A Traceable Calibration for Gaseous Elemental Mercury Measurements in Air and Water
Abstract
1. Introduction
2. Materials and Methods
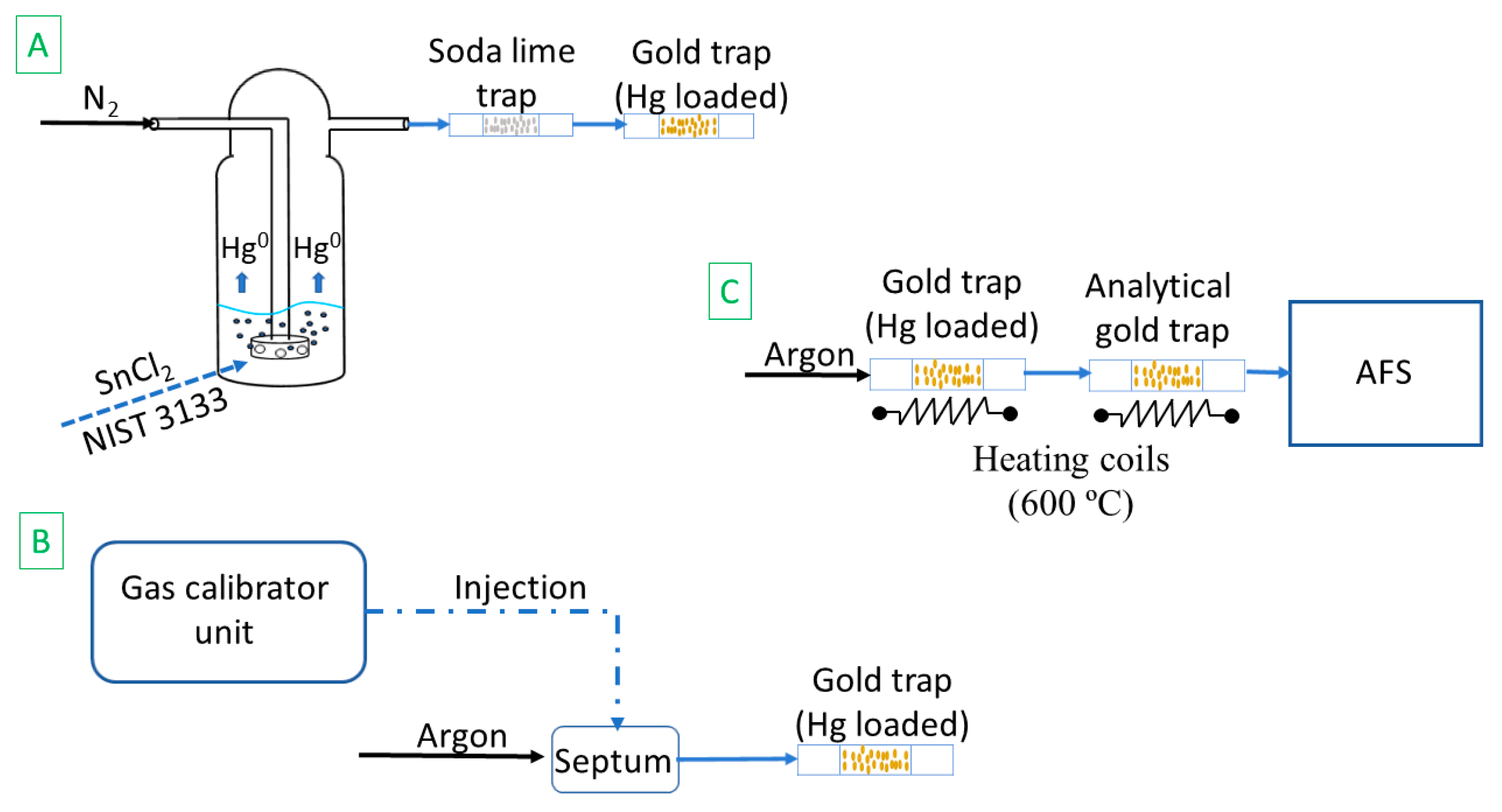
2.1. Calibration Methods
2.1.1. NIST 3133 Calibration
2.1.2. Gas Standard Calibration
2.2. Sample Analysis
2.2.1. Water Sample Analysis
2.2.2. Ambient Air Sample Analysis
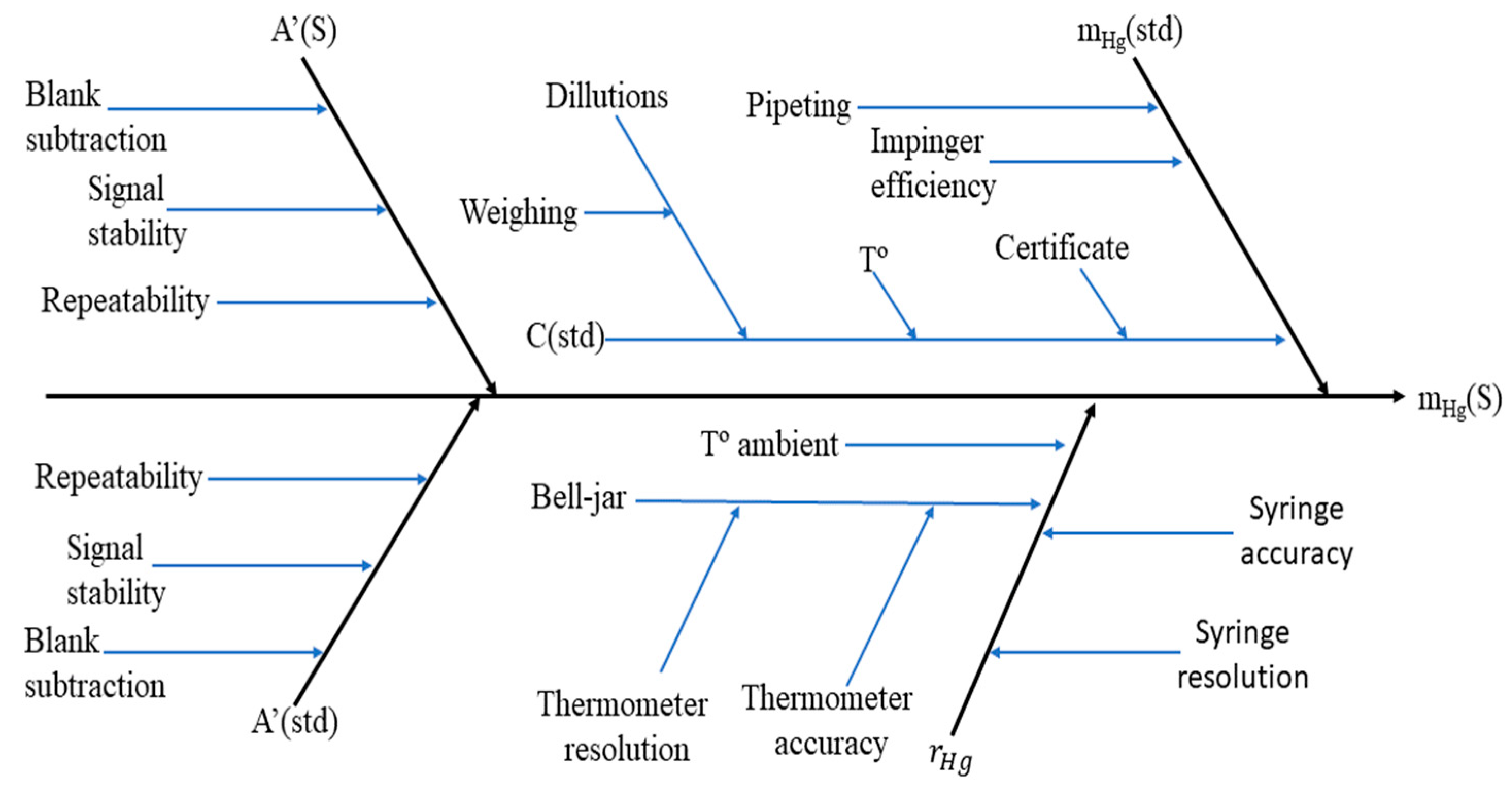
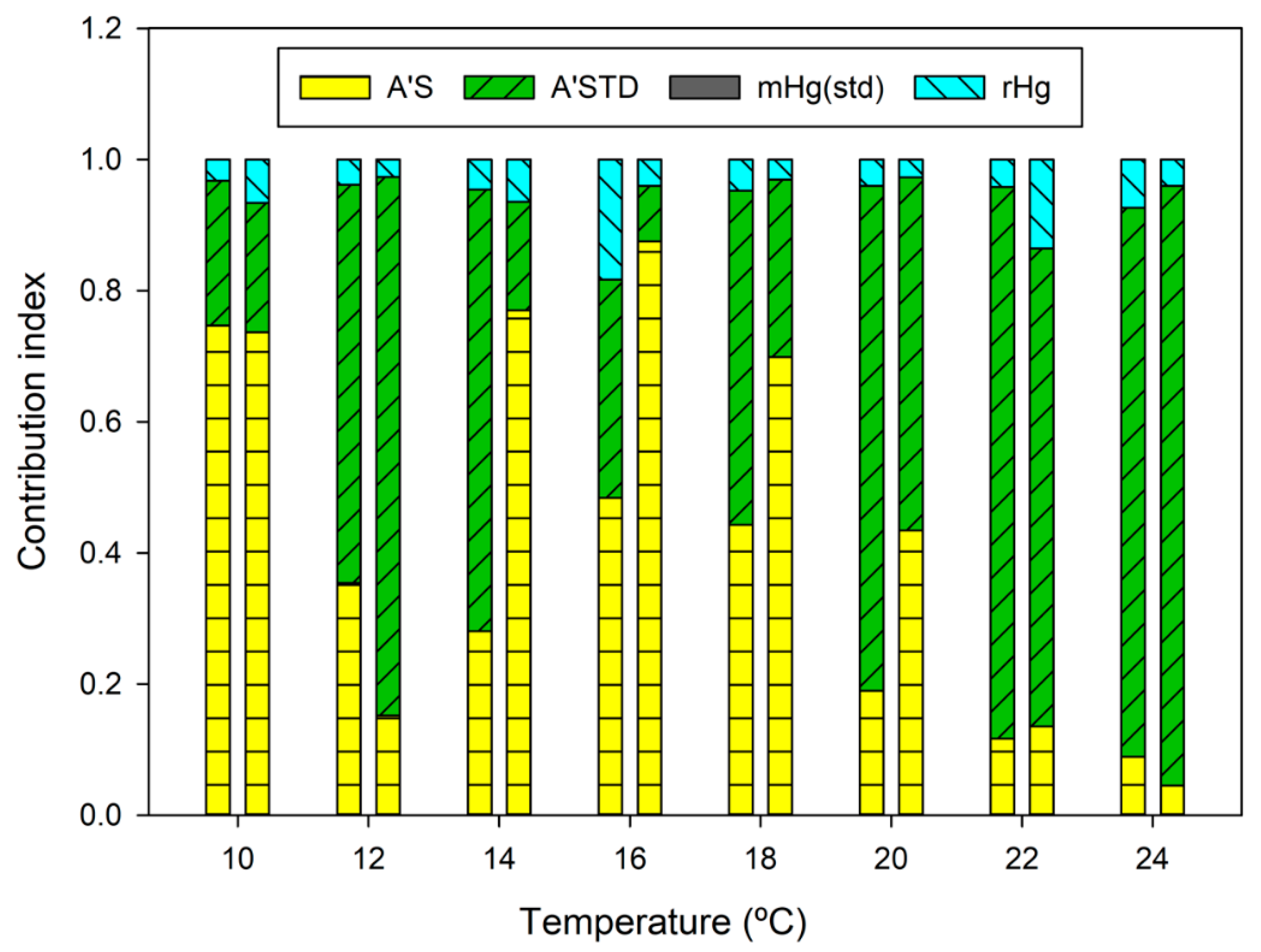
2.3. Uncertainty Budget
3. Results and Discussion
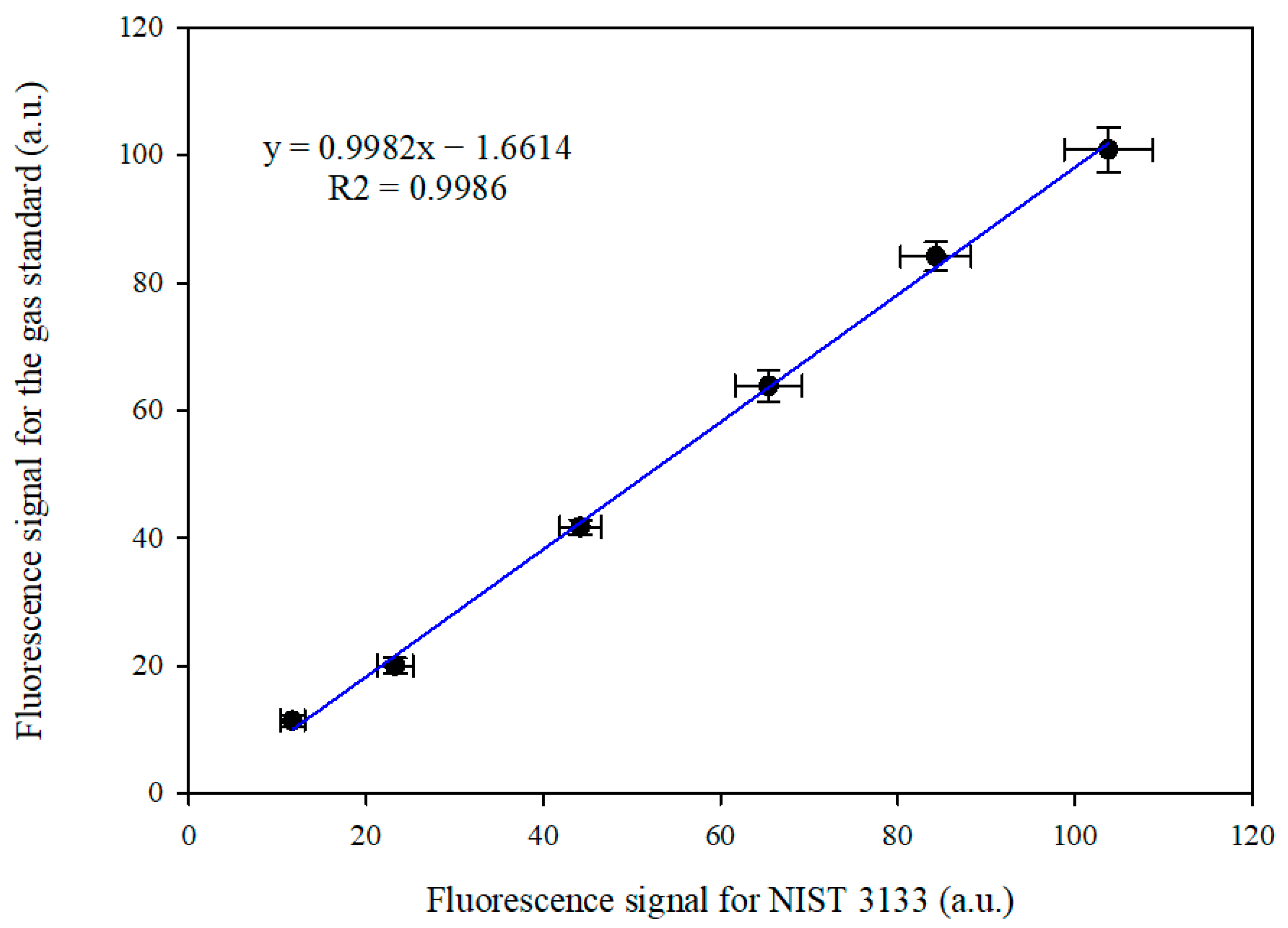
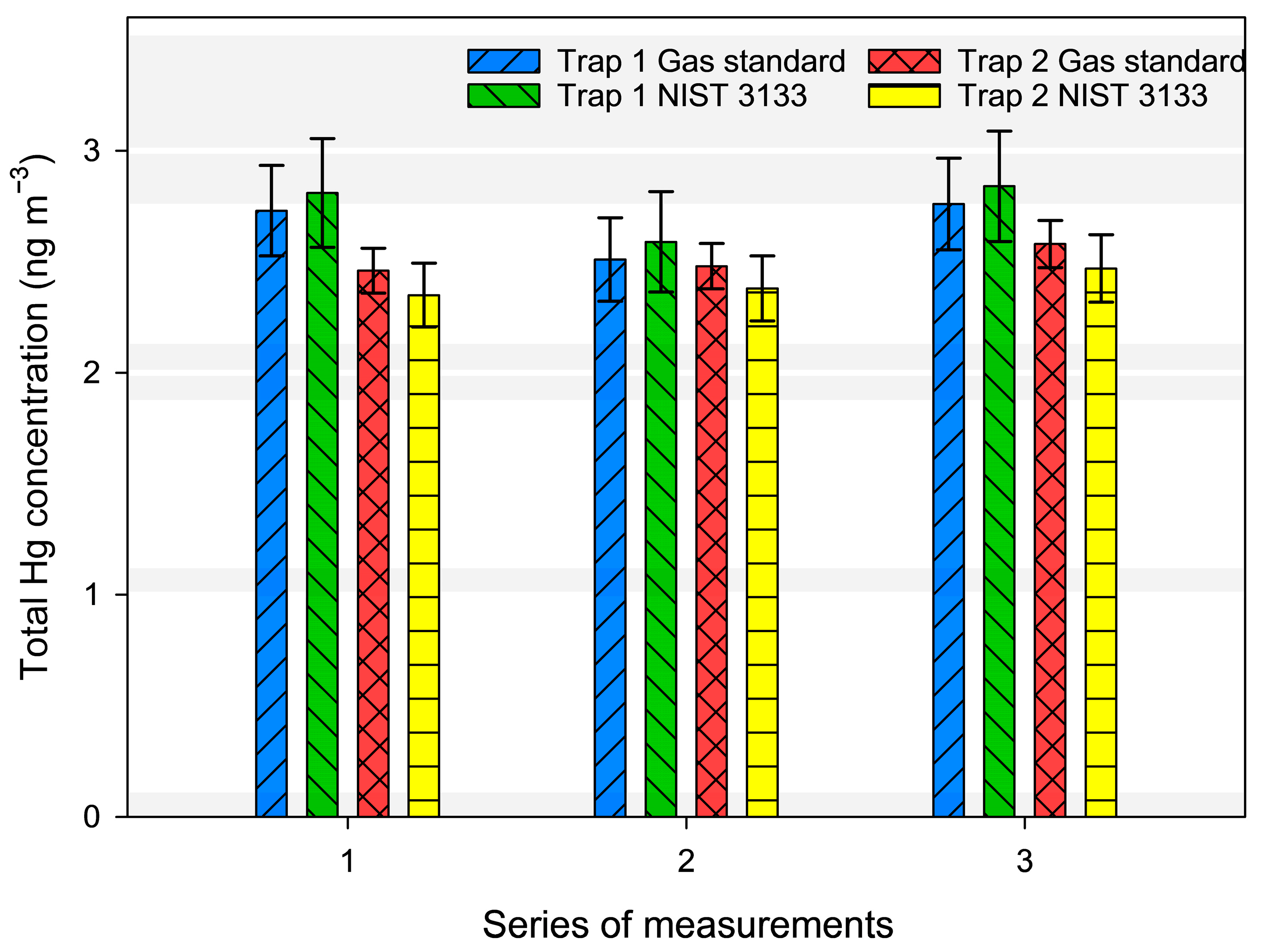
3.1. Comparison Between the Gas Standard and NIST 3133 SRM
3.2. DGM and RM Analysis from Idrijca River
3.3. Ambient Air Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Environment Programme (UNEP). The Emissions Gap Report 2013. [Online] UNEP. Available online: https://www.unep.org/resources/emissions-gap-report-2013 (accessed on 28 March 2024).
- Ciani, F.; Rimondi, V.; Costagliola, P. Atmospheric mercury pollution: The current methodological framework outlined by environmental legislation. Air Qual. Atmos. Health 2021, 14, 1633–1645. [Google Scholar] [CrossRef]
- Gustin, M.S.; Evers, D.C.; Bank, M.S.; Hammerschmidt, C.R.; Pierce, A.; Basu, N.; Blum, J.; Bustamante, P.; Chen, C.; Driscoll, C.T.; et al. Importance of Integration and Implementation of Emerging and Future Mercury Research into the Minamata Convention. Environ. Sci. Technol. 2016, 50, 2767–2770. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, T.; Brown, S.; Wong, H.; Apte, S. Assessing the impacts of scale residues from offshore oil and gas decommissioning on marine organisms. APPEA J. 2021, 61, 379. [Google Scholar] [CrossRef]
- Long, S.E.; Norris, J.E.; Carney, J.; Ryan, J.V.; Mitchell, G.D.; Dorko, W.D. Traceability of the output concentration of mercury vapor generators. Atmos. Pollut. Res. 2020, 11, 639–645. [Google Scholar] [CrossRef]
- Gačnik, J.; Živković, I.; Guevara, S.R.; Jaćimović, R.; Kotnik, J.; Horvat, M. Validating an Evaporative Calibrator for Gaseous Oxidized Mercury. Sensors 2021, 21, 2501. [Google Scholar] [CrossRef]
- Sari, S.; Timo, R.; Jussi, H.; Panu, H. Dynamic calibration method for reactive gases. Meas. Sci. Technol. 2020, 31, 034001. [Google Scholar] [CrossRef]
- Brown, A.S.; Brown, R.J.C.; Corns, W.T.; Stockwell, P.B. Establishing SI traceability for measurements of mercury vapour. Analyst 2008, 133, 946. [Google Scholar] [CrossRef]
- de Krom, I.; Bavius, W.; Ziel, R.; Efremov, E.; van Meer, D.; van Otterloo, P.; van Andel, I.; van Osselen, D.; Heemskerk, M.; van der Veen, A.M.; et al. Primary mercury gas standard for the calibration of mercury measurements. Measurement 2021, 169, 108351. [Google Scholar] [CrossRef]
- Dumarey, R.; Brown, R.J.C.; Corns, W.T.; Brown, A.S.; Stockwell, P.B. Elemental mercury vapour in air: The origins and validation of the “Dumarey equation” describing the mass concentration at saturation. Accredit. Qual. Assur. 2010, 15, 409–414. [Google Scholar] [CrossRef]
- Huber, M.L.; Laesecke, A.; Friend, D.G. Correlation for the Vapor Pressure of Mercury. Ind. Eng. Chem. Res. 2006, 45, 7351–7361. [Google Scholar] [CrossRef]
- Quétel, C.R.; Zampella, M.; Brown, R.J.C. Temperature dependence of Hg vapour mass concentration at saturation in air: New SI traceable results between 15 and 30 °C. TrAC Trends Anal. Chem. 2016, 85, 81–88. [Google Scholar] [CrossRef]
- Srivastava, A.; Hodges, J.T. Development of a High-Resolution Laser Absorption Spectroscopy Method with Application to the Determination of Absolute Concentration of Gaseous Elemental Mercury in Air. Anal. Chem. 2018, 90, 6781–6788. [Google Scholar] [CrossRef] [PubMed]
- Dumarey, R.; Heindryckx, R.; Dams, R.; Hoste, J. Determination of volatile mercury compounds in air with the coleman mercury analyzer system. Anal. Chim. Acta 1979, 107, 159–167. [Google Scholar] [CrossRef]
- ISO 14044:2003; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2003.
- ASTM C1202-03; Standard Test Method for Water Permeability of Concrete. ASTM International: West Conshohocken, PA, USA, 2003.
- O’Concubhair, R.; O’Sullivan, D.; Sodeau, J.R. Dark Oxidation of Dissolved Gaseous Mercury in Polar Ice Mimics. Environ. Sci. Technol. 2012, 46, 4829–4836. [Google Scholar] [CrossRef]
- Timonen, H.; Ambrose, J.L.; Jaffe, D.A. Oxidation of elemental Hg in anthropogenic and marine airmasses. Atmos. Chem. Phys. 2013, 13, 2827–2836. [Google Scholar] [CrossRef]
- Quétel, C.R.; Zampella, M.; Brown, R.J.C.; Ent, H.; Horvat, M.; Paredes, E.; Tunc, M. International System of Units Traceable Results of Hg Mass Concentration at Saturation in Air from a Newly Developed Measurement Procedure. Anal. Chem. 2014, 86, 7819–7827. [Google Scholar] [CrossRef]
- Ent, H.; van Andel, I.; Heemskerk, M.; van Otterloo, P.; Bavius, W.; Baldan, A.; Horvat, M.; Brown, R.J.C.; Quétel, C.R. A gravimetric approach to providing SI traceability for concentration measurement results of mercury vapor at ambient air levels. Meas. Sci. Technol. 2014, 25, 115801. [Google Scholar] [CrossRef]
- Andersson, M.E.; Gårdfeldt, K.; Wängberg, I.; Strömberg, D. Determination of Henry’s law constant for elemental mercury. Chemosphere 2008, 73, 587–592. [Google Scholar] [CrossRef]
- Brown, R.J.C.; Brown, A.S. Accurate calibration of mercury vapour measurements. Analyst 2008, 133, 1611. [Google Scholar] [CrossRef]
- Bloom, N.S.; Crecelius, E.A. Determination of mercury in seawater at sub-nanogram per liter levels. Mar. Chem. 1983, 14, 49–59. [Google Scholar] [CrossRef]
- Fitzgerald, W.F.; Gill, G.A. Subnanogram determination of mercury by two-stage gold amalgamation and gas phase detection applied to atmospheric analysis. Anal. Chem. 1979, 51, 1714–1720. [Google Scholar] [CrossRef]
- Gill, G.A.; Fitzgerald, W.F. Picomolar mercury measurements in seawater and other materials using stannous chloride reduction and two-stage gold amalgamation with gas phase detection. Mar. Chem. 1987, 20, 227–243. [Google Scholar] [CrossRef]
- JCGM 200:2012; JCGM. International Vocabulary of Metrology—Basic and General Concepts and Associated Terms (VIM). 3rd ed. Joint Committee for Guides in Metrology: Sèvres, France, 2012.
- Horvat, M.; Jereb, V.; Fajon, V.; Logar, M.; Kotnik, J.; Faganeli, J.; Hines, M.E.; Bonzongo, J.C. Mercury distribution in water, sediment and soil in the Idrijca and SoĉAhca river systems. Geochem. Explor. Environ. Anal. 2002, 2, 287–296. [Google Scholar] [CrossRef]
- Kim, K.H.; Szulejko, J.E.; Jo, H.J.; Kim, Y.H. Investigation of breakthrough behavior of volatile organic compounds on Tenax TA using a gas chromatography–mass spectrometry system. Anal. Bioanal. Chem. 2014, 406, 4031–4039. [Google Scholar]

| Stations | DGM (ng L−1) Calculated Using NIST 3133 | U (ng L−1, k = 2, Norm) | DGM (ng L−1) Calculated Using Gas Standard | U (ng L−1, k = 2, Norm) |
|---|---|---|---|---|
| S1 | 0.76 | 0.07 | 0.77 | 0.05 |
| S2 | 33.7 | 2.27 | 34 | 1.8 |
| S3 | 3.58 | 0.24 | 3.62 | 0.19 |
| S4 | 1.46 | 0.1 | 1.48 | 0.08 |
| S5 | 0.65 | 0.05 | 0.65 | 0.04 |
| S6 | 0.48 | 0.04 | 0.49 | 0.04 |
| Stations | RM (ng L−1) Calculated Using NIST 3133 | U (ng L−1, k = 2, Norm) | RM (ng L−1) Calculated Using Gas Standard | U (ng L−1, k = 2, Norm) |
|---|---|---|---|---|
| S1 | 1.16 | 0.07 | 1.18 | 0.06 |
| S2 | 49.5 | 2.75 | 50 | 2.54 |
| S3 | 6.47 | 0.35 | 6.54 | 0.33 |
| S4 | 3.59 | 0.19 | 3.63 | 0.18 |
| S5 | 1.02 | 0.06 | 1.03 | 0.06 |
| S6 | 0.77 | 0.05 | 0.78 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andron, T.D.; Corns, W.T.; Dexter, M.A.; Živković, I.; Kotnik, J.; Horvat, M. A Traceable Calibration for Gaseous Elemental Mercury Measurements in Air and Water. Atmosphere 2025, 16, 421. https://doi.org/10.3390/atmos16040421
Andron TD, Corns WT, Dexter MA, Živković I, Kotnik J, Horvat M. A Traceable Calibration for Gaseous Elemental Mercury Measurements in Air and Water. Atmosphere. 2025; 16(4):421. https://doi.org/10.3390/atmos16040421
Chicago/Turabian StyleAndron, Teodor D., Warren T. Corns, Matthew A. Dexter, Igor Živković, Jože Kotnik, and Milena Horvat. 2025. "A Traceable Calibration for Gaseous Elemental Mercury Measurements in Air and Water" Atmosphere 16, no. 4: 421. https://doi.org/10.3390/atmos16040421
APA StyleAndron, T. D., Corns, W. T., Dexter, M. A., Živković, I., Kotnik, J., & Horvat, M. (2025). A Traceable Calibration for Gaseous Elemental Mercury Measurements in Air and Water. Atmosphere, 16(4), 421. https://doi.org/10.3390/atmos16040421






