Abstract
Protected areas are crucial sanctuaries for biodiversity, strictly prohibiting the direct exploitation of natural resources and helping to maintain viable populations and communities. However, even species within these areas face challenges from climate changes. This study compared the present distribution of five woody species (Aspidosperma tomentosum, Kielmeyera coriacea, Peixotoa tomentosa, Qualea multiflora, and Senna velutina) with their projected distribution (in 2080–2100) in 30 protected Brazilian national parks. Our objectives were to evaluate ecological niche models; determine which bioclimatic variables best explain the geographic distribution of species; assess the current distribution of these species; predict changes under distinct future climatic scenarios; and analyze the potential species richness within Brazilian national parks. We overlayed binarized maps of each species and extracted statistical metrics—mean potential, standard deviation, minimum, and maximum potential—using the ‘extract’ function (raster package, v.3.5-2) in the R platform. The results revealed the dynamic nature of species distribution, each one of them being affected by a specific group of factors. All species exhibited changes in their ecological niche or distribution areas in future projections, be it losing areas (A. tomentosum: 26.27–100%; K. coriacea: 38.39–100%; P. tomentosa: 40.46–96.66%; Q. multiflora: 7.34–100%; Senna velutina: 4.52–99.99%) or gaining areas (Q. multiflora: up to 92.21%, and S. velutina: up to 22.07%). We conclude that the potential species richness within Brazilian national parks will be lower in the future. This information is crucial for biodiversity conservation efforts, offering insights into species habitat dynamics and emphasizing the need for adaptive conservation strategies. This study reinforces the urgency of preserving natural ecosystems to ensure a sustainable future for their flora and fauna.
1. Introduction
Climate change is increasingly recognized as a threat to global biodiversity, impacting the structure and functionality of ecosystems [1,2]. Climate projections based on greenhouse gas emissions indicate that by the end of the century, global temperature can increase by up to 3.1 °C [3]. However, in the case of South America, the temperature rise is even more significant, potentially reaching up to 4 °C [4]. Especially for the Brazilian Savanna, known as the Cerrado, estimates suggest temperature increases ranging from 2° to 6 °C [5]. Additionally, precipitation is expected to decrease by up to 70% compared to current levels, along with changes in the distribution of rainfall throughout the year [5,6]. These changes will impact various species of animals [7,8,9], plants [10,11,12], and seaweeds [13,14] in diverse ways and different scales [15], potentially leading to the extinction of many species [9,16].
Climatic changes can have a significant impact on plants, affecting genetic diversity [17], biotic interactions [18], phenology [18,19,20], reproductive success [18], and the geographic distribution of the species [21,22,23]. Climatic forecasts indicate that global warming will likely result in a reduced geographic distribution for several terrestrial plant species due to physiological limitations [19,23,24]. However, some species also could migrate and expand their distribution, seeking out new areas with climate conditions that are better suited for their survival, maintenance, and reproduction [25].
In the case of the Cerrado, multiple models suggest that climate change may potentially threaten plant biodiversity, leading to a significant reduction in the abundance and diversity of species [5,9,23,26]. Changes in the ecosystem’s structure can disrupt the balance within communities, affecting ecosystem services such as pollination, nutrient cycling, hydrological cycle regulation, and carbon sequestration [5].
In addition to climate change, the processes of deforestation and the increasing frequency of wildfires elevate the risk of extinction for various plant populations [27,28,29,30]. The Brazilian Cerrado has already experienced deforestation in approximately 61% of its natural areas [31] and possesses legal protection covering only 2.20% of its native territories [28], many of these protected areas belong to conservation units such as the natural parks. Due to its intense deforestation and high species richness, the Cerrado is designated as a biodiversity hotspot [32], and conservation units or protected areas play a pivotal role in mitigating the impacts of deforestation on these populations [33].
The ecological niche refers to the combination of conditions and resources that a species may exploit. It can be further divided into two principal components: the fundamental niche, which encompasses the environmental conditions that enable a species to survive, and the realized niche, which considers biological interactions, such as competition with other species [34]. The ecological niche model (ENM) has proven to be a reliable tool to predict future scenarios; however, it is not a consensus [22,35]. The ENM is based on the fundamental niche, functioning with a duality of geographic and environmental space. It utilizes data from geographic occurrences and climatic variables’ values while considering the species’ current limitations, without addressing possible adaptations [36,37]. The ENM has been widely employed to assess the influence of climate change on the geographic distribution of species, guiding conservation planning efforts by identifying climatically suitable areas with potential extinction risks [38,39,40,41,42].
The objectives of this study were as follows: (i) to evaluate ecological niche models, (ii) to determine which bioclimatic variables best explains the geographic distribution of five woody plant species, (iii) to assess the current distribution of these species, (iv) predict changes under distinct future climatic scenarios, and (v) to analyze potential species richness within the protected areas from Brazilian national parks.
2. Materials and Methods
2.1. Species Data
The Cerrado biome is home to approximately 14,000 plant species [43], with about 30% of them exhibiting anemochory as their dispersal mechanism. Anemochory is the second most common dispersion syndrome in the Cerrado [44,45]. For our study, we selected five common anemochoric species, each one from a representative Cerrado family (Table 1): Aspidosperma tomentosum Mart. & Zucc., Kielmeyera coriacea Mart. & Zucc., Peixotoa tomentosa A. Juss., Qualea multiflora Mart., and Senna velutina (Vogel) H.S. Irwin & Barnebby. These five species were chosen as they are easily found in Cerrado areas, considering that such species potentially occur in most of the protected areas mentioned in this study under current climate conditions. We compiled records of the geographic distribution of the five species from two URL sources, both on 17 January 2023: Species Link (CRIA 2014, https://specieslink.net/) and Global Biodiversity Information Facility (GBIF, https://www.gbif.org/) as other similar study [23]. To enhance data quality, we conducted a complementary search for synonyms using Reflora—Flora do Brasil 2020 for all five species. This effort resulted in a total of 11,996 points, including 2547 for A. tomentosum, 3081 for K. coriacea, 911 for P. tomentosa, 4086 for Q. multiflora and 1371 for S. velutina. We filtered the species’ location records by removing duplicate points, points lacking geographic coordinates, and points with improbable occurrences (examples: points in oceanic or Antarctic regions). Subsequently, the points were adjusted to a resolution of 5 min, which is approximately 81 km2. These occurrence points exclusively represent the realized niche and do not account for the effective niche, as they only include locations where the species have been found.

Table 1.
Characterization of the studied species lifestyle, fruit type, and restriction area.
2.2. Environmental Data
Bioclimatic variables were obtained from WorldClim version 2.0, with a 5-min resolution, and a total of 19 variables were initially considered for the study (Table S1). To mitigate multicollinearity among these variables, Pearson’s correlation was used to assess all possible pairwise correlations. Variables with a correlation equal to or greater than 75% (|r| ≥ 0.75) were identified as highly correlated and subjected to further filtering [46]. In cases of high correlation, only the variable deemed biologically significant for the five anemochorous species was retained. This approach ensured that the final set of variables accurately reflected the ecological needs of the species, minimizing redundancy and maintaining biological relevance. After the filtering process, nine variables were identified as applicable to all five species: (1) annual mean temperature, (2) mean diurnal range, (3) isothermality, (4) temperature seasonality, (5) annual precipitation, (6) precipitation of the driest month, (7) precipitation seasonality, (8) precipitation of the warmest quarter, and (9) precipitation of the coldest quarter (Table 2).

Table 2.
Nine bioclimatic variables included in the models.
2.3. Model Evaluation Methods
To evaluate each model, we used the area under the curve (AUC) of the receiver operating characteristic (ROC), which compares the model’s adequacy values with the observed data [19,47]. We further confirmed the results using the true skill statistic (TSS). AUC values range from 0 to 1, where values up to 0.6 indicate that the model has no predictive power, while a value of 1 indicates a perfect model for predicting species distribution [48]. To interpret the AUC values, we employed the following scale: AUC > 0.9 = excellent; 0.89 > AUC < 0.80 = good; 0.79 > AUC < 0.70 = fair; 0.69 > AUC < 0.60 = poor; AUC < 0.60 = failure [49]. TSS values range from −1 to 1, where values below 0 indicate a random model, and values above 0 represent an acceptable model [50].
2.4. Methodology to Detect the Best Bioclimatic Variables to Explain the Future Geographic Distribution of Species
To elucidate geographic distribution, we used the percentage contribution of the variables, as provided by MaxEnt. We considered the set of variables with higher importance values, collectively accounting for at least 70% of the model’s influence.
2.5. Methodology to Describe the Current Distribution of the Species
The Ecological Niche Models (ENMs) were constructed using the Maximum Entropy Model (MaxEnt) incorporated within R 4.0. MaxEnt employs maximum entropy techniques, relying solely on presence points [49,51]. We used the ENMeval package to assess the model’s fit parameters [52]. We designed 10 replicates of the model and calculated the average of these models. To optimize the modeling process, we incorporated two parameters into the model to enhance its predictive power: feature class (fc) and a regularization multiplier (rm). Feature class represents a mathematical transformation of the various covariates used in model construction, allowing for the modeling of complex relationships [53,54]. The regularization multiplier is a parameter that introduces news constraints, primarily aimed at preventing excessive complexity and overfitting, thereby controlling the intensity of the chosen feature classes for the model [53,55]. The available resource classes included linear (L), product (P), quadratic (Q), and hinge (H), as well as their combinations LQ, LQH, LQHP, and LQHPT. The multiplier values ranged from 0.5 to 4 (0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4). For each model, we generated 10.000 background points. The best models were selected based on the lowest Akaike Information Criterion (AIC). We employed the geographical block method to access the performance of each model [46,47,52]. For future predictions (2080–2100), we tested three climate projections: BCC-CSM2-MR—Beijing Climate Center Climate System Model [56], CNRM-ESM2-1—Evaluation of CNRM Earth System Model [57], and MIROC6—Model for Interdisciplinary Research on Climate [58]. These were evaluated along with four greenhouse gas emission scenarios: RCP2.6 (490 ppmv), RCP4.5 (650 ppmv), RCP6.0 (850 ppmv), and RCP8.5 (1370 ppmv) [3]. MIROC6 represents a milder model in terms of greenhouse gas emissions, whereas BCC-CSM2-MR is moderate and CNRM-ESM2-1 is more extreme [59].
2.6. Methodology to Investigate the Possible Changes in the Future
To analyze the area held, gained, and lost, we performed the calculations using the area function of the ‘raster’ package [60]. For each species, we conducted 12 analyses, working with the tree-tested models for future predictions and considering the four greenhouse gas emission scenarios. The matrix data were transformed into binary values: 0 for absence and 1 for presence [61]. The raster clogging limit was determined based on the tenth percentile of training presence (TPTP). TPTP excludes all regions with habitat suitability values lower than the 10% threshold based on current occurrence records. TPTP has been extensively used [62,63,64] and demonstrated superior results when compared to other thresholds, such as minimum training presence (MTP) [65]. TPTP is considered a conservative method [66,67].
2.7. Methodology to Analise the Conservation Units with the Greatest Potential for Future Species Richness
To assess the potential richness of species, we selected the park category as a basis since they are strictly protected areas, as the Brazilian law does not allow the direct use of natural resources [64]. For the analysis, we overlaid the binarized maps of the five species and extracted the mean potential, standard deviation, and minimum and maximum potential using the extract function from the raster package [60]. We focused on 30 parks located within the smallest polygon area, and we conducted 13 analyses: present, BCC-CSM2 RCP2.6, BCC-CSM2 RCP4.5, BCC-CSM2 RCP6.0, BCC-CSM2 RCP8.5, CNRM-ESM2-1 RCP2.6, CNRM-ESM2-1 RCP4.5, CNRM-ESM2-1 RCP6.0, CNRM-ESM2-1 RCP8.5, MIROC6 RCP2.6, MIROC6 RCP4.5, MIROC6-370, and MIROC6 RCP8.5 (Table 3).

Table 3.
Conservation units (protected areas inside National Parks) used, Brazilian states of localization, biome, and area (hectares).
3. Results
3.1. Model Evaluation Results
The model was considered excellent to P. tomentosa (AUC = 0.906 ± 0.004) and considered good to A. tomentosum (AUC = 0.857 ± 0.001), K. coriacea (AUC = 0.864 ± 0.002), Q. multiflora (AUC = 0.883 ± 0.001), and S. velutina (AUC = 0.805 ± 0.006). The true skill statistic values (TSSs) confirm that the models are acceptable (Table 4).

Table 4.
Ecological niche models of five Cerrado plant species. Occurrence records (N) used to perform the model parameters (feature: L: linear, Q: quadratic, H: hinge, Rm: regression models, AUC: area under the curve, TSS: true skill statistic, SD: standard deviation, P: product and their combinations; rm (0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4), and model performance with AUC train, AUC test average, AUC test variation, TSS, and logistic threshold (tenth percentile training presence—TPTP).
3.2. The Best Bioclimatic Variables to Explain the Future Geographic Distribution of Species Results
The most important bioclimatic variables that explained the geographic distribution varied among species (Table 5). For example, 80.70% of A. tomentosum’s distribution was explained by the precipitation of the coldest quarter (41.46%), precipitation of the warmest quarter (16.16%), annual precipitation (11.85%), and temperature seasonality (11.23%). For K. coriacea, 75.01% of its distribution was explained by the precipitation of the coldest quarter (26.87%), precipitation of the warmest quarter (19.04%), annual precipitation (16.15%), and temperature seasonality (12.95%). Meanwhile, 79.95% of P. tomentosa’s distribution was explained by the annual mean temperature (61.78%), the precipitation of the warmest quarter (10.23%), and precipitation seasonality (7.94%). Approximately 77.34% of Q. multiflora’s distribution was explained by the precipitation of the warmest quarter (31.00%), annual precipitation (20.00%), annual mean temperature (17.78%), and temperature seasonality (8.56). In contrast, 78.06% of S. velutina’s distribution was explained by the precipitation of the coldest quarter (47.90%), temperature seasonality (17.30%), and precipitation of the warmest quarter (12.86%).

Table 5.
Contribution of bioclimatic variables to the distribution of each species. Code refers to the bioclimatic variables considered in the statistical analysis.
3.3. The Current Distribution of the Species Results
The five species were distributed across a total of 2061 location points, with 497 belonging to A. tomentosum, 549 to K. coriacea, 108 to P. tomentosa, 606 to Q. multiflora, and 301 to S. velutina (Table 4). Our study identified areas considered climatically suitable for the survival of A. tomentosum (Figure 1a) and K. coriacea (Figure 1b). These areas were similar, spanning latitudes between −30° and −0° and longitudes between −70° and −35°, with a total extent of 3,288,314 hectares (ha) for A. tomentosum and 3,421,897 ha for K. coriacea (Table 6). Peixotoa tomentosa was located within the latitude range of −25° and −5°, and longitude range of −60° and −40°, covering an area of 1,621,865 ha (Figure 1c, Table 6). The habitat of Qualea multiflora spanned latitudes between −25° and −10° and longitudes between −75° and −40°, occupying 2,933,077 ha (Figure 1d, Table 6). Lastly, S. velutina was found within a latitude range from −25° to −0° and a longitude range from −65° to −25°, encompassing a total area of 3,281,471 ha (Figure 1e, Table 6).
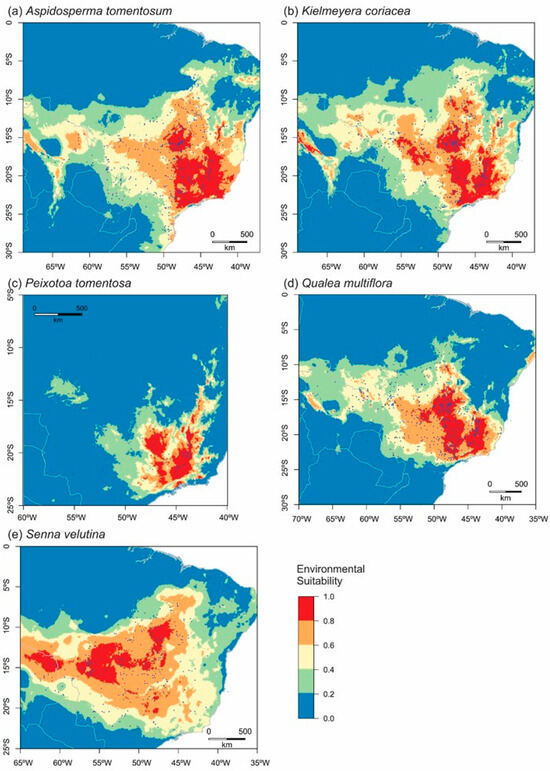
Figure 1.
Environmental suitability maps for each species varied from no suitability (represented in blue) to very good suitability (in red). Each image represents a single species: (a) Aspidosperma tomentosum, (b) Kielmeyera coriacea, (c) Peixotoa tomentosa, (d) Qualea multiflora, and (e) Senna velutina at present. The blue dots represent the selected points for each model.

Table 6.
Predicted change in the distribution of Aspidosperma tomentosum, Kielmeyera coriacea, Peixotoa tomentosa, Qualea multiflora, and Senna velutina, considering the BCC-CSM2-MR (Beijing Climate Center Climate System Model), CNRM-ESM2-1 (Evaluation of CNRM Earth System Model), and MIROC6 (Model for Interdisciplinary Research on Climate and greenhouse gas release scenarios) projections and considering RCP2.6, RCP4.5, RCP6.0, and RCP8.5 in 2080–2010.
3.4. Predictions About the Possible Changes in the Future
All five species exhibited changes in their distribution areas in the future projections (Table 6). Aspidosperma tomentosum had a loss ranging from 26.27% to 100% of its current distribution area, with a maximum gain of 2.50% (Figure S1). Kielmeyera coriacea experienced a loss ranging from 38.39% to 100% and a maximum gain of 1.61% (Figure S2). Peixotoa tomentosa’s distribution decreased by 40.46% to 96.66%, with the most significant gain being 0.16% (Figure 2). Qualea multiflora showed losses ranging from 7.34% to 100% and a substantial gain of up to 92.21% (Figure 3). Senna velutina exhibited a loss ranging from 4.52% to 99.99% of its area, with the most significant gain being 22.07% (Figure S3).
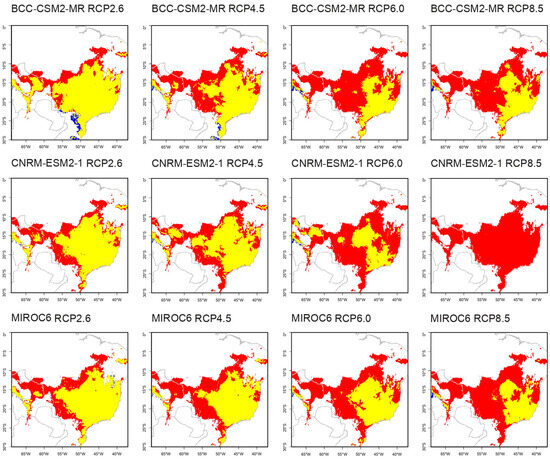
Figure 2.
Predicted new distribution of Peixotoa tomentosa under future climate scenarios. Peixotoa tomentosa is expected to maintain its individuals in the yellow areas, but to lose in the red areas. This species is not expected to expand its distribution area.
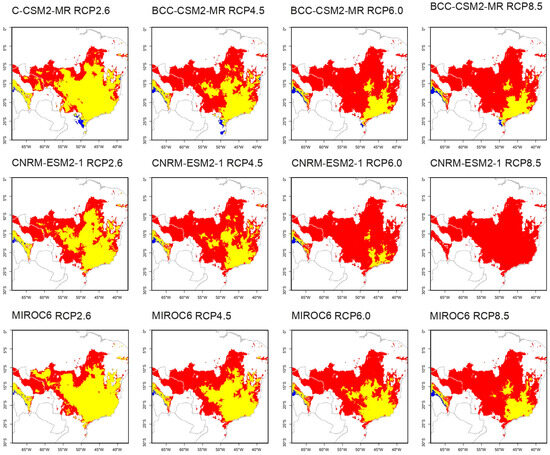
Figure 3.
Predicted new distribution of Qualea multiflora under future climate scenarios. Qualea multiflora is expected to maintain its individuals in the yellow areas, but to lose in the red areas and to expand its distribution in the blue areas.
3.5. Results About the Conservation Units with the Greatest Potential for Future Species Richness
In the current scenario, and considering the spatial projection of ecological niche models, the protected areas inside parks with the greatest potential for conservation of the five species are Parque Nacional da Chapada dos Veadeiros, Parque Nacional da Serra da Canastra, Parque Nacional da Serra do Cipó, Parque Nacional do Gandarela, Parque Nacional das Emas, Parque Nacional das Sempre-Vivas, Parque Nacional de Brasília, Parque Nacional de Caparaó, and Parque Nacional de Itatiaia (Table 7). When considering future projections, the five parks with the greatest potential for species richness are Parque Nacional do Gandarela and Parque Nacional da Serra do Cipó, both with a potential richness score of five in all projections except CNRM-ESM2-1-585. They are followed by Parque Nacional da Serra da Canastra, Parque Nacional das Sempre-Vivas, and Parque Nacional de Caparaó.

Table 7.
Average species richness potential of conversation units (protected areas), considering the present and 12 future projections. Each Parque Nacional is indicated initially as ‘PN’.
4. Discussion
Our study indicates that the climatically suitable areas for maintaining the populations of the five anemochoric species, which will be investigated by us in the future (2080–2100), will be located specifically at protected areas inside the Brazilian conservation units, in Parque Nacional da Chapada dos Veadeiros, Parque Nacional da Serra da Canastra, Parque Nacional da Serra do Cipó, Parque Nacional do Gandarela, Parque Nacional das Emas, Parque Nacional das Sempre-Vivas, Parque Nacional de Brasília, Parque Nacional de Caparaó, and Parque Nacional de Itatiaia. These areas are primarily within the Cerrado regions; however, they also extend into the Atlantic Forest, Amazon Forest, and the Caatinga, which is a unique vegetation type found in the northeastern part of Brazil. The Caatinga is characterized by shallow and stony soils, low trees, crooked trunks, and thorny and deciduous leaves during the dry season. Preserving these areas is of extreme importance for maintaining the populations of the observed species in the future. Notably, both the Cerrado and the Atlantic Forest are considered Brazilian ecosystems recognized as biodiversity hotspots worldwide [32]. These ecosystems are known for their rich biological diversity, which is threatened by species extinction and extensive habitat destruction driven by human activities [32]. The Amazon Forest, in particular, has gained international attention due to the exponential increase in deforestation [68], primarily fueled by agricultural expansion [69] and timber exploitation [70]. Preserving these areas is not only vital for conserving these ecosystems but also for safeguarding other species, including many yet to be described [19,23,71]. By protecting species from different families that play a crucial role in the Cerrado, we are also conserving phylogenetic and functional diversity, along with essential ecosystem services. These services include provisioning services that provide resources used by humans, such as water, nutrient cycling, pollination, and carbon sequestration [72].
Aspidosperma tomentosum, K. coriacea, and P. tomentosa experienced significant losses in their distribution areas across all analyzed scenarios, losing up to 100%, 100%, and 96.66%, respectively. Senna velutina also faced substantial losses, with up to 99.99% of its area being affected; however, it could potentially gain up to 22.07% of its area. Plants around the world will be affected by climate change in different regions [9]. Anthropogenic climate change is widely recognized as a major threat to biodiversity and has the potential to lead to the extinction of thousands of species over the next century [9]. Species extinction can be attributed to various factors, including physiological tolerance issues such as intolerance to high temperatures [73]. In the best-case scenario, it is estimated that 38–45% of woody plant species in the Brazilian Cerrado will cease to exist due to these climate changes. In the worst-case scenario, the estimate is as high as 56% of species [9].
Qualea multiflora generally expands its range; however, when it experiences losses, it becomes vulnerable, potentially facing extinction with a 100% reduction in its current distribution area. Notably, Q. multiflora has expanded its range into the Amazon Forest, where there was previously no record of its occurrence [43]. The establishment of a species in a new environment depends on abiotic factors, such as the variables incorporated in the model, as well as biotic factors, including competition [74]. Furthermore, new factors need to be considered, such as anthropogenic activities in the Amazon region, which have resulted in significant deforestation [75]. However, it is worth noting that Q. multi-flora previously existed in the Amazon region but later migrated to central Brazil, gaining areas further south in response to climatic fluctuations at the time [76]. We recommend further investigation into this expansion area of Q. multiflora.
The most relevant bioclimatic variable for A. tomentosum, K. coriacea, and S. velutina distribution was the precipitation of the coldest quarter (Bio 19). During the colder season, precipitation is lower, resulting in a negative relationship between fruiting and precipitation, as these three species exhibit fruit ripening between July and September [44,77]. For P. tomentosa, the most relevant bioclimatic variable was the annual mean temperature (Bio 1). Temperature is positively correlated with the flowering and fruiting of P. tomentosa [77]. For Qualea multiflora, the precipitation of the warmest quarter (Bio 18) was the most relevant variable. Precipitation during the warmest quarter corresponds to summer in the southern hemisphere, coinciding with the flowering period of Q. multiflora, which occurs between November and January [77]. The reproductive phenophase is crucial for maintaining species populations, as flowering marks the initial stage of seed generation, leading to successful seed dispersal and the establishment of new individuals [78]. Following fruiting, seed dispersal further supports population maintenance, especially during the dry season when wind intensities are higher [79].
The conservation units with the greatest potential for species development in the future are Parque Nacional do Gandarela and Parque Nacional da Serra do Cipó, followed by Parque Nacional da Serra da Canastra, Parque Nacional das Sempre-Vivas, and Parque Nacional do Caparaó. Notably, the former two parks encompass a diverse and endangered Brazilian ecosystem [80]. It is worth noting that, in general, most Brazilian parks face some form of threat. When considering the best-case scenarios, the analyzed Brazilian parks have the climatic potential to support the studied populations. However, as these scenarios worsen, we observe a concerning decline in this capacity, which poses a probable high risk of extinction. Future studies on the effects of climate change are of great importance for the main plant families and their most abundant species, particularly for species that can sustain animals as pollinators or fruit dispersers.
However, it is valid to consider the influence of human activity in future projections in addition to natural climate-forming processes. Human activities are conditioned by the forecast of the development of the world economy, especially energy, and they may be used as another crucial initial condition in the modeling process as they induce changes in the chemical composition of the atmosphere. For example, atmosphere modification includes the process of sunlight reaching the surface of the planet and the greenhouse effects. Knowledge of natural climate-forming processes and the future development of the world are the two main factors able to determine climate change. We emphasize the need for further studies focusing on these distinct factors capable of altering climatic conditions and, consequently, the distribution of species.
5. Conclusions
This study reveals significant insights into the future distribution of five important woody species within Brazilian national parks under changing climate scenarios, as it highlights that climate change will likely lead to substantial shifts in ecological niches and the distribution of these species. All species are projected to experience changes, with some, such as Aspidosperma tomentosum, Kielmeyera coriacea, and Peixotoa tomentosa, facing severe distribution losses, potentially up to 100%. Others, like Qualea multiflora and Senna velutina, may expand their ranges, though these gains are limited and often accompanied by losses elsewhere.
This analysis emphasizes the importance of Brazilian protected areas for future species conservation, particularly those in the Cerrado, Atlantic Forest, and Amazon regions. Our results underscore that these protected areas, such as Parque Nacional da Chapada dos Veadeiros and Parque Nacional da Serra do Cipó, will be crucial refuges for maintaining biodiversity. However, the potential species richness in these parks is expected to decline, which highlights the urgency for adaptive management strategies to preserve both species and ecosystems in the face of climate change.
Additionally, our study identifies critical bioclimatic variables, such as temperature and precipitation patterns, which play a central role in the distribution of species. These insights provide a foundation for further investigations into species’ phenological patterns and how they may adapt to future climate conditions. The influence of human activities, including deforestation and land-use change, also warrants further exploration, as they may exacerbate the impacts of climate change.
Ultimately, our findings call for the preservation of the identified conservation units and the need for ongoing research to monitor and mitigate the effects of climate change on biodiversity. This study reinforces the role of protected areas as essential tools for conserving natural ecosystems and highlights the importance of using proactive conservation strategies to safeguard biodiversity for future generations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos16040453/s1, Table S1. Bioclimatic variables analyzed; Figure S1: Predicted change in Aspidosperma tomentosum Mart. & Zucc. distribution under future climate scenarios; Figure S2: Predicted change in Kilmeyera coriaceae Mart. & Zucc. distribution under future climate scenarios; Table S1: Average species richness potential of conversation units. considering the present and 12 future projections.
Author Contributions
Conceptualization, L.A.-d.-L. and D.F.R.A.; methodology L.A.-d.-L., D.F.R.A. and F.A.V.; software D.F.R.A.; validation D.F.R.A.; formal analysis, D.F.R.A.; investigation L.A.-d.-L., D.F.R.A. and F.A.V.; data curation L.A.-d.-L., D.F.R.A., D.V.A. and H.M.T.-S.; writing—original draft preparation L.A.-d.-L. and D.V.A.; writing—review and editing L.A.-d.-L., D.F.R.A. and H.M.T.-S.; supervision H.M.T.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa do Estado de Minas Gerais—FAPEMIG Financial code 11589 (LAL); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance code 001 (LAL); and Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brazil (CNPQ)—Finance code 403647/2021-5 (HMTS).
Data Availability Statement
No new data were created. We used data on the geographic distribution of the species from 17 January 2023, compiled from the collection of the online websites Species Link (CRIA 2014, https://specieslink.net/) and Global Biodiversity Information Facility (GBIF, https://www.gbif.org/).
Acknowledgments
Special thanks to an introductory niche modeling course for getting us started on this project and to two anonymous reviewers for their significant improvements in the text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Zwiener, V.; Lira-Noriega, A.; Grady, C.J.; Padial, A.A.; Vitule, J.R.S. Climate Change as a Driver of Biotic Homogenization of Woody Plants in the Atlantic Forest. Glob. Ecol. Biogeogr. 2018, 27, 298–309. [Google Scholar] [CrossRef]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; Van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T.; et al. The next Generation of Scenarios for Climate Change Research and Assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Marengo, J.A. Mudanças Climáticas Globais e Seus Efeitos Sobre a Biodiversidade: Caracterização Do Clima Atual e Definição Das Alterações Climáticas Pata o Território Brasileiro Ao Longo Do Século 21; Ministério do Meio Ambiente: Brasília, Brazil, 2006.
- Bustamante, M.; Nardoto, G.; Pinto, A.; Resende, J.; Takahashi, F.; Vieira, L. Potential Impacts of Climate Change on Biogeochemical Functioning of Cerrado Ecosystems. Braz. J. Biol. 2012, 72, 655–671. [Google Scholar] [CrossRef]
- Marengo, J.A.; Ambrizzi, T.; da Rocha, R.P.; Alves, L.M.; Cuadra, S.V.; Valverde, M.C.; Torres, R.R.; Santos, D.C.; Ferraz, S.E.T. Future Change of Climate in South America in the Late Twenty-First Century: Intercomparison of Scenarios from Three Regional Climate Models. Clim. Dyn. 2010, 35, 1073–1097. [Google Scholar] [CrossRef]
- Miranda, L.S.; Imperatriz-Fonseca, V.L.; Giannini, T.C. Climate Change Impact on Ecosystem Functions Provided by Birds in Southeastern Amazonia. PLoS ONE 2019, 14, e0215229. [Google Scholar] [CrossRef] [PubMed]
- Hidasi-Neto, J.; Joner, D.C.; Resende, F.; de Macedo Monteiro, L.; Faleiro, F.V.; Loyola, R.D.; Cianciaruso, M.V. Climate Change Will Drive Mammal Species Loss and Biotic Homogenization in the Cerrado Biodiversity Hotspot. Perspect. Ecol. Conserv. 2019, 17, 57–63. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Simões, S.d.S.; Zappi, D.; Costa, G.M.d.; de Oliveira, G.; Aona, L.Y.S. Spatial Niche Modelling of Five Endemic Cacti from the Brazilian Caatinga: Past, Present and Future. Austral Ecol. 2020, 45, 35–47. [Google Scholar] [CrossRef]
- Atwater, D.Z.; Barney, J.N. Climatic Niche Shifts in 815 Introduced Plant Species Affect Their Predicted Distributions. Glob. Ecol. Biogeogr. 2021, 30, 1671–1684. [Google Scholar] [CrossRef]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a Century of Climate Change on Small-Mammal Communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef]
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A.; Graham, M.H. Effects of Climate Change on Global Seaweed Communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Wallenstein, M.D. Climate Change Alters Ecological Strategies of Soil Bacteria. Ecol. Lett. 2014, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L. Biological Consequences of Global Warming: Is the Signal Already Apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating Extinction Risk from Climate Change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Vitorino, L.C.; Reis, M.N.O.; Bessa, L.A.; de Souza, U.J.B.; Silva, F.G. Landscape and Climate Influence the Patterns of Genetic Diversity and Inbreeding in Cerrado Plant Species. Diversity 2020, 12, 421. [Google Scholar] [CrossRef]
- Vilela, A.A.; Del-Claro, V.T.S.; Torezan-Silingardi, H.M.; Del-Claro, K. Climate Changes Affecting Biotic Interactions, Phenology, and Reproductive Success in a Savanna Community over a 10-Year Period. Arthropod-Plant Interact. 2017, 12, 215–227. [Google Scholar] [CrossRef]
- Correa-Lima, A.P.A.; Varassin, I.G.; Barve, N.; Zwiener, V.P. Spatio-Temporal Effects of Climate Change on the Geographical Distribution and FLowering Phenology of Hummingbird-Pollinated Plants. Ann. Bot. 2019, 124, 389–398. [Google Scholar] [CrossRef]
- Frei, E.R.; Ghazoul, J.; Matter, P.; Heggli, M.; Pluess, A.R. Plant Population Differentiation and Climate Change: Responses of Grassland Species along an Elevational Gradient. Glob. Change Biol. 2014, 20, 441–455. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sgro, C.M. Climate Change and Evolutionary Adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Priti, H.; Aravind, N.A.; Uma Shaanker, R.; Ravikanth, G. Modeling Impacts of Future Climate on the Distribution of Myristicaceae Species in the Western Ghats, India. Ecol. Eng. 2016, 89, 14–23. [Google Scholar] [CrossRef]
- Oliveira, G.; Lima-Ribeiro, M.S.; Terribile, L.C.; Dobrovolski, R.; Telles, M.P.d.C.; Diniz-Filho, J.A.F. Conservation Biogeography of the Cerrado’s Wild Edible Plants under Climate Change: Linking Biotic Stability with Agricultural Expansion. Am. J. Bot. 2015, 102, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Atwater, D.Z.; Ervine, C.; Barney, J.N. Climatic Niche Shifts Are Common in Introduced Plants. Nat. Ecol. Evol. 2018, 2, 34–43. [Google Scholar] [CrossRef]
- Jump, A.S.; Penuelas, J. Running to Stand Still: Adaptation and the Response of Plants to Rapid Climate Change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- de Siqueira, M.F.; Peterson, A.T. Consequences of Global Climate Change for Geographic Distributions of Cerrado Tree Species. Biota Neotrop. 2003, 3, 1–14. [Google Scholar] [CrossRef]
- Costa, M.H.; Pires, G.F. Effects of Amazon and Central Brazil Deforestation Scenarios on the Duration of the Dry Season in the Arc of Deforestation. Int. J. Clim. 2010, 30, 1970–1979. [Google Scholar] [CrossRef]
- Klink, C.A.; Machado, R.B. Conservation of the Brazilian Cerrado. Conserv. Biol. 2005, 19, 707–713. [Google Scholar] [CrossRef]
- Trigueiro, W.R.; Nabout, J.C.; Tessarolo, G. Uncovering the Spatial Variability of Recent Deforestation Drivers in the Brazilian Cerrado. J. Environ. Manag. 2020, 275, 111243. [Google Scholar] [CrossRef]
- Mistry, J. Fire in the Cerrado (Savannas) of Brazil: An Ecological Review. Prog. Phys. Geogr. 1998, 22, 425–448. [Google Scholar] [CrossRef]
- Filho, I.A.M.; das Neves, A.J.; Silva, G.E.; Vieira, A.d.S. Áreas de Proteção Ambiental e a Preservação Do Bioma Cerrado. Rev. Bras. Estud. Segur. Pública 2019, 12, 10–19. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; Lamas, I.; Kasecker, T. O Papel Das Unidades de Conservação. Sci. Am. Bras. 2010, 39, 18–23. [Google Scholar]
- Hutchinson, G.E. Concluding Remarks: Animal Ecology and Demography. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Peterson, A.T.; Cobos, M.E.; Jiménez-García, D. Major Challenges for Correlational Ecological Niche Model Projections to Future Climate Conditions. Ann. N. Y. Acad. Sci. 2018, 1429, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K.; Rangel, T.F. Hutchinson’s Duality: The Once and Future Niche. Proc. Natl. Acad. Sci. USA 2009, 106, 19651–19658. [Google Scholar] [CrossRef]
- Guisan, A.; Mod, H.K.; Scherrer, D.; Münkemüller, T.; Pottier, J.; Alexander, J.M.; D’Amen, M. Scaling the Linkage between Environmental Niches and Functional Traits for Improved Spatial Predictions of Biological Communities. Glob. Ecol. Biogeogr. 2019, 28, 1384–1392. [Google Scholar] [CrossRef]
- Broennimann, O.; Thuiller, W.; Hughes, G.; Midgley, G.F.; Alkemade, J.M.R.; Guisan, A. Do Geographic Distribution, Niche Property and Life Form Explain Plants’ Vulnerability to Global Change? Glob. Change Biol. 2006, 12, 1079–1093. [Google Scholar] [CrossRef]
- Mendoza-González, G.; Mart\’\inez, M.L.; Rojas-Soto, O.R.; Vázquez, G.; Gallego-Fernández, J.B. Ecological Niche Modeling of Coastal Dune Plants and Future Potential Distribution in Response to Climate Change and Sea Level Rise. Glob. Change Biol. 2013, 19, 2524–2535. [Google Scholar] [CrossRef]
- You, J.; Qin, X.; Ranjitkar, S.; Lougheed, S.C.; Wang, M.; Zhou, W.; Ouyang, D.; Zhou, Y.; Xu, J.; Zhang, W.; et al. Response to Climate Change of Montane Herbaceous Plants in the Genus Rhodiola Predicted by Ecological Niche Modelling. Sci. Rep. 2018, 8, 5879. [Google Scholar] [CrossRef]
- Lopes, T.M.; Bailly, D.; Almeida, B.A.; Santos, N.C.L.; Gimenez, B.C.G.; Landgraf, G.O.; Sales, P.C.L.; Lima-Ribeiro, M.S.; Cassemiro, F.A.S.; Rangel, T.F.; et al. Two Sides of a Coin: Effects of Climate Change on the Native and Non-Native Distribution of Colossoma Macropomum in South America. PLoS ONE 2017, 12, e0179684. [Google Scholar] [CrossRef]
- Sen, S.; Gode, A.; Ramanujam, S.; Ravikanth, G.; Aravind, N.A. Modeling the Impact of Climate Change on Wild Piper Nigrum (Black Pepper) in Western Ghats, India Using Ecological Niche Models. J. Plant Res. 2016, 129, 1033–1040. [Google Scholar] [CrossRef]
- Reflora. Available online: https://floradobrasil.jbrj.gov.br/reflora/PrincipalUC/PrincipalUC.do;jsessionid=476FB5B3B850AA61B3CBEA61556C5CF2 (accessed on 17 January 2023).
- Pilon, N.A.L.; Udulutsch, R.G.; Durigan, G. Padrões Fenológicos de 111 Espécies de Cerrado Em Condições de Cultivo. Hoehnea 2015, 42, 425–443. [Google Scholar] [CrossRef]
- Gottsberger, G.; Silberbauer-Gottsberger, I. Dispersal and Distribution in the Cerrado Vegetation of Brazil. Sonderbd. Naturwiss. Ver. Hambg. 1983, 7, 315–352. [Google Scholar]
- Lira, F.D.A.; Amado, T.F.; Moura, T.A.; Riul, P. Vulnerable Areas to Accidents with Scorpions in Brazil. Trop. Med. Int. Health 2021, 26, 591–601. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martinez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of Species–Climate Impact Models under Climate Change. Glob. Change Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM Eval: An R Package for Conducting Spatially Independent Evaluations and Estimating Optimal Model Complexity for Maxent Ecological Niche Models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s Parameter Configuration and Small Samples: Are We Paying Attention to Recommendations? A Systematic Review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander Jr, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating Optimal Complexity for Ecological Niche Models: A Jackknife Approach for Species with Small Sample Sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The Main Progress from CMIP5 to CMIP6. Geosci. Model. Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Séférian, R.; Nabat, P.; Michou, M.; Saint-Martin, D.; Voldoire, A.; Colin, J.; Decharme, B.; Delire, C.; Berthet, S.; Chevallier, M.; et al. Evaluation of CNRM Earth System Model, CNRM-ESM2-1: Role of Earth System Processes in Present-Day and Future Climate. J. Adv. Model. Earth Syst. 2019, 11, 4182–4227. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F.; et al. Description and Basic Evaluation of Simulated Mean State, Internal Variability, and Climate Sensitivity in MIROC6. Geosci. Model. Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Van Etten, J.; Sumner, M.; Cheng, J.; Baston, D.; Bevan, A.; Bivand, R.; Busetto, L.; Canty, M.; Fasoli, B.; et al. Package ‘raster’. 2021. Available online: https://cran.r-project.org/web/packages/raster/raster.pdf (accessed on 17 January 2023).
- Freeman, E.A.; Moisen, G.G. A Comparison of the Performance of Threshold Criteria for Binary Classification in Terms of Predicted Prevalence and Kappa. Ecol. Model. 2008, 217, 48–58. [Google Scholar] [CrossRef]
- Jacobsen, C.D.; Brown, D.J.; Flint, W.D.; Pauley, T.K.; Buhlmann, K.A.; Mitchell, J.C. Vulnerability of High-Elevation Endemic Salamanders to Climate Change: A Case Study with the Cow Knob Salamander (Plethodon punctatus). Glob. Ecol. Conserv. 2020, 21, e00883. [Google Scholar] [CrossRef]
- Brambilla, M.; Ficetola, G.F. Species Distribution Models as a Tool to Estimate Reproductive Parameters: A Case Study with a Passerine Bird Species. J. Anim. Ecol. 2012, 81, 781–787. [Google Scholar] [CrossRef]
- Hernández-Lambraño, R.E.; González-Moreno, P.; Sánchez-Agudo, J.Á. Towards the Top: Niche Expansion of Taraxacum officinale and Ulex europaeus in Mountain Regions of South America. Austral Ecol. 2017, 42, 577–589. [Google Scholar] [CrossRef]
- Brito, J.C.; Acosta, A.L.; Álvares, F.; Cuzin, F. Biogeography and Conservation of Taxa from Remote Regions: An Application of Ecological-Niche Based Models and GIS to North-African Canids. Biol. Conserv. 2009, 142, 3020–3029. [Google Scholar] [CrossRef]
- Sutton, W.B.; Barrett, K.; Moody, A.T.; Loftin, C.S.; DeMaynadier, P.G.; Nanjappa, P. Predicted Changes in Climatic Niche and Climate Refugia of Conservation Priority Salamander Species in the Northeastern United States. Forests 2015, 6, 1–26. [Google Scholar] [CrossRef]
- Bosso, L.; Rebelo, H.; Garonna, A.P.; Russo, D. Modelling Geographic Distribution and Detecting Conservation Gaps in Italy for the Threatened Beetle Rosalia Alpina. J. Nat. Conserv. 2013, 21, 72–80. [Google Scholar] [CrossRef]
- Matricardi, E.A.T.; Skole, D.L.; Costa, O.B.; Pedlowski, M.A.; Samek, J.H.; Miguel, E.P. Long-Term Forest Degradation Surpasses Deforestation in the Brazilian Amazon. Science 2020, 369, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Silvério, D.V.; Brando, P.M.; Macedo, M.N.; Beck, P.S.A.; Bustamante, M.; Coe, M.T. Agricultural Expansion Dominates Climate Changes in Southeastern Amazonia: The Overlooked Non-GHG Forcing. Environ. Res. Lett. 2015, 10, 104015. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; de Souza, Á.N.; Joaquim, M.S.; Lustosa Junior, I.M.; Pereira, R.S. Concessão Florestal Na Amazônia Brasileira. Ciênc. Florest. 2020, 30, 1299–1308. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Resende, A.F.; Barlow, J.; França, F.M.; Moura, M.R.; Maciel, R.; Alves-Martins, F.; Shutt, J.; Nunes, C.A.; Elias, F.; et al. Pervasive Gaps in Amazonian Ecological Research. Curr. Biol. 2023, 33, 3495–3504.e4. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Parreira, M.R.; Nabout, J.C. The Impact of Global Climate Change on the Number and Replacement of Provisioning Ecosystem Services of Brazilian Cerrado Plants. Environ. Monit. Assess. 2021, 193, 731. [Google Scholar] [CrossRef]
- Somero, G.N. Comparative Physiology: A “Crystal Ball” for Predicting Consequences of Global Change. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 301, R1–R14. [Google Scholar] [CrossRef]
- Morin, P.J. Community Ecology, 2nd ed.; Wiley-Brackwell: Oxford, UK, 2011. [Google Scholar]
- Malhi, Y.; Roberts, J.T.; Betts, R.A.; Killeen, T.J.; Li, W.; Nobre, C.A. Climate Change, Deforestation, and the Fate of the Amazon. Science 2008, 319, 169–172. [Google Scholar] [CrossRef]
- Buzatti, R.S.d.O.; Lemos-Filho, J.P.; Bueno, M.L.; Lovato, M.B. Multiple Pleistocene Refugia in the Brazilian Cerrado: Evidence from Phylogeography and Climatic Nichemodelling of Two Qualea Species (Vochysiaceae). Bot. J. Linn. Soc. 2017, 185, 307–320. [Google Scholar] [CrossRef]
- Novaes, L.R.; Calixto, E.S.; Oliveira, M.L.; Alves-de-Lima, L.; Almeida, O.; Torezan-Silingardi, H.M. Environmental Variables Drive Phenological Events of Anemocoric Plants and Enhance Diaspore Dispersal Potential: A New Wind-Based Approach. Sci. Total Environ. 2020, 730, 139039. [Google Scholar] [CrossRef] [PubMed]
- Del-Claro, K.; Torezan-Silingardi, H.M. Ecologia Das Interações Plantas-Animais: Uma Abordagem Ecológico-Evolutiva; Technical Books Editora: Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Johansson, V.; Lönnell, N.; Rannik, Ü.; Sundberg, S.; Hylander, K. Air Humidity Thresholds Trigger Active Moss Spore Release to Extend Dispersal in Space and Time. Funct. Ecol. 2016, 30, 1196–1204. [Google Scholar] [CrossRef]
- Jacobi, C.M.; do Carmo, F.F.; Vincent, R.C.; Stehmann, J.R. Plant Communities on Ironstone Outcrops: A Diverse and Endangered Brazilian Ecosystem. Biodivers. Conserv. 2007, 16, 2185–2200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).