Abstract
Tree rings, tree needles, and moss can be used as biomonitors to evaluate atmospheric pollutant concentrations and deposition patterns spanning different timescales. This study compares output from air quality modeling and measurements to patterns observed using a combination of sulfur concentration and isotope composition in moss (using moss bags and controls) as biomonitors in a region of southern Alberta, Canada influenced by industrial emissions. Tree rings allow comparisons of historical to current sulfur deposition patterns. Moss, which integrates atmospheric nutrients during growth, allows for concurrent comparisons. The contrast of inorganic and organic sulfur within conifer tree needles provides a measure of pollutant uptake over their short lifespans. Sulfur uptake within biomonitors in a southern Alberta ecosystem allow assessment of the presence (in moss, needles) and effects (on conifer growth) of atmospheric sulfur deposition from industrial emissions. These data were examined relative to California Puff (CALPuff) model projections and traditional active and passive air quality sampling. Patterns in sulfur isotope abundance (δ34S) from moss bags placed throughout the eastern slopes of the southern Alberta foothills of the Rocky Mountains implicate local industry as the dominant atmospheric sulfur source over winter, with the tissues of conifers (needles and cores) and moss decreasing with distance from industrial emissions. This was consistent with apportionment calculations based on active and passive sampling, which also showed a surprising trend of sulfur deposition upwind of the industrial stack in the mountains to the west. δ34S values for pine needles and tree rings were consistent with greater sulfur stress and reductions in tree growth associated with increased industrial sulfur concentrations and deposition. We conclude that plant biomonitors are effective short-term (tree needles and moss) and long-term (tree cores) indicators of sulfur pollution in a complex, mountainous landscape.
1. Introduction
Quantification of the spatial patterns in pollutant dispersion and deposition in the environment from emission sources to distinguish current-day from long-term effects is useful in land management decisions and to assist with regional issues with air quality [1,2,3]. Previous research using biomonitors has considered pollutant patterns near roadways and large industrial emitters [4,5,6,7,8,9]. This earlier work has been extended to atmospheric emission attribution and spatial distribution in more pristine conditions and remote locations [10,11].
Isotope abundance measurements in plants that are used for biomonitoring research have been summarized in a series of previous studies [9,11,12,13,14,15]. Difficulties in obtaining sufficient sulfur for analysis from plant tissues that typically contain only a few percent sulfur and the necessity to establish large and consistent differences in δ34S between background and emissions have resulted in fewer environmental studies utilizing δ34S values [7,10,16]. Large differences between soil and industrial sulfur emission δ34S values have been leveraged to distinguish pollutant impacts on vegetation and surface waters [12,17,18,19]. Species-specific variations and plant tissues are important considerations for long-term biomonitoring [10]. However, biomonitoring results have not, to our knowledge, been compared to atmospheric measurements combined with high resolution modeling of spatial sulfur deposition and atmospheric SO2 concentrations. Here, we present results that demonstrate the utility of using sulfur isotopes within biomonitor tissues to distinguish historical and current industrial distributions.
Sulfur pollutants released to the atmosphere in the form of sulfur dioxide and sulfate have contributed to changes in surface water geochemistry [17,20,21]. Impacts spurred policy-makers to enact legislation and created opportunities and technologies to reduce emissions [22,23].
This study presents the results from fieldwork between 2000 and 2007 to assess the spatial footprint of emissions in a relatively pristine mountain region along the eastern slopes of the Rocky Mountains in southern Alberta, Canada. Our studies were conducted downwind and upwind of emissions and used both short- and long-term bioindicators along with passive and active air quality monitoring, and air quality and pollutant deposition modeling (CALPuff). Moss in bags, when compared to controls, provided insight into winter 2006–2007 spatial patterns in atmospheric sulfur, while first- and second-year tree needles provided insights at a slightly longer timescale. Finally, tree cores were used to assess the impacts throughout the growing period using tree ring width before and after industrial emissions commenced (~1947) by comparing sulfur content and isotope composition (δ34S). Spatial patterns from the passive samplers were compared to CALPuff model outputs. Biomonitoring results were used to assess whether additional atmospheric sulfur was primarily from industrial emissions, what the impacts on tree needles were, and whether the inorganic-to-organic sulfur ratios indicated sulfur stress. Finally, tree cores were used to examine the impacts of industrial sulfur on radial tree growth prior to and after the construction of sulfur-emitting industrial facilities in the region.
In this study we focused on how vegetation growing along the southwestern foothills of Alberta could be used to identify upwind (west) to downwind (east) differences in coniferous trees due to industrial emissions. We test whether plant tissues, specifically mosses, tree needles, and sulfur in tree cores, are potential biomonitors for the spatial footprint of pollutants released from industry over several months to decadal timescales.
2. Historical Land Use and Sulfur Emissions
Historically, emissions reductions strategies in Canada were successful in halving SOx emissions to approximately 0.26 million tonnes in 2017, compared to a decade earlier [24,25]. In Alberta, model estimates suggest between 20 and 40% of SO2 emissions remain in the province, while approximately 10% are transformed into atmospheric sulfate prior to deposition [26]. Despite these declines and the dispersion of SO2 by high winds along the eastern slopes, significant industrial sulfur can contribute to changes in soil and water chemistry near the emissions source [12,15,19,27]. Poorly developed and acidic soils supporting coniferous forests along the narrow band of forested regions along the eastern slopes of Alberta, and continued regional emissions from industrial activities, have the potential to place ecosystem functions at risk.
Tracking the effects from atmospheric deposition of pollutant sulfur from industrial emissions in the Alberta foothills has been successfully achieved using sulfur, boron, and oxygen isotopes [12,17,28]. However, previous research examined only receptors to assess environmental impacts rather than co-located atmospheric measurements and modeling and did not specifically address how sulfate deposition may have changed over time [17,19,29]. In this study we compare spatial patterns in sulfur deposition using the Lagrangian CALPuff model output to passive sampling in the winter of 2006–2007 and these measurements and modeling in turn were compared to vegetation used as biomonitors. This study reports changes in δ34S in moss, as well as tree needles and tree rings, as biomonitors to identify short- to long-term impacts from sulfur emissions on the eastern slopes of the Rocky Mountains in southern Alberta, Canada and to compare spatial patterns between what is observed in biomonitors with air quality modeling outputs.
3. Study Region and Methods
3.1. δ34S Values and Source Apportionment
The natural abundance ratio of 34S to 32S in samples was compared to the same ratio in an international standard: for sulfur isotopes, the standard is Vienna Canyon Diablo Troilite (V-CDT). δ values are reported using the following equation in parts per thousand (‰) where X is the element of interest (S) and the superscripts heavy (34) and light (32) refer to the isotopes of that element. Internal laboratory standards bracketing the sample δ34S compositions and calibrated to international references (IAEA S-1 and IAEA S-2 with δ34S values of −0.3 and +22.7‰, respectively) were used to determine the isotope composition. The precision for δ34S measurements reported here is ±0.2‰. Larger uncertainties were determined for replicate sample measurements for plant sample types based on the average from duplicates including sample preparation (±0.25‰).
δheavyX = [(heavyX/lightX)sample − (heavyX/lightX)Vstandard]/(heavyX/lightX)Vstandard
In Southern Alberta, the δ34S for SO2 from sour gas (H2S content > 4%) facilities has a value around +18‰, which is higher than sulfur in glacial till (near −18‰) and SO2 from vehicle exhaust in western Canada (+5 ± 1‰) [12,27].
3.2. Study Region
Exploration for oil and gas, primarily along the diagonal eastern slopes of the region shown in Figure 1, has intensified since 1947. Large fields of natural gas containing up to 34% H2S (sour gas) have produced marketable sales gas since 1955, when gas processing to remove H2S was developed at the southeastern gas processing facility (Figure 1). Processing sites separate sulfur from raw gas that has been piped from wells scattered along the eastern slopes and at lower elevations around the Castle and Carbondale rivers. Since 1955 much of the gas from the eastern slopes of southwestern Alberta was processed at two large facilities that in total were licensed to emit more than 52 tonnes per day of residual sulfur, primarily as SO2 from incinerators and flare stacks. The larger facility (42 tonnes S/day) is located to the south of Pincher Creek. Annual historical SO2 emissions south of Pincher Creek, shown in Figure 2, demonstrate a significant reduction in total sulfur stack emissions from the 1970s to 2009 [30]. A second gas plant processing sulfur northwest of the study area and east of Blairmore along Highway 3 was constructed in 1961. Its allowable emissions during the study period were 10 tonnes of sulfur per day. Based on this information, a date before the construction of the gas plants, 1947, was selected as the best date to represent a “before” and “after” impact in the assessment of data from tree cores in this study and the gas plant close to Pincher Creek was selected for analysis of distance effects.
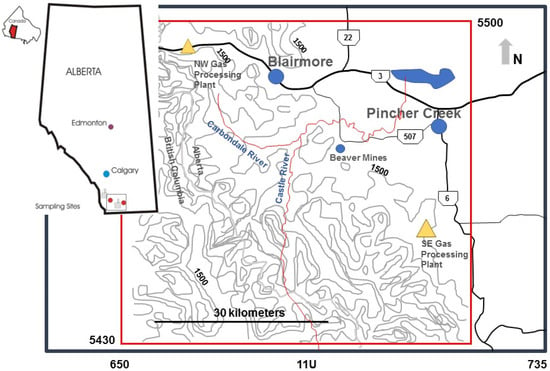
Figure 1.
The southwestern region of Alberta, Canada, where the study area is within the rectangle shown at a larger scale with digital elevation contours above 1500 m to 2500 m in 100 m increments for the 60 × 60 km2 study region of southern Alberta (Zone 11U). The location of industrial emitters of sulfur dioxide (gold triangles) and their relation to the towns of Pincher Creek and Blairmore (blue circles) as well as the Castle River are shown.
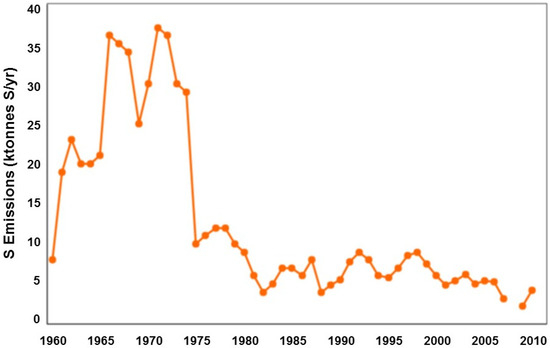
Figure 2.
Total atmospheric sulfur dioxide emissions (in tonnes of sulfur per year) from gas processing facilities south of Pincher Creek (after Eden 1996 [30]).
Sulfur dioxide is emitted after the gas has undergone refinement to remove up to 99% of the sulfur from the gas stream and is released to the atmosphere at temperatures exceeding 300 °C. It is expected, given the mountainous terrain in southwestern Alberta, that tall emission stacks (10 s to 150 m) and strong winds carry and disperse pollutants effectively downwind [22]. Predominantly westerly or southwesterly flows that characterize airflow in the region suggest that higher concentrations and sulfur deposition are expected to the east. Pollutant dispersion in complex terrain is difficult to predict, however, as complex patterns in turbulence leading to downwash conditions may occur [31]. Downwash conditions may sweep emissions high along the slopes and back westward, against prevailing wind conditions, into the mountains. Previous research on surface water and the Castle River using a multi-isotope approach has shown that significant sulfur deposition also occurs within the mountains directly west of the larger sour gas facility [19].
Sulfur dioxide is oxidized and forms secondary sulfate at ambient temperatures as it moves downwind. Flaring, where raw or partially processed gas is combusted during plant upset conditions, releases both SO2 and primary sulfate at high temperatures. Flares also occur at well sites after drilling when the size of a gas reservoir is evaluated, during uncontrolled release that can occur during drilling accidents (blowouts), or when pipeline breaks occur.
The release and deposition of sulfur within the study region is affected by strong meteorological and climate variations. The climate is characterized as humid continental, with long cool summers and a mean annual temperature of ~5 °C: mean annual precipitation ranges from 500 mm per year in the NW to 800 mm per year in the SW corner of Figure 1 [32]. The mean wind speed is 20 km/h, and the prevailing wind direction is from the west.
Atmospheric pollutant concentrations and deposition impact the vegetation in the region in a manner dependent on the terrain and underlying soil and bedrock matrix. The area is dominated by a Montane Cordillera ecoregion existing on shallow sedimentary bedrock and glacial till. Brunisols in the foothills support a mixture of alpine tundra and a dense conifer forest dominated by P. contorta (Lodgepole Pine) and Picea glauca (White Spruce), with some Populus tremuloides (Trembling Aspen) and Pseudotsuga menziesii (Douglas Fir). Dry grassland habitats have created Black Chernozem soils hosting Festuca pratensis (Fescue Grass). Vegetation used as biomonitors in this study includes moss common to the area (Pleurozem schreberi) and the two most common conifers: spruce and lodgepole pine (P. contorta).
3.3. Air Quality Modeling
California Puff Model (CALPuff: Atmospheric Studies Group (ASG), 2007, The CALPuff Modeling System, TRC Solutions, 2820 Pegasus Drive, Bakersfield, CA, USA, 1 April 2007), a Lagrangian–Gaussian puff model verified and accepted by the U.S. Environmental Protection Agency as well as the Alberta regulator, amongst others, to determine whether and when industrial emissions may exceed atmospheric concentration and deposition criteria and/or regulations, was used to evaluate non-steady state impacts from plume dispersion over a 60 km × 60 km grid at a 1 km resolution [33]. The model accounts for factors that influence emission deposition and is well suited to complex terrain (large elevation gradient) that affects wind field development and flow: this includes wet scavenging, dry deposition, simple chemical transformations, and most importantly, conditions where the air is considered stable. The distribution of SO2 and deposition of sulfate in complex terrain, with gas facility emissions of 42 and 10 tonnes of S/day, were evaluated using three-dimensional wind fields as inputs.
The land use classification parameters used to run the model for summer and winter are given in Table 1 [33]. The meteorological input file, based on Mesoscale Model (MM5) output for 2006, was divided into three subsets that used different winter, summer, and surface characteristics. The periods analyzed included 1 January–15 May, 16 May–15 October, and 16 October 16–31 December 31. Stack emission input parameters from 2006 were used to run the model and are given below in Table 2. Stability classes (0–1.8, 1.8–3.3, 3.3–5.4, 5.4–8.5, and >8.5 m/s) were assessed.

Table 1.
Seasonal variations of surface characteristic values used in adjusting CALMET files for summer and winter variation. (LAI = leaf area index).

Table 2.
Incinerator stack input parameters and SO2 emissions for 2006 from the northwest and southeast gas plants shown in Figure 1.
CALPuff was run for the entire study period of one year. Although the aim of this study was to model SO2 dispersion and sulphate deposition, the concentration of NOx emitted by processing plants was required in order to accurately account for chemical transformations. Atmospheric components that were modeled included SO2, SO4, NO, NO2, HNO3, and NO3. Background concentrations of O3 and NH3 were augmented in order to achieve a closer resemblance to the study area. Ozone was specified as 40 ppb and ammonia as 0.22 ppb. The deposition properties of each species were chosen by selecting dry or wet deposition and gas or particle phase and the default options were used. Model default settings from the CALPuff species library values (MESOPUFF 11 scheme) were used for chemical transformations for SO2, SO4, NO, NO2, HNO3, and NO3. The dry deposition velocities for SO2, NO, NO2, and HNO3 (inputs as diffusivity) were 0.151, 0.135, 0.166, and 0.163 cm2/s. The dry deposition velocities for SO4 and NO3 (diffusivity) were based on two aerosol size classes: 0.48 and 2.0 microns in diameter: default values were used. PG dispersion for rural areas was computed using the ISCST multi-segment approximation. The concentrations for species of interest were calculated using eight layers above ground level: 20, 40, 80, 160, 320, 1250, and 2500 m for 1 km × 1 km spacing, with a total of 3600 receptor points.
3.4. Air Quality Measurements
Temporal and spatial SO2 and aerosol sulfate variations were assessed using two active and one passive sampling technique combined with stable isotope apportionment [34].
High-volume samplers (HVSs, 1.3 m3/min), one for size-segregated aerosols and the other for total sulfate and SO2, were used for active air quality measurements. Two low-volume samplers (0.003 m3/min) were used to calibrate passive samplers at the sites: one nearby and directly downwind of the southeast facility shown in Figure 3, and another at a site to the northwest at the same location as the high volume air sampler. Using the high-volume sampler, air was drawn through either a six-stage cascade impactor, or through a particulate filter followed by a filter treated with a potassium carbonate—glycerol solution to collect SO2. Filters were changed approximately every two weeks (to ensure sufficient sulfur was collected for isotope determination of SO2 and sulfate) from September 2006 to mid-February 2007 with the start and stop dates and times recorded (green square near Beaver Mines in Figure 3). Measurements using high-volume samplers were also performed east of the SE plant in 2003 and 2004 using HVSs fitted with PM2.5 heads to assess emissions characteristics immediately downwind of the southeast gas processing facility.
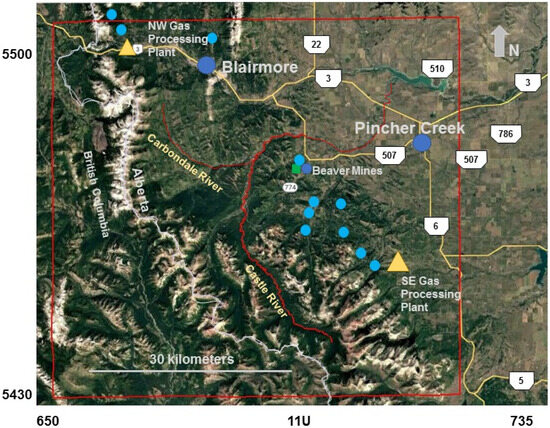
Figure 3.
Map (Google Earth) with showing 60 km × 60 km grid encompassing the region modeled (red line) relative to the towns of Blairmore and Pincher Creek and the hamlet of Beaver Mines (blue dots). Sour gas processing facilities (yellow triangles) and locations where co-located tree core and tree needle samples were collected (light blue circles) are shown. The location of the co-located high-volume and low-volume samplers for air quality measurements from 2006 are depicted by a green square. UTM coordinates (km) for Zone 11U are shown.
Passive samplers were fitted with a treated filter to capture SO2 and placed 1–1.5 m above ground level on trees within the region (sites are shown in the results section for clarity). Duplicate passive samplers were deployed at 46 locations throughout the region in order to ensure sufficient sulfur for detection was collected by combining the two filters. Due to the wide spatial distribution and field conditions, two trips were required to collect the samples, so a scaling factor for the number of days for each set was applied. Passive samplers in the northwest were exposed from mid-October 2006 to mid-March 2007, while those to the southeast were exposed from mid-October 2006 to mid-February 2007. A number of the passive monitors were missing from the field location or invalid (buried within the snow), so a total of 19 rather than 46 sites are reported here.
Filter samples, returned to the laboratory, were treated according to methods described in the literature [34,35,36]. Ion concentrations from all filter extracts were measured by Ion Chromatography calibrated against known standard concentrations spanning those of the samples. Passive sampler concentrations were calibrated to the high-volume sampler as the low-volume samplers were affected by power outages.
3.5. Sulfur in Moss
A paired “exposed versus control” study was designed using moss in bags to assess whether pollutant emissions (δ34Sa ~+18‰) throughout the relatively remote southern Alberta region were detectable through the winter months of 2006 through 2007 [37]. Moss (P. schraeberi) was collected near Spray Lakes in Kananaskis Country approximately a few hundred kilometers to the northwest of the study area in Alberta and straight west of the city of Calgary (Figure 1). The paired design contrasted moss from the control site (Kananaskis) to the same moss placed in the southwest study region between the southeast and northwest gas plants, as shown in Figure 1. Moss collected in the Kananaskis was sealed in an airtight bag and stored at 4 °C overnight before being transported to southwestern Alberta. Moss was placed in mesh bags that were secured on trees of the same species and at the same height above ground level as in the Kananaskis. Twenty sites at the Kananaskis and the southern study area were sampled: 10 at 1500 m and 10 at 1650 m elevation. Moss samples were exposed for approximately 3 months (November through January).
Moss samples were returned to the lab in ziplock bags, air-dried, and then ground and analyzed after Parr Bomb ignition to determine their sulfur contents and sulfur isotope composition [34]. Differences in total sulfur content and isotope composition between the control (Kananaskis) and the exposure were used to evaluate atmospheric sulfur from sour gas production.
3.6. Conifer Needles
Conifer tissues intercept pollutant (subscript a) and background (subscript b) SO2 and sulfate from the atmosphere (δ34Sa ~+18‰, δ34Sb ~ +5‰) and incorporate soluble sulfate from the soil (subscript s: δ34Ss ~−18‰ for pyrite dissolution) for plant growth [18,28,35]. Sample sites along the eastern slopes in the study area that were accessible from nearby road networks were chosen to be as similar as possible with respect to vegetation cover, tree density, soil type, stand elevation, and slope aspect. Sites were selected as well for the presence of both spruce (for the needle study) and large pines (for the tree core study). Elevation ranged from 1376 m to 1758 m. Spruce tree needles in moderately dense spruce and pine forests 40 years or older were collected nearby the blue circles in Figure 3 between 13 November and 17 November 2006. First- and second-year spruce needles were collected at eye level from two trees of a similar age, stand composition, and stand density [38]. Branches were bagged, labelled, and stored at 4 °C prior to analysis. Forest soil litter samples (see Supplementary Material S1: Soil), co-located with the vegetation samples, were collected and analyzed for isotope content, soluble sulfate, and total sulfur to provide information on the sulfur contributions from the local litter for comparison to the results for the study on needles.
Levels of gaseous pollutant accumulation can be detected through the analysis of the inorganic-to-organic sulfur (Si/So) ratio in the needles [38]. First- and second-year needles were pooled and dried at 70 °C overnight, ground with a Wiley Mill using a 40 micrometer mesh, and analyzed for total sulfur (So) using the Parr Bomb method described for mosses. Inorganic sulfate (Si) was determined using hydroiodic acid (HI) [38].
3.7. Tree Core Methodology
Lodgepole pine (Pinus contorta) tree cores were collected at sample sites chosen to be as similar as possible with respect to vegetation cover, tree density, soil type, stand elevation, and slope aspect: elevation ranged from 1376 m to 1758 m. At each sample site, two living specimens of P. contorta, no farther apart than 25 m, were selected for core sampling. Three core samples ~50 cm above ground level, separated 5 cm vertically, were collected from each tree using a 5 mm increment borer to ensure there was enough wood for analysis to gain insight into pre- and post-industrial conditions (1947: see Section 3.2). Cores were stored in plastic straws and refrigerated prior to laboratory analysis. Tree ring width measurements were made using an optical microscope with an ocular micrometer eyepiece and were recorded, counting inwards from the latest growing season (summer of 2006) towards the pith. Average tree age was 74 years old, while the oldest was 111 years old and the youngest 44 years old. The wood was then split at 1947 into pre- and post-industrial samples. Wood grown after 1947 to the present day was considered post-industrial, as it had grown while oil and gas development was occurring in the region. Wood grown prior to 1947 was considered pre-industrial, as it grew before the first exploratory gas well was drilled.
We expected growth to be affected by weather (average minimum temperature in the season of maximum growth (April to August), year (our samples spanned the period of industrial expansion, i.e., before and after 1947), and sulfur (two traits, measured once before and after 1947 in each tree core). We therefore analyzed radial annual growth using mixed-model regression predicting growth (ln (mm)) from year, average minimum daily temperature (April to August) from the previous year, cumulative summer precipitation (April to August) from the previous year, period-level δ34S, and period-level sulfur content, with the random effects of site, tree (site), and core (tree). We present the partial effects of year on growth as a regression line (i.e., the model fit) and supplement this with a spline fit to the partial residuals from the model to elucidate any possible non-linear trends. It is important to remember that the model has two variables (both measures of sulfur that have only two measurements for each tree core}. Therefore, we adjust the p value for the t tests involving sulfur by using smaller degrees of freedom (dfs) to reflect actual levels of replication.
4. Results and Discussion
Biomonitors reveal spatial patterns in atmospheric pollutant concentrations and the cumulative deposition of sulfur emissions across an ecosystem. The intent of this study was to compare spatial patterns in short- and long-term receptors (biomonitors) within the Castle River ecosystem to expected concentrations and deposition patterns based on the emissions from two large industrial sulfur sources based on an upwind/downwind design. Comparisons of spatial and sulfur content in biomonitors relative to air quality measurements and modeling are used to demonstrate whether biomonitor indicators, air quality measurements, and model data agree.
In addition to measuring biomonitor and atmospheric sulfur concentrations, patterns in δ34S values for tree rings, needles, and moss exposed in bags can help attribute the source of sulfur as distinct isotope compositions for industrial emissions (+18‰) and background sulfur (+5 and −18‰, respectively, for background air and soluble sulfate from pyrite dissolution in the underlying till) occur in the foothills of Alberta. Isotope apportionment models were used to identify the proportion and amount of sulfur from emissions from oil and gas activities from air quality monitoring (See Supplementary Material S2: Isotope Apportionment, Figure S4).
4.1. CALPuff Model Outputs
Predicted patterns for SO2 and sulfate deposition within the study area were determined using the receptor concentrations at 20 m and 0 m, respectively, for each 1 km × 1 km output modeled by CALPuff (Figure 4 and Figure 5).
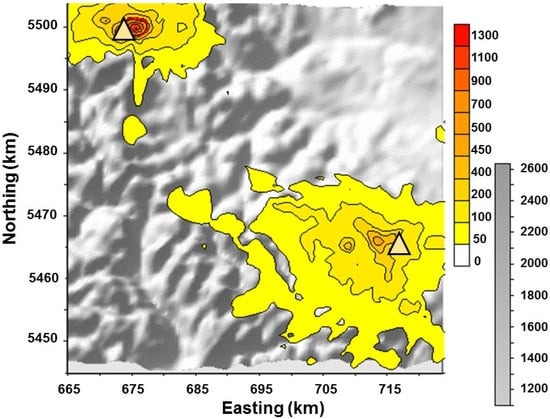
Figure 4.
Outputs from the CALPuff model at a 1 × 1 km resolution for the spatial distribution of maximum hourly SO2 concentrations over the year (in micrograms of sulfur per cubic meter: μgS/m3: yellow-to-red colour scale and overlay contours) are shown as an overlay on a digital elevation map where the terrain ranges from 1100 to 2600 masl (grey scale). The x and y axes are easting and northing (km) in UTM coordinates (Zone 11U), while the elevation is shown in greyscale as a base map. Gas plants in the NW and SE are shown as yellow triangles.
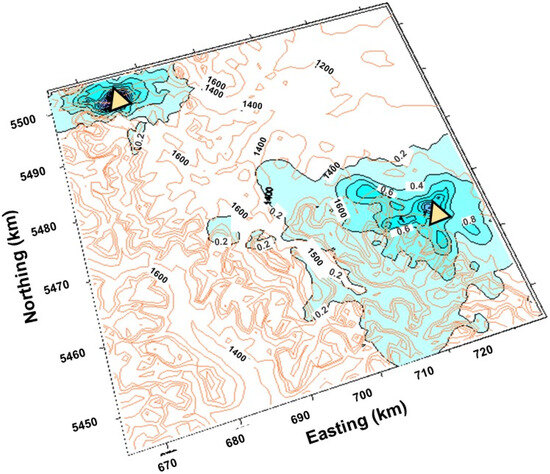
Figure 5.
Spatial patterns at 1 km × 1 km resolution in sulfate deposition based on CALPuff (μg SO4/m2/s) outputs for 2006. The x and y axes (km) are UTM coordinates (11U) for the contour map (auburn contours, black text) are shown. Emissions contours (blue overlay, grey text) are overlaid on the elevation contours. Gas plant locations are indicated by yellow triangles.
Spatial variations in hourly maximum SO2 concentrations and sulfate deposition are based on the highest concentrations over the modeled year for the Rocky Mountains in southwestern Alberta. Sulfur dioxide from industrial emission sources modeled using CALPuff is seen to be largely confined to the same valley as the processing plant in the northwest with exceedances above air quality limits (red contour) of 450 μg/m3, whereas that from the southeastern facility is largely more dispersed (Figure 4). Unexpectedly, the results do not show a distinct pattern of transport of concentrations of SO2 < 50 μg/m3 to the west and >50 μg/m3 concentrations downwind of the industrial facilities to the east. There is an elongation in the contours for SO2 concentrations between 50 to 400 μg/m3 eastward of the southeast facility, but significant upwind SO2 distribution for the 50 and 100 μg/m3 contours within the valleys to the west as well. This confounds our biomonitoring upwind/downwind sampling design. Instead of moving dominantly eastward, the emissions migrate upslope into the Rocky Mountains west of the gas facility, including into the Castle River drainage during unfavourable (easterly upslope) and downwash conditions [19].
Outputs from the CALPuff model for sulfate deposition in μgSO4/m2/s at 1 km × 1 km resolution using MM5 wind fields for 2006 are shown in Figure 5. The spatial patterns in sulfate deposition in Figure 5, and SO2 concentrations in Figure 4, are notable. As was the case for SO2, sulfate deposition appears confined to the valley around the northwest facility but reaches westward into the valleys upwind of the industrial facility at the lowest deposition contour of 0.2 μgSO4/m2/s in the southeast. Higher deposition (0.4 to a maximum of 1.0 μgSO4/m2/s) stretches immediately eastward of the southeast gas plant but also demonstrates an 0.4 μgSO4/m2/s contour stretching along the eastern slopes to the northwest and southeast.
4.2. Atmospheric Sulfur Concentrations Using HVSs
In comparison to the CALPuff model outputs, the SO2 concentrations from active high-volume samplers (HVSs) to the east of the larger gas facility, and integrated over two-weeks, ranged from 0.09 to 3.1 μg S/m3 (mean 1.3 ± 0.9 μg S/m3, n = 17). Concentrations of SO2 at the site mid-way between the SE and NW facilities (Figure 3) ranged from 0.001 to 0.25 μg S/m3 (mean 0.08 ± 0.11 μgS/m3). Note that the measured maximum two-week SO2 concentration (3.1 × (64 g/mole S)/(32 g/mole SO2) = 6.2 μgSO2/m3) to the east of the facility agrees well with the CALPuff-modeled outputs shown on a plot of daily maxima (5 μgSO2/m3) along the lowest contour in Figure S5 (see Supplementary Material S3: CALPuff Modeling Daily SO2 Maxima).
Measured sulfate concentrations, based on active HVS sampling, were similar to those for measured SO2 and size-segregated sampling. Measurements for aerosols <2.5 microns diameter showed that the aerosols affected were concentrated within the inhalable range. The average sulfate concentration on PM 2.5 to the east was 0.07 ± 0.06 μg S/m3 (n = 8) for the campaign while the sampler located mid-way between the processing facilities was almost double 0.13 ± 0.10 μg S/m3 (n = 7), suggesting a local hotspot that is not captured by the CALPuff sulfate deposition modeling shown in Figure 5. This discrepancy between the HVS-measured sulfate and CALPuff outputs could be due to a number of factors including the following:
- The inability of the model to accurately capture concentrations of sulfate at a resolution of 1 km × 1 km under complex terrain conditions for the region.
- Local industrial emissions from upstream sites that produced a higher proportion of sulfate to SO2 than are released from the tall incinerator stack at the gas processing facilities.
- The modeled conversion of SO2 to SO4 is inaccurate under the sampling conditions during the campaign.
The inability of the model to accurately capture concentrations of sulfate at a 1 km × 1 km resolution (as above) seems unlikely given the good agreement between the measured and modeled SO2 at both high-volume sampler locations close to and far from the southeastern industrial emission source. Scenarios (b) or (c) are more likely. There are three sour gas wells with a high sulfur content gas > 30% within ~2 km of the HVS site shown in Figure 3 that occasionally flare during upsets, rather than sending the gas along pipelines to the southeast facility. These events are more common on cold winter days when liquid sulfur deposits may condense on the interior of the pipes, plugging them. Flaring produces a higher proportion of sulfate to SO2 than the emissions stacks at industrial facilities that are regulated and emit >95% of their stack sulfur as SO2. Flaring also produces vaporized hydrocarbons and water vapour at flare stack temperatures that can produce effective aerosol surfaces for SO2 to quickly oxidize to sulfate. Newly formed aerosols are typically < 2.5 microns, and this is consistent with the observed aerosol size measurements from the HVS at the site mid-way between the two industrial emitters shown in Figure 3. Scenario (c) is also possible but an evaluation of SO2 to aerosol sulfate conversion is beyond the scope of the limited data produced from the set of HVS measurements performed in this study.
Sulfur isotope apportionment confirms that the majority of atmospheric sulfur in the region is industrial in origin. The percent of sulfur attributable to industry at the site shown in Figure 3 was
- 62% (51–76) of the aerosol sulfate < 0.5 microns in diameter,
- 58% (47–71) of total aerosol sulfate,
- 62% (50–75) of the SO2.
On the prairies to the east of the southeast gas facility, the proportions with an industrial origin were higher:
- 77% (64–91) of the aerosol sulfate < 2.5 microns,
- 73% (61–85) of the total aerosol sulfate,
- >95% (87–100) of the SO2.
The calculations assumed that gas plant emissions have relatively uniform δ34S values of +18 ± 2‰ and that background sulfur, composed of vehicle exhaust and wood combustion in the region, has a δ34S value of +5 ± 1‰ (Figure S4) [19,28]. Sensitivity analyses are given in parentheses. Background sulfur is present in the air at a low concentration throughout the northern hemisphere and, in the absence of anthropogenic emissions, would be derived from a mixture of lithogenic, biogenic, and volcanic sources in the study region. Air quality measurements conducted at the site of the green square in Figure 3 between 2000 and 2006 demonstrate that when the air reaches its lowest sulfur concentrations, the sulfur isotope composition is approximately +5‰. This is consistent with the isotope composition of wood combustion (unpublished results by the author for samples collected during a forest fire and during winter conditions when wood combustion markers were present in the samples) and vehicle exhaust for western Canada [39].
4.3. Passive Versus Active Sampling and CALPuff Outputs
Concentrations of SO2 from CALPuff ranged between 0.18 and 2.7 μg/m3 at the grid points containing passive samplers. The measured passive atmospheric sulfur concentrations calibrated using the high-volume sampler ranged between 0.02 and 0.14 μgS/m3 (mean 0.06 ± 0.03 μg S/m3, n = 20) during the winter months, which is more than a magnitude lower concentration than CALPuff (blue squares, Figure 6A). This was surprising given the good agreement found between the model and the HVS SO2 concentrations described in the previous Section 4.2. Therefore, passives were calibrated to the high-volume samplers instead of the intended low-volume samplers (orange squares, Figure 6A). Unaccounted for power outages in the low-volume samplers may not have been as noticeable to residents as at the HVS sites due to their lower noise levels and placement at somewhat less frequently checked locations. Although the HVS calibration of the passives resulted in better agreement in the range of concentrations (0.2 to 1 μgS/m3: 0.4 to 2 1 μgSO2/m3) with CALPuff SO2 (0.25 to 2.7 μgS/m3), it is evident in Figure 6A that the modeling and measurements do not match using the assumption that the concentrations are uniform at the individual sampling sites across a 1 km × 1 km grid scale (Figure 6A). The spatial analysis (Figure 6B), however, shows reasonable agreement with the CALPuff model output (Figure 4).
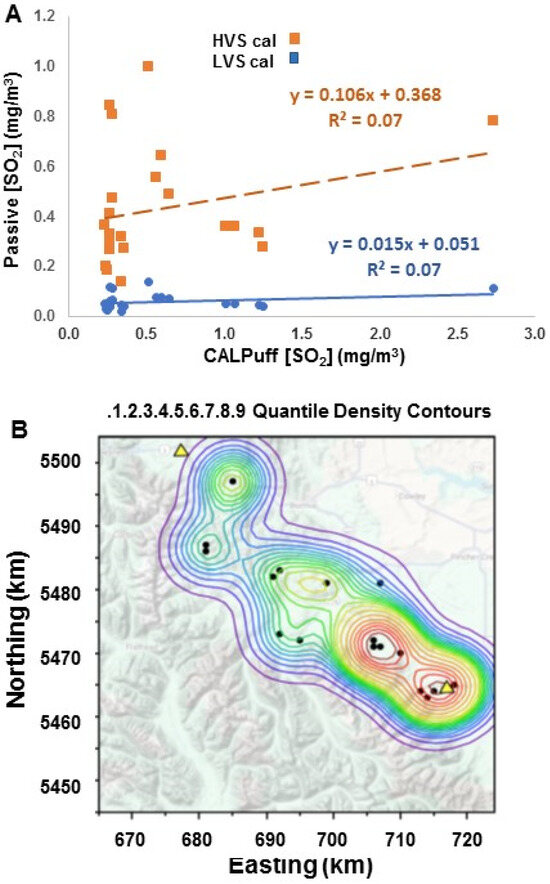
Figure 6.
(A). Passive atmospheric S concentrations calibrated to low- (blue, p = 0.00013) and high- (orange, p = 0.27) volume active samplers versus CALPuff annual SO2 concentrations at receptor sites corresponding to passive monitor locations. (B). Interpolated distribution in passive SO2 concentrations (HVS calibration) co-located with passive monitors at 19 passive sampler sites based on paired samplers between October 2006 and March 2007. (kernal smoother = 4, quantile densities are 0, 2.25, 4.08, 5.33, 6.33, 7.75, 9.42, 11.58, 14.58, 16.75, and 18.75 in parts per thousand × 10−4).
4.4. Spatial Patterns in Plant Tissues
The spatial maps reveal strong distance-based trends in atmospheric sulfur on the landscape. Were these detectable in simpler analyses of sulfur in the tissues of our bioindicators based on distance from the principal emitter (the southeast facility)? Yes, indeed. All three bioindicators showed a pattern of decreasing δ34S with distance from the southeast industrial site. The pattern for spruce needles was statistically significant (Figure 7A) while the patterns for tree cores (Figure 7B) and moss (Figure 7C) had a marginally significant relationship (p = 0.053 and 0.055, respectively). These results are consistent with the results above from the CALPuff model output, suggesting lower annual hourly SO2 concentrations and sulfur deposition along the eastern slopes to the northwest of the southeast facility (Figure 4 and Figure 5). They also corroborate the general spatial patterns observed for the passive monitors as well (Figure 6B).
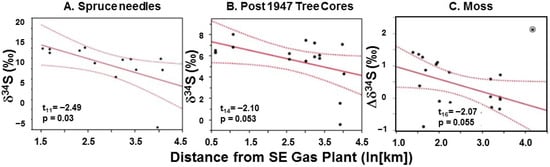
Figure 7.
First and second spruce needles (A), as well as the portion of tree cores from pines (B) post-1947 and moss (C), show spatial effects (ln (distance)) with δ34S, as a proxy for sulfur emissions/deposition attributable to industry correlating with distance from the southeast facility (p < 0.1). The moss regression excludes a significant (p < 0.05, Malhaldnobis Distance = 2.1) outlier, circled in the figure. Measurement uncertainties (δ34S ± 0.25‰) are not shown for simplicity.
All three biomonitoring approaches used here (first- and second-year spruce needles, post-industrial pine cores, and exposed bagged moss compared to controls), despite representing medium (A), long (B), and short-term (C) sulfur deposition, show similar patterns in sulfur isotope abundance with their distance from the dominant emission source. Note that when needle δ34S values are plotted against distance from the southeast (SE) gas plant, the intercept is +18.4‰, which is in good agreement with the expected value for sour gas emissions from that facility (See Supplementary Material Figure S6).
Together, these data suggest that all three approaches are useful when there is sufficient isotope discrimination between the dominant emission source and background δ34S. However, we recommend that more samples with greater spatial distribution from the main emission source are used in future studies to improve the statistical validity of these results.
4.5. Mosses as Biomonitors
Mosses and lichens are often used as bioindicators for atmospheric pollution because they lack a root system, and derive their nutrients directly from the atmosphere [10]. Sulfur uptake by moss is affected by variations in weather, the chemodynamics of each plant, rates of wet and dry deposition, and precipitation, which results in variable but minimally fractionated sulfur isotopes [5,8,15,40]. δ34S values for mosses are therefore representative of the surrounding air [5].
Additional sulfur found in mosses in the study region relative to paired controls were correlated with more positive δ34S values after exposure (r2 = 0.64, p < 0.05: Figure 8). This reinforces the findings above for atmospheric measurements and demonstrates that sulfate from gas processing accounts for the majority of sulfur above that in background air in the region.
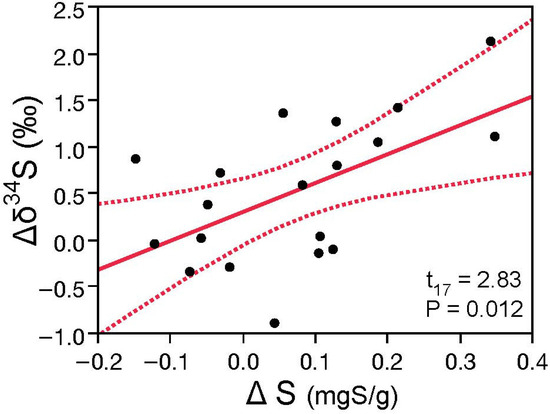
Figure 8.
Concentration and isotope differences in mosses exposed within the study and control sites are well correlated (r2 = 0.64) and reinforce industrial emissions as the source of additional sulfur in the air in the southern Alberta foothills. For clarity, measurement uncertainties of ±0.22 mgS/g and ±0.25‰ for differences in concentration and δ34S values, respectively, are not plotted.
4.6. Tree Needles as Indicators of S Stress
Sulfur dioxide can cause stress to conifers [41,42,43]. When sulfur is present in excess of what is required for plant growth, accumulation may occur within plant tissue: in conifer needles this is exhibited by an increase in inorganic sulfur [38]. This tendency of plants to accumulate sulfur makes them key indicators of industrial pollution impacts. Levels of gaseous pollutant accumulation can be detected through the analysis of the inorganic-to-organic sulfur (Si/So) ratio in the needle composition [38]. Sulfur in organic form within needle tissues does not respond to atmospheric pollutants as rapidly as inorganic sulfur in the needles; it will instead be limited by other nutrients affecting tree growth. The ratio is a useful indicator of ecosystem health in areas under sulfur pollution stress and has the potential to be an early warning environmental management tool: conifers with Si/So ratios greater than 0.4 are considered to be an indicator of incipient injury [38,43].
Combined first- and second-year needle data for the inorganic sulfur fraction and isotope composition from the two trees at each site, for each of the sites sampled in the study region, are shown in Figure 9. We see higher δ34S (HI) values at low Si concentrations and the intercept is +23‰ (the 95% confidence interval for the intercept is shown as horizontal red lines on the y axis, +11 to +35‰, which includes the value of +18‰ expected for industrial emissions, and is significantly greater than the values expected from a mix of vehicle exhaust, +5‰, and soil, −18‰). Further, the Si/So ratios found (Figure 10) suggested that trees closer to the gas processing facilities were stressed: the Si/So ratios were above 0.4 for one site immediately west of the southeast stack (Si/So = 0.70 ± 0.67) and at a site 8 km east of the northwest stack (0.56 ± 0.31). Inorganic-to-organic sulfur ratios ranged from 0.1 to 0.35 at the remainder of the sites. These data suggest spruce trees experienced more inorganic sulfur in first- and second-year needles and increased sulfur stress from industrial emissions that resulted in atmospheric sulfur deposition.
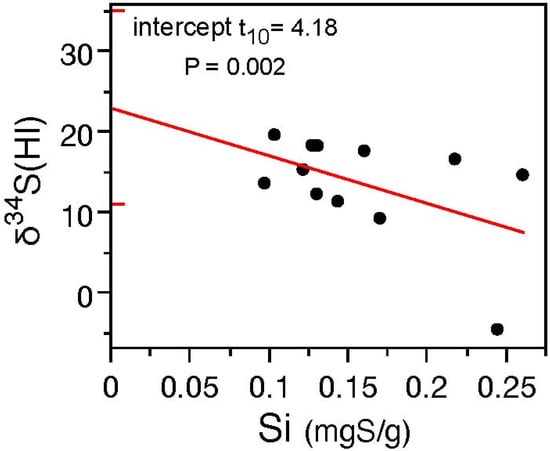
Figure 9.
The sulfur isotope composition of inorganic (HI) sulfur in conifer needles is negatively correlated with inorganic sulfur content (Si: r2 = 0.25). A positive intercept with δ34S = +23‰ roughly coincides with measurements of elemental sulfur from the industrial facility (~+18‰) and elevated atmospheric sulfur as SO2 and sulfate from the high-volume sampler filters operated downwind of the pollutant source (+18 to +21‰). Horizontal red ticks on the y axis show the 95% confidence interval of the intercept.
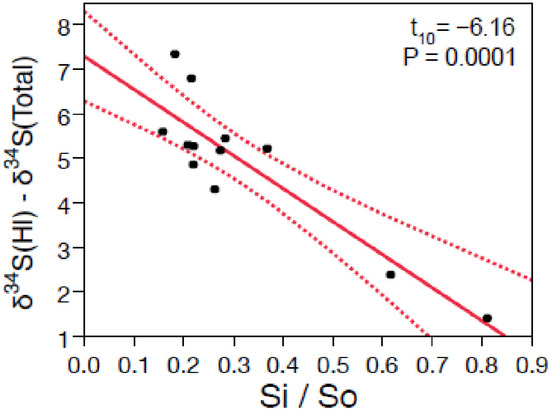
Figure 10.
The difference between tree needle inorganic and total sulfur δ34S values is significantly correlated with the inorganic (Si)-to-organic (So) sulfur ratio that indicates whether trees experience sulfur stress (Si/So > 0.4).
Tree needles display a large difference in their inorganic-to-organic sulfur isotope composition (Δδ34S) from near +1 to +8‰ (Figure 10). Δδ34S values represent the difference between the inorganic (HI) and total sulfur fractions in conifer needles, and this difference is significantly correlated with the inorganic-to-organic sulfur content (b = +7.6± 0.7‰: r2 = 0.8: p = 0.0001). Interestingly, trees that are stressed (Si/So > 0.4) display less of a difference in Δδ34S compared to those that are less stressed (Si/So < 0.4) [38]. Organic sulfur in first- and second-year needles represents the isotope composition within the growth period that is formed largely by the uptake of inorganic sulfur from the soil. Inorganic sulfur contains sulfate from the soil and sulfate that is deposited from atmospheric deposition. When atmospheric deposition is high, it is revealed as higher Si within conifer needles [38]. When the δ34S values for the inorganic sulfur in the needles closely match that of the organic sulfur in the needles (and soil), high atmospheric sulfur deposition is implicated. The data from the sites closest to the industrial facilities in the NW and SE display the lowest Δδ34S in Figure 10, and are consistent with the CALPuff model output showing that the highest sulfur deposition occurs nearby the gas plants in Figure 5.
To understand these results, it is worthwhile considering how δ34S values in plant tissues and pyrite dissolution may have been altered by industrial sulfur deposition over time. Prior to industrial sulfur emissions, soils and plant tissues are expected to reflect a mix of background atmospheric sulfur (δ34S ~+5‰) and sulfate from pyrite (δ34S ~−18‰). After industrial sulfur emissions impacted the region’s soils and vegetation, sulfur with a δ34S of ~+18‰ (See Figure S6) would become increasingly important, first to just the inorganic sulfur fraction. Over time, this sulfur would be incorporated into tissues and form litterfall and affect the surface litter layer, and then organic horizons. Finally, the lower inorganic soil horizon would progressively demonstrate higher δ34S values reflecting sulfur inputs, which are minimally affected by fractionation during soil sulfur mineralization (<1.5‰, favouring 32S in labile sulfate, [44]). In fact, this is exactly what ([12]; see Figure 7.13 Ram River) demonstrated in an intensive study of plant tissues and soils at a sulfur deposition site in central Alberta. Over time, the difference in isotope composition of the plant tissues (with atmospheric background sulfur δ34S ~+5‰ and industrial sulfur δ34S ~+18‰) and pedogenic sulfur (a mixture of background organic matter (δ34S ~+5‰) and sulfate from mineral dissolution (largely pyrite δ34S ~−18‰)) will converge to some new value that is intermediate to the end-member compositions. Further, the convergent plant tissue and soil δ34S values should be apparent closer to the source of industrial emissions, and in needles that display higher Si/So ratios. As soils develop a lower pH in response to continued atmospheric deposition, less of the mobile soil sulfate in the inorganic sulfur fraction (HI) will be retained in the organic soil layers where roots are located and be unavailable for plant uptake. The ability of soil to buffer acid deposition is measured as the cation exchange capacity (CEC). Figures S1A,B and S2 form a plausible explanation for the relationship seen in Figure 9: inorganic S needle content is lower when the δ34S value of the inorganic sulfur fraction in needles approaches the δ34S value of pollutant sulfur. Here, we posit that high δ34S (HI) in needles associated with a low Si needle concentration indicate high deposition of atmospheric pollutant (Figure 9). Soil sulfur mineralization producing soluble sulfate from organic sulfur turnover in coniferous forest soils is attended by a small sulfur isotope fractionation of <1.5‰, increasing the δ34S of the remaining organic sulfur in soil. This happens as the lighter 32S is mobilized to soluble sulfate, which is more labile, and is exported to lower soil horizons and eventually into the groundwater and surface water leaving the catchment [44]. As sulfur deposition increases, Si/So declines as nutrients including Si are leached from the soil root zone with additional acid deposition (see Figures S1 and S2 and accompanying text). This is a plausible interpretation for the results in Figure 9. At higher levels of atmospheric industrial sulfur deposition, the δ34S for inorganic sulfur in needles increasingly reflects the atmospheric source (the intercept in Figure 9). Over decadal timescales, as the proportion of inorganic-to-organic sulfur increases (higher Si/So), the sulfur incorporated into plant tissues increasingly reflects the effects from the atmospheric deposition source and the difference between the δ34S of total and inorganic sulfur will become increasingly smaller (Δδ34S decreases: Figure 9, Figure 10 and Figure S6). A slope of 0.83 is found when the δ34S values for total sulfur in litter and needles are plotted (r2 = 0.84: see Supplementary Figure S1A) and improves when the total sulfur in needles and sulfate in litter are compared (m = 0.99; r2 = 0.86). As the CEC declines, the litter sulfate δ34S values increase (Figure S2). Together these data suggest that the difference in isotope composition (inorganic—HI) versus Si/So may be a sensitive indicator of pollutant impacts on conifers in locations where atmospheric and pedogenic δ34S values are significantly distinct. The results are consistent with the long-term deposition of industrial sulfur leading to sulfur stress (high Si/So ratios) and a loss of buffering capacity in litter that potentially results in the export of soil organic matter (that retains nutrients within the root layer) downward in the soil column. Trees are able to adapt as roots shift lower in the soil column but discrepancies in the speed at which nutrients are lost in the organic horizons could plausibly affect tree growth.
4.7. Tree Cores as Biomonitors
Tree growth is well known to be affected by atmospheric SO2 as a result of deposition of sulfur into the soil [41,42,43,45]. Keller (1980) experimentally demonstrated that reduced ring width was a response to SO2 deposition [46]. Similarly, reductions in the growth of Japanese black pine (Pinus thunbergii) were mainly caused by SO2 pollution, and δ34S values in tree rings have been a useful proxy for evaluating the source of atmospheric sulfur deposition [47,48].
Here we used a mixed-model regression of tree ring width (i.e., radial growth) that included the mean δ34S values as well as sulfur content to test whether the negative effects of sulfur on tree growth could be observed in this study, while also considering the long-term pattern of growth (i.e., year) and the effect of two features of weather experienced during the growing season in the previous year known to affect tree growth: average minimum daily temperature and summed precipitation (both measured in the April to August time period). The model identified the year, sulfur content, mean δ34S, and mean minimum daily air temperature (April to August) for the region as the top four components that explained 29.5% of the variation in ring width. The radial growth rates of trees decreased linearly through the 90-year period of study (Figure 11A), but a spline fit through the adjusted values (the blue line in Figure 11A) shows that the growth rate was roughly constant until around 1950, after which it decreased relatively linearly. Tree growth decreased with increased sulfur content, higher δ34S values, and mean daily minimum temperature (Figure 11B–D). Trees impacted by atmospheric pollutant deposition from industrial sources would be expected to show decreased growth with sulfur content and δ34S in this study, as the organic layer in the soil becomes increasingly impacted (as also hypothesized for tree needles).
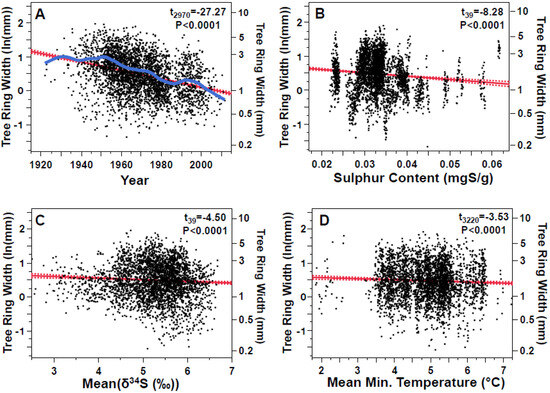
Figure 11.
Partial effects plots for a mixed-model regression (r2 = 0.56), described in the text, explaining width of tree rings (i.e., growth, ln-transformed to normality) in cross-sections of lodgepole pine trees. (A). Growth decreased with time (slope = −0.012). A spline (blue line, λ = 1000), not part of the model fit, allows visualization of any non-linear trends in the data attributed to changes in the industrial landscape. (B). Growth decreased with sulfur content (slope = −9.115). (C). Growth decreased with δ34S (slope = −0.048). (D). Growth decreased with average daily minimum temperature in April to August of the previous year (slope = −0.032). Lines show regression slopes with their 95% confidence intervals.
In general, we expect warmer summer temperatures to increase the growth of temperate trees [49], including the growth of lodgepole pines [50]. The decrease in tree growth associated with warmer minimum summer temperatures is novel, and contrasts with the positive effect of higher summer temperatures in low-elevation lodgepole pines reported by [49] Lo et al., 2010. The negative effect of minimum daily temperature is unlikely to be explained by late-summer water stress (e.g., [51]), as we found no effect of summer precipitation on radial growth (t3211 = 0.93, p = 0.35).
5. Conclusions
Moss bags deployed in the winter and spruce needles and lodgepole pine tree cores provided insight into the detection and impacts of industrial sulfur deposition on biomonitors on the eastern slopes of the Rocky Mountains in southern Alberta. The results were consistent with patterns based on modeled SO2 concentrations and sulfate deposition within the 60 × 60 km2 study region. Outputs from the CALPuff model showed emissions deposition was high downwind to the east of the southeast sour gas processing facility as well as upwind into the more sensitive mountain region to the west. Sulfur in the tissues of our biomonitors decreased with distance from the largest local industrial point source. Moss as a bioindicator showed that the majority of atmospheric sulfur in the region at the time of the study was from industrial sources.
Spruce tree needles showed that trees were under increased sulfur stress (Si/So > 0.4 as an indicator) when differences in the inorganic-to-organic sulfur isotope ratios (Δδ34S) were low, and this occurs when deposition of industrial sulfur is high. Radial tree growth decreased with sulfur in tissues, warmer summers, and time (particularly time since 1950, representing the start of industrial gas extraction in the study area). These results demonstrate that changes in sulfur deposition and sulfur stress in vegetation can be quantitatively assessed using isotope apportionment and that the spatial distribution of sulfur impacts on vegetation is in broad agreement with CALPuff-modeled SO2 and sulfate deposition as well as air quality measurements of atmospheric SO2 and sulfate. This study suggests that the utilization of biomonitors in remote and mountainous terrain using sulfur isotopes is appropriate to gain insights into spatial patterns in pollutant sulfur distribution and deposition, and potentially allows for investigation in grid-scale patterns for comparison with model outputs in the future. Additionally, biomonitoring measurements could be enhanced in the future by comparing data collected for moss bags in the growth season versus wintertime as any potential emissions from soil and/or plants that could confound the sulfur isotope measurements used for source apportionment are absent during the winter months.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/atmos16101149/s1, S1. Soil. Figure S1. δ34S values for total (orange) and inorganic (blue) sulfur in tree needles compared to litter (A). Better agreement is observed between δ34S values for tree needle sulfur (total) and extractable sulfate in litter (B). Figure S2. δ34S values for sulfate in litter compared to Cation Exchange Capacity (CEC). S2. Isotope Apportionment. Figure S3. Isotope apportionment assuming two-source mixing. The isotope composition of the varying source can be found from a plot of d values versus the inverse of concentration. Figure S4. The sulfur isotope composition of SO2 versus the inverse of SO2 concentration for 8 HVS sampling periods of two weeks, where each are plotted as grey circles. The accompanying total aerosol sulfate sample data (orange squares) are also plotted against the inverse of SO2 concentration. The entire suite of data lie within two mixing lines. The upper border is bounded by the isotope composition of SO2 at high (LHS) and low (RHS) atmospheric concentrations. The lower border is bounded by the δ34S of total sulfate when SO2 is high (LHS) and low (RHS). S3. CALPuff Modeling. Figure S5. Spatial patterns in daily maximum SO2 concentrations based on conditions using CALPuff model outputs throughout 2006 using Mesoscale Model 5 (MM5) wind fields. The NW and SE industrial facilities are shown as yellow rectangles; the x and y axes are Easting and Northing (km) in UTM coordinates (Zone 11U) while the z axis is elevation. S4. Tree Needles. Figure S6. δ34S values for first- and-second year spruce needles are shown versus ln(distance) from the southeast (SE) facility that is the dominant sulfur emission source for atmospheric sulfur in the region. Note that the intercept (b = +18.4‰ with confidence intervals +9.4‰ to +27.5‰ (CI not shown)) corroborates the expected δ34S value for sulfur emissions at the gas plant: +18‰. Note that this graph differs from Figure 9 in the text that shows the sulfur isotope composition of inorganic sulfur in tree needles (δ34S(HI)) with distance. References [52,53,54,55] are cited in the supplementary materials.
Author Contributions
A.-L.N. conceptualized and validated the project and provided context for students to define topics for their research study. The students were responsible for the research design, sample collection, analysis, and interpretation with assistance from A.-L.N. and their group mentor. Student co-authors (S.L., E.E.C., P.M.B., J.M.) contributed to methodology, investigation, data curation R.C. contributed to methodology, formal analysis, writing-original draft an review and editing, visualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was used to produce this research. All research was performed as part of a research course where students learned how to perform and report on environmental topics of interest to the public within the Environmental Science Program at the University of Calgary.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This study could not have been performed without the contributions of the enthusiastic and highly motivated students enrolled in the 2003 and 2006 Special Problems in Environmental Research course (ENSC 502) at the University of Calgary. These students include: Air Quality Modeling: R. Chandrasekaran, A. Jevne, S. Li, B. Morrice, and D. Sappier; Air Quality Measurements: A. Bowen, D. Couroux, K.D. Farrelly, C. Kwok, A.C. Milner, M.J. Nicoud, E. Sorenson, T.J. Tarnoczi, and K.A. White; Sulfur in Moss: E. Caldwell, J. Eustace, H. Firla, R. Hunt, and J. Matic; Tree Needle Sulfur: D. Birk, C. Enevold, Y. Ma, F. Mohamed, and E. Mullinger; Tree Ring Sulfur: P. Blancher, S. Le Gallou, E. Newton, J. Ouellet, and L. Wu. The mentors for group projects were P. Staniasek (CALPuff modeling), A. Legge (tree needle sampling and logistics), and R.Cartar (tree core analysis and interpretation). A.-L.Norman mentored the remainder of the groups whose work is shown here. The students wrote and summarized their data in formal reports and submitted metadata for the class project, which was then used by A.-L.Norman and R.Cartar to create this manuscript and interpret the results. Farzin Malekani was instrumental in providing technical, analytical (IC), and logistical support. Jesusa Pontoy provided valuable assistance for the students in sample preparation for isotope analysis. P. Staniasek and A. Legge (Biosphere Solutions) provided guidance on model parameter inputs and operation (CALPuff modeling), study design, and sampling (tree needles) to each of the groups mentioned in parentheses.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Benedict, K.B.; Day, D.; Schwandner, F.M.; Kreidenweis, S.M.; Schichtel, B.; Malm, W.C.; Collett, J.L., Jr. Observations of atmospheric reactive nitrogen species in Rocky Mountain National Park and across northern Colorado. Atmos. Environ. 2013, 64, 66–76. [Google Scholar]
- Driscoll, C.T.; Lawrence, G.B.; Bulger, A.J.; Butler, T.J.; Cronan, C.S.; Eagar, C.; Lambert, K.F.; Likens, G.E.; Stoddard, J.L.; Weathers, K.C. Acidic deposition in the Northeastern United States: Sources and inputs, ecosystem effects, and management strategies. BioScience 2001, 51, 180. [Google Scholar] [CrossRef]
- Tichomirowa, M.; Haubrich, F.; Klemm, W.; Matschullat, J. Regional and temporal (1992–2004) evolution of air-borne sulphur isotope composition in Saxony, southeastern Germany, central Europe. Isot. Environ. Health Stud. 2007, 43, 295–305. [Google Scholar]
- Batts, J.E.; Calder, L.J.; Batts, B.D. Utilizing stable isotope abundances of lichens to monitor environmental change. Chem. Geol. 2004, 204, 345–368. [Google Scholar] [CrossRef]
- Wadleigh, M.A. Lichens and atmospheric sulphur: What stable isotopes reveal. Environ. Pollut. 2003, 126, 345–351. [Google Scholar] [CrossRef]
- Case, J.; Krouse, H.R. Variations in sulphur content and stable sulfur isotope composition of vegetation near a SO2 source at Fox Creek, Alberta, Canada. Oecologia 1980, 44, 247–257. [Google Scholar]
- Winner, W.E.; Bewley, I.D.; Krouse, H.R.; Brown, H.M. Stable sulphur isotope analysis of SO2 pollution impact on vegetation. Oecologia 1978, 36, 352–361. [Google Scholar] [CrossRef]
- Zechmeister, H.G.; Hohenwallner, D. A comparison of biomonitoring methods for the estimation of atmospheric pollutants in an industrial town in Austria. Environ. Monit. Assess. 2006, 117, 245–259. [Google Scholar] [CrossRef]
- Savard, M. Tree-ring stable isotopes and historical perspectives on pollution—An overview. Environ. Pollut. 2010, 158, 2007–2013. [Google Scholar] [PubMed]
- Dolegowska, S.; Galuszka, A.; Migaszewski, Z.M. Significance of the long-term biomonitoring studies for understanding the impact of pollutants on the environment based on a synthesis of 25-year biomonitoring in the Holy Cross Mountains, Poland. Environ. Sci. Pollut. Res. 2021, 28, 10413–10435. [Google Scholar] [CrossRef] [PubMed]
- Stojanowska, A.; Gorka, M.; Lewandowska, A.U.; Wisniewska, K.; Modelska, M.; Widory, D. Can Abies alba needles be used as bio-passive samplers to assess air quality? Aerosol Air Qual. Res. 2021, 21, 210097. [Google Scholar] [CrossRef]
- Krouse, H.R.; Stewart, J.W.B.; Grinenko, V.A. Chapter 7. Pedosphere and Biosphere. In Stable Isotopes: Natural and Anthropogenic Sulphur in the Environment; Krouse, H.R., Grinenko, V.A., Eds.; Wiley: Chichester, UK, 1992; pp. 267–306. [Google Scholar]
- Niepsch, D.; Clarke, L.J.; Newton, J.; Tzoulas, K.; Cavan, G. High spatial resolution of air quality in urban centres using lichen carbon, nitrogen and sulfur contents and stable-isotope-ratio signatures. Environ. Sci. Pollut. Res. 2023, 30, s8731–s8754. [Google Scholar] [CrossRef]
- Snyder, K.A.; Robinson, S.A.; Schmidt, S.; Hultine, K.R. Stable isotope approaches and opportunities for improving plant conservation. Conserv. Physiol. 2022, 10, 1–24. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Glooschenko, W.A. Isotopic composition of sulfur in mosses across Canada. Environ. Sci. Technol. 1992, 26, 85–89. [Google Scholar] [CrossRef]
- Prietzel, J.; Mayer, B.; Legge, A.H. Cumulative impact of 40 years of industrial sulfur emissions on a forest soil in west-central Alberta (Canada). Environ. Pollut. 2004, 132, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Rock, L.; Mayer, B. Tracing nitrate and sulfate in river basins using isotope techniques. Water Sci. Technol. 2006, 53, 209–217. [Google Scholar]
- Rock, L.; Mayer, B. Identifying the influence of geology, land use, and anthropogenic activities on riverine sulfate on a watershed scale by combining hydrometric, chemical and isotopic approaches. Chem. Geol. 2009, 262, 121–130. [Google Scholar] [CrossRef]
- Norman, A.L.; Xie, J.; Wieser, M. Chapter 6. Using isotopic abundances to follow anthropogenic emissions: An example of sulfur, oxygen, and boron isotopes in a Canadian watershed. In Biochemical Toxicology; Gailer, J., Turner, R., Eds.; DeGruyter Publishers: Berlin, Germany, 2023; 20p. [Google Scholar]
- Galloway, J.N.; Norton, S.A.; Church, M.R. Freshwater acidification from atmospheric deposition of sulfuric acid: A conceptual model. Environ. Sci. Technol. 1983, 17, 541–545. [Google Scholar] [CrossRef]
- Rochelle, B.P.; Church, M.R.; David, M.B. Sulphur retention at intensively studies sites in the US and Canada. Water Air Soil Pollut. 1987, 33, 73–83. [Google Scholar]
- Cheng, L.; Angle, R.P.; Peake, H.; Sandhu, S. Effective acidity modelling to establish acidic deposition objectives and manage emissions. Atmos. Environ. 1995, 29, 383–392. [Google Scholar] [CrossRef]
- Summers, P.W.; Whelpdale, D.M. Acid precipitation in Canada. Water Air Soil Pollut. 1976, 6, 447–455. [Google Scholar] [CrossRef]
- GOC. Government of Canada (GOC) Air Pollutant Emissions; Summary. 2019. Available online: www.canada.ca/en/environment-climate-change/services/environmental-indicators/air-pollutant-emissions.html (accessed on 23 July 2025).
- GOC. Government of Canada (GOC) Air Pollutant Emissions from the Oil and Gas Industry. 2019. Available online: www.canada.ca/en/environment-climate-change/services/environmental-indicators/air-pollutant-emissions.html (accessed on 23 July 2025).
- NPRI 2019. Environment Canada: National Pollutant Release Inventory (NPRI) Environment Canada Canada’s Pollutant Emissions Inventory Report: Chapter 2, Emissions and Trends. Available online: www.canada.ca/en/environment-climate-change/services/air-pollution/publications/emissions-inventory-report-2019/air-pollutant-emissions.html (accessed on 23 July 2025).
- Krouse, H.R. Sulfur Isotope Tracing of the Fate of Emission from Sour Gas Processing in Alberta, Canada. In Stable Isotopes: Natural and Anthropogenic Sulphur in the Environment; Krouse, H.R., Grinenko, V.A., Eds.; Wiley: Chichester, UK, 1992; pp. 309–326. [Google Scholar]
- Xie, G.; Norman, A.L.; Wieser, M. (2010) S and B isotope variations to track air pollutant deposition in the Castle River of southern Alberta, Canada. In Environmental Forensics: Proceedings of the 2009 INEF Annual Conference; Morrison, R.D., O’Sullivan, G., Eds.; Royal Society of Chemistry: London, UK, 2010; pp. 176–184. [Google Scholar]
- Krouse, H.R.; Legge, A.; Brown, H.M. Sulfur gas emissions in the boreal forest: The West Whitecourt case study: V. Stable sulfur isotopes. Water Air Soil Pollut. 1984, 22, 321–347. [Google Scholar] [CrossRef]
- Eden, G.E. Sources of Sulphur in Post—Industrial Sediments of Waterton Reservoir, S.W. Alberta. Master’s Thesis, The University of Calgary, Calgary, AB, Canada, 1996; 147p. [Google Scholar]
- De Roo, F.; Mauder, M. The influence of idealized surface heterogeneity on virtual turbulent flux measurements. Atmos. Chem. Phys. 2018, 18, 5059–5074. [Google Scholar] [CrossRef]
- Environment Canada: Historical Climate Data. Available online: https://climate.weather.gc.ca/ (accessed on 15 July 2025).
- Scire, J.S.; Strimaitis, D.G.; Yamartino, R. A User’s Guide for the CALPuff Dispersion Model. 2000. Available online: www.calpuff.org (accessed on 21 July 2025).
- Norman, A.L.; Krouse, H.R.; MacLeod, J. Aerosol sulphate in Calgary, Alberta: An isotope study of atmospheric sulphur. In Air Pollution Modeling and Its Application XVI; Borrego, C., Incecik, S., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; pp. 107–125. [Google Scholar]
- Krouse, H.R.; Mayer, B.; Schoenau, J.J. Applications of Stable Isotope Techniques to Soil Sulfur Cycling; Marcel Decker: New York, NY, USA, 1996; p. 247. [Google Scholar]
- Norman, A.L.; Belzer, W.; Barrie, L. Insights into the biogenic contribution to total sulphate in aerosol and precipitation in the Fraser Valley afforded by isotopes of sulphur and oxygen. J. Geophys. Res. 2004, 109, D05311. [Google Scholar] [CrossRef]
- Tretiach, M.; Adamo, P.; Bargagli, R.; Baruffo, L.; Carletti, L.; Crisafulli, P.; Giordano, S.; Modenesi, S.; Pittao, E. Lichen and moss bags as monitoring devices in urban areas. Part I: Influence of exposure on sample vitality. Environ. Pollut. 2007, 146, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Legge, A.H.; Bogner, J.C. Foliar sulphur species in pine: A new indicator of a forest ecosystem under air pollution stress. Environ. Pollut. 1988, 55, 15–27. [Google Scholar] [CrossRef]
- Norman, A.L.; Anlauf, K.; Hayden, K.; Thompson, B.; Brook, J.R.; Li, S.M.; Bottenheim, J. Aerosol sulphate and its oxidation on the Pacific NW coast: S and O isotopes in PM2.5. Atmos. Environ. 2006, 40, 2676–2689. [Google Scholar] [CrossRef]
- Tekko, S. Bioindication of sulphur distribution in Estonia using mosses. Proc. Est. Acad. Sci. Ecol. 1991, 1, 179–182. [Google Scholar] [CrossRef]
- Watmough, S.A.; Aherne, J.; Alewell, C.; Arp, P.; Bailey, S.; Clair, T.; Dillon, P.; Duchesne, L.; Eimers, C.; Fernandez, I.; et al. Sulphate, nitrogen and base cation budgets at 21 forested catchments in Canada, the United States and Europe. Environ. Monit. Assess. 2005, 109, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Rautio, P.; Huttunen, S.; Kukkola, E.; Peura, R.; Lamppu, J. Deposited particles, element concentrations and needle injuries on Scots pines along an industrial pollution transect in northern Europe. Environ. Pollut. 1998, 103, 81–89. [Google Scholar] [CrossRef]
- Amundson, R.G.; Walker, R.B.; Schellhase, H.U.; Legge, A.H. Sulphur gas emissions in the boreal forest: The West Whitecourt case study VIII: Pine tree mineral nutrition. Water Air Soil Pollut. 1990, 50, 219–232. [Google Scholar] [CrossRef]
- Norman, A.L.; Giesemann, A.; Krouse, H.R.; Jager, H.J. Sulfur isotope fractionation during sulfur mineralization: Results of an incubation-extraction experiment with Black Forest soils. Soil Biol. Biogeochem. 2002, 34, 1425–1438. [Google Scholar] [CrossRef]
- Howarth, R.W.; Stewart, J.W.B. The interactions of sulphur with other element cycles in ecosystems. In Scope 48: Sulphur Cycling on the Continents; Howarth, R.W., Stewart, J.W.B., Ivanov, M.V., Eds.; Wiley: New York, NY, USA, 1992. [Google Scholar]
- Keller, T. The effect of a continuous springtime fumigation with sulphur dioxide and carbon dioxide uptake and structure of the annual ring in spruce. Can. J. For. Res. 1980, 10, 1–6. [Google Scholar] [CrossRef]
- Hirano, T.; Morimoto, K. Growth reduction of the Japanese black pine corresponding to an air pollution episode. Environ. Pollut. 1999, 106, 5–12. [Google Scholar] [CrossRef]
- Kawamura, H.; Matsuoka, N.; Koike, M.; Takashima, Y. Isotopic evidence in tree rings for historical changes in atmospheric sulfur sources. Environ. Sci. Technol. 2006, 40, 5750–5754. [Google Scholar] [CrossRef]
- Lo, Y.H.; Blanco, J.A.; Seely, B.; Welham, C.; Kimmins, J.H. Relationships between climate and radial tree growth in interior British Columbia, Canada. For. Ecol. Manag. 2010, 259, 932–942. [Google Scholar]
- McLane, S.C.; Daniels, L.D.; Aitken, S.N. Climate impacts on lodgepole pine (Pinus contorta) radial growth in a provenance experiment. For. Ecol. Manag. 2011, 262, 115–123. [Google Scholar] [CrossRef]
- Huang, J.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Change Biol. 2010, 16, 711–731. [Google Scholar]
- Carter, M.R. (Ed.) Soil Sampling and Methods of Analysis, Canadian Society of Soil Science; Lewis Publishers: Ann Arbor, MI, USA, 1993; p. 141. [Google Scholar]
- Krouse, H.R. Sulphur isotope abundances elucidate uptake of atmospheric sulphur emissions by vegetation. Nature 1976, 265, 45–46. [Google Scholar] [CrossRef]
- Oulehle, F.; Hofmeister, J.; Cudlin, P.; Hruska, J. The effects of reduced atmospheric deposition on soil solution chemistry at a site subjected to long-term acidification, Načetín, Czech Republic. Sci. Total Environ. 2006, 370, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Krouse, H.R.; Stewart, J.W.B.; Grinenko, V.A. Pedosphere and Biosphere Stable Isotopes: Natural and Anthropogenic Sulphur in the Environment, Scope 43: Chapter 7; Krouse, H.R., Grinenko, V.A., Eds.; Wiley: Chichester, UK, 1992; pp. 267–306. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).