Bioaerosol Exposure during Sorting of Municipal Solid, Commercial and Industrial Waste: Concentration Levels, Size Distribution, and Biodiversity of Airborne Fungal
Abstract
1. Introduction
2. Materials and Methods
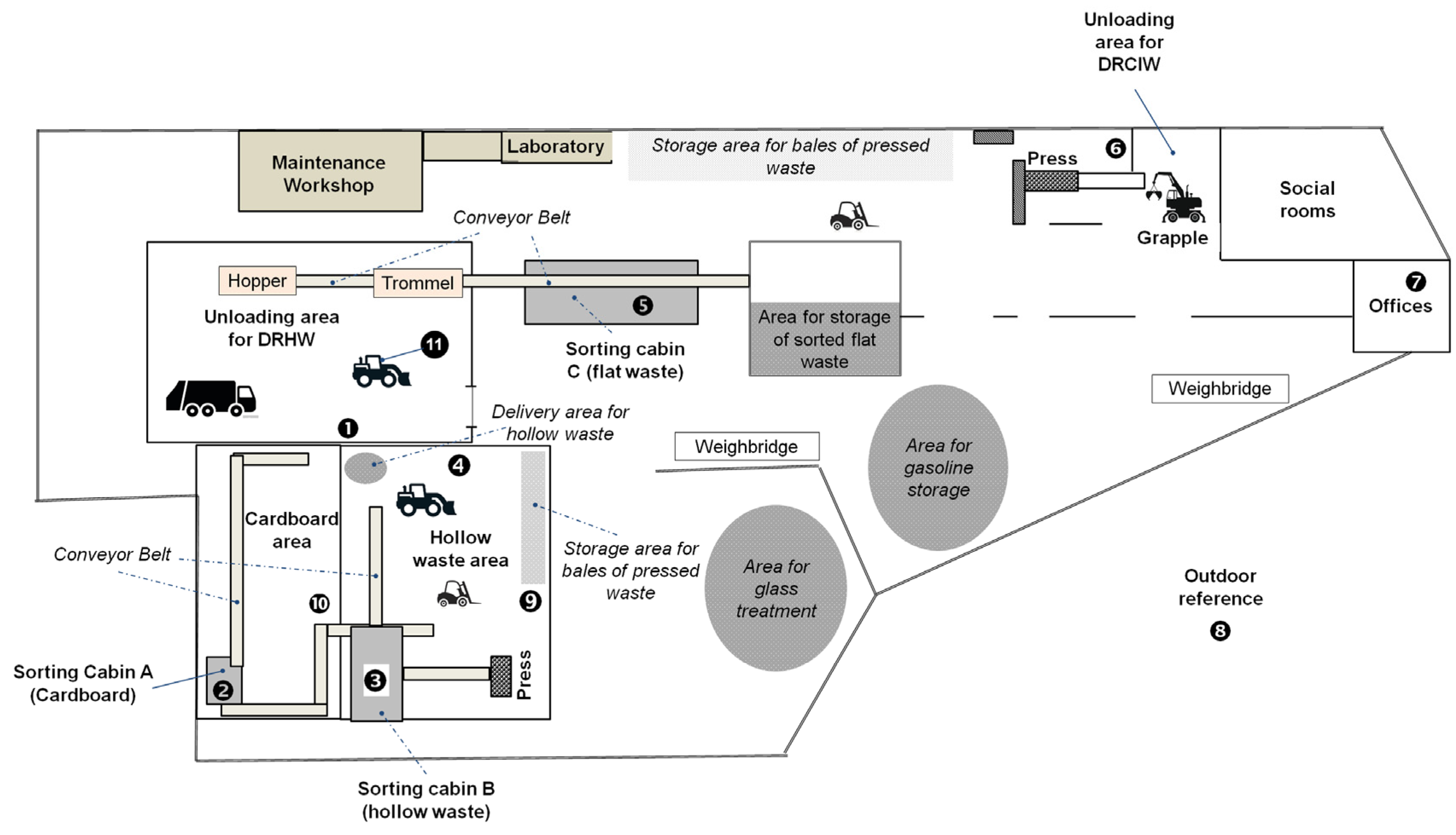
2.1. General Information about the Investigated Waste Sorting Plant (WSP)
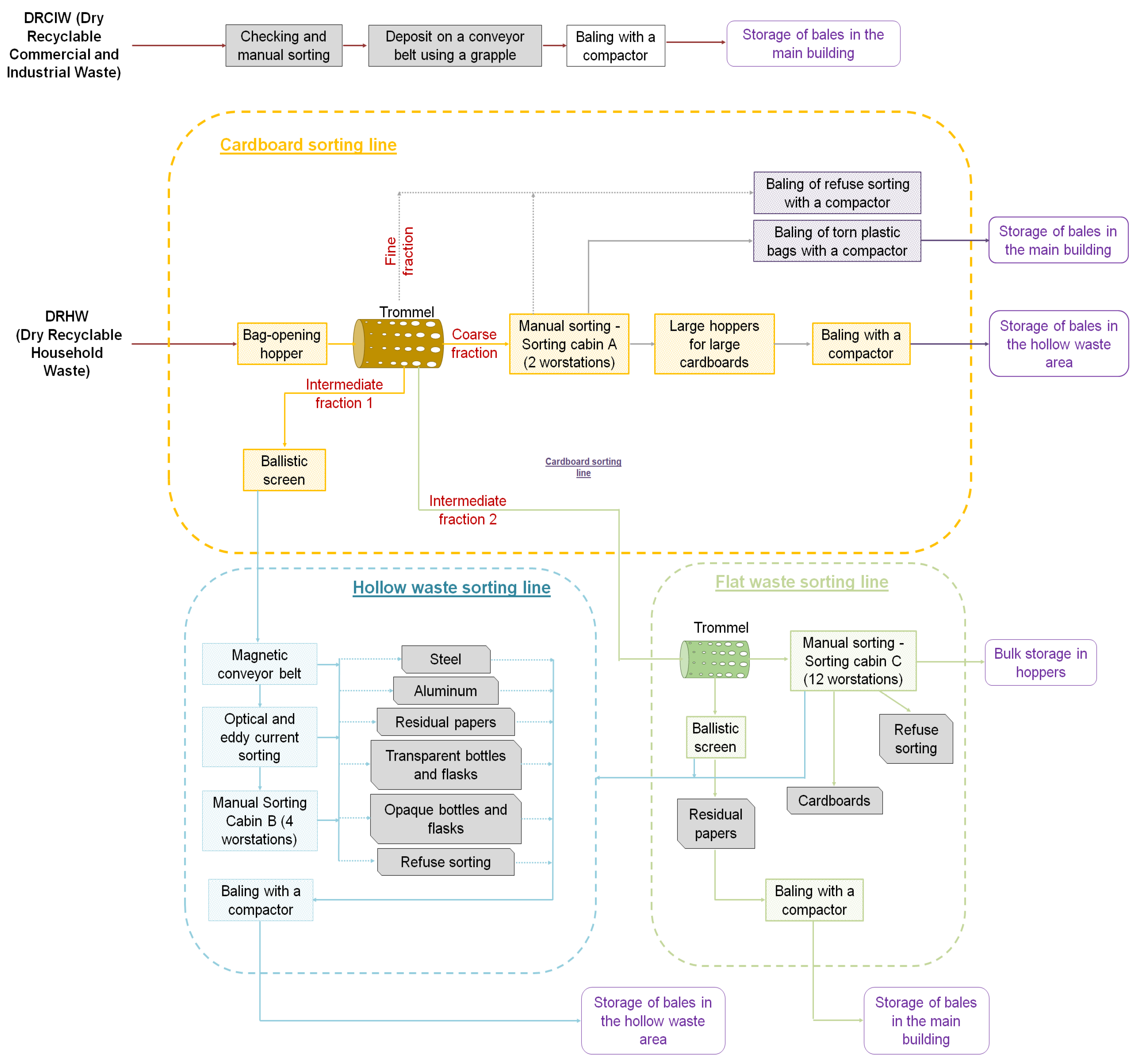
2.2. Description of the WSP and the Sorting Process
2.3. Ventilation System in the WSP
2.4. Measurement Strategy
2.4.1. Stationary Measurements
2.4.2. Personal Measurements
2.5. Air Sampling Methods
2.6. Real-Time Measurement of Airborne Particles Number Concentration and Size Distribution
2.7. Sampling of Settled Dust
2.8. Transport and Preservation of Samples
2.9. Measurement of Temperature and Relative Humidity
2.10. Sample Analysis
2.10.1. Cullturable Microorganisms
2.10.2. Analysis of Fungal Biodiversity in Samples
2.10.3. Gravimetric Dust Analysis
2.11. Data Analysis
3. Results
3.1. Sampling Conditions
3.2. Real-Time Number Concentration of Airborne Particles
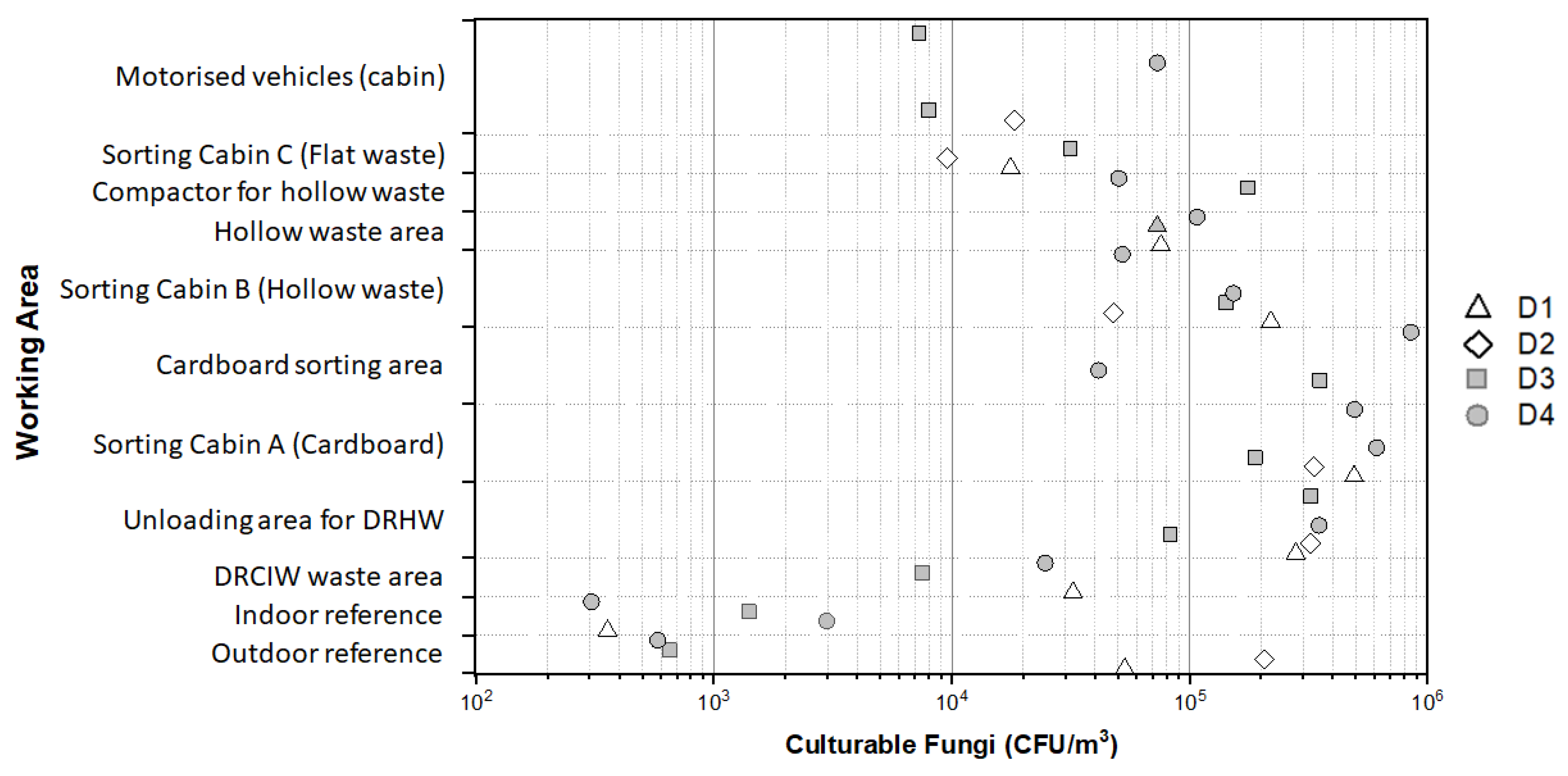
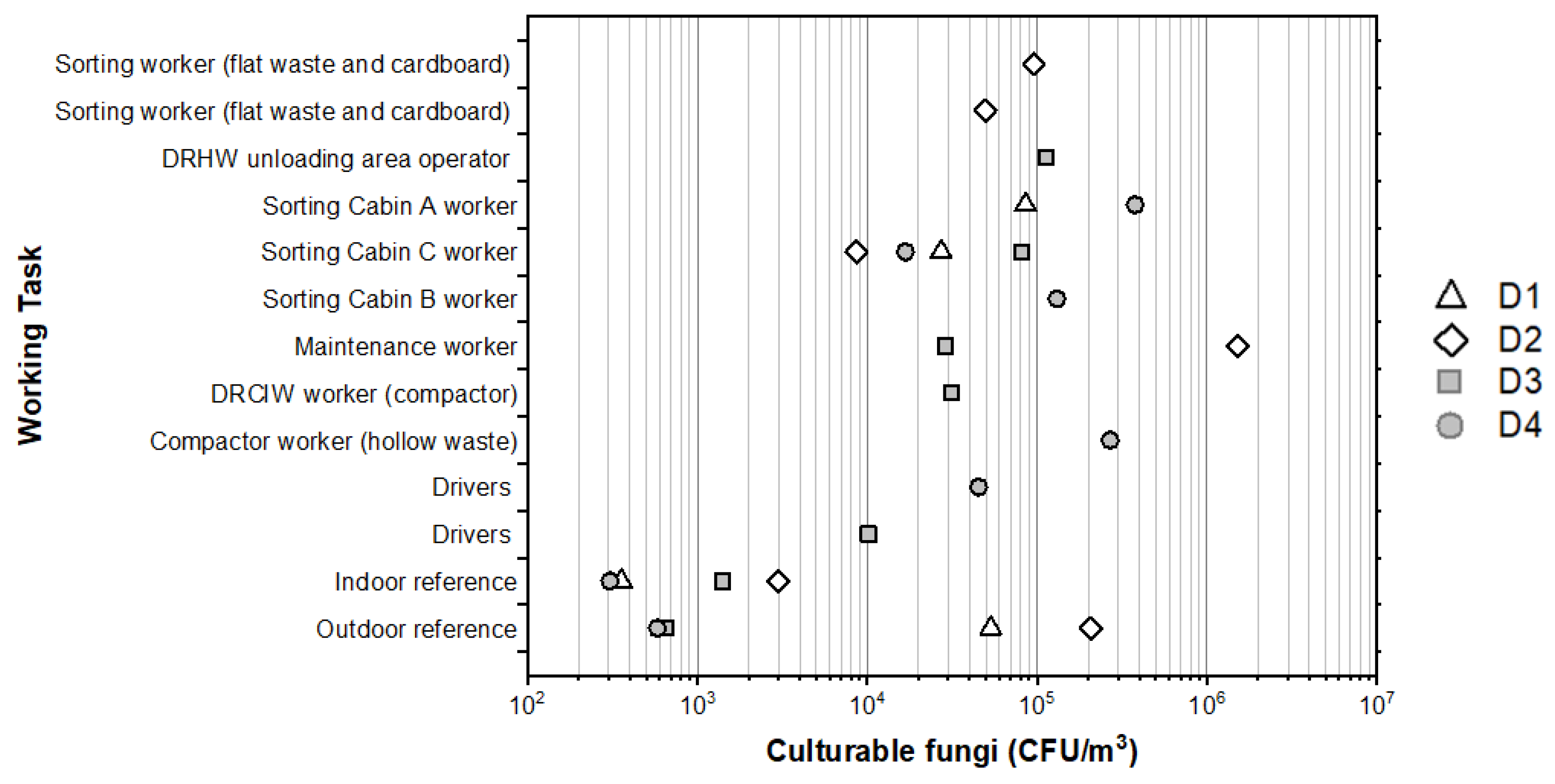
3.3. Exposure Levels to Airborne Fungi
3.4. Exposure Levels to Inhalable Dust
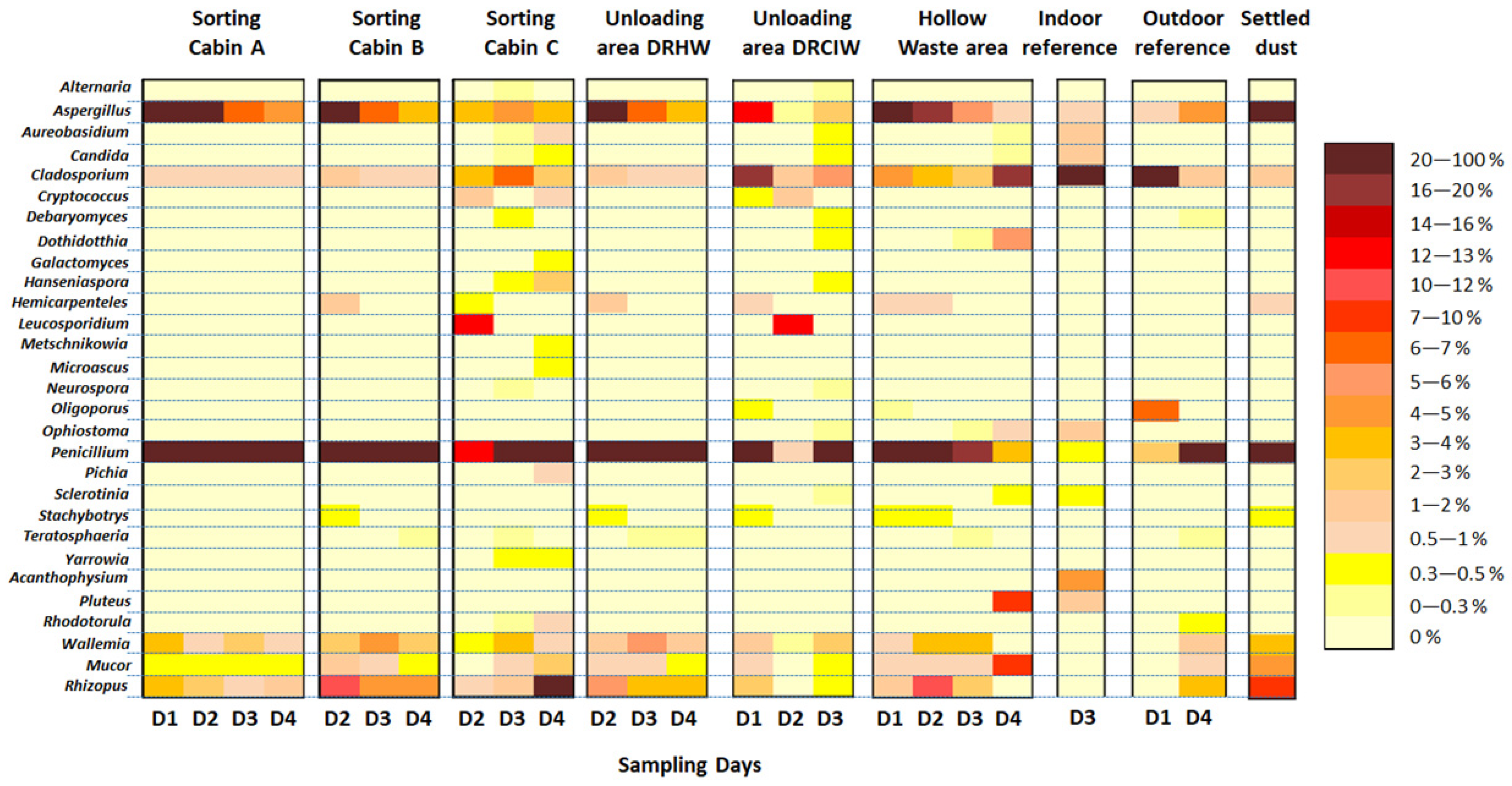
3.5. Biodiversity among Fungal Communities in the Emitted Bioaerosols
3.5.1. General Data and Alpha Biodiversity
3.5.2. Biodiversity among Eukaryota Communities
3.6. Size Distribution of Airborne Culturable Fungi
4. Discussion
4.1. Ambient Concentration Levels in the WSP
4.1.1. Level of Total Airborne Particles Measured with the OPC
4.1.2. Airborne Inhalable Gravimetric Dust
4.1.3. Airborne Culturable Fungi
4.2. Biodiversity of Airborne Fungal Communities
4.2.1. Overview of Fungal Biodiversity in the Air of the WSP
4.2.2. Dominant Fungal Taxa and Possible Origins
4.2.3. Spatio-Temporal Variation in Biodiversity
4.3. Size Distribution of Bioaerosols
4.4. Personal Exposure Levels
4.4.1. Personal Exposure to Inhalable Dust
4.4.2. Personal Exposure to Culturable Fungi
4.5. Risks Associated with the Measured Exposures
4.5.1. Risks Associated to the Exposure to Airborne Dust
4.5.2. Risks Associated to the Exposure to Airborne Fungi
4.6. The Benefits of Joint Measurement of Concentration, Biodiversity and Size Distribution
4.7. Prevention Means
4.8. Synthesis of Findings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EU, (European Union). Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. 2008. Available online: http://data.europa.eu/eli/dir/2008/98/oj (accessed on 23 March 2024).
- ADEME (Agence De l’Environnement et de la Maîtrise de l’Energie). Déchets: Chiffres-Clés. In Faits et Chiffres; ADEME (Agence De l’Environnement et de la Maîtrise de l’Energie): Anger, France, 2020; pp. 1–28. [Google Scholar]
- Cabaret, M.; Folley, S. État Des Lieux Du Parc des Centres De Tri De Recyclables Secs Ménagers En France; TERRA, ADEME: Anger, France, 2013; p. 51. [Google Scholar]
- Poole, C.J.M.; Basu, S. Systematic Review: Occupational illness in the waste and recycling sector. Occup. Med. 2017, 67, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Walser, S.M.; Gerstner, D.G.; Brenner, B.; Bünger, J.; Eikmann, T.; Janssen, B.; Kolb, S.; Kolk, A.; Nowak, D.; Raulf, M.; et al. Evaluation of exposure–response relationships for health effects of microbial bioaerosols—A systematic review. Int. J. Hyg. Environ. Health 2015, 218, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hebisch, R.; Linsel, G. Workers’ Exposure to Hazardous Substances and Biological Agents in Recycling Enterprises. Arbeitsplatzbelastung 2012, 72, 163–169. [Google Scholar]
- Schlosser, O.; Robert, S.; Noyon, N. Airborne Mycotoxins in Waste Recycling and Recovery Facilities: Occupational Exposure and Health Risk Assessment. Waste Manag. 2020, 105, 395–404. [Google Scholar] [CrossRef]
- Kozajda, A.; Jeżak, K.; Cyprowski, M.; Szadkowska-Stańczyk, I. Inhalable Dust, Endotoxins and (1-3)-Β-D-Glucans as Indicators of Exposure in Waste Sorting Plant Environment. Aerobiologia 2017, 33, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Cyprowski, M.; Ławniczek-Wałczyk, A.; Górny, R.L. Occupational Exposure to Anaerobic Bacteria in a Waste Sorting Plant. J. Air Waste Manag. Assoc. 2021, 71, 1292–1302. [Google Scholar] [CrossRef]
- Duquenne, P.; Facon, B. Exposition Aux Bioaérosols Dans Les Centres De Tri Des Déchets Ménagers Recyclables. Hygiène Sécurité Trav. 2018, 252, 44–50. [Google Scholar]
- Lehtinen, J.; Tolvanen, O.; Nivukoski, U.; Veijanen, A.; Hänninen, K. Occupational Hygiene in Terms of Volatile Organic Compounds (Vocs) and Bioaerosols at Two Solid Waste Management Plants in Finland. Waste Manag. 2013, 33, 964–973. [Google Scholar] [CrossRef]
- Breum, N.O.; Würtz, H.; Midtgaard, U.; Ebbehøj, N. Dustiness and Bio-Aerosol Exposure in Sorting Recyclable Paper. Waste Manag. Res. 1999, 17, 100–108. [Google Scholar] [CrossRef]
- Madsen, A.M.; Frederiksen, M.W.; Mahmoud Kurdi, I.; Sommer, S.; Flensmark, E.; Tendal, K. Expanded cardboard waste sorting and occupational exposure to microbial species. Waste Manag. 2019, 87, 345–356. [Google Scholar] [CrossRef]
- Degois, J.; Clerc, F.; Simon, X.; Bontemps, C.; Leblond, P.; Duquenne, P. First metagenomic survey of the microbial diversity in bioaerosols emitted in waste sorting plants. Ann. Work. Expo. Health 2017, 61, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Degois, J.; Simon, X.; Clerc, F.; Bontemps, C.; Leblond, P.; Duquenne, P. One-year follow-up of microbial diversity in bioaerosols emitted in a waste sorting plant in France. Waste Manag. 2021, 120, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Bragoszewska, E. The dose of fungal aerosol inhaled by workers in a waste-sorting plant in Poland: A case study. Int. J. Environ. Res. Public Health 2020, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Bulski, K.; Frączek, K.; Chmiel, M. Microbiological air quality at municipal waste sorting plant. Environ. Prot. Nat. Resour. 2016, 27, 24–27. [Google Scholar] [CrossRef][Green Version]
- Madsen, A.M.; Beswick, A.; Oppliger, A.; Kolk, A.; Crook, B.; Tendal, K.; Hinker, M.; Cyprowsky, M.; Raulf, M.; Duquenne, P.; et al. Occupational exposure to microorganisms as related to new waste sorting instructions—A PEROSH project. In Proceedings of the European OSH Symposium “Vision Zero in the Waste Industry”, Hamburg, Germany, 23–24 October 2019. [Google Scholar]
- Madsen, A.M.; Frederiksen, M.W.; Jacobsen, M.H.; Tendal, K. Towards a risk evaluation of workers’ exposure to handborne and airborne microbial species as exemplified with waste collection workers. Environ. Res. 2020, 183, 109177. [Google Scholar] [CrossRef] [PubMed]
- Cyprowski, M.; Stobnicka-Kupiec, A.; Górny, R.L.; Gołofit-Szymczak, M.; Ptak-Chmielewska, A.; Ławniczek-Wałczyk, A. Across-shift changes in upper airways after exposure to bacterial cell wall components. Ann. Agric. Environ. Med. 2019, 26, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Osteresch, B.; Almeida, A.; Cramer, B.; Humpf, H.-U.; Viegas, C. Enniatin B and ochratoxin A in the blood serum of workers from the waste management setting. Mycotoxin Res. 2018, 34, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Simon, X.; Duquenne, P. Assessment of workers’ exposure to bioaerosols in a french cheese factory. Ann. Occup. Hyg. 2014, 58, 677–692. [Google Scholar] [CrossRef][Green Version]
- Görner, P.; Wrobel, R.; Simon, X. High efficiency CIP 10-I personal inhalable aerosol sampler. J. Phys. Conf. Ser. 2009, 151, 012061. [Google Scholar] [CrossRef]
- NF X 43-262; AFNOR. Air Quality—Workplace Air—Solid Aerosol Sampling with a Rotating Dish (Respirable, Thoracic and Inhalable Fractions). Normes Européennes: Occitanie, France, 2012.
- Simon, X.; Bau, S.; Boivin, A.; Duquenne, P.; Witschger, O.; Görner, P. Physical performances and kinetics of evaporation of the CIP 10-M Personal Sampler’s Rotating Cup Containing Aqueous or Viscous Collection Fluid. Aerosol Sci. Technol. 2016, 50, 507–520. [Google Scholar] [CrossRef]
- Burkart, J.; Steiner, G.; Reischl, G.; Moshammer, H.; Neuberger, M.; Hitzenberger, R. Characterizing the performance of two optical particle counters (Grimm OPC1.108 and OPC1.109) under urban aerosol conditions. J. Aerosol Sci. 2010, 41, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Görner, P.; Simon, X.; Bémer, D.; Lidén, G. Workplace aerosol mass concentration measurement using optical particle counters. J. Environ. Monit. 2012, 14, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, P.; Simon, X.; Koehler, V.; Goncalves-Machado, S.; Greff, G.; Nicot, T.; Poirot, P. Documentation of bioaerosol concentrations in an indoor composting facility in France. J. Environ. Monit. 2012, 14, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.H.; Park, C.W.; Yoon, K.Y.; Park, J.H.; Hwang, J. Size distributions of total airborne particles and bioaerosols in a municipal composting facility. Bioresour. Technol. 2008, 99, 5150–5154. [Google Scholar] [CrossRef]
- Galès, A.; Bru-Adan, V.; Godon, J.-J.; Delabre, K.; Catala, P.; Ponthieux, A.; Chevallier, M.; Birot, E.; Steyer, J.-P.; Wéry, N. Predominance of single bacterial cells in composting bioaerosols. Atmos. Environ. 2015, 107, 225–232. [Google Scholar] [CrossRef]
- Wikuats, C.F.H.; Duarte, E.H.; Prates, K.V.M.C.; Janiaski, L.L.L.; de Oliveira Gabriel, B.; da Cunha Molina, A.; Martins, L.D. Assessment of airborne particles and bioaerosols concentrations in a waste recycling environment in Brazil. Sci. Rep. 2020, 10, 14812. [Google Scholar] [CrossRef]
- Tolvanen, O.K. Airborne bio-aerosols and noise in a dry waste treatment plant in Pietarsaari, Finland. Waste Manag. Res. 2001, 19, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hussein, T.; Hruška, A.; Dohányosová, P.; Džumbová, L.; Hemerka, J.; Kulmala, M.; Smolík, J. Deposition rates on smooth surfaces and coagulation of aerosol particles inside a test chamber. Atmos. Environ. 2009, 43, 905–914. [Google Scholar] [CrossRef]
- Tolvanen, O.K. Exposure to bioaerosols and noise at a Finnish dry waste treatment plant. Waste Manag. Res. 2004, 22, 346–357. [Google Scholar] [CrossRef]
- Karamkhani, M.; Asilian-Mahabadi, H.; Daraei, B.; Seidkhani-Nahal, A.; Noori-Zadeh, A. Liver and kidney serum profile abnormalities in workers exposed to aflatoxin B1 in urban solid waste management centers. Environ. Monit. Assess. 2020, 192, 472. [Google Scholar] [CrossRef]
- Marchand, G.; Lavoie, J.; Lazure, L. Evaluation of bioaerosols in a municipal solid waste recycling and composting plant. J. Air Waste Manag. Assoc. 1995, 45, 778–781. [Google Scholar] [CrossRef]
- Van Tongeren, M.; Van Amelsvoort, L.; Heederik, D. Exposure to organic dusts, endotoxins, and microorganisms in the municipal waste industry. Int. J. Occup. Environ. Health 1997, 3, 30–36. [Google Scholar] [CrossRef]
- Sigsgaard, T.; Malmros, P.; Nersting, L.; Petersen, C. Respiratory disorders and atopy in Danish refuse workers. Am. J. Respir. Crit. Care Med. 1994, 149, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.; Guertin, S. Evaluation of health and safety risks in municipal solid waste recycling plants. J. Air Waste Manag. Assoc. 2001, 51, 352–360. [Google Scholar] [CrossRef]
- Rahkonen, P.; Ettala, M.; Laukkanen, M.; Salkinoja-Salonen, M. Airborne microbes and endotoxins in the work Environment of two sanitary landfills in Finland. Aerosol Sci. Technol. 1990, 13, 505–513. [Google Scholar] [CrossRef]
- Nadal, M.; Inza, I.; Schuhmacher, M.; Figueras, M.J.; Domingo, J.L. Health risks of the occupational exposure to microbiological and chemical pollutants in a municipal waste organic fraction treatment plant. Int. J. Hyg. Environ. Health 2009, 212, 661–669. [Google Scholar] [CrossRef]
- Atalia, K.R.; Buha, D.M.; Joshi, J.J.; Shah, N.K. Microbial biodiversity of municipal solid waste of Ahmedabad. J. Mater. Environ. Sci. 2015, 6, 1914–1923. [Google Scholar]
- Gladding, T.L.; Gwyther, C.L. A study of the potential release of bioaerosols from containers as a result of reduced frequency residual waste collections. Sci. Total Environ. 2017, 576, 481–489. [Google Scholar] [CrossRef]
- Schlosser, O.; Déportes, I.Z.; Facon, B.; Fromont, E. Extension of the sorting instructions for household plastic packaging and changes in exposure to bioaerosols at materials recovery facilities. Waste Manag. 2015, 46, 47–55. [Google Scholar] [CrossRef]
- Viegas, C.; Gomes, A.Q.; Abegão, J.; Sabino, R.; Graça, T.; Viegas, S. Assessment of fungal contamination in waste sorting and incineration—Case study in portugal. J. Toxicol. Environ. Health Part A 2014, 77, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Malta-Vacas, J.; Viegas, S.; Sabino, R.; Viegas, C. Fungal and microbial volatile organic compounds exposure assessment in a waste sorting plant. J. Toxicol. Environ. Health Part A 2012, 75, 1410–1417. [Google Scholar] [CrossRef]
- Černá, K.; Wittlingerová, Z.; Zimová, M.; Janovský, Z. Exposure to airborne fungi during sorting of recyclable plastics in waste treatment facilities. Med. Pr. 2017, 68, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duquenne, P. On the identification of culturable microorganisms for the assessment of biodiversity in bioaerosols. Ann. Work. Expo. Health 2018, 62, 139–146. [Google Scholar] [CrossRef]
- Mbareche, H.; Brisebois, E.; Veillette, M.; Duchaine, C. Bioaerosol sampling and detection methods based on molecular approaches: No pain no gain. Sci. Total Environ. 2017, 599, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Caetano, L.A.; Cox, J.; Korkalainen, M.; Haines, S.R.; Dannemiller, K.C.; Viegas, S.; Reponen, T. The effects of waste sorting in environmental microbiome, THP-1 cell viability and inflammatory responses. Environ. Res. 2020, 185, 109450. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Raulf, M.; Duquenne, P.; Graff, P.; Cyprowski, M.; Beswick, A.; Laitinen, S.; Rasmussen, P.U.; Hinker, M.; Kolk, A.; et al. Review of biological risks associated with the collection of municipal wastes. Sci. Total Environ. 2021, 791, 148287. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Sonjak, S.; Zalar, P.; Frisvad, J.C.; Diderichsen, B.; Plemenitaš, A. Extremophilic fungi in arctic ice: A relationship between adaptation to low temperature and water activity. Phys. Chem. Earth Parts A/B/C 2003, 28, 1273–1278. [Google Scholar] [CrossRef]
- Das, M.K.L.; Prasad, J.S.; Ahmad, S.K. Endoglucanase production by paper-degrading mycoflora. Lett. Appl. Microbiol. 1997, 25, 313–315. [Google Scholar] [CrossRef]
- Zalar, P.; Sybren de Hoog, G.; Schroers, H.-J.; Frank, J.M.; Gunde-Cimerman, N. Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie Van Leeuwenhoek 2005, 87, 311–328. [Google Scholar] [CrossRef]
- Zajc, J.; Gunde-Cimerman, N. The genus Wallemia—From contamination of food to health threat. Microorganisms 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiu, Y.; Gustafsson, Å.; Krais, A.M.; Weiss, J.M.; Lundh, T.; Bergman, Å. Characterization of residential household dust from Shanghai by particle size and analysis of organophosphorus flame retardants and metals. Environ. Sci. Eur. 2019, 31, 94. [Google Scholar] [CrossRef]
- Zeng, Q.; Westermark, S.; Rasmuson-Lestander, A.; Wang, X. Detection and quantification of Wallemia sebi in aerosols by real-time PCR, conventional PCR, and cultivation. Appl. Environ. Microbiol. 2004, 70, 7295–7302. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Nowoisky, J.; Pickersgill, D.A.; Després, V.R.; Pöschl, U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA 2009, 106, 12814–12819. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.M.; McCubbin, I.B.; Hallar, A.G.; Fierer, N. Seasonal variability in airborne bacterial communities at a high-elevation site. Atmos. Environ. 2012, 50, 41–49. [Google Scholar] [CrossRef]
- Kumari, P.; Woo, C.; Yamamoto, N.; Choi, H.-L. Variations in abundance, diversity and community composition of airborne fungi in swine houses across seasons. Sci. Rep. 2016, 6, 37929. [Google Scholar] [CrossRef] [PubMed]
- Nehme, B.; Gilbert, Y.; Letourneau, V.; Forster, R.J.; Veillette, M.; Villemur, R.; Duchaine, C. Culture-independent characterization of Archaeal biodiversity in swine confinement building bioaerosols. Appl. Environ. Microbiol. 2009, 75, 5445–5450. [Google Scholar] [CrossRef] [PubMed]
- Brągoszewska, E. Exposure to Bacterial and Fungal Aerosols: Microorganism Indices in A Waste-Sorting Plant in Poland. Int. J. Environ. Res. Public Health 2019, 16, 3308. [Google Scholar] [CrossRef]
- Clauß, M. Particle size distribution of airborne microorganisms in the environment—A review. Landbauforsch Appl. Agric. For. Res. 2015, 65, 77–100. [Google Scholar] [CrossRef]
- Sturm, R. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract—A review. J. Adv. Res. 2012, 3, 295–304. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Krajewski, J.A.; Tarkowski, S.; Cyprowski, M.; Szarapińska-Kwaszewska, J.; Dudkiewicz, B. Occupational exposure to organic dust associated with municipal waste collection and management. Int. J. Occup. Med. Env. Health 2002, 15, 289–301. [Google Scholar]
- Gladding, T.; Thorn, J.; Stott, D. Organic dust exposure and work-related effects among recycling workers. Am. J. Ind. Med. 2003, 43, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-U.; Ryu, S.-H.; Kim, S.-B.; Yoon, C.-S. An assessment of dust, endotoxin, and microorganism exposure during waste collection and sorting. J. Air Waste Manag. Assoc. 2011, 61, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Guillou, P.; Brunet, D.; Rousselle, C.; Binet, S. Recommandation de l’Anses en vue de la révision des valeurs limites d’exposition professionnelle (VLEP) pour les poussières dites sans effet spécifique (PSES). Arch. Mal. Prof. L’environnement 2020, 81, 676–677. [Google Scholar] [CrossRef]
- Mandal, J.; Brandl, H. Bioaerosols in indoor environment—A review with special reference to residential and occupational locations. Open Environ. Biol. Monit. J. 2011, 4, 83–96. [Google Scholar] [CrossRef]
- SUVA (Schweizerische Unfallversicherungsanstalt). Valeurs Limites D’exposition Aux Postes de Travail 2015, edition 2015; Référence 1903.f; SUVA: Luzern, Switzerland, 2024; Available online: http://www.suva.ch (accessed on 2 February 2024).
- Arbeit und Soziales im Gemeinsamen Ministerialblatt. Trba (Technische Regel für Biologische Arbeitsstoffe) 214—Abfallbehandlungsanlagen. In Technische Regeln für Biologische Arbeitsstoffe; Federal Institute for Occupational Safety and Health (BAuA): Dortmund, Germany, 2013; pp. S978–S989. [Google Scholar]
- David, C.; Emili, A.; Alonso, L.; Loison, P.; Mater, G.; Duquenne, P.; Cheron, J.; Durand-Billaud, E.; Facon, B.; Renevot, V.; et al. Valeurs guides bactéries et moisissures: Interprétation des résultats de métrologie des bioaérosols. Hygiène Sécurité Trav. 2023, 271, 55–63. [Google Scholar]
- Eduard, W. A health-based criteria document on fungal spore exposure in the working population. Is it relevant for the general population? Indoor Air 2008, 18, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Rouzaud, P.; Soulat, J.M.; Trela, C.; Fraysse, P.; Recco, P.; Carles, P.; Lauque, D. Symptoms and serum precipitins in workers exposed to dry sausage mould: Consequences of exposure to sausage mould. Int. Arch. Occup. Environ. Health 2001, 74, 371–374. [Google Scholar]
- Winck, J.C.; Delgado, L.; Murta, R.; Lopez, M.; Marques, J.A. Antigen characterization of major cork moulds in Suberosis (cork worker’s pneumonitis) by immunoblotting. Allergy 2004, 59, 739–745. [Google Scholar] [CrossRef]
- Latge, J.-P. Aspergillus fumigatus and Aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Broadbridge, C.; Murray, S.; Waghorn, D.; Mahoney, A. Gardening can seriously damage your health. Lancet 2008, 371, 2056. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.P.; Bush, R.K.; Demain, J.G.; Denning, D.W.; Dixit, A.; Fairs, A.; Greenberger, P.A.; Kariuki, B.; Kita, H.; Kurup, V.P.; et al. Fungi and allergic lower respiratory tract diseases. J. Allergy Clin. Immunol. 2012, 129, 280–291. [Google Scholar] [CrossRef]
- Jančič, S.; Zalar, P.; Kocev, D.; Schroers, H.-J.; Džeroski, S.; Gunde-Cimerman, N. Halophily reloaded: New insights into the extremophilic life-style of Wallemia with the description of Wallemia hederae sp. nov. Fungal Divers. 2016, 76, 97–118. [Google Scholar] [CrossRef]
- Weber, R.W. Allergen of the Month—Mucor. Ann. Allergy Asthma Immunol. 2015, 115, A15. [Google Scholar] [CrossRef] [PubMed]
- Rydjord, B.; Eduard, W.; Stensby, B.; Sandven, P.; Michaelsen, T.E.; Wiker, H.G. Antibody response to long-term and high-dose mould-exposed sawmill workers. Scand. J. Immunol. 2007, 66, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Quirce, S.; Vandenplas, O.; Campo, P.; Cruz, M.J.; de Blay, F.; Koschel, D.; Moscato, G.; Pala, G.; Raulf, M.; Sastre, J.; et al. Occupational hypersensitivity pneumonitis: An EAACI position paper. Allergy 2016, 71, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Egbuta, M.A.; Mwanza, M.; Babalola, O.O. Health Risks Associated with Exposure to Filamentous Fungi. Int. J. Environ. Res. Public Health 2017, 14, 719. [Google Scholar] [CrossRef] [PubMed]
- Burzoni, S.; Duquenne, P.; Mater, G.; Ferrari, L. Workplace biological risk assessment: Review of existing and description of a comprehensive approach. Atmosphere 2020, 11, 741. [Google Scholar] [CrossRef]
- Madsen, A.M.; Alwan, T.; Ørberg, A.; Uhrbrand, K.; Jørgensen, M.B. Waste workers’ exposure to airborne fungal and bacterial species in the truck cab and during waste collection. Ann. Occup. Hyg. 2016, 60, 651–668. [Google Scholar] [CrossRef]
- Schlosser, O.; Huyard, A.; Rybacki, D.; Do Quang, Z. Protection of the vehicle cab environment against bacteria, fungi and endotoxins in composting facilities. Waste Manag. 2012, 32, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; de Oliveira, A.C.; Caetano, L.A.; Carolino, E.; Quintal-Gomes, A.; Twaruzek, M.; Kosicki, R.; Soszczynska, E.; Viegas, S. A new approach to assess occupational exposure to airborne fungal contamination and mycotoxins of forklift drivers in waste sorting facilities. Mycotoxin Res. 2017, 33, 285–295. [Google Scholar] [CrossRef] [PubMed]



| Sampling Point | Number of Samples | Reads Number * | OTUs Number ** | Shannon Index ** | Simpson Index ** |
|---|---|---|---|---|---|
| Unloading area DHRW | 3 | 7566 (2.8) | 376 (46–422) | 4.02 (1.54–4.17) | 0.05 (0.05–0.4) |
| Sorting cabin A | 4 | 15,227 (1.6) | 42 (38–436) | 1.30 (1.30–3.89) | 0.4 (0.06–0.5) |
| Sorting cabin B | 3 | 11,242 (1.5) | 271 (31–316) | 3.75 (1.48–3.98) | 0.07 (0.05–0.4) |
| Sorting cabin C | 3 | 5115 (1.5) | 248 (51–324) | 4.36 (2.80–4.39) | 0.04 (0.03–0.09) |
| Hollow waste area | 4 | 5573 (4.3) | 157 (39–330) | 1.73 (1.35–4.09) | 0.4 (0.3–0.4) |
| Unloading area DRCIW | 3 | 6276 (1.8) | 43 (40–46) | 2.34 | 0.2 (0.05–0.2) |
| Indoor reference | 1 | 2211 | 67 | 3.03 | 0.09 |
| Outdoor reference | 2 | 6282 (1.1) | 15–286 | 1.83–4.12 | 0.05–0.2 |
| Settled dust | 1 | 10,015 | 65 | 1.79 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duquenne, P.; Simon, X.; Coulais, C.; Koehler, V.; Degois, J.; Facon, B. Bioaerosol Exposure during Sorting of Municipal Solid, Commercial and Industrial Waste: Concentration Levels, Size Distribution, and Biodiversity of Airborne Fungal. Atmosphere 2024, 15, 461. https://doi.org/10.3390/atmos15040461
Duquenne P, Simon X, Coulais C, Koehler V, Degois J, Facon B. Bioaerosol Exposure during Sorting of Municipal Solid, Commercial and Industrial Waste: Concentration Levels, Size Distribution, and Biodiversity of Airborne Fungal. Atmosphere. 2024; 15(4):461. https://doi.org/10.3390/atmos15040461
Chicago/Turabian StyleDuquenne, Philippe, Xavier Simon, Catherine Coulais, Véronique Koehler, Jodelle Degois, and Brigitte Facon. 2024. "Bioaerosol Exposure during Sorting of Municipal Solid, Commercial and Industrial Waste: Concentration Levels, Size Distribution, and Biodiversity of Airborne Fungal" Atmosphere 15, no. 4: 461. https://doi.org/10.3390/atmos15040461
APA StyleDuquenne, P., Simon, X., Coulais, C., Koehler, V., Degois, J., & Facon, B. (2024). Bioaerosol Exposure during Sorting of Municipal Solid, Commercial and Industrial Waste: Concentration Levels, Size Distribution, and Biodiversity of Airborne Fungal. Atmosphere, 15(4), 461. https://doi.org/10.3390/atmos15040461







