Abstract
Vortex fluids are often present in natural and artificial aquatic environments and are also widely used in industrial water treatment and product manufacturing processes. Vortex processes have been studied quite extensively; however, little attention has been paid to the potential release of biological aerosols to the ambient air in common situations involving microbial-contaminated vortex liquids. The model organism was Escherichia coli, a common Gram-negative coliform bacterium widely present in the aquatic and air environments. This study examines the influence of various parameters, including liquid rotation speed, column height, temperature, surface tension and vessel size, on the rate of bioaerosol formation. A commonly used single-stage bioaerosol impactor was employed to collect microbial aerosols at different process parameters under controlled laboratory conditions. The main results show that bioaerosol production increases markedly with increasing rotation speed, reaching a maximum rate at the highest value used in this project (1300 rpm). The tallness of the liquid column is strongly responsible for the bioaerosol production efficiency reaching a difference of almost one order of magnitude along the range between 45 mm (highest bioaerosol release) and 110 mm used in this research. Fluid temperature and surface tension are also very influential parameters responsible for bioaerosol generation during fluid vortex motion; corresponding results are discussed in this manuscript.
1. Introduction
Bioaerosols are airborne particles with a typical size range of 0.05 to 10 µm, originating from biological sources. Numerous biological components, including bacteria, fungi, viruses, and other elements, such as endotoxins and hazardous chemicals, are present in these particles. Bioaerosols encompass a diverse range of microscopic biological entities and associated substances that can be dispersed in the air [1].
Various epidemiological studies have linked exposure to bioaerosols to increased rates of respiratory symptoms, respiratory infections, and gastrointestinal illnesses among workers and nearby residents [2]. The inhalation of bioaerosols is one of the main routes of their transmission, causing respiratory diseases, allergic reactions, and other health issues [3]. In addition, individuals representing higher-risk groups, such as children, the elderly, and people with pre-existing respiratory conditions, may be more vulnerable to the health effects of exposure to bioaerosols [4]. In addition to human diseases, the release of bioaerosols into the environment can have ecological impacts, including the transmission of diseases among wildlife and the contamination of water sources.
Bioaerosols emitted by wastewater treatment plants (WWTPs) contain various harmful active substances [5]. This situation contributes to an illness commonly referred to as “sewage worker’s syndrome” among individuals working at WWTPs [6]. Several investigations have demonstrated the presence of pathogenic microorganisms in bioaerosols, including bacteria such as Escherichia coli, Legionella spp., Mycobacterium spp. and Pseudomonas aeruginosa, as well as viruses such as norovirus and rotavirus. These microorganisms can survive and remain infectious in an aerosolised form, posing a risk to employees and individuals residing in the vicinity of WWTPs [7]. WWTPs contain various microorganisms that actively contribute to the treatment process. These microbial communities are composed of bacteria, viruses, fungi and other microbes that function together to break down organic matter and remove nutrients and contaminants [8]. Various levels of wastewater treatment, including primary, secondary and tertiary, have different microbial community compositions [9], which could also be potentially aerosolised. In general, various activities in wastewater treatment plants, such as aeration, mixing and sludge handling, can generate aerosols that contain microorganisms. The inhalation of these bioaerosols may lead to respiratory infections, allergies and other adverse health effects [10].
Aerosol formation typically occurs during the disruption of a liquid phase containing microorganisms [11]. This disruption can be achieved through various mechanisms including bubbling, shaking, vibrating, swirling, vortex and others. Aeration, or the introduction of air into a liquid medium, is intrinsically linked to many processes and plays a crucial role in bioaerosol generation [12]. For example, the dynamics of aeration in bubbling processes, characterised by the formation of bubbles of varying sizes within the liquid phase, can significantly influence the outcome of the aerosolisation processes. Both large and small bubbles have been identified as having distinct effects on bioaerosol production [13]. Large bubbles, which are frequently linked to increased gas–liquid mass transfer rates, are essential for capturing and moving microorganisms from the liquid phase into the air. It is easier for microorganisms to be released into the atmosphere when they are transported to the liquid–air interface by these large bubbles as they ascend through the liquid medium [14]. Conversely, small bubbles contribute to the generation of smaller aerosol particles, which can suspend in the air for extended time periods and enter deeper parts of the human respiratory tract. Smaller aerosol particles may pose a greater risk for human inhalation and subsequent health effects [15].
In addition to bubble size, previous studies have explored various parameters that affect aerosol formation and behaviour in bubbling processes, such as temperature, the surface tension of the liquid medium, the rotational speed (rpm) of vortex, the use of antifoaming agents as surfactants and the volume of the microbial suspension being processed [13,16,17]. These factors were found to have a significant impact on the aerosolisation process and the characteristics of the resulting bioaerosols.
It should be emphasised that although the volume of literature reporting the results of studies on bubbling processes is quite large, the literature on the study of aerosol release as a result of vortex processes is practically absent. On this basis, the main objective of this project was to address the lack of comprehensive studies investigating the release of bioaerosols from microbial suspensions during vortex under controlled laboratory conditions. The relationships between the vortex aeration dynamics, the properties of the liquid media and the final characteristics of the produced bioaerosol will be investigated. The contribution of key process parameters, including liquid temperature, rotating speed, surface tension and microbial suspension height, towards bioaerosol generation capabilities will be extensively investigated, and recommendations towards the minimisation of the bioaerosol formation rate will be discussed. This research is essential for expanding knowledge about bioaerosol behaviour in such situations and for informing the health and safety measures of employees and adjacent communities regarding their potential release into the environment.
2. Materials and Methods
2.1. Culture Preparation
Experiments were carried out with Escherichia coli, which are widely distributed in atmospheric and aquatic environments, and Gram-negative coliform bacteria, both indoors and outdoors [18]. E. coli was shown to be quite sensitive to aerosolisation and other physical and biological stresses [19]. All details of the microbial preparation procedure could be found in [13]. In brief, a single colony of an E. coli bacterial culture (ATCC 11303) was used to inoculate 200 mL of sterile nutrient broth (1.3 g/100 mL) (#CM0001, OXOID Ltd., Basingstoke, Hampshire, UK), followed by shaking at 150 rpm for 18 h at 37 °C. Then, the culture was centrifugated at 7000 rpm for 15 min (Centrifuge model 5810, Eppendorf, Hamburg, Germany), the supernatant was removed from the tube and the remaining cells were resuspended in 200 mL of sterile deionised water. To minimise the presence of bacterial clumps that resulted from the centrifugation, the suspension was treated in a sonic bath for 10 min to loosen potential microbial agglomerates.
2.2. Normalisation of Microbial Suspension
Based on the assumption that the rate of aerosolised microorganisms is highly dependent on the concentration of the microbial suspension, for the consistency of all the experimental results, the microbial suspension concentration of exactly 107 CFU/mL was selected for the entire experimental procedure (within 5% inter-batch discrepancy). To ensure that such a concentration was provided for all experimental runs, the following simple procedure was employed. First, it ought to be noticed that this number was selected as the concentration after overnight bacterial harvesting always varied between 7.8 × 107 and 1.2 × 108 CFU/mL and could be diluted to produce one litre of microbial suspension with the bacterial concentration of 107 CFU/mL. On completion of the 18 h microbial harvesting procedure described above, a sample of the microbial suspension (1 mL) was collected, and the remaining liquid was refrigerated and stored overnight at 4 °C. The sample was 10-fold diluted with sterile water, and a 100 µL aliquot of an appropriate dilution was spread onto the surface of the nutrient agar plates (#CM0003B, OXOID Ltd., Basingstoke, Hampshire, UK). Then, the plates were incubated at 37 °C overnight with the following counting colonies by a colony counter (Biolab, Clayton, VIC, Australia). Based on the counting results, one litre of the suspension with the bacterial concentration of exactly 107 CFU/mL was produced by diluting the original suspension, providing the integrity of all experimental runs. Upon completion of the experiments, the concentration was re-checked by the same procedure to ensure that no significant microbial inactivation occurred during the experimental runs. The difference in the results was always within 5%, which could certainly be taken as acceptable.
2.3. Experimental Setup
The laboratory setup is shown in Figure 1. It consists of a heat-resistant cylindrical glass vessel located at the bottom of the plant and a transparent plastic top equipped with a specially designed holder capable of accommodating a single-stage 400-hole BioStage impactor (SKC Ltd., Eighty Four, PA, USA). The plastic top was made to fit tightly onto the glass cylinder. A series of holes were strategically located around the periphery of the plastic part approximately 30 mm above the liquid level. They were used as make-up air supply inlets, ensuring smooth device operation at the required level of 28.3 L/min, and they played an important role in aerosol transportation to the impactor. The air flow was generated by a BioLite+ vacuum pump (SKC Ltd., Eighty-Four, PA, USA). Most of the experiments were undertaken by using 100 mm diameter vessel; however, for the final series of the experiments, a 140 mm vessel of similar height was used.
Figure 1.
Schematic diagram of the experimental setup.
Two magnetic bar stirrers, including a long (50 mm) and short (30 mm) one, were used for vortex generation in the experiments to investigate the bioaerosol release at different intensities of vortex processes. Both agitators were used to operate the system at three rotational speeds, 900, 1100 and 1300 rpm, which were selected to provide slow, medium and violent vortex regimes. A hot plate equipped with a stirring module (Model PC-420D, Labnet International Inc., Edison, NJ, USA) was used to provide the required stirrers’ speeds and corresponding vortex intensity.
The temperature range that was specifically selected to investigate the effects of different liquid temperatures on the release of bioaerosols was from 4 °C to 38 °C. To simulate winter conditions found in both natural and artificial aquatic systems, as well as the lowest possible temperature in industrial environments, 4 °C was chosen as a representative value. Finally, the upper limit of the temperature range used in this project was selected at the level of 38 °C as it is very close to the highest temperature at which bacteria could still reliably survive. Obviously, the temperature in industrial dwellings could be higher; however, a higher temperature would have led to the massive inactivation of the bacteria and a decrease in their potential release into the atmosphere in live forms. To run the temperature-related investigations, the suspension was kept in the fridge and immediately used for experiments undertaken at 4 °C. Then, the microbial suspension was very slowly heated on a hot plate until the next target temperature of 10 °C was reached and stabilised. The experiments at this temperature were correspondingly undertaken at the two highest rotational speeds of the large stirring bar. Then, the procedure was repeated with 5 °C steps up to the final value of 38 °C.
In the next stage of the research, the effect of the height of the microbial suspension column on the efficiency of bioaerosol generation was investigated. A size range from 45 to 110 mm was selected. To carry out this series, the experimental glass vessel was first filled with 400 mL of suspension (corresponding to the height of 45 mm) and then adjusted to 67 mm, 90 mm and 110 mm (corresponding to volumes of 600, 800 and 1000 mL, respectively). The experiments were carried out with both stirrers operating at all three rotational velocities outlined above.
The effect of surface tension on the bioaerosol generation in liquid vortex processes was investigated by adding antifoaming agent Antifoam A (A6582 Sigma-Aldrich, St. Louis, MO, USA) to the bacterial suspension. In particular, the antifoaming agent was added to the suspension to achieve the concentrations of ~1.0 g/L and ~1.5 g/L of the bacterial suspension to reduce the surface tension for consecutive experimental runs.
Note that, considering the strong sensitivity of the BioStage impactor to the microbial concentration and the corresponding possibility of either under- or over-loading the agar plates, several preliminary experiments were conducted to determine the optimal sampling time for all experimental conditions to achieve a countable concentration of bacterial aerosols for the experiments described above. Then, at least three repeats were undertaken for each set of experimental conditions.
2.4. Bioaerosol Monitoring Procedure
As discussed, bioaerosols generated in the swirling process were mainly monitored by a single-stage 400-hole BioStage Impactor at the flow rate of 28.3 L/min. In addition, some experiments related to the size distribution of the bioaerosol were undertaken using a 6-stage bioaerosol impactor [20], which operates at the same flow rate, providing d50 of 7 µm, 4.7 µm, 3.3 µm, 2.1 µm, 1.1 µm and 0.65 µm, for the 6 stages. It should be noted that considering identical air inlet nozzles of both devices, no additional hardware was required when the impactors were altered in the experiments.
Prior to each experimental run, a fresh agar plate was placed into the impactor (6 plates in the cases of 6-stage device utilisation), and after assembly, the device was located upside down at the top of the aerosol chamber, as schematically shown in Figure 1. Then, the pump was switched on and operated during a corresponding period of time (usually between 15 and 180 s). On completion, the plate was removed from the impactor, labelled and incubated at 37 °C overnight. The number of culturable bacteria was assessed upon completion of incubation using the colony counter; the results were statistically adjusted using positive-hole conversion tables [21], and the concentration of aerosolised culturable bacteria was calculated and presented in CFU/m3.
Two very important issues must be noted. First, the entire laboratory setup was located in a Class II Biohazard Cabinet to eliminate any extraneous particles from the experimental flows and to prevent any potential bioaerosol leaking within the laboratory. Second, the “blank” experiments were undertaken to ensure that no cross contamination could potentially occur. In particular, the stirrer was switched off and the impactor was run for over 90 min. Upon completion of the run, the plate was handled as discussed above, and the average result of three repeats was 2.7 CFU/m3. This number is a few orders of magnitude below the average experimental results, eliminating any influence of the natural environment on the results of the experiments.
3. Results and Discussions
3.1. Process Visualisation
Using high-speed photography, to illustrate the process, dozens of photographs were taken at different process parameters. Selected representative images are shown in Figure 2. Series A shows the process driven by a short stirrer at rotation speeds of 900, 1100 and 1300 rpm from left to right, respectively. Series B shows the process occurring at the corresponding rotation speeds caused by a longer stirrer. As it can be seen for the short stirrer, at the lowest speed, the vortex had a distinctive conical shape with almost no bubbles produced. Increasing the rotational speed to 1100 rpm caused more violent dynamics of the vortex and increased the number of bubbles due to the elevation of the interlayer friction in the liquid responsible for the bubble formation. At the highest rotational speed, the air cone rather became frustum; its bottom point reached the spinning stirrer, increasing the efficiency of the aeration and producing a large number of air bubbles around 1 mm in diameter.
Figure 2.
Images of the process at different rotational speeds (column height—110 mm). (A)—small stirrer, (B)—large stirrer.
A very similar picture is observed for the process driven by the longer stirrer (B), with the same trend for bubble formation remaining. Slightly larger cones were observed for the two lowest rotation speeds compared to those with the smaller stirrer. Similarly, for the highest speed, the bottom part of the formed frustum had a bigger diameter, and the edge of the upper part was much less stable (no distinctive surface shape was observed, especially at the highest value of rpm).
As is seen for both cases, the stirrer being touched by the bottom point of the vortex cone and the following conversion of the shape to frustum could cause very turbulent interaction between phases, leading to extensive aeration and the corresponding formation of the bubbles. Their following unavoidable bursting enables the enhanced production of aerosols which, in cases of microbial suspensions, could have substantial microbial content.
3.2. Effect of Rotational Speed
The concentrations of the bioaerosols produced in microbial suspensions at different rotational speeds are illustrated in Figure 3. The results revealed that the concentrations of bioaerosols increase with the rotational velocity increase, reaching the maximum value for the highest flow used in this study (1300 rpm). Similar trends were reported in the literature [12] that the concentration of culturable bacteria in the air increased as the rotational speed of the brush aerator increased. Of course, the different natures of the operations do not allow for direct comparison; however, the observed trends were very similar. It is worth emphasising that the production of bioaerosols by vortex fluids at higher speeds using a larger stirring bar capable of generating an elevated number of small bubbles bursting at the surface level is one of the mechanisms responsible for enhanced bioaerosol generation. This observation has previously been reported in relation to bubbling processes, highlighting that bubble bursting is a very efficient way to produce significant quantities of biological aerosols from microbial suspensions [13].
Figure 3.
Effect of rotational speed on the bioaerosol emission (error bars represent the standard deviation of at least 3 measurements).
The increase in rotational velocity assisted in the generation of shear force in the boundary layers due to the strong turbulence effect. The presence of shear force and turbulent effects promoted the eddies which forced a higher amount of bioaerosol to leave the surface. Han and colleagues [22] demonstrated that bioaerosols produced via bubble bottom aeration are predominantly composed of smaller particles, contrasting with the larger particle composition observed in the case of brush surface aeration, and suggested that increased rotational speeds and increased levels of turbulence may contribute to the release of bioaerosols.
Regarding practical applications, it should be emphasised that since bioaerosols are still produced in considerable quantities using an even smaller stirring bar operating at 900 RPM (non-violent, bubble-free mode, as seen in Figure 2), this confirms that the generation of bioaerosol in swirling processes occurs due to both interlayer friction and the bursting of bubbles. This finding means that even using a low rotational velocity would not eliminate the release of microbial contaminants, requiring much greater attention to the safety of the operations involved. Obviously, a reduction in bioaerosol emissions could be achieved by further reducing the rotation speeds, but this is not considered in this project since the mixing efficiency would not be very high, making this approach not very practical.
The other interesting observation is related to the fact that very similar-looking processes observed for the smaller stirrer at 1100 RPM and the larger stirrer at 900 RPM (Figure 2) produced very similar amounts of bioaerosol (Figure 3), which allows us to assume that the process could be mathematically generalised for different types of hardware.
A very important measure of the process is the size distribution of bioaerosols leaving the bulk liquid. Figure 4 demonstrates the results obtained by the six-stage impactor for the long stirring bar used. The results demonstrated a very similar trend for the small bar and are not shown here to minimise the wasting of valuable journal space. As is seen, for all RPMs, the numbers of large-sized particles with the diameters of >3.3 µm are almost an order of magnitude smaller as compared to the numbers of fine ones. The last two stages, being fine particles (<1.1 µm), show much higher concentrations of bioaerosols produced, which is another strong warning factor, as smaller particles might remain in the ambient air for longer periods of time, causing a more significant biological threat.
Figure 4.
Size distribution of the bioaerosols at various rotational speeds.
Some interesting issues could be discussed based on the results shown in Figure 4. Considering that the size of E. coli is known to be of around 0.9 µm [13] and taking into account the fact that most bioaerosols produced in these experiments were <1.1 µm in size, it can be concluded that bacterial aerosols produced in vortex processes are quite naked and have a minimal amount of liquid on their surface. This finding is unique because most processes for the generation of bioaerosols from liquid suspensions for laboratory applications encounter significant amounts of surface moisture, usually removed by evaporation as a result of mixing the bioaerosol stream with the dry make-up air stream. This result can be very useful for the development of simple and cost-effective bioaerosol generators with minimal air-drying requirements.
3.3. Effect of Height of Column on Bioaerosol Emissions
The results of bioaerosol emissions from swirling microbial suspensions using various column heights are presented in Figure 5. Figure 5A illustrates the outcomes obtained for the short stirring bar, and Figure 5B—those for the long one. As is seen, the aerosol production was more efficient with lower heights of the suspension column, reaching minimal values at the 45 mm height used for both stirrers. This observation is of particular significance, as it suggests that a smaller height of the microbial suspension column enables a much more violent “bubble” regime to be reached at lower rotation speeds, enabling elevated bioaerosol emissions at lower RPMs. This statement is supported by Figure 6, which illustrates the vortex of a 45 mm high fluid column driven by a short stirring bar. The extensive bubble formation observed even in Figure 6A, obtained at 900 RPM, which is clearly the “bubble-free” mode in cases where taller columns are used (Figure 2), becomes truly severe with the increase in the velocity to commonly used values of 1100 and 1300 RPM (Figure 6B,C, respectively). This violent operation is clearly associated with increased bioaerosol production, releasing up to 5 times the number of microorganisms compared to the release obtained for the tallest liquid column used in this project (110 mm). The results obtained for both stirrers at intermediate column heights demonstrate a linear trend, as can be clearly seen in Figure 5.
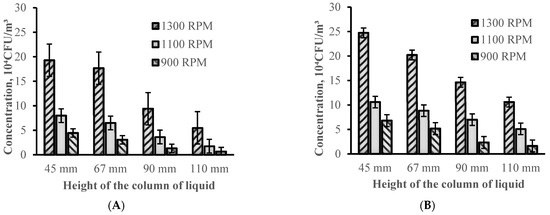
Figure 5.
Effect of the liquid column height on the bioaerosol release; (A)—small stirrer, (B)—large stirrer (error bars represent the standard deviation of at least 3 measurements).

Figure 6.
Images of the process at different rotational speeds (column height—45 mm) and with small stirrer. (A) 900 RPM (B) 1100 RPM (C) 1300 RPM.
Interestingly, for short columns, significant numbers of large millimetre-size drops could be observed on the walls of the vessel above the liquid level (Figure 6). Such droplets were not detected during experiments with taller columns and could be associated with much larger shear forces (shorter distances between the stirrer and the liquid surface) in the closer vicinity to the liquid surface levels, providing sufficient energy to release larger droplets from the surface. With the increase in the column height, this effect seems to be attenuated.
3.4. Effect of Temperature on Release of Bioaerosols
The effects of bioaerosol emissions from microbial suspensions during the vortex of fluid at different temperatures were observed and are depicted in Figure 7. As discussed above, the microbial suspension temperature range is bounded by physical constraints: the freezing point sets the lower limit, whereas temperatures exceeding 38 °C risk microbial inactivation. The results were obtained for the larger stirring bar operating at the two highest rotational velocities. As is seen, both rotational speeds exhibited a linear trend and a notable increase in bioaerosol emissions as the temperature increased. This effect is usually related to the efficiency of the heat and mass exchange processes, which rises with the temperature rise. In addition, the increase in the temperature causes an increase in fluxes from hotter liquid to the clean gas carrier. The results are in good agreement with reports recently published in the literature; Sun and colleagues [23] reported that the temperature increase in the liquid phase from 25 °C to 65 °C led to a 30% increase in bubble detachment volume attributed to evaporation.
Figure 7.
Temperature-related bioaerosol release (error bars represent standard deviation of at least 3 measurements).
3.5. Effect of the Reactor Size on Bioaerosol Emissions
The effect of the reactor size on the bioaerosol emissions from the vortex fluid has been investigated, and the results for all rotational speeds produced by the longer stirring bar in both the 100 mm and 140 mm diameters are presented in Figure 8. In addition, the process was visualised, and the corresponding images are shown in Figure 9 (A—140 mm diameter, B—100 mm diameter). As is seen, the process in both reactors looks relatively similar regarding the vortex shape and the presence of small bubbles. However, the results demonstrated in Figure 8 are very interesting and deserve analysis. First, the bioaerosol emission at the lowest rotation velocity is quite similar for both devices, which is simply described by the fact that in both reactors, the diameter of the vortex is small enough; so, the size of the vessel does not seem to matter. Then, with the increase in the rotational velocity, the difference in the bioaerosol emission increases, reaching about 40% at the highest velocity of 1300 RPM. Such an outcome looks quite unexpected; however, a simple analysis of Figure 9 gives an answer to this question. In particular, as discussed above and confirmed in Figure 9, bubbles start to be produced at 1100 RPM, and their amount, as expected, is increased at 1300 RPM. Then, to produce droplets, they are supposed to burst at the liquid–air interface, which, in the case of the larger rector, is approximately two times bigger, capable of creating more bioaerosols. Of course, such an estimate is quite coarse; however, more accurate results should be site-specific and obtained for each reactor of interest. It should also be noticed that these findings are of particular importance if the reported results are to be used to evaluate microbial release from industrial-scale devices. Considering that the area of the liquid–gas interface could be measured/estimated for each reactor diameter and shape/size of the vortex cone, the release could be evaluated as a ratio of the surface of the industrial device to the laboratory plant presented in this report. Again, such an estimation is quite coarse, and some additional monitoring might be required to verify outcomes.
Figure 8.
Effect of the vessel diameter on bioaerosol release (error bars represent the standard deviation of at least 3 measurements).
Figure 9.
Images of the process at different rotational speeds and with a large stirrer; (A)—140 mm diameter vessel, (B)—100 mm diameter vessel.
3.6. Impact of Surface Tension on Bioaerosol Emissions
Finally, the influence of surfactants on bioaerosol release from swirling bacterial suspensions has been investigated, and the results are shown in Figure 10. As discussed above, the antifoaming agent was added to the microbial suspension in quantities of 1.0 g/L and 1.5 g/L. These concentrations were used in our numerous projects (see, for example, [13]). Also, the results for a concentration of zero for the agent are copied here from Figure 3 for the readership’s convenience. It must be noted that numerous images of the process were taken at different concentrations of the surfactant; however, no visual difference was observed (no images are given here, as they do not show any interesting features). The results show a slight decrease in the aerosol production for the cases of operation with a surfactant. This finding is quite interesting, as it contradicts the results previously obtained by [13,16,24], who reported that adding surfactants reduces surface tension and increases the mass of released particles per bubble. However, the nature of the bubbling process is quite different as compared to the vortex processes. In particular, the bubbling processes operating without surfactants produce quite substantial amounts of foam on the surface. It was shown that this foam could act as a filter supressing aerosol production; so, the addition of antifoam minimises the amount of foam, enhancing aerosol production and transportation across the liquid–air interface. In contrast, vortex processes due to different dynamics do not produce excessive amounts of foam, and the influence of antifoaming agents on this type of process is rather positive, causing the rupture of liquid films and minimising the bursting of bubbles.
Figure 10.
Effect of the liquid surface tension on the bioaerosol emission (error bars represent the standard deviation of at least 3 measurements).
4. Conclusions
This research investigated bioaerosol emissions using E. coli microbial suspensions under controlled laboratory conditions by the swirling of microbial suspensions. Key findings revealed, under various parameters, that a higher rotation velocity is associated with increased bioaerosol production rates, and the influence of the stirring device size plays a significant role; more bioaerosols are produced by larger devices. Elevated temperatures, the smaller height of the liquid column and a larger vessel size also led to higher bioaerosol emissions. In contrast, using antifoam agents could decrease the bioaerosol concentration in the ambient air. These research findings can improve workplace safety by optimising conditions in industries where similar processes are employed. One of the main aims of this research is just to “ring the bell”, as the potential hazard associated with this type of process is certainly underestimated. Of course, the process is very complicated for mathematical generalisation; so, an optimal operation procedure for both hardware and process parameters must be developed for each particular case, along with the use of site-representative microbial strains.
Author Contributions
M.S.: conceptualisation, experimentation, data acquisition/analysis, manuscript writing; I.E.A.: conceptualisation, data analysis/validation, general supervision; manuscript writing, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated in this research are presented in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schlosser, O. Bioaerosols and Health: Current Knowledge and Gaps in the Field of Waste Management. Detritus 2019, 5, 111–125. [Google Scholar] [CrossRef]
- Nair, A.T. Bioaerosols in the landfill environment: An overview of microbial diversity and potential health hazards. Aerobiologia 2021, 37, 185–203. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, C.; Mou, X.; Wang, X.C. Bioaerosol in a typical municipal wastewater treatment plant: Concentration, size distribution, and health risk assessment. Water Sci. Technol. 2020, 82, 1547–1559. [Google Scholar] [CrossRef]
- Masclaux, F.G.; Hotz, P.; Gashi, D.; Savova-bianchi, D.; Oppliger, A. Assessment of airborne virus contamination in wastewater treatment plants. Environ. Res. 2014, 133, 260–265. [Google Scholar] [CrossRef]
- Mirskaya, E.; Agranovski, I.E. Sources and mechanisms of bioaerosol generation in occupational environments. Crit. Rev. Microbiol. 2018, 44, 739–758. [Google Scholar] [CrossRef]
- Han, Y.; Yang, T.; Yan, X.; Li, L.; Liu, J. Effect of aeration mode on aerosol characteristics from the same wastewater treatment plant. Water Res. 2020, 170, 115324. [Google Scholar] [CrossRef]
- Madhwal, S.; Prabhu, V.; Sundriyal, S.; Shridhar, V. Distribution, characterization and health risk assessment of size fractionated bioaerosols at an open land fill site in Dehradun, India. Atmos. Pollut. Res. 2020, 11, 156–169. [Google Scholar] [CrossRef]
- Kozajda, A.; Jeżak, K. Occupational exposure to Staphylococcus aureus in the wastewater treatment plants environment. Med. Pr. 2020, 71, 265–278. [Google Scholar] [CrossRef]
- Kim, K.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef]
- Lou, M.; Liu, S.; Gu, C.; Hu, H.; Tang, Z.; Zhang, Y.; Xu, C.; Li, F. The bioaerosols emitted from toilet and wastewater treatment plant: A literature review. Environ. Sci. Pollut. Res. Int. 2021, 28, 2509–2521. [Google Scholar] [CrossRef]
- Burdsall, A.C.; Xing, Y.; Cooper, C.W.; Harper, W.F., Jr. Bioaerosol emissions from activated sludge basins: Characterization, release, and attenuation. Sci. Total Environ. 2021, 753, 141852. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Song, H.; Ba, Y.; Li, L.; Hong, Q.; Wang, Y. The changing pattern of bioaerosol characteristics, source, and risk under diversity brush aerator speed. Ecotoxicol. Environ. Saf. 2022, 236, 113478. [Google Scholar] [CrossRef]
- Kruglyakova, E.; Mirskaya, E.; Agranovski, I.E. Bioaerosol Release from Concentrated Microbial Suspensions in Bubbling Processes. Atmosphere 2022, 13, 2029. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Thomas, R.J. Particle size and pathogenicity in the respiratory tract. Virulence 2013, 4, 847–858. [Google Scholar] [CrossRef]
- Néel, B.; Erinin, M.A.; Delke, L. Role of Contamination in Optimal Droplet Production by Collective Bubble Bursting. Geophys. Res. Lett. 2022, 49, e2021GL096740. [Google Scholar] [CrossRef]
- Sofieva, S.; Asmi, E.; Atanasova, N.S.; Heikkinen, A.E.; Vidal, E.; Duplissy, J.; Romantschuk, M.; Kouznetsov, R.; Kukkonen, J.; Bamford, D.H.; et al. Effects of temperature and salinity on bubble-bursting aerosol formation simulated with a bubble-generating chamber. Atmos. Meas. Tech. 2022, 15, 6201–6219. [Google Scholar] [CrossRef]
- Pyankov, O.; Agranovski, I.E.; Huang, R.; Mullins, B. Removal of biological aerosols on oil coated filters. CLEAN-Soil Air Water 2008, 36, 609–614. [Google Scholar] [CrossRef]
- Agranovski, I.E.; Agranovski, V.; Reponen, T.; Willeke, K.; Grinshpun, S.A. Development and evaluation of a new personal sampler for culturable airborne microorganisms. Atmos. Environ. 2002, 36, 889–898. [Google Scholar] [CrossRef]
- Andersen, A.A. New sampler for the collection, sizing, and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef]
- Macher, J. Positive-hole correction of multiple-jet impactors for collecting viable microorganisms. Am. Ind. Hyg. Assoc. J. 1989, 50, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, T.; Han, C.; Li, L.; Liu, J. Study of the generation and diffusion of bioaerosol under two aeration. Environ. Pollut. 2020, 267, 115571. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Gu, H.; Yu, X.; Yin, W. Bubble Formation Characteristic of Submerged Single-Hole Orifice in Aerosol Suspension. Front. Energy Res. 2019, 7, 92. [Google Scholar] [CrossRef]
- Ke, W.R.; Kuo, Y.M.; Lin, C.W.; Huang, S.H.; Chen, C.C. Characterization of aerosol emissions from single bubble bursting. J. Aerosol Sci. 2017, 109, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).