Long-Term and Seasonal Changes in Emission Sources of Atmospheric Particulate-Bound Pyrene and 1-Nitropyrene in Four Selected Cities in the Western Pacific
Abstract
1. Introduction
2. Materials and Methods
2.1. Airborne Particulate Samples
2.2. Data of PAH and NPAH Concentrations
| City (Period) | PM Size | Ref. |
|---|---|---|
| Kanazawa, Kitakyushu, Shenyang, Shanghai (summer 1997–winter 2014) | TSP | [5] |
| Kanazawa (summer 2017 and winter 2018) | PM≤2.1 + PM >2.1 | [21] |
| Kanazawa (winter 2020–winter 2021) | PM2.5 | This study |
| Shanghai (summer 2015–winter 2018) | PM2.5 | [22] |
| Shenyang (autumn 2018 and spring 2019) | PM2.5 | [23] |
2.3. Calculation of Source Contributions
3. Results
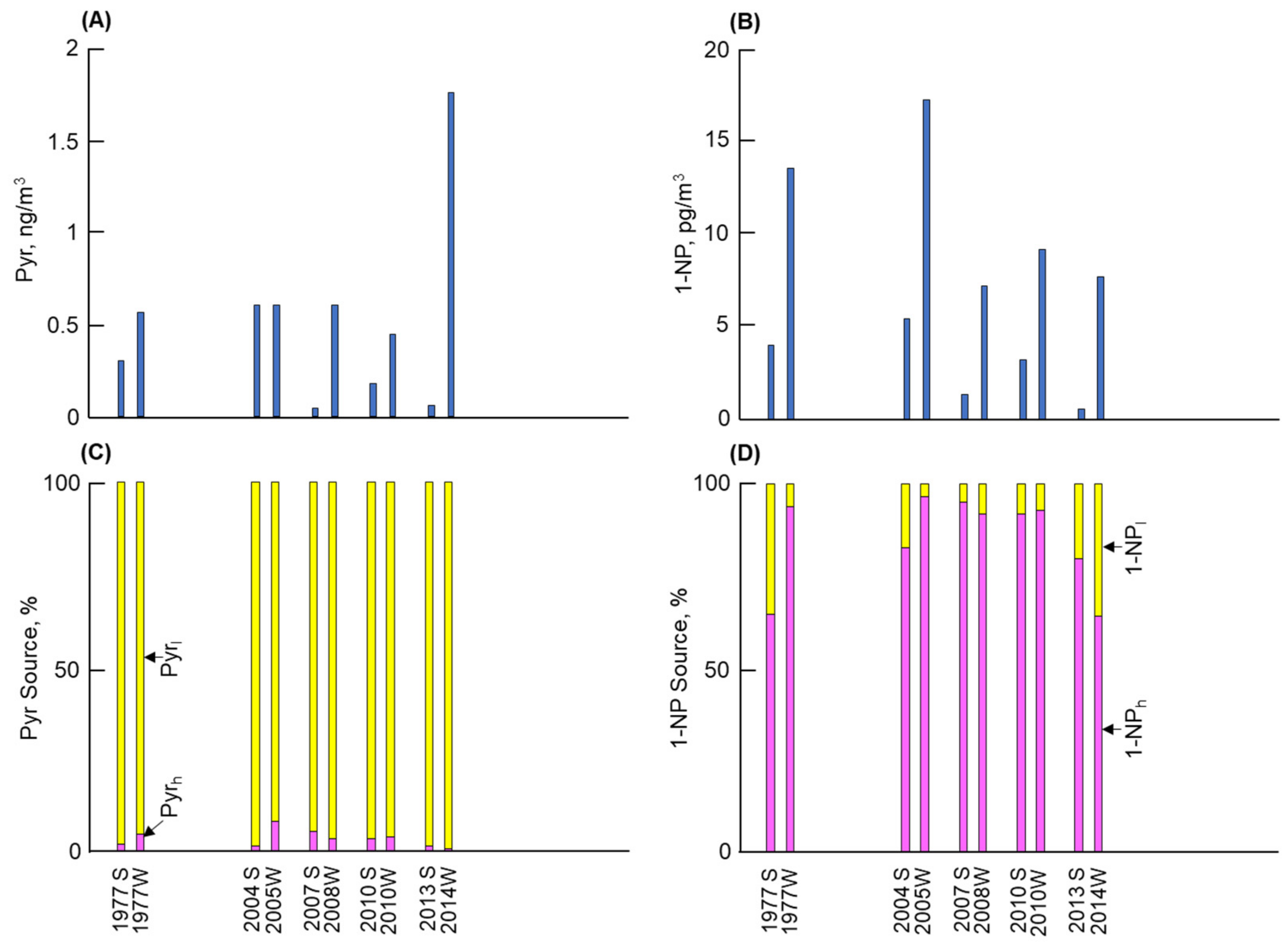
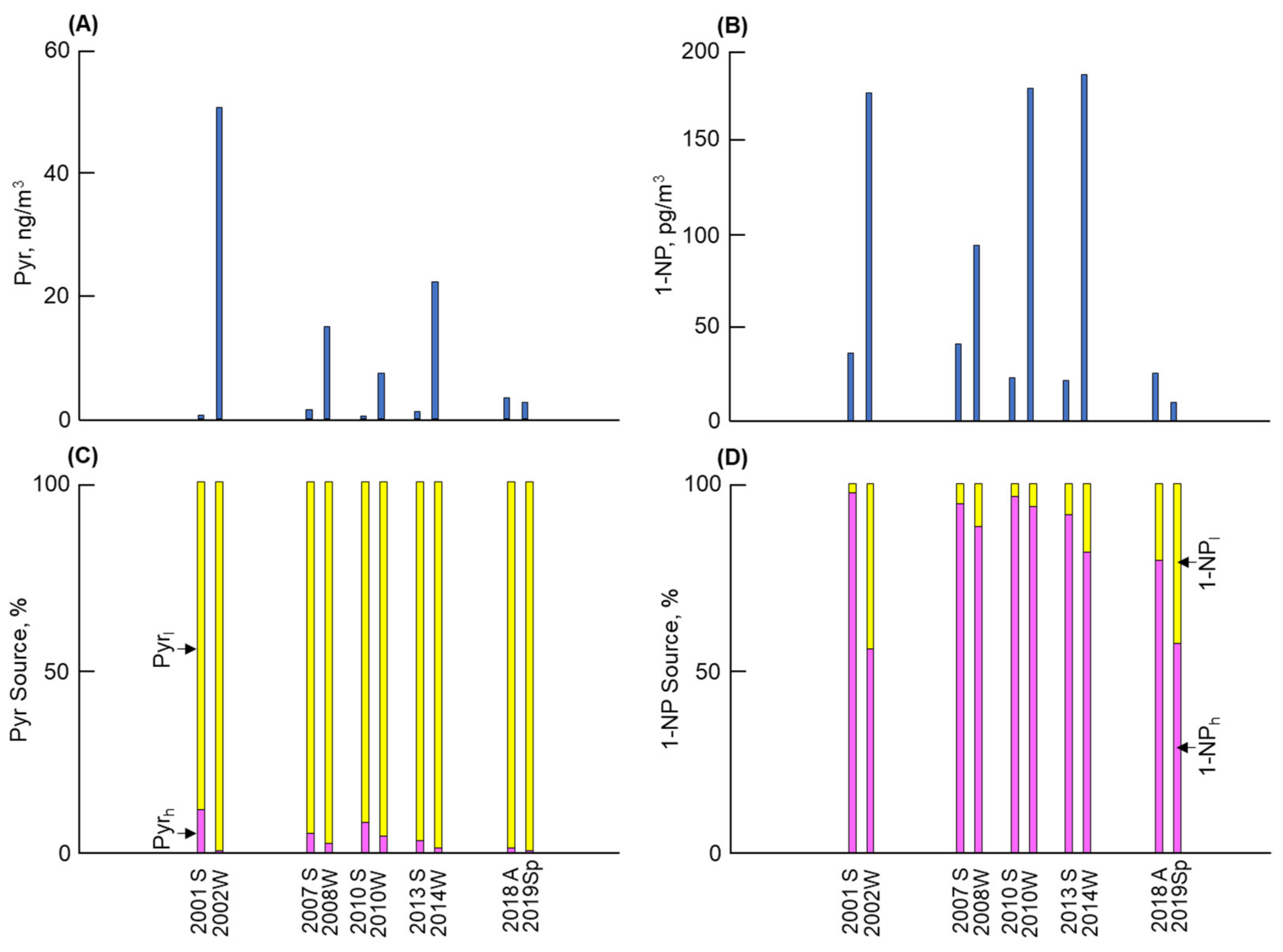
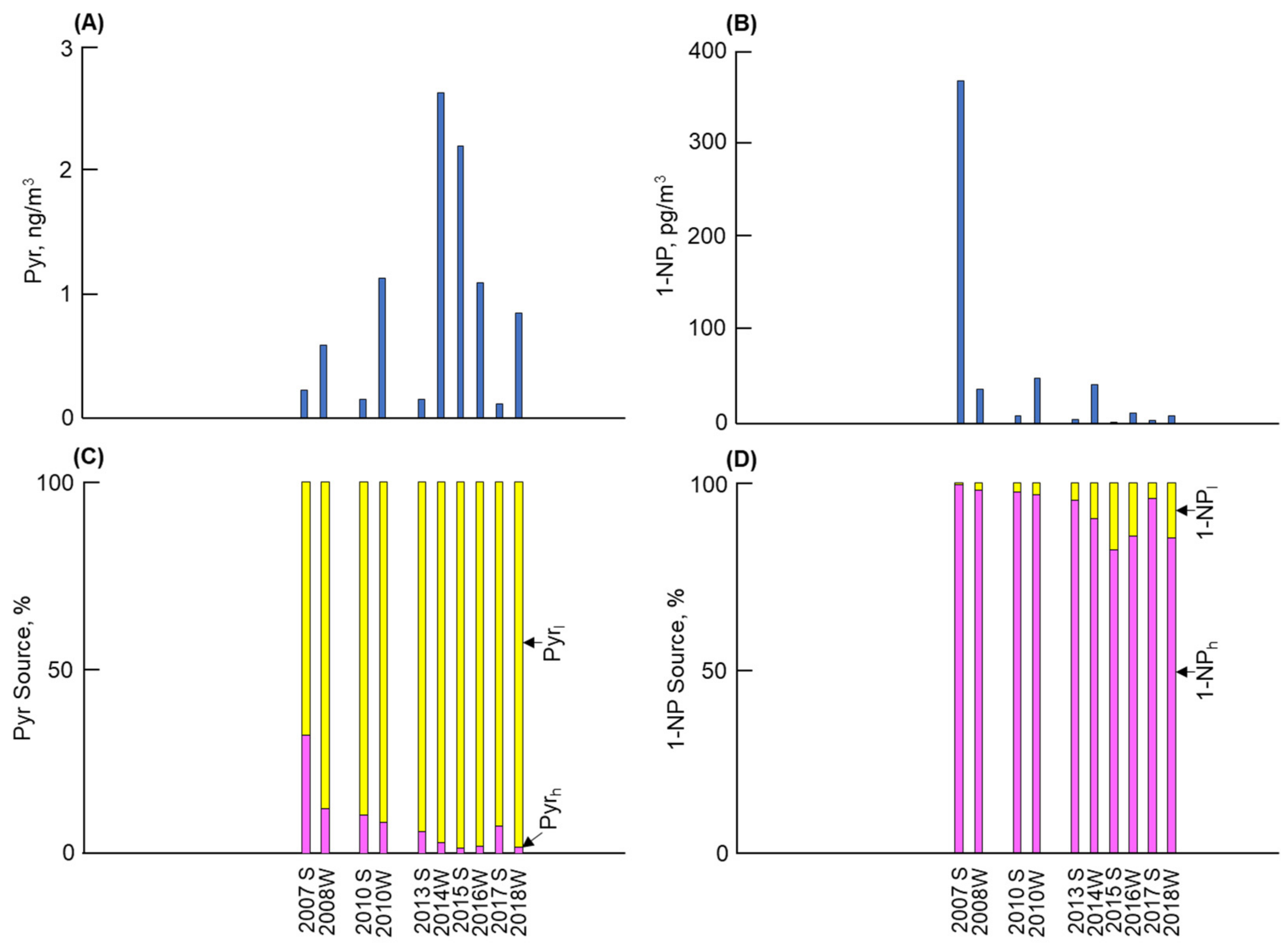
3.1. Long-Term and Seasonal Changes in Pyr and 1-NP
3.2. Long-Term and Seasonal Change in Source Contributions
4. Discussion
5. Conclusions
- (1)
- Kanazawa demonstrated the lowest Pyr concentration among the four cities. The contributions of vehicles to Pyr before and after 2010 were 35% and 5%, respectively. By contrast, the atmospheric 1-NP concentration, of which more than 95% was emitted from vehicles, markedly decreased after 2010 by a factor of more than 1/10. These changes can be attributed to the effective control of PM/NOx emissions from vehicles.
- (2)
- The second-lowest Pyr concentration and the lowest 1-NP concentration were observed in Kitakyushu. The contribution of coal combustion was more than 96% to the atmospheric Pyr, while that to 1-NP before and after 2010 was 9% and 19%, respectively. The large contribution of coal combustion to Pyr and 1-NP can be attributed to iron manufacturers, where a large amount of coal was consumed in coke oven plants.
- (3)
- The highest Pyr concentration with the largest seasonal change (winter/summer) was found in Shenyang. Although vehicles were the major contributor to 1-NP, coal combustion, including winter coal heating, contributed 97% or more to the atmospheric Pyr and around 14–27% to the atmospheric 1-NP.
- (4)
- Shanghai revealed the second-highest atmospheric Pyr and 1-NP concentrations, but the former concentration was substantially lower than that in Shenyang. Coal combustion was the major contributor to Pyr and 1-NP, but vehicles still contributed 18% to the atmospheric Pyr before 2010. However, their contribution decreased to 1% after 2010 due to the development of technology for vehicle emissions.
- (5)
- Strong correlations between the Pyr and PAH concentrations and between the 1-NP and NPAH concentrations in the four cities indicate that the above results for Pyr and 1-NP can be considered in terms of the contributions of vehicles and coal combustion to PAHs and NPAHs, respectively.
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Air Pollution and Child Health: Prescribing Clean Air. WHO Reference Number: WHO/CED/PHE/18.01.2018. Available online: https://who.int/bitstraam/handle/10665/275545/WHO-CED-PHE-18.1-erg.pdf (accessed on 2 April 2024).
- International Agency for Research on Cancer. Diesel and Gasoline Engine Exhausts and Some Nitroarenes. In IARC Monographs on the Evaluation on Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2014; Volume 105, ISBN 978-92-832-01434. [Google Scholar]
- Hayakawa, K. Chapter 22, Polycyclic aromatic hydrocarbons. In Handbook of Air Quality and Climate Change; Akimoto, H., Tanimoto, H., Eds.; eBook; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 978-981-15-2527-8. [Google Scholar] [CrossRef]
- Japan Society of Atmospheric Environment. History of Atmospheric Pollution in Japan (Jpn: Nippon No Taikiosen No Rekishi); Kouken-Kyokai: Tokyo, Japan, 2000; ISBN 978484420101. [Google Scholar]
- Hayakawa, K.; Tang, N.; Nagato, E.G.; Toriba, A.; Sakai, S.; Kano, F.; Goto, S.; Endo, O.; Arashidani, K.; Kakimoto, H. Long term trends in atmospheric concentrations of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons: A study of Japanese cities from 1997 to 2014. Environ. Pollut. 2018, 233, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Tang, N.; Nagato, E.; Toriba, A.; Lin, J.M.; Zhao, L.; Zhou, Z.; Qing, W.; Yang, X.; Mishukov, V.; et al. Long-term trends in urban atmospheric polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons: China, Russia and Korea from 1999 to 2014. Int. J. Environ. Res. Public Heath 2020, 17, 431. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K. Review: Environmental behaviors and toxicities of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbon. Chem. Pharm. Bull. 2016, 64, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Eberly, S.; Paatero, P.; Norris, G.A. Methods for estimating uncertainty in PMF solutions: Examples with ambient air and water quality data and guidance on reporting PMF results. Sci. Total Environ. 2015, 518, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Walker, T.R.; Adams, M.; Willis, R. Estimating PAH sources in harbor sediments using diagnostic ratios. Remediat. J. 2019, 29, 51–62. [Google Scholar] [CrossRef]
- Masclet, P.; Bresson, M.A.; Mouvier, G. Polycyclic aromatic hydrocarbons emitted by power stations and influence of combustion conditions. Fuel 1987, 66, 556–562. [Google Scholar] [CrossRef]
- Manoli, E.; Kouras, A.; Samura, C. Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three cites in northern Greece. Chemosphere 2004, 56, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Schauer, J.J.; Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R.; Simoneit, B.T. Source appointment of airborne particulate matter using organic compounds as tracers. Atmos. Environ. 1996, 30, 3837–3855. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Nimiesnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tang, N.; Toriba, A.; Nagato, E.G. Calculating sources of combustion-derived particulates using 1-nitropyrene and pyrene as markers. Environ. Pollut. 2020, 265, 114730. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Tang, N.; Matsuki, A.; Inomata, Y.; Toriba, A.; Nagato, E.G. Calculating source contributions to urban atmospheric polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons using 1-nitropyrene and pyrene: An application to an Asian dust event. Chemosphere 2021, 280, 130662. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Tang, N.; Toriba, A. Long-term and seasonal changes in sources of urban atmospheric particulates in the Western Pacific. Appl. Sci. 2022, 12, 2149. [Google Scholar] [CrossRef]
- Wise, S.A.; Phinney, K.W.; Sander, L.C.; Schantz, M.M. Review: Role of chromatography in the development of standard reference materials for organic analysis. J. Chromatogr. A 2012, 1261, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Murahashi, T.; Butoh, M.; Miyazaki, M. Determination of 1,3-, 1,6-, and 1,8-dinitropyrenes and 1-nitropyrene in urban air by high-performance liquid chromatography using chemiluminescence detection. Environ. Sci. Technol. 1995, 29, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Taga, R.; Hattori, T.; Toriba, A.; Kizu, R.; Hayakawa, K. Simultaneous determination of twenty-one mutagenic nitropolycyclic aromatic hydrocarbons by high-performance liquid chromatography with chemiluminescence detection. In Proceedings of the 13th International Symposium, Bioluminescence and Chemiluminescence Progress and Perspective, Pacifico Yokihama, Japan, 2–6 August 2004; Tsuji, A., Maeda, M., Matsumoto, M., Krica, L., Stanley, P., Eds.; World Science: London, UK, 2005; pp. 441–444. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tang, N.; Xing, W.; Oanh, P.K.; Hara, A.; Nakamura, H. Concentrations and sources of atmospheric PM, polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in Kanazawa, Japan. Atmosphere 2021, 12, 256. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, X.; Xing, W.; Zhou, Q.; Zhang, L.; Wu, Q.; Zhou, Z.; Chen, R.; Toriba, A.; Hayakawa, K.; et al. Yearly variation in characteristics and health risk of polycyclic aromatic hydrocarbons and nitro-PAHs in urban Shanghai from 2010–2018. J. Environ. Sci. 2021, 99, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, L.; Kashiwakura, K.; Zhao, L.; Chen, L.; Han, C.; Nagao, S.; Tang, N. Autumn and spring observations of PM2.5-bound polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons in China and Japan. Environ. Pollut. 2024, 343, 123139. [Google Scholar] [CrossRef] [PubMed]
- Zajemska, M.; Poskart, A.; Musial, D. The kinetics of nitrogen oxides formation in the flame gas. Econ. Environ. Stud. 2015, 15, 445–460. Available online: https://www.econstor.eu/bitstream/10419/178898/1/ees_15_4_08.pdf (accessed on 2 April 2024).
- Hayakawa, K. Review: Recent research progress on nitropolycyclic aromatic hydrocarbons in outdoor and indoor environments. Appl. Sci. 2022, 12, 12544. [Google Scholar] [CrossRef]
- Miller-Schulze, J.P.; Paulsen, M.; Kameda, T.; Toriba, A.; Hayakawa, K.; Cassidy, B.; Naeher, L.; Villalobos, M.A.; Simpson, C.D. Nitro-PAH exposures of occupationally-exposed traffic workers and associated urinary 1-nitropyrene metabolite concentrations. J. Environ. Sci. 2016, 49, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R. Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environ. Sci. Technol. 1993, 27, 636–651. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare (MHLW), Japan. Demographic Statistics. 2022. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai03/index.html (accessed on 2 April 2024).
- Hama, H.; Tokuda, T.; Izaki, A.; Ohno, T.; Watanabe, Y.; Kanda, T.; Tang, N.; Kameda, T.; Toriba, A.; Hayakawa, K. Variation in polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in airborne particulates collected in urban Kanazawa, Japan, in last 12 years. J. Jpn. Soc. Atmos. Environ. 2012, 47, 1–8. [Google Scholar] [CrossRef]
- Yasuhiro, M. District heating in China and Japan: What both sides should learn. Dist. Heat. Coolong 2018, 119, 18–23. Available online: https://www.dhcjp.or.jp/cms_admin/wp-content/uploads/2018/03/119.pdf (accessed on 3 March 2024).
- Zhang, H.; Yang, L.; Zhang, X.; Xing, W.; Wang, Y.; Bai, P.; Zhang, L.; Li, Y.; Hayakawa, K.; Toriba, A.; et al. Characteristics and health risks of polycyclic aromatic hydrocarbons and nitro-PAHs in Xinxiang, China in 2015 and 2017. Int. J. Environ. Res. Public Health 2021, 18, 3017. [Google Scholar] [CrossRef] [PubMed]
- Kalisa, E.; Nagato, E.; Bizuru, E.; Lee, K.; Tang, N.; Pointing, S.; Hayakawa, K.; Archer, S.; Lacap-Bugler, D. Characterization and risk assessment of atmospheric PM2.5 and PM10 particulate-bound PAHs and NPAHs in Rwanda, Central-East Africa. Environ. Sci. Technol. 2018, 52, 12179–12187. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Q.; Zhang, H.; Zhang, X.; Xing, W.; Wang, Y.; Bai, P.; Yamaguchi, M.; Choji, T.; Zhang, L.; et al. Atmospheric behaviour of polycyclic and nitro-polycyclic aromatic hydrocarbons and water-soluble inorganic ions in winter in Kirishima, a typical Japanese commercial city. Int. J. Environ. Res. Public Health 2021, 18, 688. [Google Scholar] [CrossRef] [PubMed]
- Kalisa, E.; Nagato, E.; Bizura, E.; Lee, K.; Tang, N.; Pointing, S.; Hayakawa, K.; Archer, S.; Lacap-Bugler, D. Pollution characteristics of ambient PM2.5-bound PAHs and NPAHs in typical Japanese and New Zealand cities and rural sites. Atmos. Pollut. Res. 2019, 10, 1396–1403. [Google Scholar] [CrossRef]
- Shen, H.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.; Wang, B.; Zhang, Y.; Chen, Y.; Lu, Y.; et al. Global atmospheric emissions of polycyclic aromatic hydrocarbons from 1960 to 2008 and future predictions. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K. Polycyclic aromatic hydrocarbons from vegetation burning and health effects: Special Issue “Air Pollution from vegetation fire and its health effects in Asia”. Glob. Environ. Res. 2023, 27, 13–20. [Google Scholar]
- Hayakawa, K.; Kitamura, R.; Butoh, M.; Imaizumi, N.; Miyazaki, M. Determination of diamino- and aminopyrenes by high-performance liquid chromatography with chemiluminescence detection. Anal. Sci. 1991, 7, 573–577. [Google Scholar] [CrossRef]

| City | Pyr | Pyrh | Pyrh/Pyr | |||||
|---|---|---|---|---|---|---|---|---|
| (c) ≤2008 pg/m3 | (d) ≥2010 pg/m3 | d/c | (e) ≤2008 pg/m3 | (f) ≥2010 pg/m3 | f/e | ≤2008 % | ≥2010 % | |
| Kanazawa | 338 ± 197 | 154 ± 128 | 0.46 | 119 ± 88 | 7.4 ± 7.3 | 0.06 | 35 | 5 |
| Kitakyushu | 456 ± 234 | 611 ± 783 | 1.3 | 18 ± 17 | 8.0 ± 6.3 | 0.44 | 4 | 1 |
| Shenyang | 16,900 ± 23,400 | 7430 ± 8600 | 0.44 | 173 ± 129 | 193 ± 118 | 1.1 | 1 | 3 |
| Shanghai | 405 | 1040 ± 950 | 2.6 | 71 | 33 ± 32 | 0.46 | 18 | 3 |
| City | Pyr | Pyrl | ||||
|---|---|---|---|---|---|---|
| S, pg/m3 | W, pg/m3 | W/S | S, pg/m3 | W, pg/m3 | W/S | |
| Kanazawa | 169 ± 178 | 304 ± 176 | 1.8 | 122 ± 110 | 240 ± 115 | 2.0 |
| Kitakyushu | 239 ± 231 | 797 ± 542 | 3.3 | 234 ± 228 | 774 ± 550 | 3.3 |
| Shenyang | 971 ± 473 | 2380 ± 1880 | 25 | 913 ± 468 | 2360 ± 1880 | 26 |
| Shanghai | 567 ± 914 | 1260 ± 797 | 2.2 | 540 ± 911 | 1200 ± 787 | 2.2 |
| City | 1-NP | 1-NPl | 1-NPl/1-NP | |||||
|---|---|---|---|---|---|---|---|---|
| (c) ≤2008 pg/m3 | (d) ≥2010 pg/m3 | d/c | (e) ≤2008 pg/m3 | (f) ≥2010 pg/m3 | f/e | ≤2008 % | ≥2010 % | |
| Kanazawa | 55 ± 45 | 4.5 ± 3.2 | 0.08 | 0.29 ± 0.21 | 0.24 ± 0.19 | 0.83 | 1 | 5 |
| Kitakyushu | 8.3 ± 6.1 | 5.2 ± 4.0 | 0.63 | 0.76 ± 0.42 | 0.98 ± 1.24 | 1.3 | 9 | 19 |
| Shenyang | 88 ± 66 | 85 ± 90 | 0.97 | 24 ± 38 | 12 ± 14 | 0.50 | 27 | 14 |
| Shanghai | 16 ± 18 | 1.2 ± 1.3 | 8 | |||||
| City | 1-NP | 1-NPl | ||||
|---|---|---|---|---|---|---|
| S, pg/m3 | W, pg/m3 | W/S | S, pg/m3 | W, pg/m3 | W/S | |
| Kanazawa | 22 ± 39 | 33 ± 49 | 1.5 | 0.13 ± 0.08 | 0.36 ± 0.21 | 2.8 |
| Kitakyushu | 2.9 ± 2.0 | 11 ± 4.3 | 3.8 | 0.6 ± 0.6 | 1.1 ± 1.0 | 2.0 |
| Shenyang | 31 ± 9.8 | 161 ± 44 | 5.1 | 1.5 ± 0.7 | 34 ± 32 | 23 |
| Shanghai | 4.8 ± 2.7 | 30 ± 18 | 6.2 | 0.23 ± 0.05 | 1.9 ± 1.3 | 8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayakawa, K. Long-Term and Seasonal Changes in Emission Sources of Atmospheric Particulate-Bound Pyrene and 1-Nitropyrene in Four Selected Cities in the Western Pacific. Atmosphere 2024, 15, 634. https://doi.org/10.3390/atmos15060634
Hayakawa K. Long-Term and Seasonal Changes in Emission Sources of Atmospheric Particulate-Bound Pyrene and 1-Nitropyrene in Four Selected Cities in the Western Pacific. Atmosphere. 2024; 15(6):634. https://doi.org/10.3390/atmos15060634
Chicago/Turabian StyleHayakawa, Kazuichi. 2024. "Long-Term and Seasonal Changes in Emission Sources of Atmospheric Particulate-Bound Pyrene and 1-Nitropyrene in Four Selected Cities in the Western Pacific" Atmosphere 15, no. 6: 634. https://doi.org/10.3390/atmos15060634
APA StyleHayakawa, K. (2024). Long-Term and Seasonal Changes in Emission Sources of Atmospheric Particulate-Bound Pyrene and 1-Nitropyrene in Four Selected Cities in the Western Pacific. Atmosphere, 15(6), 634. https://doi.org/10.3390/atmos15060634





