Mitigating Climate Warming: Mechanisms and Actions
Abstract
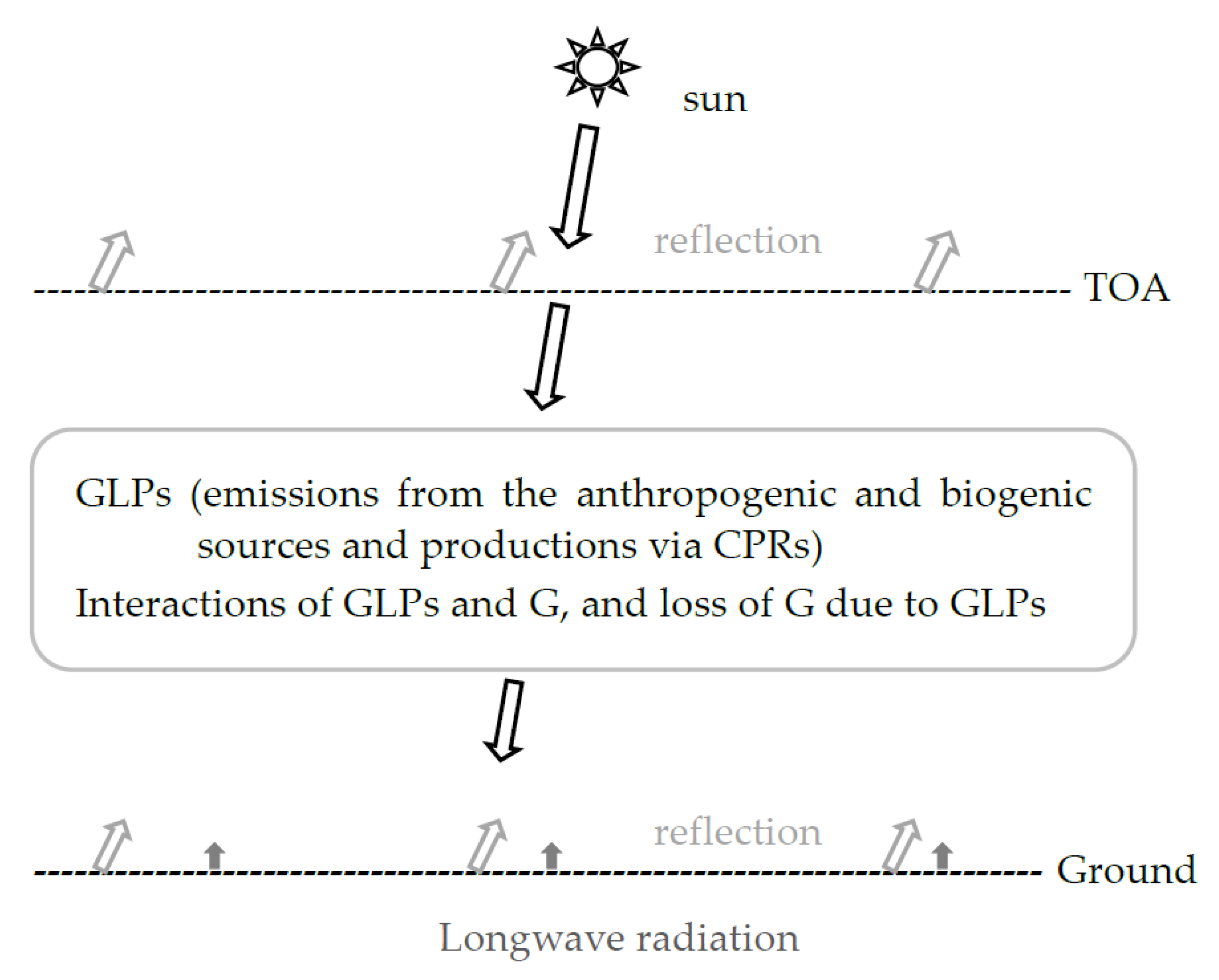
1. Introduction
2. Data and Methodology
2.1. Data
2.2. Methodology
3. Results
3.1. A Strong Positive Relationship Between T and GLPs Under Relatively Clear Sky Conditions (S/G < 0.5 and S/G < 0.6)
3.2. A Relationship Between T and Atmospheric Substances at 29 Sites Under All Skies (S/G = 0–1.0)
3.3. A Relationship Between T and Atmospheric Substances Under All Skies (S/G = 0–1.0) Using Polynomial Fitting
3.4. Application of the Relationship Between T and S/G (S/G < 0.6, h > 20°)
3.5. Mechanism Analysis of the Relationship Between T and S/G
4. Discussions
Phenomena of Air Temperature Change at More Sites Under All Sky Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; McKay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. 1995, 100, e8873–e8892. [Google Scholar] [CrossRef]
- Sekiyama, T.T.; Kiyotaka, S.; Deushi, M.; Kodera, K.; Lean, J. Stratospheric ozone variation induced by the 11-year solar cycle: Recent 22-year simulation using 3-D chemical transport model with reanalysis data. Geophys. Res. Lett. 2006, 33, L17812. [Google Scholar] [CrossRef]
- Bai, J.; de Leeuw, G.; De Smedt, I.; Theys, N.; Van Roozendael, M.; Sogacheva, L.; Chai, W.H. Variations and photochemical transformations of atmospheric constituents in North China. Atmos. Environ. 2018, 189, 213–226. [Google Scholar] [CrossRef]
- Madronich, S.; Flocke, S. The Role of Solar Radiation in Atmospheric Chemistry. In Environmental Photochemistry, Vol. 2/2L of the Handbook of Environmental Chemistry; Boule, P., Ed.; Springer: Berlin, Heidelberg, 1999; pp. 1–26. [Google Scholar]
- Vaughan, D.G.; Marshall, G.J.; Connolley, W.M.; Parkinson, C.; Mulvaney, R.; Hodgson, D.A.; King, J.C.; Pudsey, C.J.; Turner, J. Recent rapid regional climate warming on the Antarctic Peninsula. Clim. Chang. 2003, 60, 243–274. [Google Scholar] [CrossRef]
- Solanki, S.K. Solar variability and climate change: Is there a link? Astron. Geophys. 2002, 43, 5–9. [Google Scholar] [CrossRef]
- Elminir, H.K. Relative influence of weather conditions and air pollutants on solar radiation—Part 2: Modification of solar radiation over urban and rural sites. Meteorol. Atmos. Phys. 2007, 96, 257–264. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Fasullo, J.T.; Kiehl, J. Earth’s global energy budget. Bull. Am. Meteorol. Soc. 2009, 90, 311–323. [Google Scholar] [CrossRef]
- Lean, J.; Rind, D. Climate forcing by changing solar radiation. J. Clim. 2010, 11, 3069–3094. [Google Scholar] [CrossRef]
- Andreae, M.O.; Ramanathan, V. Climate’s Dark Forcings. Sciences 2013, 340, 280–281. [Google Scholar] [CrossRef]
- Rosenfeld, D.; Sherwood, S.; Wood, R.; Donner, L. Climate Effects of Aerosol-Cloud Interactions. Sciences 2014, 343, 379–380. [Google Scholar] [CrossRef]
- Calabrò, E.; Magazù, S. Correlation between Increases of the Annual Global Solar Radiation and the Ground Albedo Solar Radiation due to Desertification—A Possible Factor Contributing to Climatic Change. Climate 2016, 4, 64. [Google Scholar] [CrossRef]
- Salmon, A.; Marzo, A.; Polo, J.; Ballestrín, J.; Carra, E.; Alonso-Montesinos, J. World map of low-layer atmospheric extinction values for solar power tower plants projects. Renew. Energy 2022, 201, 876–888. [Google Scholar] [CrossRef]
- Bilbao, J.S.; Vill’an, D.M.; de M Castrillo, A. Analysis and cloudiness influence on UV total irradiation. J. Climatol. 2011, 31, 451–460. [Google Scholar]
- Bai, J.H. Analysis of ultraviolet radiation in clear skies in Beijing and its affecting factors. Atmos. Environ. 2011, 45, 6930–6937. [Google Scholar] [CrossRef]
- Bai, J.H. Photosynthetically active radiation loss in the atmosphere in North China. Atmos. Pollut. Res. 2013, 4, 411–419. [Google Scholar] [CrossRef]
- Bai, J.H. UV extinction in the atmosphere and its spatial variation in North China. Atmos. Environ. 2017, 154, 318–330. [Google Scholar] [CrossRef]
- Du, J.; Huang, L.; Min, Q.; Zhu, L. The Influence of Water Vapor Absorption in the 290–350 nm Region on Solar Radiance: Laboratory Studies and Model Simulation. Geophys. Res. Lett. 2013, 40, 4788–4792. [Google Scholar] [CrossRef]
- Li, S.P.; Matthews, J.; Sinha, A. Atmospheric hydroxyl radical production from electronically excited NO2 and H2O. Science 2008, 319, 1657–1660. [Google Scholar] [CrossRef]
- Matthews, J.; Sinha, A.; Francisco, J.S. The importance of weak absorption features in promoting tropospheric radical production. Proc. Natl. Acad. Sci. USA 2005, 102, 7449–7452. [Google Scholar] [CrossRef]
- Wilson, E.M.; Wenger, J.C.; Venables, D.S. Upper limits for absorption by water vapor in the near-UV. J. Quantitat. Spectrosc. Radiat. Transf. 2016, 170, 194–199. [Google Scholar] [CrossRef]
- Kondratyev, K. Solar Energy; Science Press: Beijing, China, 1962; pp. 123–132. [Google Scholar]
- Bai, J.H.; Zong, X.M.; Ma, Y.M.; Wang, B.B.; Zhao, C.F.; Yang, Y.K.; Guang, J.; Cong, Z.Y.; Li, K.L.; Song, T. Long-term variations in global solar radiation and its interaction with atmospheric substances at Qomolangma. Int. J. Environ. Res. Public Health 2022, 19, 8906. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zong, X. Global Solar Radiation Transfer and its Loss in the Atmosphere. Appl. Sci. 2021, 11, 2651. [Google Scholar] [CrossRef]
- Bai, J.H.; Wan, X.W.; Arslan, E.; Zong, X.M. Global solar radiation and its interactions with atmospheric substances, and their effects on air temperature change in Ankara Province. Climate 2024, 12, 35. [Google Scholar] [CrossRef]
- Driemel, A.; Augustine, J.; Behrens, K.; Colle, S.; Cox, C.; Cuevas-Agulló, E.; Denn, F.M.; Duprat, T.; Fukuda, M.; Grobe, H.; et al. Baseline Surface Radiation Network (BSRN): Structure and data description (1992–2017). Earth Syst. Sci. Data 2018, 10, 1491–1501. [Google Scholar] [CrossRef]
- Grigioni, P.; Camporeale, G.; Ciardini, V.; De Silvestri, L.; Iaccarino, A.; Proposito, M.; Scarchilli, C. Dati Meteorologici Della Stazione Meteorologica CONCORDIA Presso la Base CONCORDIA STATION (Dome C). ENEA; Osservatorio Meteo-Climatologico Antartico: Bologna, Italy, 2022. [Google Scholar] [CrossRef]
- Grigioni, P.; Antonelli, A.; Camporeale, G.; Ciardini, V.; De Silvestri, L.; Dolci, S.; Iaccarino, A.; Proposito, M.; Scarchilli, C. YOPP-SH Meteorological Observations from Automatic Weather Station Concordia_AWS, Antarctica. Laboratory for Observations and Analyses of Earth and Climate, PANGAEA; Data Publisher for Earth & Environmental Science: Bremerhaven, Germany, 2019. [Google Scholar] [CrossRef]
- Six, D.; Fily, M.; Alvain, S.; Henry, P.; Benoist, J.P. Surface characterisation of the Dome Concordia area (Antarctica) as a potential satellite calibration site, using Spot 4 Vegetation instrument. Remote Sens. Environ. 2004, 89, 83–94. [Google Scholar] [CrossRef]
- Candidi, M.; Lori, A. Status of the Antarctic Base at Dome C. Mem. S. A. It. 2003, 74, 29–36. [Google Scholar]
- Scambos, T.A.; Campbell, G.G.; Pope, A.; Haran, T.; Muto, A.; Lazzara, M.; Reijmer, C.H.; van den Broeke, M.R. Ultralow surface temperatures in East Antarctica from satellite thermal infrared mapping: The coldest places on Earth. Geophys. Res. Lett. 2018, 45, 6124–6133. [Google Scholar] [CrossRef]
- Argentini, S.; Pietroni, I.; Mastrantonio, G.; Viola, A.P.; Dargaud, G.; Petenko, I. Observations of near surface wind speed, temperature and radiative budget at Dome C, Antarctic Plateau during 2005. Antarct. Sci. 2014, 26, 104–112. [Google Scholar] [CrossRef]
- Lanconelli, C.; Busetto, M.; Dutton, E.G.; König-Langlo, G.; Maturilli, M.; Sieger, R.; Vitale, V.; Yamanouchi, T. Polar baseline surface radiation measurements during the International Polar Year 2007–2009. Earth Syst. Sci. Data 2011, 3, 1–8. [Google Scholar] [CrossRef]
- Merali, Z. Quantum gas goes below absolute zero. Nature 2013. [Google Scholar] [CrossRef]
- Carlton, A.G.; Wiedinmyer, C.; Kroll, J.H. A review of secondary organic aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 2009, 9, 4987–5005. [Google Scholar] [CrossRef]
- Horowitz, A.; Meller, R.; Moortgat, G.K. The UV-VIS absorption cross sections of the a- dicarbonyl compounds: Pyruvic acid, biacetyl and glyoxal. J. Photochem. Photobiol. A Chem. 2001, 146, 19–27. [Google Scholar] [CrossRef]
- Hansel, A.K.; Ehrenhauser, F.S.; Richards-Henderson, N.K.; Anastasio, C.; Valsaraj, K.T. Aqueous-phase oxidation of green leaf volatiles by hydroxyl radical as a source of SOA: Product identification from methyl jasmonate and methyl salicylate oxidation. Atmos. Environ. 2015, 102, 43–51. [Google Scholar] [CrossRef]
- Jacobson, M. Isolating nitrated and aromatic aerosols and nitrated aromatic gases as sources of ultraviolet light absorption. J. Geophys. Res. 1999, 104, 3527–3542. [Google Scholar] [CrossRef]
- Jonsson, Å.M.; Hallquist, M.; Ljungström, E. Influence of OH Scavenger on the Water Effect on Secondary Organic Aerosol Formation from Ozonolysis of Limonene, Δ3-Carene, and α-Pinene. Environ. Sci. Technol. 2008, 42, 5938–5944. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.H.; Zong, X.M.; Lanconelli, C.; Lupi, A.; Driemel, A.; Vitale, V.; Li, K.L.; Song, T. Long-Term Variations of Global Solar Radiation and Its Potential Effects at Dome C (Antarctica). Int. J. Environ. Res. Public Health 2022, 19, 3084. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Heikkilä, A.; Zong, X. Long-Term Variations of Global Solar Radiation and Atmospheric Constituents at Sodankylä in the Arctic. Atmosphere 2021, 12, 749. [Google Scholar] [CrossRef]
- Zhang, X.; Sander, S.P.; Cheng, L.; Thimmakondu, V.S.; Stanton, J.F. Matrix-isolated infrared absorption spectrum of CH2BrOO radical. Chem. Phys. Lett. 2016, 657, 131–134. [Google Scholar] [CrossRef]
- Shindell, D.T.; Chin, M.; Dentener, F.; Fiore, A.M.; Hess, P.; MacKenzie, I.A.; Sanderson, M.G.; Schultz, M.G.; Schulz, M.; Stevenson, D.S.; et al. A multi-model assessment of pollution transport to the Arctic. Atmos. Chem. Phys. 2008, 8, 5353–5372. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Climate response of fossil fuel and biofuel soot, accounting for soot’s feedback to snow and sea ice albedo and emissivity. J. Geophys. Res. 2004, 109, D2120. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Effects of externally-through-internally-mixed soot inclusions within clouds and precipitation on global climate. J. Phys. Chem. 2006, 110, 6860. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, V.; Feng, Y. Air pollution, greenhouse gases and climate change: Global and regional perspectives. Atmos. Environ. 2009, 43, 37–50. [Google Scholar] [CrossRef]
- Von Schneidemesser, E.; Monks, P.S.; Allan, J.D.; Bruhwiler, L.; Forster, P.; Fowler, D.; Lauer, A.; Morgan, W.T.; Paasonen, P.; Righi, M.; et al. Chemistry and the linkages between air quality and climate change. Chem. Rev. 2015, 115, 3856–3897. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Wang, H.; Lu, J.; Fu, Q.; Jonko, A.K.; Lee, Y.J.; Ma, W.; Maslowski, W.; Qin, Y. Assessing radiative feedbacks and their contribution to the Arctic amplification measured by various metrics. J. Geophys. Res. Atmospheres 2024, 129, e2024JD040880. [Google Scholar] [CrossRef]
- Valero, F.P.J.; Pope, S.K.; Bush, B.C.; Nguyen, Q.; Marsden, D.; Cess, R.D.; Simpson-Leitner, A.S.; Bucholtz, A.; Udelhofen, P.M. Absorption of solar radiation by the clear and cloudy atmosphere during the Atmospheric Radiation Measurement Enhanced Shortwave Experiments (ARESE) I and II: Observations and models. J. Geophys. Res. Atmos. 2003, 108, 4016. [Google Scholar] [CrossRef]
- Fried, A.; McKeen, S.; Sewell, S.; Harder, J.; Henry, B.; Goldan, P.; Kuster, W.; Williams, E.; Baumann, K.; Shetter, R.; et al. Photochemistry of formaldehyde during the 1993 tropospheric OH photochemistry experiment. J. Geophys Res. 1997, 102, 6283–6289. [Google Scholar] [CrossRef]
- Lanza, B.; Jiménez, E.; Ballesteros, B.; Albaladejo, J. Absorption cross section determination of biogenic C5-aldehydes in the actinic region. Chem. Phys. Lett. 2008, 454, 184–189. [Google Scholar] [CrossRef]
- Ghanshyam, V.; Ravishankara, R. Absorption cross sections of CH3OOH, H2O2, and D2O2 vapors between 210 and 365 nm at 297 K. J. Geophys Res. 1989, 94, 3487–3492. [Google Scholar]
- Bruhl, C.; Crutzen, P.J. On the disproportionate role of tropospheric ozone as a filter against solar UV-B radiation. Geophys. Res. Lett. 1989, 16, 703–706. [Google Scholar] [CrossRef]
- Zerefos, C.; Isaksen, I.; Ziomas, I. Chemistry and Radiation Changes in the Ozone Layer; ASI Series; Springer: Berlin/Heidelberg, Germany, 2012; Volume 557. [Google Scholar]
- Bond, T.C. Spectral dependence of visible light absorption by carbonaceous particles emitted from coal combustion. Geophys. Res. Lett. 2001, 28, 4075–4078. [Google Scholar] [CrossRef]
- Bai, J.H. UV attenuation in the cloudy atmosphere. J. Atmos. Chem. 2009, 62, 211–228. [Google Scholar] [CrossRef]
- Buckley, P.T.; Birks, J.W. Evaluation of visible–light photolysis of ozone–water cluster molecules as a source of atmospheric hydroxyl radical and hydrogen–peroxide. Atmos. Environ. 1995, 29, 2409–2415. [Google Scholar] [CrossRef]
- Lonardo, G.; Masciarelli, G. Infrared absorption cross-sections and integrated absorption intensities of HFC-125 and HFC-143a. J. Quant. Spectrosc. Radiat. Transf. 2000, 66, 129–142. [Google Scholar] [CrossRef]
- Gratien, A.; Nilsson, E.; Doussin, J.-F.; Johnson, M.S.; Nielsen, C.J.; Stenstrøm, Y.; Picquet-Varrault, B. UV and IR Absorption Cross-sections of HCHO, HCDO, and DCDO. J. Phys. Chem. A. 2007, 111, 11506–11513. [Google Scholar] [CrossRef]
- Brauer, C.S.; Blake, T.A.; Guenther, A.B.; Sharpe, S.W.; Sams, R.L.; Johnson, T.J. Quantitative infrared absorption cross sections of isoprene for atmospheric measurements. Atmos. Meas. Tech. 2014, 7, 3839–3847. [Google Scholar] [CrossRef]
- Harrison, J.J.; Allen, N.D.C.; Bernath, P. Infrared absorption cross sections for acetone (propanone) in the 3 μm region. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 53–58. [Google Scholar] [CrossRef]
- Vaida, V.; Daniel, J.S.; Kjaergaard, H.G.; Goss, L.M.; Tuck, A.F. Atmospheric absorption of near infrared and visible solar radiation by the hydrogen bonded water dimer. Q. J. R. Meteorol. Soc. 2001, 127, 1627–1643. [Google Scholar] [CrossRef]
- Gorchakova, G.I.; Gushchina, R.A.; Kopeikina, V.M.; Karpova, A.V.; Semoutnikovab, E.G.; Datsenkoa, O.I.; Ya, T. Ponomarevac. Anomalous Absorption of Smoke Aerosol in the Visible and Near-Infrared Regions of the Spectrum. Dokl. Earth Sci. 2023, 510, 317–322. [Google Scholar] [CrossRef]
- Kirchstetter, T.W.; Thatcher, T.L. Contribution of organic carbon to wood smoke particulate matter absorption of solar radiation. Atmos. Chem. Phys. 2012, 12, 6067–6072. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Zheng, G.J.; Wei, C.; Mu, Q.; Zheng, B.; Wang, Z.B.; Gao, M.; Zhang, Q.; He, K.B.; Carmichae, G.; et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2016, 2, e1601530. [Google Scholar] [CrossRef] [PubMed]
- Kambezidis, H.D. The Solar Radiation Climate of Greece. Climate 2021, 9, 183. [Google Scholar] [CrossRef]
- DiCarlo, P.; Brune, W.H.; Martinez, M.; Harder, H.; Lesher, R.; Ren, X.R.; Thornberry, T.; Carroll, M.A.; Young, V.; Shepson, P.B.; et al. Missing OH reactivity in a forest: Evidence for unknown biogenic VOCs. Science 2004, 304, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Wild, M.; Gilgen, H.; Roesch, A.; Ohmura, A.; Long, C.; Dutton, E.; Forgan, B.; Kallis, A.; Russak, V.; Tsvetkov, A. From dimming to brightening: Decadal changes in solar radiation at the Earth’s surface. Science 2005, 308, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.H.; Yang, F.T.; Wang, H.M.; Yao, L.; Xu, M.J. Multiple-Win Effects and Beneficial Implications from Analyzing Long-Term Variations of Carbon Exchange in a Subtropical Coniferous Plantation in China. Atmosphere 2024, 15, 1218. [Google Scholar] [CrossRef]
- Zhou, H.; Yue, X.; Dai, H.; Geng, G.; Yuan, W.; Chen, J.; Shen, G.; Zhang, T.; Zhu, J.; Liao, H. Recovery of ecosystem productivity in China due to the Clean Air Action plan. Nat. Geosci. 2024, 17, 1233–1239. [Google Scholar] [CrossRef]
- Klinger, L.F.; Li, Q.J.; Guenther, A.B.; Greenberg, J.P.; Baker, B.; Bai, J.H. Assessment of volatile organic compound emissions from ecosystems of China. J. Geophy. Res. 2002, 107, 4603. [Google Scholar] [CrossRef]
- Bai, J.H.; Guenther, A.; Turnipseed, A.; Duhl, T.; Greenberg, J. Seasonal and interannual variations in whole-ecosystem BVOC emissions from a subtropical plantation in China. Atmos. Environ. 2017, 161, 176–190. [Google Scholar] [CrossRef]
- Bai, J.H.; Wu, Z.X.; Yang, C.; Guenther, A. Seasonal variations in whole–ecosystem BVOC emissions and ozone fluxes from a tropical rubber tree plantation in China. Atmos. Environ. 2025, 351, 121182. [Google Scholar] [CrossRef]
- Yin, L.; Bai, B.; Zhang, B.; Zhu, Q.; Di, Q.; Requia, W.J.; Schwartz, J.D.; Shi, L.; Liu, P. Regional-specific trends of PM2.5 and O3 temperature sensitivity in the United States. npj Clim. Atmos. Sci. 2025, 8, 12. [Google Scholar] [CrossRef]
- Shikhovtsev, M.Y.; Makarov, M.M.; Aslamov, I.A.; Tyurnev, I.N.; Molozhnikova, Y.V. Application of Modern Low-Cost Sensors for Monitoring of Particle Matter in Temperate Latitudes: An Example from the Southern Baikal Region. Sustainability 2025, 17, 3585. [Google Scholar] [CrossRef]
- Pfannerstill, E.Y.; Arata, C.; Zhu, Q.; Schulze, B.C.; Ward, R.; Woods, R.; Harkins, C.; Schwantes, R.H.; Seinfeld, J.H.; Bucholtz, A.; et al. Temperature-dependent emissions dominate aerosol and ozone formation in Los Angeles. Science 2024, 384, 1324–1329. [Google Scholar] [CrossRef]
- Zavalishin, N.N. Reasons for Modern Warming: Hypotheses and Facts. J. Atmos. Sci. Res. 2022, 5, 11–17. [Google Scholar] [CrossRef]
- Ershkov, S.; Leshchenko, D.; Prosviryakov, E. Revisiting long-time dynamics of Earth’s angular rotation depending on quasiperiodic solar activity. Mathematics 2023, 11, 2117. [Google Scholar] [CrossRef]





| Station Full Name | Abbreviation | Location | Latitude | Longitude | Elevation | First Dataset in Archive | Surface Type | Topography Type | Rural/Urban | Köppen–Geiger Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| Alert | ALE | Canada, Lincoln Sea | 82.5 | −62.4 | 127 | 16 August 2004 | tundra | hilly | rural | ET (Polar, tundra) |
| Barrow | BAR | AK, USA | 71.3 | −156.6 | 8 | 1 January 1995 | tundra | flat | rural | ET (Polar, tundra) |
| Billings | BIL | OK, USA | 36.6 | −97.5 | 317 | 1 June 1993 | grass | flat | rural | Cfa (Temperate, no dry season, hot summer) |
| Boulder | BOU | CO, USA | 40.1 | −105.0 | 1577 | 1 January 1992 | grass | flat | rural | BSk (Arid, steppe, cold) |
| Cabauw | CAB | Netherlands | 52.0 | 4.9 | 0 | 1 December 2005 | grass | flat | rural | Cfb (Temperate, no dry season, warm summer) |
| Carpentras | CAR | France | 44.1 | 5.1 | 100 | 1 August 1996 | cultivated | hilly | rural | Csa (Temperate, dry summer, hot summer) |
| Chesapeake Light | CLH | North Atlantic Ocean, USA | 36.9 | −75.7 | 37 | 1 June 2000 | water, ocean | flat | rural | NaN (NaN) |
| Cener | CNR | Spain, Navarra | 42.8 | −1.6 | 471 | 1 July 2009 | asphalt | mountain valley | urban | Cfb (Temperate, no dry season, warm summer) |
| Darwin | DAR | Australia | −12.4 | 130.9 | 30 | 1 June 2002 | grass | flat | rural | Aw (Tropical, savannah) |
| Concordia Station, Dome C | DOM | Antarctica | −75.1 | 123.3 | 3233 | 1 January 2006 | glacier, accumulation area | flat | rural | EF (Polar, frost) |
| Fukuoka | FUA | Japan | 33.6 | 130.4 | 3 | 1 April 2010 | asphalt | flat | urban | Cfa (Temperate, no dry season, hot summer) |
| Gobabeb | GOB | Namibia, Namib Desert | −23.6 | 15.0 | 407 | 15 May 2012 | desert gravel | flat | rural | BWh (Arid, desert, hot) |
| Georg von Neumayer | GVN | Antarctic, Dronning Maud Land | −70.7 | −8.3 | 42 | 1 January 1992 | iceshelf | flat | rural | NaN (NaN) |
| Ishigakijima | ISH | Japan | 24.3 | 124.2 | 5.7 | 1 April 2010 | asphalt | flat | rural | Af (Tropical, rainforest) |
| Izaña | IZA | Spain, Tenerife | 28.3 | −16.5 | 2372.9 | 1 March 2009 | rock | mountain top | rural | Csb (Temperate, dry summer, warm summer) |
| Kwajalein | KWA | Marshall Islands | 8.7 | 167.7 | 10 | 1 March 1992 | water, ocean | flat | rural | Af (Tropical, rainforest) |
| Lindenberg | LIN | Germany | 52.2 | 14.1 | 125 | 1 September 1994 | cultivated | hilly | rural | Dfb (Cold, no dry season, warm summer) |
| Langley Research Center | LRC | VA, USA | 37.1 | −76.4 | 3 | 1 December 2014 | grass | flat | urban | Cfa (Temperate, no dry season, hot summer) |
| Momote | MAN | Papua New Guinea | −2.1 | 147.4 | 6 | 1 September 1996 | grass | flat | rural | Af (Tropical, rainforest) |
| Minamitorishima | MNM | Japan, Minami-Torishima | 24.3 | 154.0 | 7.1 | 1 April 2010 | water (ocean) | flat | rural | NaN (NaN) |
| Nauru Island | NAU | Nauru | −0.5 | 166.9 | 7 | 1 November 1998 | rock | flat | rural | Af (Tropical, rainforest) |
| Ny-Ålesund | NYA | Norway, Spitsbergen | 78.9 | 11.9 | 11 | 1 August 1992 | tundra | mountain valley | rural | ET (Polar, tundra) |
| Palaiseau, SIRTA Observatory | PAL | France | 48.7 | 2.2 | 156 | 1 May 2003 | concrete | flat | urban | Cfb (Temperate, no dry season, warm summer) |
| Payerne | PAY | Switzerland | 46.8 | 6.9 | 491 | 1 September 1992 | cultivated | hilly | rural | Dfb (Cold, no dry season, warm summer) |
| Petrolina | PTR | Brazil | −9.1 | −40.3 | 387 | 1 December 2006 | concrete, since 2015: shrub | flat | rural | BSh (Arid, steppe, hot) |
| Sonnblick | SON | Austria | 47.1 | 13.0 | 3108.9 | 1 January 2013 | rock | mountain top | rural | ET (Polar, tundra) |
| South Pole | SPO | Antarctica | −90.0 | −24.8 | 2800 | 1 January 1992 | glacier, accumulation area | flat | rural | EF (Polar, frost) |
| Syowa | SYO | Antarctica | −69.0 | 39.6 | 29 | 1 January 1994 | sea ice | hilly | rural | NaN (NaN) |
| Tateno | TAT | Japan | 36.1 | 140.1 | 25 | 1 February 1996 | grass | flat | urban | Cfa (Temperate, no dry season, hot summer) |
| Station (Abbr.) | h > 5° | h > 10° | h > 15° | h > 20° | all |
|---|---|---|---|---|---|
| ALE | 8198 | 7112 | 5437 | 3231 | 8469 |
| BAR | 11,631 | 10,265 | 8451 | 6624 | 11,817 |
| BIL | 53,574 | 51,040 | 47,511 | 43,653 | 54,282 |
| BOU | 38,292 | 36,125 | 33,856 | 30,390 | 39,446 |
| CAB | 20,514 | 19,530 | 17,592 | 15,218 | 20,686 |
| CAR | 52,251 | 49,383 | 45,082 | 40,193 | 53,754 |
| CLH | 27,930 | 26,716 | 24,933 | 22,843 | 28,621 |
| CNR | 22,063 | 20,828 | 19,428 | 17,560 | 22,552 |
| DAR | 30,137 | 29,023 | 27,680 | 26,251 | 30,165 |
| DOM | 33,298 | 26,620 | 20,578 | 15,362 | 36,139 |
| FUA | 16,516 | 16,178 | 15,414 | 14,531 | 16,550 |
| GVN | 30,675 | 25,600 | 20,929 | 16,444 | 33,828 |
| KWA | 20,397 | 20,227 | 19,696 | 19,270 | 20,442 |
| LIN | 33,388 | 31,812 | 28,272 | 25,179 | 33,681 |
| LRC | 16,534 | 15,779 | 14,706 | 13,488 | 16,847 |
| MAN | 25,130 | 24,596 | 24,316 | 23,013 | 25,233 |
| MNM | 29,361 | 28,732 | 27,400 | 25,551 | 29,512 |
| NAU | 30,160 | 29,338 | 28,957 | 27,888 | 30,298 |
| NYA | 24,520 | 21,164 | 15,340 | 10,556 | 25,024 |
| PAL | 22,463 | 21,444 | 19,386 | 17,039 | 22,769 |
| PAY | 43,132 | 41,059 | 38,379 | 34,077 | 43,540 |
| PTR | 10,803 | 10,456 | 9957 | 9242 | 11,065 |
| SON | 12,327 | 11,263 | 9913 | 8096 | 13,154 |
| SPO | 35,387 | 31,561 | 250,39 | 159,99 | 36,242 |
| SYO | 30,856 | 26,149 | 21,655 | 17,472 | 33,900 |
| TAT | 39,228 | 37,133 | 35,224 | 31,731 | 39,641 |
| Station (Abbr.) | h > 5° | h > 10° | h > 15° | h > 20° | all |
|---|---|---|---|---|---|
| ALE | 9060 | 7759 | 5885 | 3491 | 9559 |
| BAR | 13,653 | 11,653 | 9495 | 7454 | 14,105 |
| BIL | 58,809 | 55,512 | 51,214 | 46,949 | 60,524 |
| BOU | 42,123 | 39,341 | 36,766 | 32,930 | 44,037 |
| CAB | 20,514 | 19,530 | 17,592 | 15,218 | 20,686 |
| CAR | 56,453 | 52,916 | 48,047 | 42,691 | 58,993 |
| CLH | 30,891 | 29,247 | 27,168 | 24,773 | 32,087 |
| CNR | 24,878 | 23,288 | 21,627 | 19,455 | 25,740 |
| DAR | 33,909 | 32,099 | 30,471 | 28,865 | 34,154 |
| DOM | 34,724 | 27,535 | 21,209 | 15,776 | 38,270 |
| FUA | 19,833 | 19,166 | 18,070 | 16,955 | 19,987 |
| GVN | 33,387 | 27,651 | 22,518 | 17,671 | 37,750 |
| KWA | 23,721 | 23,319 | 22,405 | 21,763 | 23,863 |
| LIN | 40,013 | 37,555 | 33,112 | 29,383 | 40,871 |
| LRC | 18,184 | 17,182 | 15,970 | 14,584 | 18,783 |
| MAN | 29,766 | 28,776 | 28,325 | 26,696 | 30,100 |
| MNM | 32,805 | 31,682 | 29,894 | 27,672 | 33,306 |
| NAU | 34,783 | 33,179 | 32,590 | 31,185 | 35,161 |
| NYA | 27,975 | 23,730 | 17,056 | 11,706 | 29,033 |
| PAL | 26,736 | 25,239 | 22,686 | 19,864 | 27,548 |
| PAY | 49,337 | 46,364 | 43,099 | 37,988 | 50,308 |
| PTR | 12,091 | 11,632 | 11,052 | 10,256 | 12,650 |
| SON | 13,487 | 12,313 | 10,824 | 8869 | 14,473 |
| SPO | 38,288 | 33,791 | 26,562 | 16,869 | 39,498 |
| SYO | 33,361 | 28,083 | 23,197 | 18,695 | 37,624 |
| TAT | 45,290 | 42,617 | 40,177 | 36,132 | 46,207 |
| Station (Abbr.) | h > 5° | h > 10° | h > 15° | h > 20° | All | NY |
|---|---|---|---|---|---|---|
| ALE | 26,837 | 22,477 | 16,775 | 9701 | 29,096 | 9 |
| BAR | 54,411 | 43,576 | 34,667 | 26,787 | 58,556 | 18 |
| BIL | 92,444 | 85,903 | 78,087 | 70,720 | 97,495 | 25 |
| BOU | 66,698 | 60,764 | 55,975 | 49,179 | 70,791 | 18 |
| CAB | 64,379 | 57,834 | 48,700 | 39,847 | 67,255 | 17 |
| CAR | 83,285 | 76,735 | 68,461 | 59,765 | 88,693 | 22 |
| CLH | 51,734 | 47,863 | 43,806 | 39,279 | 54,562 | 15 |
| CNR | 47,385 | 43,140 | 39,292 | 34,300 | 49,535 | 12 |
| DAR | 48,643 | 45,307 | 42,505 | 39,344 | 50,663 | 12 |
| DOM | 40,616 | 31,178 | 23,629 | 17,413 | 46,260 | 15 |
| FUA | 46,925 | 44,104 | 40,244 | 37,192 | 48,485 | 12 |
| GOB | 40,627 | 37,883 | 34,784 | 31,901 | 43,854 | 10 |
| GVN | 96,807 | 76,637 | 60,922 | 47,050 | 100,000 | 29 |
| ISH | 47,256 | 44,727 | 41,447 | 37,852 | 48,941 | 12 |
| IZA | 50,369 | 46,252 | 43,113 | 38,556 | 53,973 | 13 |
| KWA | 40,388 | 37,389 | 34,613 | 33,205 | 41,682 | 17 |
| LIN | 98,076 | 88,264 | 73,008 | 61,264 | 1E+05 | 26 |
| LRC | 30,987 | 28,717 | 26,351 | 23,687 | 32,461 | 8 |
| MAN | 60,523 | 56,086 | 53,985 | 50,152 | 63,419 | 16 |
| MNM | 48,699 | 45,653 | 42,078 | 38,393 | 51,204 | 12 |
| NAU | 52,476 | 46,847 | 45,667 | 43,166 | 54,295 | 14 |
| NYA | 100,000 | 87,202 | 60,691 | 41,313 | 1E+05 | 30 |
| PAL | 61,414 | 56,334 | 48,863 | 40,410 | 64,501 | 19 |
| PAY | 100,000 | 98,768 | 88,449 | 73,483 | 100,000 | 29 |
| PTR | 17,619 | 16,668 | 15,512 | 14,378 | 18,840 | 7 |
| SON | 37,042 | 33,650 | 29,437 | 24,638 | 39,488 | 10 |
| SPO | 56,269 | 47,349 | 36,405 | 22,789 | 61,194 | 19 |
| SYO | 80,104 | 64,264 | 51,129 | 39,791 | 92,184 | 27 |
| TAT | 54,411 | 43,576 | 34,667 | 26,787 | 58,556 | 26 |
| Station (Abbr.) | T | G | S/G | Mean S/G |
|---|---|---|---|---|
| ALE | −0.2332 | −0.4839 | −0.0081 | 0.73 |
| BAR | 0.0032 | 1.3839 | −0.0005 | 0.82 |
| BIL | 0.0179 | 0.1726 | −0.0009 | 0.52 |
| BOU | −0.0836 | 0.2169 | 0.0032 | 0.51 |
| CAB | 0.0492 | 1.4756 | −0.0036 | 0.70 |
| CAR | −0.0279 | 0.1826 | 6E−05 | 0.47 |
| CLH | 0.0564 | 1.821 | 0.0011 | 0.54 |
| CNR | 0.0493 | 0.5838 | 0.0007 | 0.58 |
| DAR | −0.0044 | −0.2085 | 0.0017 | 0.48 |
| DOM | 0.005 | 0.7969 | −0.0008 | 0.45 |
| FUA | 0.0889 | 2.6255 | −0.0058 | 0.67 |
| GOB | 0.0697 | −0.7316 | −3 × 10−14 | 0.29 |
| GVN | 0.0068 | 0.1383 | 0.0011 | 0.73 |
| ISH | 0.066 | 1.1379 | −0.003 | 0.67 |
| IZA | 0.0249 | −1.0228 | −0.0008 | 0.28 |
| KWA | −0.0092 | −3.1484 | −0.0031 | 0.53 |
| LIN | 0.0786 | 0.8227 | −0.0024 | 0.69 |
| LRC | 0.0777 | 0.8246 | −0.0014 | 0.54 |
| MAN | −0.0077 | −1.614 | 0.0023 | 0.62 |
| MNM | 0.1083 | 0.7437 | 0.1083 | 0.49 |
| NAU | −0.0413 | −0.7612 | −0.0413 | 0.51 |
| NYA | 0.0516 | 0.1928 | 0.0011 | 0.79 |
| PAL | 0.0473 | 6.8274 | −0.0242 | 0.73 |
| PAY | 0.0714 | 1.2654 | −0.0013 | 0.64 |
| PTR | 0.0193 | −1.0556 | 0.0027 | 0.49 |
| SON | 0.0361 | 0.2437 | −0.0062 | 0.69 |
| SPO | −0.0257 | −1.5954 | 0.0043 | 0.48 |
| SYO | 0.0197 | 0.6473 | 0.0007 | 0.67 |
| TAT | 0.0152 | 0.6856 | −0.0018 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Wan, X.; Lupi, A.; Zong, X.; Arslan, E. Mitigating Climate Warming: Mechanisms and Actions. Atmosphere 2025, 16, 1170. https://doi.org/10.3390/atmos16101170
Bai J, Wan X, Lupi A, Zong X, Arslan E. Mitigating Climate Warming: Mechanisms and Actions. Atmosphere. 2025; 16(10):1170. https://doi.org/10.3390/atmos16101170
Chicago/Turabian StyleBai, Jianhui, Xiaowei Wan, Angelo Lupi, Xuemei Zong, and Erhan Arslan. 2025. "Mitigating Climate Warming: Mechanisms and Actions" Atmosphere 16, no. 10: 1170. https://doi.org/10.3390/atmos16101170
APA StyleBai, J., Wan, X., Lupi, A., Zong, X., & Arslan, E. (2025). Mitigating Climate Warming: Mechanisms and Actions. Atmosphere, 16(10), 1170. https://doi.org/10.3390/atmos16101170






