Explainable Artificial Intelligence and Machine Learning for Air Pollution Risk Assessment and Respiratory Health Outcomes: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Questions
- What machine learning techniques are used for air pollutants risk assessment and respiratory health outcomes?
- To what extent is xAI integrated into machine learning models for air pollution risk assessment and respiratory health outcomes?
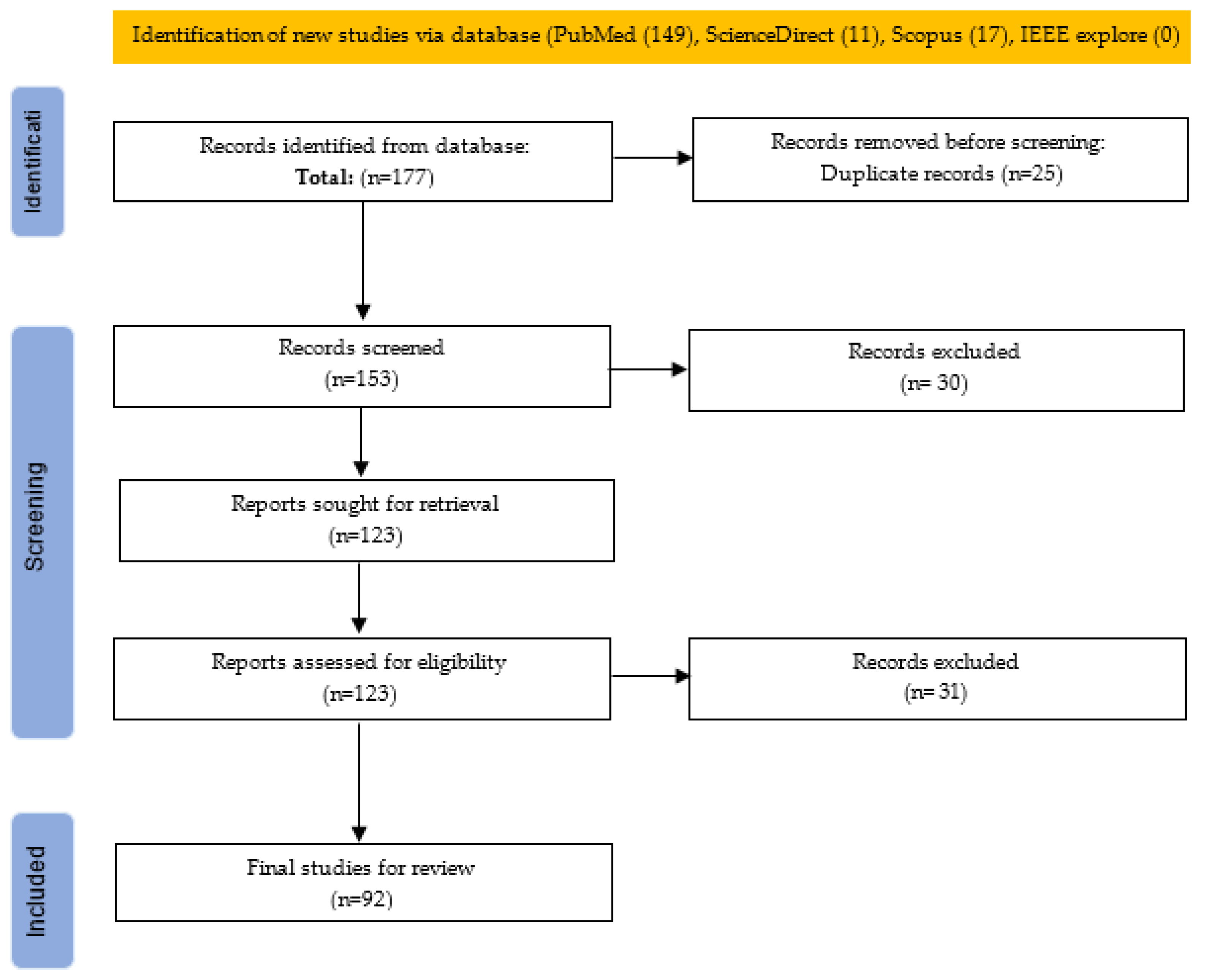
2.2. Literature Search Strategy
2.3. Risk of Bias Assessment and Reproducibility Strategy
2.4. Articles Extraction
3. Results and Discussion
3.1. Machine Learning for Air Pollution Risk Assessment and Respiratory Health Outcomes
3.2. Explainable AI with Machine Learning Models for Air Pollution Risk Assessment and Respiratory Health Outcomes
3.3. Challenges and Limitations
3.4. Uncertainty Quantification
3.5. Implications of the Research Finding
3.6. Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| AA | Acceleration of Aging |
| WHO | World Health Organization |
| EC | Elemental Carbon |
| AI | Artificial Intelligence |
| xAI | Explainable Artificial Intelligence |
| ML | Machine Learning |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| OPEs | organophosphates |
| APS | Average Particle Size |
| KPGT | Knowledge-guided Pre-training of Graph |
| LDSA | Lung Deposited Surface Area |
| PNC | Particle Number Concentration |
| PAEs | Phthalates |
| RMSE | Root-Mean-Square Error |
| MERS | Middle East respiratory syndrome |
| SARS | Severe Acute Respiratory Syndrome |
| UFPs | Ultrafine Particles |
| BC | Black Carbon |
| BD | Burden of Disease |
| DT | Decision Tree |
| KKN | K-Nearest Neighbors |
| NB | Naive Bayesian |
| HAP | Hazardous Air Pollutants |
| AQI | Air Quality Index |
| COPD | Chronic Obstructive Pulmonary Disease |
| LPO-XGBoost | Lung Performance Optimization-based XGBoost |
| PEP | Population Exposure to PM2.5 |
| CARI | Comprehensively Integrated Air-Risk Index |
| MP | Mycoplasma Pneumoniae |
| XGBoost | eXtreme Gradient Boosting |
| GCN | Graph Convolutional Neural Network |
| ERT | Extremely Randomized Tree |
| SEA | Socioeconomic Activity |
| SARIMA | Seasonal Auto-Regressive Integrated Moving Average |
| DNN | Deep Neural Networks |
| Deep-CNN | Deep Convolutional Neural Networks |
| BaP | Benzopyrene |
| DLNMs | Distributed Lag Nonlinear Models |
| EPA | Environmental Protection Agency |
| LECR | Lung Excess Cancer Risk |
| VOCs | Volatile Organic Compounds |
| ERA | E-Waste Recycling Areas |
| PLS | partial least squares regression |
| phyMTDNN | Physics-informed multi-task deep neural network |
| CT | Computed Tomography |
| HOCs | Hydrophobic Organic Compounds |
| AR | Androgen Receptors |
| CTM | Chemical Transport Modeling |
| MMF | Measurement Model Fusion |
| QSAR | Quantitative Structure–Activity Relationship |
| LIME | Local Interpretable Model-Agnostic Explanations |
| GAM | Generalized Additive Model |
| HAQI | Health-Risk Air Quality Index |
| HQ | Hazard Quotients |
| RF | Random Forest |
| LR | Logistic Regression |
| RT-PCR | Reverse transcription–polymerase chain reaction |
Appendix A
Appendix A.1. Search String
Appendix A.2. Summary of Related Literature on ML for Air Pollution Risk Assessment
| Authors | Air Pollutant | Focus | Machine Learning Models | Risks | Respiratory Health Outcome | Findings | Best Model |
|---|---|---|---|---|---|---|---|
| Famiyeh et al. [76] | PAHs pollution | Employed a component-based potency factor approach to estimate LECR (lung excess cancer risk) in Ningbo. Potency factors are BaP unit risk values from both the WHO and the Environmental Protection Agency (EPA). | ML algorithms: RF, extremely randomized trees (ERT), and extreme gradient boosting (XGBoost), to enhance the accuracy of source-specific LECR assessments. | Lung excess cancer risk | LECR estimation can effectively mitigate PAH pollution and reduce lung cancer risks. | ERT identified the primary contributors to elevated LECR in Ningbo from sources such as industrial emissions, coal combustion, and gasoline engine exhaust. A moderate PAH exposure risk level > 1.0 × 10−6 | ERT |
| Abbafati et al. [77] | PM2.5, PM10 | Investigate relationship between type 2 diabetes (T2DM) and exposure to PM2.5 and PM10. | Random effects models and non-parametric methods to assess association between air pollution and T2DM. | Type-2 diabetes (T2DM) | - | Statistically significant relationship between T2DM and PM2.5. T2DM incidence rates were significantly negatively associated with time (coefficient = −0.07961, p < 0.01), indicating a decreasing trend over time. Increases in the ratio of PM2.5 to PM10 (pwratio) were significantly positively associated with increases in T2DM incidence rates (coefficient = 0.52304, p < 0.01). | - |
| Weichenthal et al. [78] | ultrafine particles (UFPs, <0.1 μm) and black carbon (BC) | Examine long-term health consequences of traffic pollutants (ultrafine particles) and BC in urban areas. Estimate associations between long-term exposures to outdoor UFPs and BC and non-accidental and cause-specific mortality. | exposure assessment models: (1) land use regression (LUR) models; (2) CNN trained with mobile monitoring data and aerial images; (3) combined LUR and CNN. Cox proportional hazard model estimated association between long-term exposures to outdoor UFPs and BC, and potential confounding factors (that is, socio-demographic factors and co-pollutants identified) were adjusted. | Mortality | - | Outdoor UFP were positively associated with mortality, independent of other outdoor air pollutants like PM2.5 mass concentrations and oxidant gases (e.g., nitrogen dioxide (NO2) and O3). | Combined LUR and CNN performed slightly better than LUR model. |
| Wang et al. [79] | Volatile Organic Compounds (VOCs) | Examine health risks from exposure to volatile organic compounds (VOCs) in e-waste recycling areas (ERA) and predict the presence of e-waste pollution | Non-machine learning model (ultrahigh performance liquid chromatography) and Bayesian kernel machine regression model | - | - | Significantly higher levels of VOC exposure and oxidative damage biomarkers (ODBs) among e-waste workers | - |
| Wang et al. [80] | PM2.5 bound mercury (PBM2.5) | Explore the driving factors, spatiotemporal pollution distribution and associated health risks of PBM2.5 | RF applied to predict PBM2.5 concentration. Health risk assessment model | Non-carcinogenic risk of PBM2.5 was negligible in the year 2020, with HQ value mostly < 0.02 during winter. | Population density and PM2.5 from power generation stations contributed mainly to PBM2.5 concentration. | RF | |
| Wang et al. [81] | Airborne trace element (TE) in PM2.5 | quantify variations in pollution characteristics and health risks of TEs | Weather normalization and health risk assessment models. | Threat to human health and ecosystems. | TEs show dual effects (i.e., a tendency towards higher TE concentrations due to meteorological factors and its health-related risks during polluted period). Selenium (Se), manganese (Mn), and lead (Pb) are the most meteorologically influenced TEs, whereas chromium (Cr) and manganese (Mn) are the dominant TEs that pose health risks | - | |
| Chen et al. [82] | Fourteen common air pollutants | Examine habitual cooking, indoor air pollutants and the risk of lung cancer. | Gas chromatography–mass spectrometry. | risk of lung adenocarcinoma | lung cancer | Frequent cooking and indoor incense burning increase the risk of lung adenocarcinoma. | - |
| Yang et al. [83] | PM1, PM2.5, PM10, and NO2 | Examine air pollution and visual impairment | Machine learning methods. | visual impairment | Exposure to air pollution were positively associated with the odds of visual impairment. However, changes in a child’s age, gender, and area of residence, parent level of education and smoking of cigarette may change the association | ||
| Yang et al. [84] | PM1 and PM2.5 | Examine long-term exposure to PM1, PM2.5 and children’s lung function. | - | Lung function | Lung function | PM1 may be very hazardous to children’s respiratory health than PM2.5 exposure. | |
| Al Noaimi et al. [85] | PM2.5, NO2, SO2 | Maternal exposure of air pollutants | - | Higher birth defect (BD) risk, neural tube defects (NTD), genitourinary defects (GUD) risk | Exposure to PM2.5 during the first trimester is significantly associated with a higher overall. Maternal exposure to NO2 during BD Gestational Time Window of Risk (GTWR) showed a significant protective effect for neural tube defects (NTD). Maternal exposure to SO2 during GTWR showed a significant association with a higher genitourinary defects (GUD) risk | ||
| Li et al. [6] | Air toxics (that is, hazardous air pollutants) | Examine multi-air toxic combinations and its association with disease like asthma. | Machine learning | Asthma outcomes | multi-air toxic significantly associated with asthma outcomes | ||
| Wang et al. [86] | Prenatal ambient fine particulate matter PM2.5 | Prenatal ambient fine particulate matter PM2.5 exposure on early childhood neurodevelopment | General linear mixed model with binomially distributed errors | Risk of suspected developmental delay (SDD) in children, specifically in problem-solving context for girls. | Prenatal PM2.5 exposure affected early childhood neurodevelopment. | ||
| Xu et al. [87] | Chemical constituents (that is, organic matter OM, black carbon (BC), sulfate (SO42−), nitrate (NO3−), ammonium (NH4+), and soil dust (Dust-PM2.5)). | investigate association between prenatal exposure to PM2.5 and neurodevelopment in infants (1 year) | Machine learning model estimates daily concentration. Geospatial-statistical model evaluates average concentration of the chemical constituents. | Risk of a child’s non-optimal gross motor development. | - | Prenatal exposure to PM2.5, and with high SO42− concentration, were related to children’s non-optimal gross motor development. However, short- and long-term effects of perinatal PM2.5 exposure on children’s neurodevelopment merit further investigation. | |
| Zhang et al. [88] | PM2.5 | Estimate spatiotemporal heterogeneity in fine particle concentration and its health risks exposure and inhalation of PM2.5. | Combine three-dimensional landscape pattern index (TDLPI) and extreme gradient boosting (XGBOOST) to improve LUR model (LTX) | The health risks of human exposure to fine particles were moderately high in winter in the study area. | LTX (RMSE of 8.73 μg/m3 | ||
| Huang et al. [20] | PM2.5, PM10, sulfur dioxide (SO2) and nitrogen dioxide (NO2) | Examine association between long-term exposure to multiple air pollutants and cardiopulmonary mortality. Identify air pollutant contributing to mortality risk | Satellite-derived machine learning model. Time-varying Cox proportional hazards model evaluated individual association between air pollutants and mortality from non-accidental causes, cardiovascular diseases (CVDs), non-malignant respiratory diseases (RDs) and lung cancer, accounting for demographic and socioeconomic factors. | Mortality risks linked to air pollutant mixture | lung cancer | PM2.5 regularly contributed the most to high mortality risks associated with air pollutant mixture, followed by SO2 or PM10. There was strong positive association of long-term individual and joint exposure to PM10, PM2.5, SO2, and NO2. PM2.5 is potentially the main contributor to mortalities from non-accidental causes, CVDs, non-malignant RDs and lung cancer in high-exposure settings. | |
| Sun et al. [65] | PM2.5, SO4, NO3, ammonium (NH4+), and chloride (Cl−) | investigate the individual and joint mortality risks related to PM2.5 inorganic chemical compositions, and identify primary contributors | Satellite-based machine learning model calculated the chemical compositions. Time-varying Cox proportional hazards model analyzed associations between the chemical compositions | Cardiopulmonary mortality. | Risk of cardiorespiratory mortality. | Higher incomes earners with lower level of education were more vulnerable to inorganic chemical exposure. Long-term exposure to higher levels of PM2.5 inorganic compositions was connected to significantly increase cardiopulmonary mortality, with SO42− as the potential primary contributor. PM2.5 sources impact health. Joint exposure model shows that simultaneous rise in one IQR in all four compositions, increased the risk of cardiorespiratory mortality by at least 36.3%, with long-term exposure to SO42− contributing the most to non-accidental and cardiopulmonary deaths. | |
| Gao et al. [89] | Chemical components (sulfate(SO2−4), ammonium (NH4+)), PM2.5 | Examine Chemical components and the expiratory airflow limitation (EAL) in adult. | Land use regression model predicts the exposure to six air pollutants. PM2.5 was determined using a validated machine-learning algorithm. Logistic regression model employed to estimate effect sizes. Pulmonary function evaluated with medical-grade pulmonary function analyzer. | Air pollution score (APS) was associated with a 25% higher risk of EAL. | - | PM2.5 exposure had the sturdiest link with the risk of EAL. Combined effects of air pollution increased the risk of EAL in youth, where SO2−4 and NH4+ predominantly contributed to chemical components |
Appendix A.3. xAI Technique Integrated in ML for Air Pollution Risk Assessment in the Literature
| Author | Air Pollutant | Focus | Respiratory Health Outcome | ML Model | xAI Model | Accuracy Measure | Findings |
|---|---|---|---|---|---|---|---|
| Abdillah et al. [90] | UFPs, PM0.1 | Assessed UFP number concentrations (UFPs PNC) exposure dose for healthy adults and children. | Inhaled UFPs were mainly deposited in tracheobronchial (TB) respiratory fraction for adults (67.7%) and in alveoli (ALV) fraction for children (67.5%) | Multiple linear regression (MLR), XGBoost and RF. | SHAP analysis with the XGBoost model | XGBoost has highest estimation performance with RMSE (0.79) | Spatial variability was successfully pointed out for each roadside. |
| Xu et al. [91] | PM2.5, organic matter (OM), nitrate (NO3−) and ammonium (NH4+),black carbon (BC) and sulfate (SO42−) | Assessed the concentration thresholds at which components of PM2.5 affect mortality. | - | LightGBM model based on Bayesian. | SHAP algorithms to identify the concentration thresholds at which the components of PM2.5 affect mortality | - | The mortality rates influenced by five PM2.5 components suggesting a consistent downward trend. Relative importance OM, NO3− and NH4+ in influencing mortality increased by 6.3%, 17.4% and 4%, respectively. Relative importance of BC and SO42− in influencing mortality decreased rapidly to approx. 2%. |
| Jing et al. [92] | nature park visits and environmental factors (PM2.5) | Investigate the associations between nature park visits and adult asthma risk in urbanized areas. | Exposure–response relationship shows that increasing park visits reduces asthma risk and enhance respiratory health in urban settings, but the protective effect plateaus when visits exceed 51.94 per year. | XGBoost, NDVI (Normalized Difference Vegetation Index) | SHAP | - | High urban heat island index (UHI) and high PM2.5 levels increased asthma risk. High PM2.5 levels (AP = 0.24, 95% CI: 0.15 to 0.32) increased asthma risk. |
| Wang et al. [64] | PM2.5, PM10, | Effects of airborne particulate matter on climate and human health | - | LightGBM, XGBoost | SHAP used to separate meteorological contributions because of the strong influence on PM concentration. | lightGBM model trains 45 times faster than the XGBoost | The SHAP technique had good agreement with meteorological normalization approach to separate meteorological contributions (R2 > 0.5). |
References
- Jitkajornwanich, K.; Vijaranakul, N.; Jaiyen, S.; Srestasathiern, P.; Lawawirojwong, S. Enhancing risk communication and environmental crisis management through satellite imagery and AI for air quality index estimation. MethodsX 2024, 12, 102611. [Google Scholar] [CrossRef]
- Hang, Y.; Meng, X.; Li, T.; Wang, T.; Cao, J.; Fu, Q.; Dey, S.; Li, S.; Huang, K.; Liang, F.; et al. Assessment of long-term particulate nitrate air pollution and its health risk in China. iScience 2022, 25, 104899. [Google Scholar] [CrossRef]
- Pajot, A.; Yapo, M.; Coulibaly, S.; Doumbia, M.; Gnamien, S.; Kouao, K.; Ahoua, S.; Adjoua Dje, S.; Liousse, C.; Moh, R.; et al. Air pollution exposure, respiratory consequences, and perceptions among urban African children living in poor conditions—A case study in Abidjan, Côte d’Ivoire. PLoS Glob. Public Health 2025, 5, e0003703. [Google Scholar] [CrossRef]
- Sankar, L.; Akrasu, K. Efficient multi-station air quality prediction in Delhi with wavelet and optimization-based model. PLoS ONE 2025, 20, e0330465. [Google Scholar] [CrossRef]
- Zaben, S.O.; Zainon, W.M.N.W.; Sabry, A.H. Machine learning-based methods for detecting respiratory abnormalities using audio and visual analysis: A review. Results Eng. 2025, 26, 104744. [Google Scholar] [CrossRef]
- Li, Y.C.; Hsu, H.L.; Chun, Y.; Chiu, P.H.; Arditi, Z.; Claudio, L.; Pandey, G.; Bunyavanich, S. Machine learning-driven identification of early-life air toxic combinations associated with childhood asthma outcomes. J. Clin. Investig. 2021, 131, e152088. [Google Scholar] [CrossRef]
- Neo, E.X.; Hasikin, K.; Mokhtar, M.I.; Lai, K.W.; Azizan, M.M.; Razak, S.A.; Hizaddin, H.F. Towards Integrated Air Pollution Monitoring and Health Impact Assessment Using Federated Learning: A Systematic Review. Front. Public Health 2022, 10, 851553. [Google Scholar] [CrossRef]
- Ye, Y.; Aizezi, N.; Feng, J.; Han, B.; Li, X.; Su, Z.; Li, L.; Liu, Y. Advanced Characterization of Industrial Smoke: Particle Composition and Size Analysis with Single Particle Aerosol Mass Spectrometry and Optimized Machine Learning. Anal. Chem. 2025, 97, 5554–5562. [Google Scholar] [CrossRef]
- Zylowski, T. Study on Criteria for Explainable AI for Laypeople. In CEUR Workshop Proceedings, Proceedings of the Joint of 45th German Conference on Artificial Intelligence Workshops, Tutorials and Doctoral Consortium, KI-JP 2022, Trier, Germany, September 19–23 2022; CEUR-WS: Aachen, Germany, 2022. [Google Scholar]
- Leivaditis, V.; Maniatopoulos, A.A.; Lausberg, H.; Mulita, F.; Papatriantafyllou, A.; Liolis, E.; Beltsios, E.; Adamou, A.; Kontodimopoulos, N.; Dahm, M. Artificial Intelligence in Thoracic Surgery: A Review Bridging Innovation and Clinical Practice for the Next Generation of Surgical Care. J. Clin. Med. 2025, 14, 2729. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lin, S.; Neamtiu, I.A.; Ye, B.; Csobod, E.; Fazakas, E.; Gurzau, E. Predicting environmental risk factors in relation to health outcomes among school children from Romania using random forest model—An analysis of data from the SINPHONIE project. Sci. Total Environ. 2021, 784, 147145. [Google Scholar] [CrossRef] [PubMed]
- Chadalavada, S.; Faust, O.; Salvi, M.; Seoni, S.; Raj, N.; Raghavendra, U.; Gudigar, A.; Barua, P.D.; Molinari, F.; Acharya, R. Application of artificial intelligence in air pollution monitoring and forecasting: A systematic review. Environ. Model. Softw. 2025, 185, 106312. [Google Scholar] [CrossRef]
- Masood, A.; Ahmad, K. A review on emerging artificial intelligence (AI) techniques for air pollution forecasting: Fundamentals, application and performance. J. Clean. Prod. 2021, 322, 129072. [Google Scholar] [CrossRef]
- Brereton, P.; Kitchenham, B.A.; Budgen, D.; Turner, M.; Khalil, M. Lessons from applying the systematic literature review process within the software engineering domain. J. Syst. Softw. 2007, 80, 571–583. [Google Scholar] [CrossRef]
- Subramaniam, S.; Raju, N.; Ganesan, A.; Rajavel, N.; Chenniappan, M.; Prakash, C.; Pramanik, A.; Basak, A.K.; Dixit, S. Artificial Intelligence Technologies for Forecasting Air Pollution and Human Health: A Narrative Review. Sustainability 2022, 14, 9951. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, K.; Huang, K.; Ding, H.; Xu, T.; Chen, Z.; Sun, Y. Visualization and Analysis of Air Pollution and Human Health Based on Cluster Analysis: A Bibliometric Review from 2001 to 2021. Int. J. Environ. Res. Public Health 2022, 19, 12723. [Google Scholar] [CrossRef]
- Ye, T.; Yu, P.; Wen, B.; Yang, Z.; Huang, W.; Guo, Y.; Abramson, M.J.; Li, S. Greenspace and health outcomes in children and adolescents: A systematic review. Environ. Pollut. 2022, 314, 120193. [Google Scholar] [CrossRef]
- Vachon, J.; Kerckhoffs, J.; Buteau, S.; Smargiassi, A. Do machine learning methods improve prediction of ambient air pollutants with high spatial contrast? A systematic review. Environ. Res. 2024, 262 Pt 2, 119751. [Google Scholar] [CrossRef] [PubMed]
- Kibria, H.B.; Hossain, M.A.; Rehman, S.; Alahakoon, D.; Rahman, M.A. An explainable lightweight parallel depth-wise separable model for lung infection detection from chest X-rays. Neural Comput. Appl. 2025, 37, 4545–4566. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, Y.; Chen, X.; Zeng, X.; Knibbs, L.D.; Zhang, Y.; Jalaludin, B.; Dharmage, S.C.; Morawska, L.; Guo, Y.; et al. Individual and joint associations of long-term exposure to air pollutants and cardiopulmonary mortality: A 22-year cohort study in Northern China. Lancet Reg. Health West. Pac. 2023, 36, 100776. [Google Scholar] [PubMed]
- Liu, K.; Li, S.; Qian, Z.M.; Dharmage, S.C.; Bloom, M.S.; Heinrich, J.; Jalaludin, B.; Markevych, I.; Morawska, L.; Knibbs, L.D.; et al. Benefits of influenza vaccination on the associations between ambient air pollution and allergic respiratory diseases in children and adolescents: New insights from the Seven Northeastern Cities study in China. Environ. Pollut. 2020, 256, 113434. [Google Scholar] [CrossRef]
- Atzeni, M.; Cossu, L.; Gaiotti, S.; Cappon, G.; Tine, M.; Previtero, D.; Padrin, Y.; Baraldo, S.; Semenzato, U.; Vettoretti, M. AirPredict: An eHealth platform for asthma management leveraging wearable sensors, digital diaries, and air quality monitoring to optimize patient outcomes. Front. Digit. Health 2025, 7, 1573342. [Google Scholar] [CrossRef]
- Rowland, S.N.; Green, C.G.; Halliwill, J.R.; Singanayagam, A.; Heaney, L.M. Gut feelings on short-chain fatty acids to regulate respiratory health. Trends Endocrinol. Metab. 2025. [Google Scholar] [CrossRef]
- Renouf, B.; Sutanto, E.N.; Kidd, C.; Lim, J.; Amin, M.; Berry, L.; Hoyne, G.F.; D’Vaz, N.; Kicic-Starcevich, E.; Stick, S.M.; et al. Profiling epithelial viral receptor expression in amniotic membrane and nasal epithelial cells at birth. Placenta 2025, 160, 82–88. [Google Scholar] [CrossRef]
- Xu, J.; Yang, W.; Bai, Z.; Zhang, R.; Zheng, J.; Wang, M.; Zhu, T. Modeling spatial variation of gaseous air pollutants and particulate matters in a Metropolitan area using mobile monitoring data. Environ. Res. 2022, 210, 112858. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Tao, Y.; Zhao, X.; Wang, D.; Wei, T.; Hou, Y.; Xu, X. Mapping the personal PM2.5 exposure of China’s population using random forest. Sci. Total Environ. 2023, 871, 162090. [Google Scholar] [CrossRef]
- Maio, S.; Fasola, S.; Marcon, A.; Angino, A.; Baldacci, S.; Bilò, M.B.; Bono, R.; La Grutta, S.; Marchetti, P.; Sarno, G.; et al. Relationship of long-term air pollution exposure with asthma and rhinitis in Italy: An innovative multipollutant approach. Environ. Res. 2023, 224, 115455. [Google Scholar] [CrossRef]
- Tang, C.X.; Dong, Y.; Yuan, X.Y.; Wang, R.; Wu, C.C.; Bao, L.J.; Zeng, E.Y. Effects of organic carbon/elemental carbon and particle size on inhalation bioaccessibility of particle-bound PAHs. Sci. Total Environ. 2023, 889, 164225. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yuan, Q.; Gao, M.; Li, T. A new perspective to satellite-based retrieval of ground-level air pollution: Simultaneous estimation of multiple pollutants based on physics-informed multi-task learning. Sci. Total Environ. 2023, 857 Pt 2, 159542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhang, K.; Zhang, Y.; Ji, Y.; Zhu, B.; Liang, Z.; Wang, H.; Ge, X. Machine learning assesses drivers of PM2.5 air pollution trend in the Tibetan Plateau from 2015 to 2022. Sci. Total Environ. 2023, 878, 163189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Guo, Y.; Su, T.; Chen, G.; Liu, H.; Li, Q.; Bao, H.; Ji, Y.; Luo, S.; Liu, Z.; et al. Individual and joint effect of indoor air pollution index and ambient particulate matter on fetal growth: A prospective cohort study. Int. J. Epidemiol. 2023, 52, 690–702. [Google Scholar] [CrossRef]
- Ahmadian, F.; Rajabi, S.; Maleky, S.; Baghapour, M.A. Spatiotemporal analysis of airborne pollutants and health risks in Mashhad metropolis: Enhanced insights through sensitivity analysis and machine learning. Environ. Geochem. Health 2025, 47, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, S.; Wang, P.; Zheng, Z.; Shi, S.; Li, X.; Xu, C.; Yu, K.; Chen, R.; Kan, H.; et al. Predicting particulate matter, nitrogen dioxide, and ozone across Great Britain with high spatiotemporal resolution based on random forest models. Sci. Total Environ. 2024, 926, 171831. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Ma, J.; Chen, J.; Chen, S.; Zheng, Y.; Huang, B.; Yu, J.; Li, T.; He, S. Ambient fine particulate matter and its constituents may exacerbate the acceleration of aging in adults. Environ. Int. 2024, 192, 109019. [Google Scholar] [CrossRef]
- Jiang, P.; Gao, C.; Zhao, J.; Li, F.; Ou, C.; Zhang, T.; Huang, S. An exploration of urban air health navigation system based on dynamic exposure risk forecast of ambient PM2.5. Environ. Int. 2024, 190, 108793. [Google Scholar] [CrossRef]
- Lv, L.; Wei, P.; Hu, J.; Chu, Y.; Liu, X. High-spatiotemporal-resolution mapping of PM2.5 traffic source impacts integrating machine learning and source-specific multipollutant indicator. Environ. Int. 2024, 183, 108421. [Google Scholar] [CrossRef]
- Masseran, N.; Safari, M.A.M.; Tajuddin, R.R.M. Probabilistic classification of the severity classes of unhealthy air pollution events. Environ. Monit. Assess. 2024, 196, 523. [Google Scholar] [CrossRef]
- Chen, L.; Yousaf, M.; Xu, J.; Ma, X. Ultrafine particles: Sources, toxicity, and deposition dynamics in the human respiratory tract —experimental and computational approaches. J. Environ. Manag. 2025, 376, 124458. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Bergmann, M.L.; Taghavi Shahri, S.M.; Tayebi, S.; Cole-Hunter, T.; Kerckhoffs, J.; Khan, J.; Meliefste, K.; Lim, Y.H.; Mortensen, L.H.; et al. Harnessing AI to unmask Copenhagen’s invisible air pollutants: A study on three ultrafine particle metrics. Environ. Pollut. 2024, 346, 123664. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zhao, X.; Fu, X.; Jin, Y. Computational insights into exploring the potential effects of environmental contaminants on human health. Sci. Rep. 2025, 15, 11779. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Achebak, H.; Petetin, H.; Méndez Turrubiates, R.F.; Guo, Y.; Pérez García-Pando, C.; Ballester, J. Trends in population exposure to compound extreme-risk temperature and air pollution across 35 European countries: A modelling study. Lancet Planet. Health 2025, 9, e384–e396. [Google Scholar] [CrossRef]
- Gao, K.; Hua, K.; Wang, S.; Chen, X.; Zhu, T. Exploring the reproductive exposure risks of phthalates and organophosphates in atmospheric particulate matter based on quantitative structure-activity relationships and network toxicology models. J. Hazard. Mater. 2025, 488, 137395. [Google Scholar] [CrossRef]
- Gao, K.; Hua, K.; Chen, X.; Zheng, C.; Li, X.; Wu, Q.; Ji, L.; Wang, L.; Wei, W.; Lu, L. Occurrence, Characteristics, and Mixed Reproductive Exposure Risk Assessment of Traditional Phthalates and Their Novel Alternatives in Campus Indoor Dust. Environ. Sci. Technol. 2025, 59, 6708–6718. [Google Scholar] [CrossRef]
- Holloway, T.; Bratburd, J.R.; Fiore, A.M.; Kerr, G.H.; Mao, J. Satellite data to support air quality assessment and management. J. Air Waste Manag. Assoc. 2025, 75, 429–463. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.P.; Fu, J.S.; Liu, Y. Perspective improvement of regional air pollution burden of disease estimation by machine intelligence. Front. Public Health 2025, 13, 1436838. [Google Scholar] [CrossRef]
- Lee, S.J.; Cho, I.G.; Lee, H.Y.; Ju, J.T.; Shin, H.J.; Choi, S.D. Development of a comprehensive air risk index and its application to high spatial-temporal health risk assessment in a large industrial city. Environ. Pollut. 2025, 367, 125545. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, B.; Zhang, X.; Liu, X.; Wang, T.; Thuy Dinh, V.N.; Jaffrezo, J.L.; Uzu, G.; Dominutti, P.; Darfeuil, S.; et al. Source apportionment of PM(10) particles in the urban atmosphere using PMF and LPO-XGBoost. Environ. Res. 2025, 278, 121659. [Google Scholar] [CrossRef] [PubMed]
- Wenjie, W.; Rui, L.; Pengpeng, Z.; Chao, D.; Donglin, Z. Integrated network toxicology, machine learning and molecular docking reveal the mechanism of benzopyrene-induced periodontitis. BMC Pharmacol. Toxicol. 2025, 26, 118. [Google Scholar] [CrossRef]
- Yang, H.M.; Wang, J.Y.; Li, C.; Zhang, Y.Q.; Wang, R.; Yang, Q.; Yao, Y.; Wang, Z.; Xu, S.L.; Huang, H.H.; et al. Is there an association between eye-level greenness and childhood hypertension using street view? Findings from the Seven Northeastern Cities study in China. Environ. Res. 2025, 268, 120768. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, P.; Zhan, Z.; Dai, Q.; Casuccio, G.S.; Gao, J.; Li, J.; He, Y.; Qian, H.; Bi, X.; et al. Advancing Source Apportionment of Atmospheric Particles: Integrating Morphology, Size, and Chemistry Using Electron Microscopy Technology and Machine Learning. Environ. Sci. Technol. 2025, 59, 3645–3655. [Google Scholar] [CrossRef]
- Paul, N.; Yao, J.; McLean, K.E.; Stieb, D.M.; Henderson, S.B. The Canadian Optimized Statistical Smoke Exposure Model (CanOSSEM): A machine learning approach to estimate national daily fine particulate matter PM2.5 exposure. Sci. Total Environ. 2022, 850, 157956. [Google Scholar] [CrossRef]
- Brooks, N.; Biswas, D.; Hossin, R.; Yu, A.; Saha, S.; Saha, S.; Saha, S.K.; Luby, S.P. Health consequences of small-scale industrial pollution: Evidence from the brick sector in Bangladesh. World Dev. 2023, 170, 106318. [Google Scholar] [CrossRef]
- Hu, C.Y.; Gutierrez-Avila, I.; He, M.Z.; Lavigne, É.; Alcala, C.S.; Yitshak-Sade, M.; Lamadrid-Figueroa, H.; Tamayo-Ortiz, M.; Mercado-Garcia, A.; Just, A.C.; et al. Windows of susceptibility and joint effects of prenatal and postnatal ambient air pollution and temperature exposure on asthma and wheeze in Mexican children. Environ. Int. 2024, 193, 109122. [Google Scholar] [CrossRef]
- Okello, G.; Nantanda, R.; Tatah, L.; Sserunjogi, R.; Johnson, O.; Awokola, B.; Okure, D.; Thondoo, M.; Green, P.; Babajide, O.; et al. Association between ambient air pollution and respiratory health in Kampala, Uganda: Implications for policy and practice. Urban Clim. 2024, 58, 102128. [Google Scholar] [CrossRef]
- Bouma, F.; Hoek, G.; Koppelman, G.H.; Vonk, J.M.; Janssen, N.A.H.; van Ratingen, S.; Hendricx, W.; Wesseling, J.; Kerckhoffs, J.; Vermeulen, R.; et al. Comparison of air pollution exposure assessment methods and the association with children’s respiratory health. Environ. Int. 2025, 198, 109407. [Google Scholar] [CrossRef]
- Leão, M.L.P.; Zhang, L.; da Silva Júnior, F.M.R. Effect of particulate matter PM2.5 and PM10 on health indicators: Climate change scenarios in a Brazilian metropolis. Environ. Geochem. Health 2023, 45, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Shams, S.R.; Choi, Y.; Singh, D.; Kayastha, S.; Park, J. Deep learning-based forecasting of daily maximum ozone levels and assessment of socioeconomic and health impacts in South Korea. Sci. Total Environ. 2025, 983, 179684. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, W.; Mfarrej, M.F.B.; Saleem, M.H.; Khan, K.A.; Ma, J.; Raposo, A.; Han, H. Breathing in danger: Understanding the multifaceted impact of air pollution on health impacts. Ecotoxicol. Environ. Saf. 2024, 280, 116532. [Google Scholar] [CrossRef] [PubMed]
- Teyton, A.; Baer, R.J.; Benmarhnia, T.; Bandoli, G. Exposure to Air Pollution and Emergency Department Visits during the First Year of Life among Preterm and Full-term Infants. JAMA Netw. Open 2023, 6, E230262. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Yazdi, M.; Wang, Y.; Di, Q.; Wei, Y.; Requia, W.J.; Shi, L.; Sabath, M.B.; Dominici, F.; Coull, B.A.; Evans, J.S. Long-Term Association of Air Pollution and Hospital Admissions among Medicare Participants Using a Doubly Robust Additive Model. Circulation 2021, 143, 1584–1596. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, C.; Li, M.; Huang, Q. High-resolution monthly assessment of population exposure to PM2.5 and its relationship with socioeconomic activities using multisource geospatial data. Environ. Monit. Assess. 2025, 197, 342. [Google Scholar] [CrossRef]
- Pei, S.; Yang, L.; Gao, H.; Liu, Y.; Dai, E.; Feng, F.; Lu, J. Pollutants-mediated viral hepatitis in different types: Assessment of different algorithms and time series models. Sci. Rep. 2024, 14, 21141. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Liu, Y.; Li, G.; An, T. A review of disrupted biological response associated with volatile organic compound exposure: Insight into identification of biomarkers. Sci. Total Environ. 2024, 948, 174924. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Zhang, R.; Meng, X.; Kan, H.; Zhang, H. Estimating particulate matter concentrations and meteorological contributions in China during 2000–2020. Chemosphere 2023, 330, 138742. [Google Scholar] [CrossRef]
- Sun, H.; Chen, X.; Huang, W.; Wei, J.; Yang, X.; Shan, A.; Zhang, L.; Zhang, H.; He, J.; Pan, C.; et al. Association between Long-Term Exposure to PM2.5 Inorganic Chemical Compositions and Cardiopulmonary Mortality: A 22-Year Cohort Study in Northern China. Environ. Health 2024, 2, 530–540. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, H.; Hao, Y.; Zhao, L.; Pan, W.; Xue, Q.; Liu, X.; Fu, J.; Zhang, A. Deciphering exogenous chemical carcinogenicity through interpretable deep learning: A novel approach for evaluating atmospheric pollutant hazards. J. Hazard. Mater. 2024, 465, 133092. [Google Scholar] [CrossRef]
- Rajesh, M.; Babu, R.G.; Moorthy, U.; Easwaramoorthy, S.V. Machine learning driven framework for realtime air quality assessment and predictive environmental health risk mapping. Sci. Rep. 2025, 15, 28801. [Google Scholar] [CrossRef]
- Razavi-Termeh, S.V.; Sadeghi-Niaraki, A.; Ali, F.; Naqvi, R.A.; Choi, S.-M. Spatio-temporal modeling of asthma-prone areas: Exploring the influence of urban climate factors with explainable artificial intelligence (XAI). Sustain. Cities Soc. 2024, 116, 105889. [Google Scholar] [CrossRef]
- Agbehadji, I.E.; Obagbuwa, I.C. Integration of Explainable Artificial Intelligence into Hybrid Long Short-Term Memory and Adaptive Kalman Filter for Sulfur Dioxide (SO2) Prediction in Kimberley, South Africa. Atmosphere 2025, 16, 523. [Google Scholar] [CrossRef]
- Agbehadji, I.E.; Obagbuwa, I.C. Spatiotemporal Graph Convolutional Network-Based Long Short-Term Memory Model with A* Search Path Naviga-tion and Explainable Artificial Intelligence for Carbon Mon-oxide Prediction in Northern Cape Province, South Africa. Atmosphere 2025, 16, 1107. [Google Scholar] [CrossRef]
- Sunder, M.S.S.; Tikkiwal, V.A.; Kumar, A.; Tyagi, B. Unveiling the Transparency of Prediction Models for Spatial PM2.5 over Singapore: Comparison of Different Machine Learning Approaches with eXplainable Artificial Intelligence. AI 2023, 4, 787–811. [Google Scholar] [CrossRef]
- Chang, T.H.; Liu, Y.C.; Lin, S.R.; Chiu, P.H.; Chou, C.C.; Chang, L.Y.; Lai, F.P. Clinical characteristics of hospitalized children with community-acquired pneumonia and respiratory infections: Using machine learning approaches to support pathogen prediction at admission. J. Microbiol. Immunol. Infect. 2023, 56, 772–781. [Google Scholar] [CrossRef]
- Chi, C.Y.; Moghadas-Dastjerdi, H.; Winkler, A.; Ao, S.; Chen, Y.P.; Wang, L.W.; Su, P.I.; Lin, W.S.; Tsai, M.S.; Huang, C.H. Clinical Validation of Explainable Deep Learning Model for Predicting the Mortality of In-Hospital Cardiac Arrest Using Diagnosis Codes of Electronic Health Records. Rev. Cardiovasc. Med. 2023, 24, 265. [Google Scholar] [CrossRef]
- Li, C.; Xia, Y.; Wang, L. Household unclean fuel use, indoor pollution and self-rated health: Risk assessment of environmental pollution caused by energy poverty from a public health perspective. Environ. Sci. Pollut. Res. Int. 2024, 31, 18030–18053. [Google Scholar] [CrossRef] [PubMed]
- Commodore, S.; Christopher, S.; Wolf, B.; Svendsen, E. Assessment of trace elements directly from archived total suspended particulate filters by laser ablation ICP-MS: A case study of South Carolina. J. Trace Elem. Miner. 2023, 3, 100041. [Google Scholar]
- Famiyeh, L.; Chen, K.; Tesema, F.B.; Kelly, C.; Ji, D.; Xiao, H.; Tong, L.; Wang, Z.; He, J. Refining source-specific lung cancer risk assessment from PM2.5-bound PAHs: Integrating component-based potency factors and machine learning in Ningbo, China. Ecotoxicol. Environ. Saf. 2025, 297, 118174. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Nieddu, L.; Cattani, G.; Reatini, M.A.; Ponzani, P. Association between air pollution and type II diabetes in Italy from clinical data and population-weighted exposure at the municipality level. Sci. Rep. 2025, 15, 28326. [Google Scholar] [CrossRef] [PubMed]
- Weichenthal, S.; Lloyd, M.; Ganji, A.; Simon, L.; Xu, J.; Venuta, A.; Schmidt, A.; Apte, J.; Chen, H.; Lavigne, E.; et al. Long-Term Exposure to Outdoor Ultrafine Particles and Black Carbon and Effects on Mortality in Montreal and Toronto, Canada. Res. Rep. Health Eff. Inst. 2024, 2024, 217. [Google Scholar]
- Wang, J.R.; Kuang, H.X.; Liu, Y.; Li, X.Y.; Chen, T.H.; Zhu, X.H.; Fan, R.F.; Xiang, M.D.; Yu, Y.J. Associations between volatile organic compounds exposure and multiple oxidative damage biomarkers: Method development, human exposure, and application for e-waste pollution prediction. Sci. Total Environ. 2024, 956, 177402. [Google Scholar]
- Wang, H.; Li, T.; Wang, G.; Peng, Y.; Zhang, Q.; Wang, X.; Ren, Y.; Liu, R.; Yan, S.; Meng, Q.; et al. Significant spatiotemporal changes in atmospheric particulate mercury pollution in China: Insights from meta-analysis and machine-learning. Sci. Total Environ. 2024, 955, 177184. [Google Scholar]
- Wang, H.; Guan, X.; Li, J.; Peng, Y.; Wang, G.; Zhang, Q.; Li, T.; Wang, X.; Meng, Q.; Chen, J.; et al. Quantifying the pollution changes and meteorological dependence of airborne trace elements coupling source apportionment and machine learning. Sci. Total Environ. 2024, 948, 174452. [Google Scholar]
- Chen, K.C.; Tsai, S.W.; Shie, R.H.; Zeng, C.; Yang, H.Y. Indoor Air Pollution Increases the Risk of Lung Cancer. Int. J. Environ. Res. Public Health 2022, 19, 1164. [Google Scholar] [CrossRef]
- Yang, B.Y.; Guo, Y.; Zou, Z.; Gui, Z.; Bao, W.W.; Hu, L.W.; Chen, G.; Jing, J.; Ma, J.; Li, S.; et al. Exposure to ambient air pollution and visual impairment in children: A nationwide cross-sectional study in China. J. Hazard. Mater. 2021, 407, 124750. [Google Scholar] [CrossRef]
- Yang, M.; Guo, Y.M.; Bloom, M.S.; Dharmagee, S.C.; Morawska, L.; Heinrich, J.; Jalaludin, B.; Markevychd, I.; Knibbsf, L.D.; Lin, S.; et al. Is PM1 similar to PM2.5? A new insight into the association of PM1 and PM2.5 with children’s lung function. Environ. Int. 2020, 145, 106092. [Google Scholar] [CrossRef]
- Al Noaimi, G.; Yunis, K.; El Asmar, K.; Abu Salem, F.K.; Afif, C.; Ghandour, L.A.; Hamandi, A.; Dhaini, H.R. Prenatal exposure to criteria air pollutants and associations with congenital anomalies: A Lebanese national study. Environ. Pollut. 2021, 281, 117022. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Li, J.; Zhou, Y.; Luo, R.; Meng, X.; Zhang, Y. Prenatal exposure to ambient fine particulate matter and early childhood neurodevelopment: A population-based birth cohort study. Sci. Total Environ. 2021, 785, 147334. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tao, S.; Huang, L.; Du, J.; Liu, C.; Jiang, Y.; Jiang, T.; Lv, H.; Lu, Q.; Meng, Q.; et al. Maternal PM2.5 exposure during gestation and offspring neurodevelopment: Findings from a prospective birth cohort study. Sci. Total Environ. 2022, 842, 156778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, L.; Ma, W.; Wang, N.; Wen, F.; Liu, Q. Spatiotemporal estimation of the PM2.5 concentration and human health risks combining the three-dimensional landscape pattern index and machine learning methods to optimize land use regression modeling in Shaanxi, China. Environ. Res. 2022, 208, 112759. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Ding, H.; Bao, Y.; Zhang, C.; Chi, B.; Xia, Y.; Zhao, Y.; Zhang, H. Air pollution exposure, chemical compositions, and risk of expiratory airflow limitation in youth in Northeast China. Ecotoxicol. Environ. Saf. 2024, 285, 117055. [Google Scholar] [CrossRef]
- Abdillah, S.F.I.; You, S.J.; Wang, Y.F. Characterizing sector-oriented roadside exposure to ultrafine particles (PM0.1) via machine learning models: Implications of covariates influences on sectors variability. Environ. Pollut. 2024, 359, 124595. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, W.; Dai, C.; Zhao, S.; Qian, R.; Dong, B.; Li, Z.; Ma, J. Spatiotemporal evolution and risk thresholds of PM2.5 components in China from the human health perspective. Environ. Pollut. 2025, 373, 126194. [Google Scholar] [CrossRef]
- Jing, F.; Ma, W.; Li, Z.; Zhou, S.; Ruan, Y.; He, G.; Hu, J.; Liu, T. Nationwide analysis of the association between nature park visits and adult asthma risk in urbanized neighborhoods. Cities 2025, 166, 106301. [Google Scholar] [CrossRef]
| Author(s) | Research Focus | Methodology | Data Repositories | Number of Articles Selected | Year Range |
|---|---|---|---|---|---|
| Chadalavada et al. [12] | Monitoring and prediction. | Systematic review: PRISMA guidelines. | PubMed (43), Scopus (224) and Web of Science (145). | 64 | 2019 to 2023 |
| Masood et al. [13] | Application, fundamentals and performance. | Systematic review based on the three-phased approach suggested by [14]: plan, conduct review and report the review. | Total of 300 articles from Scopus, Web of Science, Ei Compendex, PubMed and ScienceDirect, Harvard University library catalog (HOLLIS). | 90 | 2003 to 2021 |
| Subramaniam et al. [15] | Forecasting and human health. | Classic narrative review that follows the systematic review technique. | Google Scholar, Springer, PubMed, ScienceDirect, IEEE explore, Scopus and Web of Science (WoS). | January 2015 through June 2022 | |
| Liu et al. [16] | Air pollution and health. | Bibliometric techniques. | - | 14,955 | 2001 to 2021 |
| Leivaditis et al. [10] | Applications, benefits, limitations and future direction of AI focusing on thoracic surgery. | PRISMA and risk of bias assessment were conducted using the Cochrane Risk of Bias Tool and ROBINS-I for non-randomized studies. | PubMed, Scopus, WoS and Cochrane Library. | 36 | Studies published up to January 2025 |
| Ye et al. [17] | Measurement methods of greenspace, their health outcomes and potential mechanisms. | Systematic review. | MEDLINE and EMBASE. | 140 | Up to 11 April 2022 |
| Vachon et al., 2024 [18] | Compared statistical and machine learning models to predict NO2, ultrafine particles (UFPs) and black carbon (BC). | Systematic review. | WoS and Scopus. | 38 | Up to 13 June 2024 |
| Model Type | Type of Algorithm | Typical Datasets Used | Validation Approach | Comparability |
|---|---|---|---|---|
| Tree-based models | Random Forest (RF), XGBoost, LightGBM | Air quality monitoring station data; meteorological variables. | K-fold cross-validation; train/test split | Widely used; strong performance, but feature importance varies across studies. |
| Deep learning models | CNN, LSTM, DNN [57] | Spatiotemporal pollution data; satellite-based remote sensing; health outcomes. | Hold-out validation; temporal split | Handle complex patterns; often limited transparency; high data demands. |
| Hybrid models | LUR + ML, SARIMA + ML | Land use regression data; time series pollution data. | Rolling-origin or temporal CV | Improve accuracy but add complexity; results are difficult to generalize. |
| Statistical baselines | SARIMA, regression models | Time series pollutant concentration. | Train/test split; rolling validation | Serve as benchmark; often outperforms ML in small datasets. |
| Author | Model(s) | Dataset/Application | xAI Technique(s) | Outcome Focus | Key Findings |
|---|---|---|---|---|---|
| Zhang et al. [66] | GCN (CarcGC) | Chemical carcinogenicity (molecular structural graphs) | Model-intrinsic interpretability | Air pollution and lung cancer | GCN-based interpretability identified structural predictors of carcinogenicity. |
| Rajesh et al. [67] | Gradient Boosting, RF, XGBoost, LSTM | Real-time multi-location AQ and health risk | SHAP | Air pollutant prediction + AQ classification | Accurate AQ prediction with interpretable pollutant contributions. |
| Razavi-Termeh et al. [68] | XGBoost + Bat algorithm | Asthma-prone locations (spatiotemporal) | SHAP | Asthma risk + seasonal pollutant drivers. | Identified seasonal pollutant importance (e.g., PM2.5 in spring, CO in summer). |
| Agbehadji et al. [69] | LSTM + Adaptive Kalman Filter | SO2 concentration prediction | SHAP | Air pollution (no direct health outcomes) | Improved interpretability of SO2 estimates; highlighted noise impact. |
| Agbehadji et al. [70] | (GeoxAI-GCN-LSTM-A*) | CO prediction. | LIME | Air pollution | Optimal navigation route with RMSE (0.4059 for 8 h and 0.4124 for 16 h). |
| Kibria et al. [19] | LW-PDS-CovidNet (CNN) | Lung infection detection (CT, X-ray, RT-PCR) | SHAP, Grad-CAM | Respiratory infections (COVID-19, SARS, MERS, etc.) | High accuracy (>90%); highlighted key imaging features. |
| Sunder et al. [71] | RF, Gradient Boosting, TP AutoML. | PM2.5 temporal prediction | xAI (feature importance, SHAP-like methods) | Air pollution | Meta-heuristic TP AutoML achieved highest GPI; interpretability aided temporal feature analysis. |
| Chang et al. [72] | ML classifiers | Pediatric ARI prediction | SHAP | Respiratory pathogens (adenovirus, influenza, etc.) | Identified biomarkers (CRP most important for adenovirus). |
| Chi et al. [73] | D-SHAP (Deep SHAP) | Clinical diagnosis codes | Deep SHAP | Respiratory failure, sepsis, pneumonia, etc. | Explanations aligned with physician judgment, improving trust in predictions. |
| Model(s) | Dataset/Task | Reported Metric(s) | Performance | Findings |
|---|---|---|---|---|
| RF, XGBoost, LSTM [68] | Real-time AQ and health risk prediction | R2 (0.82–0.89), RMSE (between 4.2 and 6.1 µg/m3) | High temporal accuracy. | SHAP improved interpretability of pollutant drivers. |
| XGBoost + Bat Alg. [69] | Asthma-prone locations | AUC (between 0.973 and 0.984) | Seasonal variations noted. | PM2.5, CO, O3, PM10 dominant factors. |
| LSTM + Adaptive Kalman Filter [70] | SO2 prediction | RMSE (0.79) | Reduced noise error. | No direct health outcomes. |
| CNN (LW-PDS-CovidNet) [19] | Lung infection detection | Accuracy between 92–98%. | High diagnostic accuracy. | xAI (SHAP, Grad-CAM) explained imaging features |
| Pediatric ARI ML [72] | Clinical infections prediction. | AUC between 0.86 and 0.92. | Event patterns predictive. | SHAP highlighted biomarkers. |
| D-SHAP model [73] | Diagnosis codes and mortality risk. | AUC > 0.90 | Clinician-aligned explanations. | Improved clinical trust. |
| xAI Technique | Strengths | Limitations | Best-Use Scenarios |
|---|---|---|---|
| SHAP (Shapley Additive Explanations) | Provides consistent, game-theoretic feature attributions; both global and local interpretability; widely adopted in environmental/epidemiological studies. | Computationally expensive; sensitive to correlated features; explanations may be complex for non-technical users. | Tabular spatiotemporal data; epidemiological studies; policy translation requiring robust feature attribution. |
| LIME (Local Interpretable Model-Agnostic Explanations). | Fast; flexible; model-agnostic; intuitive local approximations. | Unstable across runs; explanations vary with sampling; less reliable for high-stakes contexts. | Quick interpretability checks; exploratory analysis; low-stakes decision support. |
| Grad-CAM (Gradient-weighted Class Activation Mapping). | Visual explanations overlayed on images; intuitive for clinicians; highlights regions of interest. | Limited to CNNs; qualitative rather than quantitative; can miss non-visual factors. | Medical imaging (X-rays, CT scans); radiology applications. |
| Integrated Gradients. | Provides attribution scores along a path from baseline to input; less noisy than saliency maps; theoretically grounded. | Requires careful baseline selection; computationally intensive. | Deep learning models with structured or sequential data (e.g., respiratory signals, spatiotemporal patterns). |
| Counterfactual Explanations. | Intuitive “what-if” reasoning; directly actionable; useful for clinical/policy interventions. | Computationally complex; less standardized; limited adoption in air pollution. | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbehadji, I.E.; Obagbuwa, I.C. Explainable Artificial Intelligence and Machine Learning for Air Pollution Risk Assessment and Respiratory Health Outcomes: A Systematic Review. Atmosphere 2025, 16, 1154. https://doi.org/10.3390/atmos16101154
Agbehadji IE, Obagbuwa IC. Explainable Artificial Intelligence and Machine Learning for Air Pollution Risk Assessment and Respiratory Health Outcomes: A Systematic Review. Atmosphere. 2025; 16(10):1154. https://doi.org/10.3390/atmos16101154
Chicago/Turabian StyleAgbehadji, Israel Edem, and Ibidun Christiana Obagbuwa. 2025. "Explainable Artificial Intelligence and Machine Learning for Air Pollution Risk Assessment and Respiratory Health Outcomes: A Systematic Review" Atmosphere 16, no. 10: 1154. https://doi.org/10.3390/atmos16101154
APA StyleAgbehadji, I. E., & Obagbuwa, I. C. (2025). Explainable Artificial Intelligence and Machine Learning for Air Pollution Risk Assessment and Respiratory Health Outcomes: A Systematic Review. Atmosphere, 16(10), 1154. https://doi.org/10.3390/atmos16101154





