applications. AGU Fall Meet. Abstr. 2022, 2022, A45I-1943. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Benzene. In Chemical Agents and Related Occupations; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2012. [Google Scholar]
- Jang, Y.C.; Lee, S.; Shin, Y.; Kim, H.; Lee, J. Human health risk assessment of benzene from industrial complexes of Chungcheong and Jeonla province. J. Environ. Impact Assess. 2011, 20, 497–507. [Google Scholar]
- Goho, S.A. Advances in Air Monitoring Opportunities and Challenges for Addressing Race, Poverty, and Environmental Justice. Am. J. Law Equal. 2023, 3, 53–63. [Google Scholar] [CrossRef]
- Stephanie, T.; Edwards, S.J.; Walker, T.R. A toxicity-based analysis of Canada’s National Pollutant Release Inventory (NPRI): A case study in Nova Scotia. Environ. Sci. Pollut. Res. 2020, 27, 2238–2247. [Google Scholar]
- Available online: https://laws-lois.justice.gc.ca/eng/regulations/SOR-2020-231/page-4.html?wbdisable=true (accessed on 31 March 2025).
- Seok, H.J.; Kahn, J.B.; Kim, Y.L.; Seo, J.W.; Hong, S.Y.; Kim, H.J. Discharge impact analysis of air pollutants for integrated environmental management. J. Environ. Anal. Health Toxicol. 2020, 23, 240–247. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, S.J.; Lee, D.G. Enhancing the applicability and improvement direction of integrated environmental permit system. J. Korean Soc. Water Environ. 2018, 34, 339–345. [Google Scholar]
- Lee, H.E.; Kim, J.H.; Seo, D.; Yoon, S.J. Prioritization of Volatile Organic Compound Reduction in the Tire Manufacturing Industry through Speciation of Volatile Organic Compounds Emitted at the Fenceline. Atmosphere 2024, 15, 223. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, J.H.; Yoon, S.J.; Cho, S.H.; Yu, J.U.; Kang, C.W.; Moon, K.W.; Lee, H.E. Evaluating the feasibility of air environment management system for VOCs through ‘VOCs specification’ of petroleum refining industry. J. Air Waste Manag. Assoc. 2023, 73, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Han, J.S.; Yun, H.K.; Cho, S.Y. Evaluation of incremental reactivity and ozone production contribution of VOCs using the PAMS data in Seoul metropolitan area. J. Korean Soc. Atmos. Environ. 2007, 23, 286–296. [Google Scholar] [CrossRef]
- Walgraeve, C.; Demeestere, K.; Dewulf, J.; Van Huffel, K.; Van Langenhove, H. Uptake rate behavior of tube-type passive samplers for volatile organic compounds under controlled atmospheric conditions. Atmos. Environ. 2011, 45, 5872–5879. [Google Scholar] [CrossRef]




| Facility | Characteristics of the Facility | Site Area |
|---|---|---|
| Study Facility A | Manufacture of synthetic resins, such as PE/PP, and petrochemical basic chemicals, including styrene monomer (SM), by-product fuels, and solvents from basic fractions | 3,047,914 m2 (753.15 ac) |
| Study Facility B | Petroleum refining and manufacture of petrochemical basic chemical compounds | 3,039,476.79 m2 (751.07 ac) |
| Study Facility A | Study Facility B | ||||
|---|---|---|---|---|---|
| Process Division (Abbreviation) | Process Overview | VOC Emission Substances | Process Division (Abbreviation) | Process Overview | VOC Emission Substances |
| LD/LDD | Production of synthetic resins utilizing ethylene and styrene monomer | Ethylene, Styrene Monomer, etc. | CDU, VDU, HCR, HDS, DCU | Processes including Atmospheric Distillation, Vacuum Distillation, Hydrocracking | Trace Olefins, Paraffinic Fractions |
| ADL PE | Manufacturing of PE and PP from ethylene and propylene | Ethylene, Propylene, Polyethylene, Polypropylene | CDU, HDS | Processes including Atmospheric Distillation, Hydrodesulfurization | Paraffinic Fractions |
| EVA | Polypropylene production using ethylene and propylene | Ethylene, Propylene, Polyethylene | BTX Process | Production of aromatic compounds | Refined and Separated Fractions |
| PP4 | PP manufacturing from ethylene | Ethylene, Polypropylene, etc. | WWT Process (Wastewater Treatment) | Treatment of wastewater generated within the facility | None (Acid/Alkali Chemical Treatment, Odor from Biological Treatment Process) |
| CFU/ARO#2 | Use of heavy naphtha for producing benzene, gasoline, solvents, alongside crude oil refining | Gasoline, Benzene, Paraxylene, LPG, Kerosene, Jet Fuel, Diesel, etc. | FCC, RDS Process | Catalytic Cracking of Residual Oil (B/C) using advanced equipment | Some Olefins, Paraffinic Fractions |
| PTA | Producing high-purity terephthalic acid through the distillation or hydrogenation of xylene | Xylene, Terephthalic Acid, etc. | CDU, BTX Process | Distillation and Aromatic Processing | Aromatic Compounds, Paraffinic Fractions |
| TANK YARD | Storage process for crude oil and related products | Crude Oil | Crude Oil/Products, etc. TANK FARM | Storage facilities for crude oil, naphtha, gasoline, kerosene, diesel | Crude Oil |
| SM #1,#2 | Synthesis of styrene monomer or PE resin from ethylene | Ethylene, Styrene Monomer | |||
| NCC | Production of various products using light naphtha from the refinery (CFU) | Ethylene, Propylene, C4 Synthetic Rubber, etc. | |||
| BTX | Aromatic product production | Benzene, Toluene, Xylene, etc. | |||
| Category | Measurement Technique |
|---|---|
| Measured Substances | EPA Method 325B, Validated Sorbents and Uptake Rates for Selected Clean Air Act Compounds, including 18 types of ozone precursor VOCs, 57 types, and TO-14A target substances, 31 types |
| Sampling Area | Identical to Active Sampling |
| Sampling Duration | 14 days |
| Analysis Method | Solid Adsorbent Technique |
| Adsorbent Tube and Passive Type | Carbopak X |
| Facility | Date | Measurement Method |
|---|---|---|
| Study Facility A | 31 May–14 June (15 days) | Passive |
| 31 October–1 November (2 days) | Active | |
| Study Facility B | 3 June–17 June (15 days) | Passive |
| 29 August–30 August (2 days) | Active |
| No. | Substance Name | Cas No. | No. | Substance Name | Cas No. |
|---|---|---|---|---|---|
| 1 | Ethylene | 74-85-1 | 30 | 3-Methylhexane | 589-34-4 |
| 2 | Acetylene | 74-86-2 | 31 | 2,2,4-Trimethylpentane | 50-84-1 |
| 3 | Ethane | 74-84-0 | 32 | n-Heptane | 142-82-5 |
| 4 | Propylene | 115-07-1 | 33 | Methylcyclohexane | 108-87-2 |
| 5 | Propane | 74-98-6 | 34 | 2,3,4-Trimethylpentane | 565-75-3 |
| 6 | Isobutane | 75-28-5 | 35 | Toluene | 108-88-3 |
| 7 | 1-Butene | 106-98-9 | 36 | 2-Methylheptane | 592-27-8 |
| 8 | n-Butane | 106-97-8 | 37 | 3-Methylheptane | 589-81-1 |
| 9 | trans-2-Butene | 624-64-6 | 38 | n-Octane | 111-65-9 |
| 10 | cis-2-Butene | 590-18-1 | 39 | Ethylbenzene | 100-41-4 |
| 11 | Isopentnae | 78-78-4 | 40 | m-Xylene | 108-38-3 |
| 12 | 1-Pentene | 109-67-1 | 41 | p-Xylene | 106-42-3 |
| 13 | n-Pentane | 109-66-0 | 42 | Styrene | 100-42-5 |
| 14 | Isoprene | 78-79-50 | 43 | o-Xylene | 95-47-6 |
| 15 | trans-2-Pentene | 646-04-8 | 44 | n-Nonane | 111-84-2 |
| 16 | cis-2-Pentene | 627-20-3 | 45 | Isopropylbenzene | 98-82-8 |
| 17 | 2,2-Dimethylbutane | 75-83-2 | 46 | n-Propylbenzene | 103-65-1 |
| 18 | Cyclopentane | 287-92-3 | 47 | m-Ethyltoluene | 620-14-4 |
| 19 | 2,3-Dimethylbutane | 79-29-8 | 48 | p-Ethyltoluene | 622-96-8 |
| 20 | 2-Methylpentane | 107-83-5 | 49 | 1,3,5-Trimethylbenzene | 108-67-8 |
| 21 | 3-Methylpentane | 96-14-0 | 50 | o-Ehtyltoluene | 611-14-3 |
| 22 | 1-Hexene | 592-41-6 | 51 | 1,2,4-Trimethylbenzene | 95-63-6 |
| 23 | n-Hexane | 110-54-3 | 52 | n-Decane | 124-18-5 |
| 24 | Methylcyclopentane | 96-37-7 | 53 | 1,2,3-Trimethylbenzene | 526-73-8 |
| 25 | 2,4-Dimethylpentane | 108-08-7 | 54 | m-Diehtylbenzene | 141-93-5 |
| 26 | Benzene | 71-43-2 | 55 | p-Diehtylbenzene | 105-05-5 |
| 27 | Cyclohexane | 110-82-7 | 56 | n-Undecane | 1120-21-4 |
| 28 | 2-Methylhexane | 591-76-4 | 57 | n-Dodecane | 112-40-3 |
| 29 | 2,3-Dimethylpentane | 565-59-3 | - | - | - |
| Compounds | Spiked Concentration (ng/mL) | Average (ng/mL) | Deviation | Precision (%) | Accuracy (%) | Detection Limit (ng/mL) | Limit of Quantification (ng/mL) |
|---|---|---|---|---|---|---|---|
| Benzene | 32.91 | 42.15 | 0.788 | 1.870 | 99.430 | 1.748 | 5.567 |
| Toluene | 39.19 | 50.62 | 0.782 | 1.544 | 97.560 | 1.804 | 5.744 |
| Ethylbenzene | 44.29 | 57.36 | 0.473 | 0.825 | 96.450 | 0.704 | 2.242 |
| m,p-Xylene | 88.58 | 104.19 | 1.668 | 1.601 | 97.580 | 2.320 | 7.388 |
| Styrene | 43.02 | 55.37 | 0.895 | 1.617 | 97.240 | 1.858 | 5.917 |
| o-Xylene | 44.72 | 59.15 | 2.143 | 3.622 | 97.390 | 1.500 | 4.779 |
| Substance Name | Community Notification Concentration | Calculated Average Concentration | ΔC Concentration |
|---|---|---|---|
| Benzene | 9 µg/m3 (Action level) 25.8 µg/m3 | 3.60 µg/m3 | 17.9 µg/m3 |
| Toluene | 37,540 µg/m3 | 7.84 µg/m3 | 56.87 µg/m3 |
| Ethylbenzene | - | 1.26 µg/m3 | 2.66 µg/m3 |
| m,p-Xylene | 22,326 µg/m3 | 3.59 µg/m3 | 7.3 µg/m3 |
| Styrene | 25,510 µg/m3 | 1.51 µg/m3 | 5.42 µg/m3 |
| o-Xylene | 22,326 µg/m3 | 0.80 µg/m3 | 1.05 µg/m3 |
| Substance Name | Community Notification Concentration | Calculated Average Concentration | ΔC Concentration |
|---|---|---|---|
| 1,2-Dichloropropane | 4 µg/m3 (Action level) | 0.04 | 0.04 |
| Benzene | 9 µg/m3 (Action level) 25.8 µg/m3 | 1.24 | 4.71 |
| Toluene | 37,540 µg/m3 | 1.99 | 3.31 |
| Ethylbenzene | - | 1.51 | 0.75 |
| m,p-Xylene | 22,326 µg/m3 | 2.42 | 7.06 |
| Styrene | 25,510 µg/m3 | 0.02 | 0.2 |
| o-Xylene | 22,326 µg/m3 | 1.14 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.E.; Yoon, S.J.; Kim, J.H. Analysis of VOC Emission Characteristics in the Petrochemical Industry and Study on Fenceline Monitoring Techniques. Atmosphere 2025, 16, 543. https://doi.org/10.3390/atmos16050543
Lee HE, Yoon SJ, Kim JH. Analysis of VOC Emission Characteristics in the Petrochemical Industry and Study on Fenceline Monitoring Techniques. Atmosphere. 2025; 16(5):543. https://doi.org/10.3390/atmos16050543
Chicago/Turabian StyleLee, Hyo Eun, Seok J. Yoon, and Jeong Hun Kim. 2025. "Analysis of VOC Emission Characteristics in the Petrochemical Industry and Study on Fenceline Monitoring Techniques" Atmosphere 16, no. 5: 543. https://doi.org/10.3390/atmos16050543
APA StyleLee, H. E., Yoon, S. J., & Kim, J. H. (2025). Analysis of VOC Emission Characteristics in the Petrochemical Industry and Study on Fenceline Monitoring Techniques. Atmosphere, 16(5), 543. https://doi.org/10.3390/atmos16050543






