A New Method Proposed for the Estimation of Exposure to Atmospheric Pollution through the Analysis of Black Pigments on the Lung Surface
Abstract
1. Introduction
2. Design and Method
2.1. Setting
- (1)
- Private interview room: Face-to-face interviews with the NOK occurred in this private space, providing a comfortable and confidential setting.
- (2)
- Autopsy room: This room was used for capturing images of the lungs (and for tissue collection when appropriate for the study design). The collection team collaborated with technicians and pathologists to ensure accurate data.
- (3)
- Office: An area designated for data analysis, archiving research documents, and coordinating various aspects of the study, located outside the autopsy service. In our research, this area was situated within the university.
2.2. Materials
- computer;
- printer;
- copy paper;
- spreadsheet editor (i.e., Excel);
- statistical program (i.e., SPSS);
- ImageJ program (NIH Image).
- high-resolution camera (with charged battery);
- glass Petri dishes (90 mm or 100 mm);
- gauze;
- personal protective equipment (PPE): long-sleeved fluid-repellent gown, gloves, safety googles, disposable bouffant caps, waterproof knee-high safety boots
2.3. Procedure
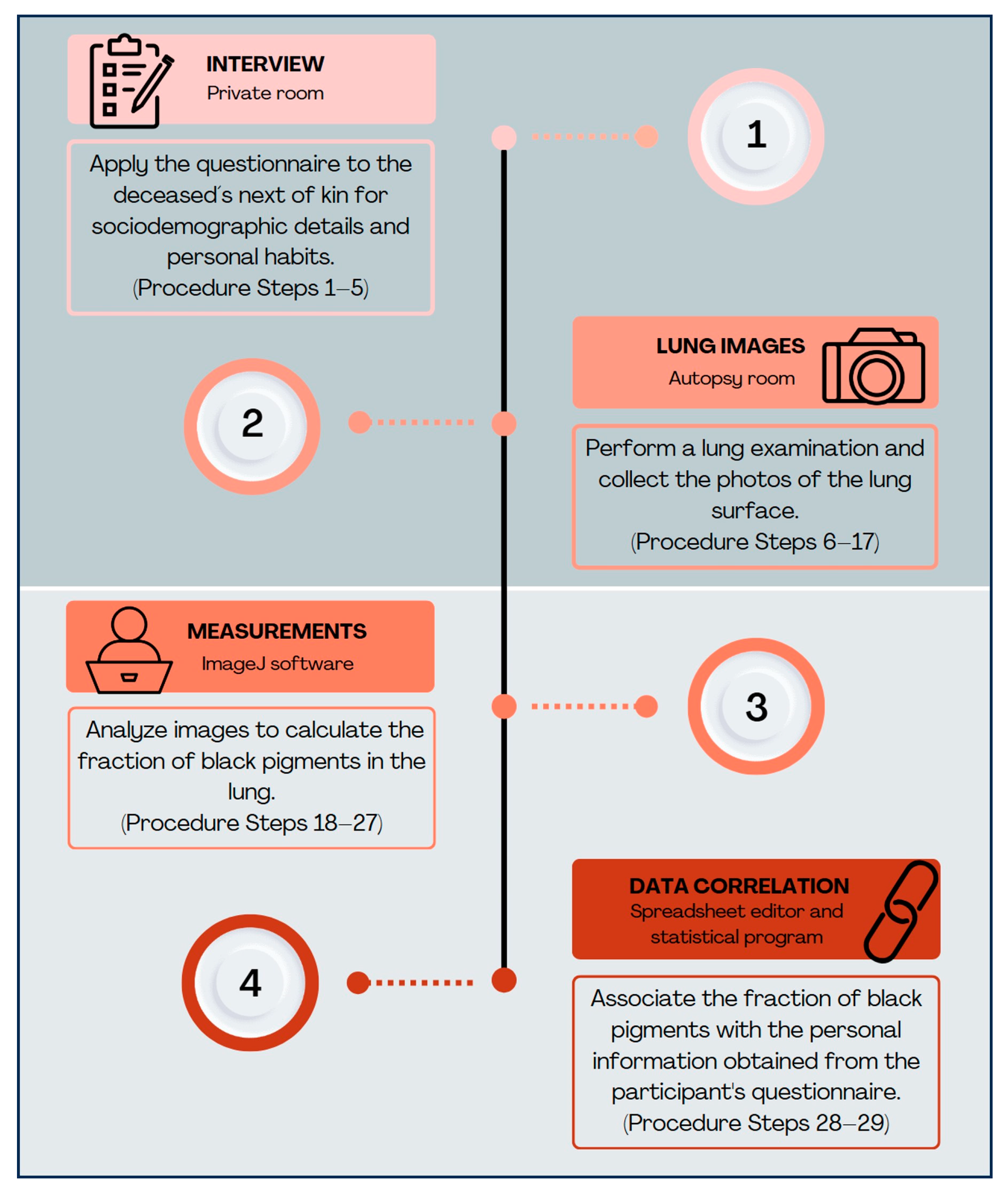
2.3.1. Stage 1—Interview for Collecting Sociodemographic and Personal Data
- The autopsy service front desk employees receive the NOK and, as soon as the authorization for the body to proceed to the autopsy is registered, inform the inter-viewer of the research procedure;
- Before the autopsy procedure, the interviewer must appropriately approach the NOK. The objective of the study and a summary of the procedures must be explained to the NOK to obtain their consent for providing information and collecting images/samples from all deceased subjects intended to be included in the study;
- The interviewer should administer a questionnaire to the NOK to collect reliable and complete information about the deceased. The questionnaire should include questions about residential addresses, duration of residence in the city, occupation, daily commuting, smoking status (including the occurrence of environmental tobacco smoking in the residence), and chronic comorbidities;
- 4.
- After completing this part of the protocol, the collected data must be registered in a study database, allowing future export to a spreadsheet editor for analysis of all subjects. At this point, if the information obtained is complete, it is possible to confirm the eligibility of the case for proceeding in the study;
- 5.
- The interviewer must then communicate the case identification number to the team responsible for collecting lung images (and potentially tissue samples) during the autopsy procedure. Then, proceed to the pathological step of the protocol;
2.3.2. Stage 2—Collection of Pathological Data from Lung Images
- 6.
- Put on the necessary personal protective equipment (PPE) for entering the autopsy room. Proceed to the autopsy room with the basic materials necessary for collecting images;
- 7.
- Inform the autopsy technician of the case identification, for them to start the autopsy procedure and provide the lungs from the body;
- 8.
- While waiting for the organs, take the first photo of the tag identifying the number of the autopsied body;
- 9.
- Upon receiving the lungs, carefully remove excess blood from the lung surface with water and wipe with gauze;
- 10.
- Position the lungs in an antero–lateral view on a bench under good illumination for examination;
- 11.
- Position the glass Petri dish (applying light pressure to flatten the lung surface) on the superior lobe of the right lung and take the second picture;
- 12.
- Position the glass Petri dish on the inferior lobe of the right lung to take the third picture;
- 13.
- Repeat steps 11 and 12 on the left lung to obtain the fourth and fifth pictures, respectively;
- 14.
- Check the quality of the photos immediately after taking them. If necessary, repeat the sequence of photos;
- 15.
- If tissue collection is required for studies associating exposure to environmental particles with respiratory or other organ/system outcomes, perform this step now and proceed with specific sample processing protocols;
- 16.
- Return the lungs carefully to the technician for the standard autopsy procedures;
- 17.
- Exit the autopsy room, remove PPE, and proceed to the data analysis office;
2.3.3. Stage 3—Measurements and Analysis of Observed Black Particle Deposition
- 18.
- Transfer the photos to the computer and save them in folders labeled with the case identification.
- 19.
- Open the ImageJ software(ImageJ https://imagej.net/ij/index.html) (the original ImageJ, NIH Image), which is used to generate the point test system for the images of the four lobes;
- 20.
- Open the four images of the lung surfaces collected for the case. Analyze them with the following steps, one at a time;
- 21.
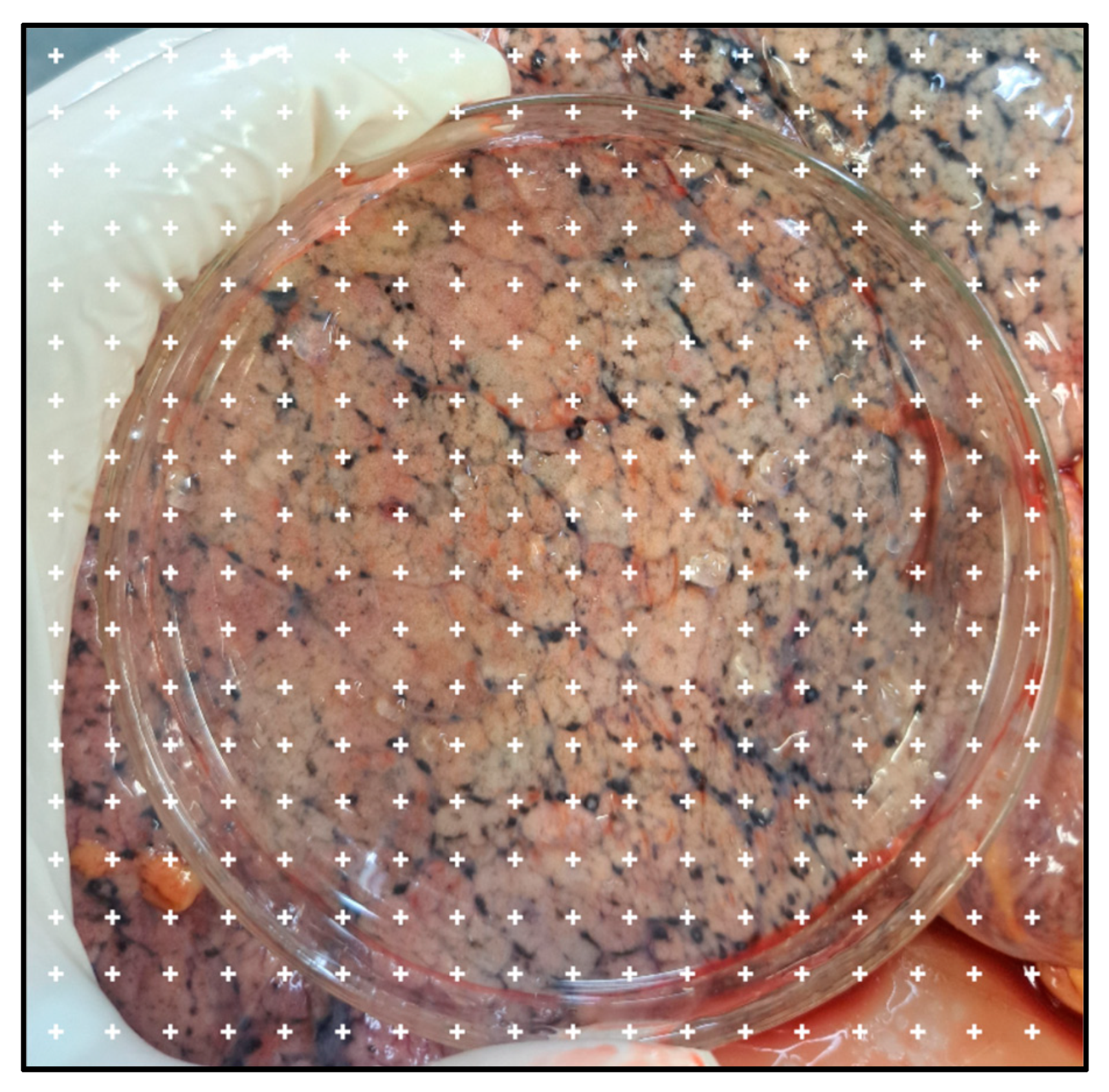
- Apply the point system to each image using the following steps: Plugins > Analyze > Grid;
- 22.
- Open the “Grid” tab for configuration. Select the following settings: Type: “Crosses”; Area per point: 30,000; select “Bold and Apply.” The result of these steps is demonstrated in Figure 2;
- 23.
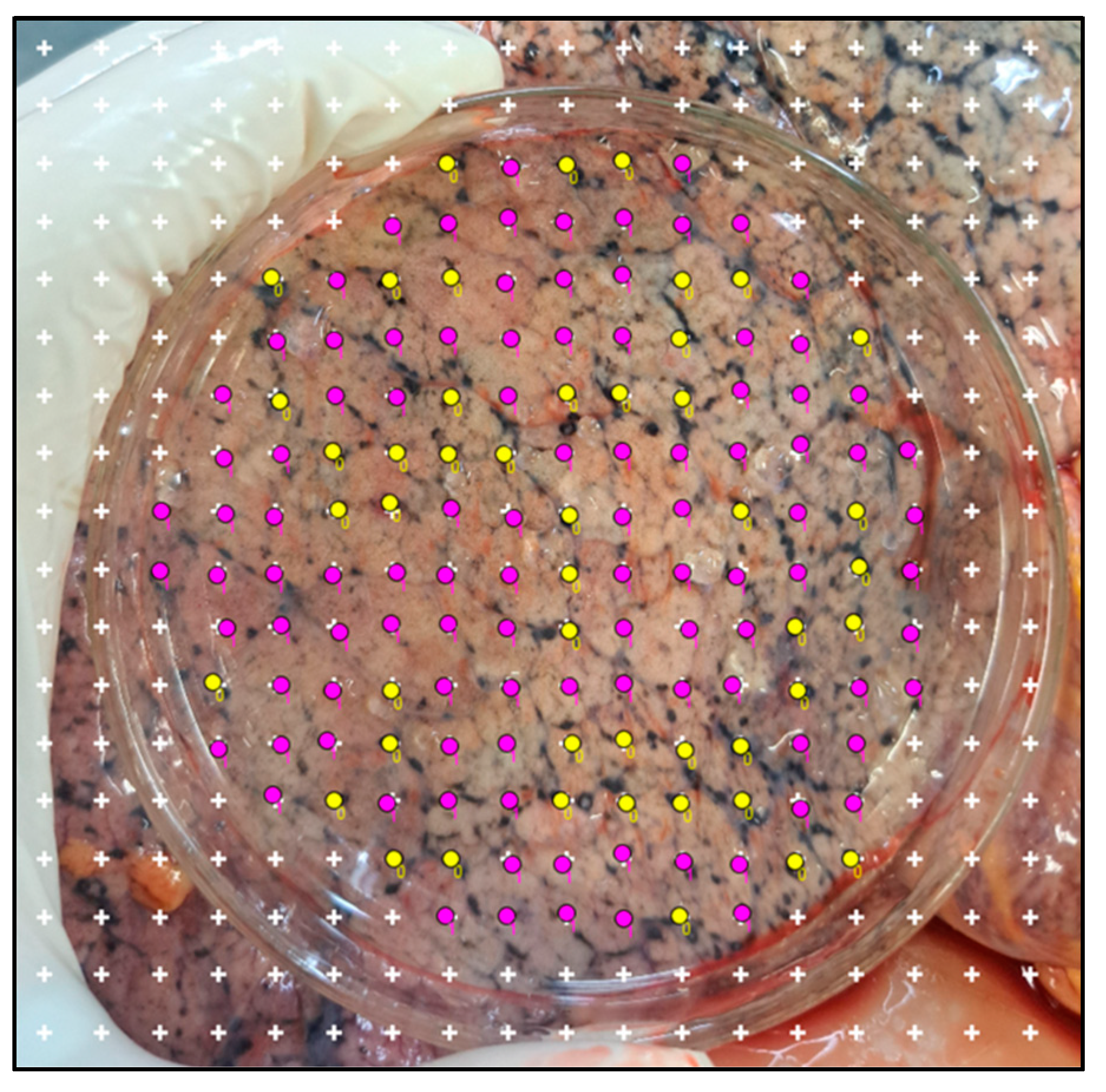
- Start the point count by going to: Plugins > Analysis > Cell counter.
- 24.
- Open the point tools tab. Start counting with counter “0” to quantify the black particles’ points, and switch to counter “1” to count points on tissue without black particles, or vice versa.
- 25.
- Considering only the points that fall on the lung surface image covered by the glass Petri dish, count the points over areas with black particles as well as the points over the rest of the lung tissue (Figure 3). The total number quantified by counter “0” and counter “1” is available in the point tool tab.
- 26.
- Calculate the proportion of black patches by dividing the number of black patches by the total number of black patches plus the number of clean pleura, to obtain the fraction of black pigments for each lobe. An example is shown in Figure 3;
- 27.
- Calculate the mean of the values obtained from the four lobes. Use this final value to estimate the complex mixture of lifelong urban ambient particles retained in the lung;
2.3.4. Stage 4—Data Correlation
- 28.
- Transfer the values to the spreadsheet editor for analysis, associating the fraction of black pigments with the personal information obtained from each participant’s questionnaire;
- 29.
- Conduct specific statistical analyses in accordance with the study’s objectives.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, R.; Parsche, F.; Bierbrier, M.; McKerrow, J.H. Tissue identification and histologic study of six lung specimens from Egyptian mummies. Am. J. Phys. Anthropol. 1987, 72, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Isidro, A.; Malgosa, A.; Prats-Munoz, G. Anthracosis in a Coptic mummy. Arch. Bronconeumol. 2014, 50, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.P.C.; Justo, L.T.; Dos Santos, N.V.; Marquezini, M.V.; de André, P.A.; da Rocha, F.M.M.; Pasqualucci, C.A.; Barrozo, L.V.; Singer, J.M.; De André, C.D.S.; et al. Pleural anthracosis as an indicator of lifetime exposure to urban air pollution: An autopsy-based study in Sao Paulo. Environ. Res. 2019, 173, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Pryor, J.T.; Cowley, L.O.; Simonds, S.E. The physiological effects of air pollution: Particulate matter, physiology and disease. Front. Public Health 2022, 10, 882569. [Google Scholar] [CrossRef]
- Konduracka, E.; Rostoff, P. Links between chronic exposure to outdoor air pollution and cardiovascular diseases: A review. Environ. Chem. Lett. 2022, 20, 2971–2988. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Hoek, G.; van Vliet, P.; Meliefste, K.; Fischer, P.; Gehring, U.; Heinrich, J.; Cyrys, J.; Bellander, T.; Lewne, M.; et al. Estimating long-term average particulate air pollution concentrations: Application of traffic indicators and geographic information systems. Epidemiology 2003, 14, 228–239. [Google Scholar]
- Van Donkelaar, A.; Martin, R.V.; Brauer, M.; Boys, B.L. Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environ. Health Perspect. 2015, 123, 135–143. [Google Scholar] [CrossRef]
- Gruzieva, O.; Georgelis, A.; Andersson, N.; Johansson, C.; Bellander, T.; Merritt, A.S. Comparison of personal exposure to black carbon levels with fixed-site monitoring data and with dispersion modelling and the influence of activity patterns and environment. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 538–545. [Google Scholar]
- Mirowsky, J.; Gordon, T. Noninvasive effects measurements for air pollution human studies: Methods, analysis, and implications. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 354–380. [Google Scholar] [CrossRef]
- Takano, A.P.C.; Rybak, J.; Veras, M.M. Bioindicators and human biomarkers as alternative approaches for cost-effective assessment of air pollution exposure. Front. Environ. Eng. 2024, 3, 1346863. [Google Scholar]
- Bai, Y.; Brugha, R.E.; Jacobs, L.; Grigg, J.; Nawrot, T.S.; Nemery, B. Carbon loading in airway macrophages as a biomarker for individual exposure to particulate matter air pollution—A critical review. Environ. Int. 2015, 74, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Bové, H.; Nawrot, T.S.; Nemery, B. Carbon load in airway macrophages as a biomarker of exposure to particulate air pollution; a longitudinal study of an international Panel. Part Fibre Toxicol. 2018, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.S.; Prudon, B.; Panditi, S.L.; Abebe, Y.; Grigg, J. Carbon loading of alveolar macrophages in adults and children exposed to biomass smoke particles. Sci. Total Environ. 2005, 345, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Rallis, G.N.; Boumba, V.A.; Sakkas, V.A.; Fragkouli, K.; Siozios, G.; Albanis, T.A.; Vougiouklakis, T. Residues of selected polychlorinated biphenyls (PCB) and organochlorine pesticides (OCP) in postmortem lungs from Epirus, northwestern Greece. J. Toxicol. Environ. Health A 2014, 77, 767–775. [Google Scholar]
- Domingo, J.L.; García, F.; Nadal, M.; Schuhmacher, M. Autopsy tissues as biological monitors of human exposure to environmental pollutants. A case study: Concentrations of metals and PCDD/Fs in subjects living near a hazardous waste incinerator. Environ. Res. 2017, 154, 269–274. [Google Scholar]
- Brauer, M.; Avila-Casado, C.; Fortoul, T.I.; Vedal, S.; Stevens, B.; Churg, A. Air pollution and retained particles in the lung. Environ. Health Perspect. 2001, 109, 1039–1043. [Google Scholar] [CrossRef]
- Churg, A.; Brauer, M.; del Carmen Avila-Casado, M.; Fortoul, T.I.; Wright, J.L. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ. Health Perspect. 2003, 111, 714–718. [Google Scholar] [CrossRef]
- de André, C.D.S.; Bierrenbach, A.L.; Barroso, L.P.; de André, P.A.; Justo, L.T.; Pereira, L.A.A.; Taniguchi, M.T.; Minto, C.M.; Takecian, P.L.; Kamaura, L.T.; et al. Validation of physician certified verbal autopsy using conventional autopsy: A large study of adult non-external causes of death in a metropolitan area in Brazil. BMC Public Health 2022, 22, 748. [Google Scholar] [CrossRef]
- Griffin, C.P.; Bowen, J.R.; Walker, M.M.; Lynam, J.; Paul, C.L. Understanding the value of brain donation for research to donors, next-of-kin and clinicians: A systematic review. PLoS ONE 2023, 18, e0295438. [Google Scholar] [CrossRef]
- Desmedt, C.; Carey, L.A. Global post-mortem tissue donation programmes to accelerate cancer research. Nat. Rev. Cancer 2024, 24, 289–290. [Google Scholar] [CrossRef] [PubMed]
- da Motta Singer, J.; Saldiva de André, C.D.; Afonso de André, P.; Monteiro Rocha, F.M.; Waked, D.; Vaz, A.M.; Gois, G.F.; de Fátima Andrade, M.; Veras, M.M.; Nascimento Saldiva, P.H.; et al. Assessing socioeconomic bias of exposure to urban air pollution: An autopsy-based study in São Paulo, Brazil. Lancet Reg. Health Am. 2023, 22, 100500. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.M.; Tsai, F.J.; Lee, Y.L.; Chang, J.H.; Chang, L.T.; Chang, T.Y.; Chung, K.F.; Kuo, H.P.; Lee, K.Y.; Chuang, K.J.; et al. The impact of air pollution on respiratory diseases in an era of climate change: A review of the current evidence. Sci. Total Environ. 2023, 898, 166340. [Google Scholar] [CrossRef] [PubMed]
- Bălă, G.P.; Râjnoveanu, R.M.; Tudorache, E.; Motișan, R.; Oancea, C. Air pollution exposure-the (in)visible risk factor for respiratory diseases. Environ. Sci. Pollut. Res. Int. 2021, 28, 19615–19628. [Google Scholar] [CrossRef] [PubMed]
- Bevan, G.H.; Al-Kindi, S.G.; Brook, R.D.; Münzel, T.; Rajagopalan, S. Ambient Air Pollution and Atherosclerosis: Insights Into Dose, Time, and Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 628–637. [Google Scholar]
- Sanidas, E.; Papadopoulos, D.P.; Grassos, H.; Velliou, M.; Tsioufis, K.; Barbetseas, J.; Papademetriou, V. Air pollution and arterial hypertension. A new risk factor is in the air. J. Am. Soc. Hypertens. 2017, 11, 709–715. [Google Scholar] [CrossRef]
- Takano, A.P.C.; de André, C.D.S.; de Almeida, R.; Waked, D.; Veras, M.M.; Saldiva, P.H.N. Association of pulmonary black carbon accumulation with cardiac fibrosis in residents of São Paulo, Brazil. Environ. Res. 2024, 248, 118380. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Zeidberg, L.D.; Prindle, R.A. The Nashville air pollution study. II. Pulmonary anthracosis as an index of air pollution. Am. J. Public Health Nations Health 1963, 53, 185–199. [Google Scholar] [CrossRef]
- Geiser, M.; Kreyling, W.G. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010, 7, 2. [Google Scholar] [CrossRef]
- Wang, W.; Lin, Y.; Yang, H.; Ling, W.; Liu, L.; Zhang, W.; Lu, D.; Liu, Q.; Jiang, G. Internal Exposure and Distribution of Airborne Fine Particles in the Human Body: Methodology, Current Understandings, and Research Needs. Environ. Sci. Technol. 2022, 56, 6857–6869. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.S.; Toro, P.; Corredor, G.; Mukhopadhyay, S.; Madabhushi, A. The state of the art for artificial intelligence in lung digital pathology. J. Pathol. 2022, 257, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Hu, C.J.; Rowe, C.L.; Kang, H.; Gong, X.; Dagucon, C.P.; Wang, J.; Lin, Y.; Sood, A.; Guo, Y.; et al. Application of artificial intelligence in quantifying lung deposition dose of black carbon in people with exposure to ambient combustion particles. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 529–537. [Google Scholar] [PubMed]
- Yu, W.; Ye, T.; Zhang, Y.; Xu, R.; Lei, Y.; Chen, Z.; Yang, Z.; Zhang, Y.; Song, J.; Yue, X.; et al. Global estimates of daily ambient fine particulate matter concentrations and unequal spatiotemporal distribution of population exposure: A machine learning modelling study. Lancet Planet. Health 2023, 7, e209–e218. [Google Scholar] [CrossRef]
- Song, J. Towards space-time modelling of PM2.5 inhalation volume with ST-exposure. Sci. Total Environ. 2024, 948, 174888. [Google Scholar] [CrossRef]
- Song, J.; Stettler, M.E.J. A novel multi-pollutant space-time learning network for air pollution inference. Sci. Total Environ. 2022, 811, 152254. [Google Scholar] [CrossRef]
- ICRP. Human respiratory tract models for radiological protection. ICRP Publ. 1994, 66, 1–3. [Google Scholar]
- Chauhan, B.V.S.; Corada, K.; Young, C.; Smallbone, K.L.; Wyche, K.P. Review on Sampling Methods and Health Impacts of Fine (PM2.5, ≤2.5 µm) and Ultrafine (UFP, PM0.1, ≤0.1 µm) Particles. Atmosphere 2024, 15, 572. [Google Scholar] [CrossRef]
- Morawska, L.; Buonanno, G. The physics of particle formation and deposition during breathing. Nat. Rev. Phys. 2021, 3, 300–301. [Google Scholar] [CrossRef]
- Mühlfeld, C.; Ochs, M. Functional Aspects of Lung Structure as Related to Interaction with Particles. In Particle-Lung Interactions, 2nd ed.; Gehr, P., Mühlfeld, C., Rothen-Rutishauser, B., Blank, F., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 9–12. [Google Scholar]
- Saieg, M.A.; Cury, P.M.; Godleski, J.J.; Stearns, R.; Duarte, L.G.; D’Agostino, L.; Kahn, H.; Pinto, E.M.; Mauad, T.; Saldiva, P.H.; et al. Differential elemental distribution of retained particles along the respiratory tract. Inhal. Toxicol. 2011, 23, 459–467. [Google Scholar] [CrossRef]
- Althubaiti, A. Information bias in health research: Definition, pitfalls, and adjustment methods. J. Multidiscip. Healthc. 2016, 9, 211–217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waked, D.; Veras, M.M.; Saldiva, P.H.N.; Takano, A.P.C. A New Method Proposed for the Estimation of Exposure to Atmospheric Pollution through the Analysis of Black Pigments on the Lung Surface. Atmosphere 2024, 15, 1126. https://doi.org/10.3390/atmos15091126
Waked D, Veras MM, Saldiva PHN, Takano APC. A New Method Proposed for the Estimation of Exposure to Atmospheric Pollution through the Analysis of Black Pigments on the Lung Surface. Atmosphere. 2024; 15(9):1126. https://doi.org/10.3390/atmos15091126
Chicago/Turabian StyleWaked, Dunia, Mariana Matera Veras, Paulo Hilário Nascimento Saldiva, and Ana Paula Cremasco Takano. 2024. "A New Method Proposed for the Estimation of Exposure to Atmospheric Pollution through the Analysis of Black Pigments on the Lung Surface" Atmosphere 15, no. 9: 1126. https://doi.org/10.3390/atmos15091126
APA StyleWaked, D., Veras, M. M., Saldiva, P. H. N., & Takano, A. P. C. (2024). A New Method Proposed for the Estimation of Exposure to Atmospheric Pollution through the Analysis of Black Pigments on the Lung Surface. Atmosphere, 15(9), 1126. https://doi.org/10.3390/atmos15091126








