Carbon Sequestration Potential of Agroforestry versus Adjoining Forests at Different Altitudes in the Garhwal Himalayas
Abstract
1. Introduction
2. Materials and Methods
3. Methodology Adopted
3.1. Diversity
3.2. Carbon Sequestration
3.3. Carbon Credit
3.4. Soil Analysis
4. Results
4.1. Diversity Assessment of Plants at Different Altitudes
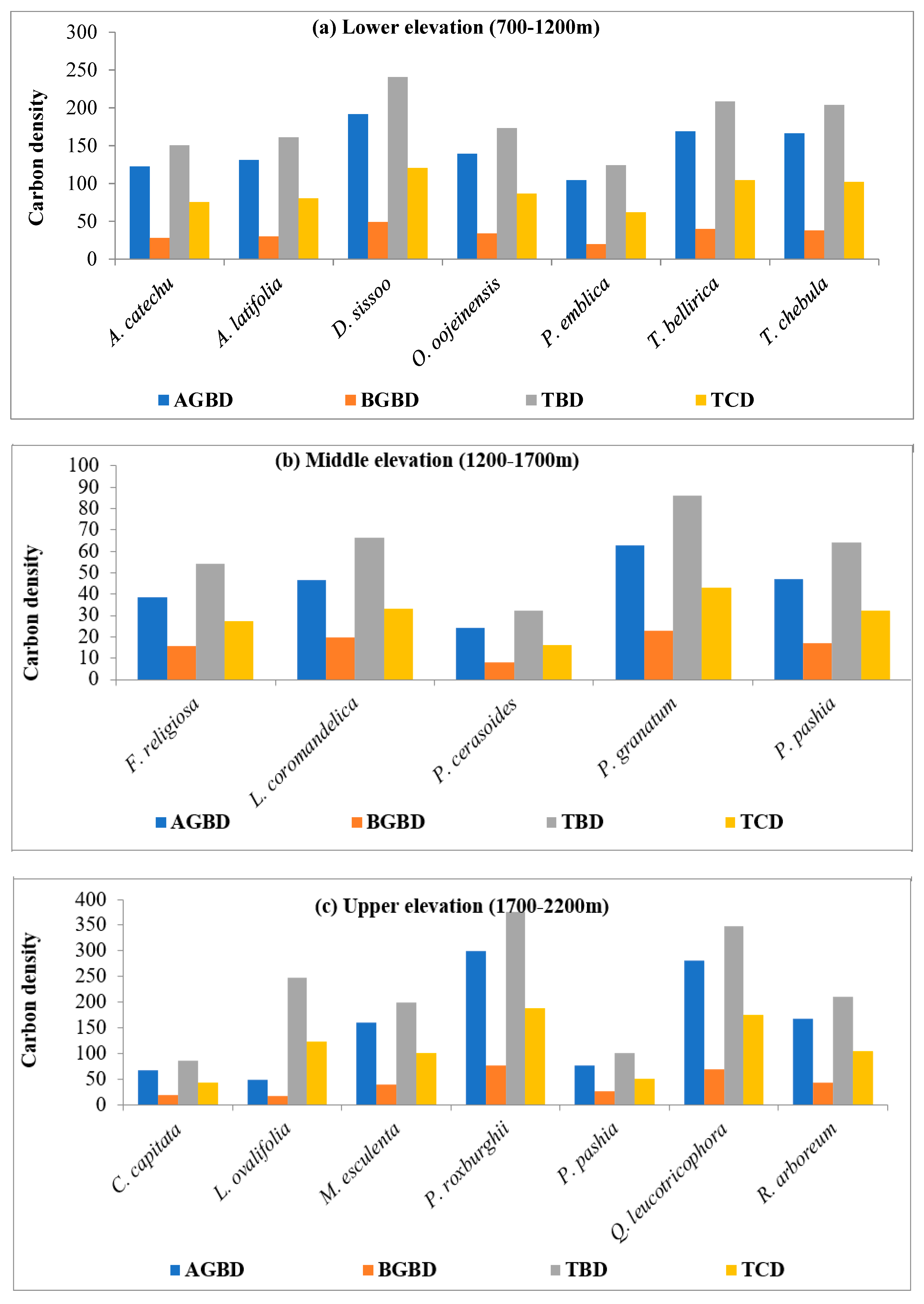
4.2. Species Wise Carbon Density
4.3. Oxygen (O2) Production Potential and Carbon Credits of Trees in Different Altitudes
4.4. Species Diversity
4.5. Carbon Sequestration
4.6. Soil Properties
5. Discussion
5.1. Diversity Assessment
5.2. Diversity Indices
5.2.1. Margalef Index
5.2.2. Menhenik Index
5.2.3. Shannon-Weiner Index
5.2.4. Simpson Index
5.2.5. Pielou Equitability
5.3. Carbon Sequestration
5.4. Soil Organic Carbon (SOC)
5.5. Bulk Density (BD)
5.6. Soil Organic Carbon Stock
5.6.1. O2 Production Potential
5.6.2. Carbon Credit
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aynekulu, E.; Suber, M.; van Noordwijk, M.; Arango, J.; Roshetko, J.M.; Rosenstock, T.S. Carbon Storage Potential of Silvopastoral Systems of Colombia. Land 2020, 9, 309. [Google Scholar] [CrossRef]
- Besar, N.A.; Suaedi, H.; Phua, M.H.; James, D.; Mokhtar, M.B.; Ahmed, M.F. Carbon Stock and Carbon Sequestration Potential of an Agroforestry System in Sabah. Malays. For. 2020, 11, 210. [Google Scholar]
- Sarkar, P.K.; Sarkar, P.; Kumar, A.; Pala, N.A.; Kumar, M. Carbon Storage Potential of a Waterlogged Agroforestry System of Tripura, India. Water Air Soil Pollut. 2021, 232, 151. [Google Scholar] [CrossRef]
- Dhyani, S.; Murthy, I.K.; Kadaverugu, R.; Dasgupta, R.; Kumar, M.; Gadpayle, K.A. Agroforestry to Achieve Global Climate Adaptation and Mitigation Targets: Are South Asian countries sufficiently prepared? Forests 2021, 12, 303. [Google Scholar] [CrossRef]
- Mey, C.B.J.; Gore, M.L. Biodiversity Conservation and Carbon Sequestration in Agroforestry Systems of the Mbalmayo Forest Reserve. J. Environ. Sci. 2021, 37, 91–103. [Google Scholar]
- Komal, N.; Zaman, Q.; Yasin, G.; Nazir, S.; Ashraf, K.; Waqas, M.; Ahmad, M.; Batool, A.; Talib, I.; Chen, Y. Carbon Storage Potential of Agroforestry System near Brick Kilns in Irrigated Agro-Ecosystem. Agriculture 2022, 12, 295. [Google Scholar] [CrossRef]
- Nath, P.C.; Thangjam, U.; Kalita, S.S.; Sahoo, U.K.; Giri, K.; Nath, A.J. Tree Diversity and Carbon Important Species vary with Traditional Agroforestry Managers in the Indian Eastern Himalayan Region. Environ. Sci. Pollut. Res. 2022, 29, 64732–64744. [Google Scholar] [CrossRef]
- Vijay, R.E. The Efficiency of Agricultural Crops in the Present Agri-Horti-Silvicultural System in India. Int. J. Agrofor. Silvic. 2016, 3, 197–202. [Google Scholar]
- Nagar, A.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Singh, I. Altitudinal Variation in Morphometric Traits of Pod, Seed, and Seedling growth of Bauhinia variegata L. in Garhwal Himalaya. Ann. Silvic. Res. 2022, 47, 85–94. [Google Scholar]
- Singh, B.; Kumar, M.; Cabral-Pinto, M.M.S.; Bhatt, B.P. Seasonal and altitudinal variation in chemical composition of Celtis australis L. Tree Foliage. Land 2022, 11, 2271. [Google Scholar] [CrossRef]
- Reang, D.; Hazarika, A.; Sileshi, G.W.; Pandey, R.; Das, A.K.; Nath, A.J. Assessing Tree Diversity and Carbon Storage During Land Use Transitioning from Shifting Cultivation to Indigenous Agroforestry Systems: Implications for REDD+ Initiatives. J. Environ. Manag. 2021, 298, 113470. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, B.; Rawat, D.; Singh, A.P.; Tewari, S. Physico-chemical Properties of Soil and Productivity of Lentil (Lens culinaris Medic.) and Wheat (Triticum aestivum L.) Under Existing agri-Horticulture System in Mid Hills of Uttarakhand Himalaya. J. Tree Sci. 2020, 39, 24–32. [Google Scholar] [CrossRef]
- Satapathy, S.R.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Kumar, S.; Kumar, P.; Rawat, D. Allelopathic Potential of Ficus auriculata and Ficus semicordata on Growth of Four Traditional Food Crops of Garhwal Himalaya. J. Agric. Food Res. 2022, 9, 100352. [Google Scholar]
- Padu, K.; Khanduri, V.P.; Singh, B.; Rawat, D.; Riyal, M.K.; Kumar, K.S. Phytotoxicity of Common Weeds on Germination, Seedling Growth, NPK Uptake and Chlorophyll Content of Four Hill Crops of Garhwal Himalaya. J. Agric. Food Res. 2023, 12, 100539. [Google Scholar] [CrossRef]
- Kishwan, J.; Pandey, R.; Dadhwal, V.K. Emission Removal Capability of India’s Forest and Tree Cover. Small-Scale For. 2012, 11, 61–72. [Google Scholar] [CrossRef]
- Kumar, S.; Bijalwan, A.; Singh, B.; Rawat, D.; Yewale, A.G.; Riyal, M.K.; Thakur, T.K. Comparison of Carbon Sequestration Potential of Quercus leucotrichophora–based Agroforestry Systems and Natural Forest in Central Himalaya, India. Water Air Soil Pollut. 2021, 232, 250. [Google Scholar] [CrossRef]
- Tiwari, A.; Khanduri, V.P.; Rawat, D.; Singh, B.; Riyal, M.K.; Mishra, G.; Kumar, M. Vegetation Composition, Soil Properties, and Carbon Stock of Montane Forests Along Disturbance in the Garhwal Himalaya, India. Front. For. Glob. Chang. 2023, 6, 1193792. [Google Scholar] [CrossRef]
- Nunes, L.J.R. The Rising Threat of Atmospheric CO2: A Review on the Causes, Impacts, and Mitigation Strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Dey, D.C.; Knapp, B.O.; Battaglia, M.A.; Deal, R.L.; Hart, J.L.; O’Hara, K.L.; Schweitzer, C.J.; Schuler, T.M. Barriers to natural regeneration in temperate forests across the USA. New For. 2019, 50, 11–40. [Google Scholar] [CrossRef]
- Quandt, A.; Neufeldt, H.; Gorman, K. Climate Change Adaptation Through Agroforestry: Opportunities and Gaps. Curr. Opin. Environ. Sustain. 2023, 60, 101244. [Google Scholar] [CrossRef]
- Watts, M.; Hutton, C.; Mata Guel, E.O.; Suckall, N.; Peh, K.S.H. Impacts of Climate Change on Tropical Agroforestry Systems: A Systematic Review for Identifying Future Research Priorities. Front. For. Glob. Chang. 2022, 5. [Google Scholar] [CrossRef]
- Jhariya, M.K.; Yadav, D.K.; Banerjee, A. (Eds.) Agroforestry and Climate Change: Issues and Challenges; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Gebre, A.B. Potential Effects of Agroforestry Practices on Climate Change Mitigation and Adaptation Strategies: A Review. J. Nat. Sci. Res. 2016, 6, 83–89. [Google Scholar]
- De Zoysa, M.; Inoue, M. Climate Change Impacts, Agroforestry Adaptation and Policy Environment in Sri Lanka. Open J. For. 2014, 4, 439–456. [Google Scholar] [CrossRef]
- Prajapati, A.; Pachauri, S.; Srivastava, P.; Pathak, A.; Rawat, D. Status of Some Extractable Macro- and Micro-Nutrients in Soils of Tehri Garhwal District of Uttarakhand. Pantnagar J. Res. 2021, 19, 171. [Google Scholar]
- Curtis, J.T.; Mcintosh, R.P. The Interrelations of Certain Analytic and Synthetic Phytosociological Characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Phillips, E.A. Methods of Vegetation Study; Rinehart & Winston: New York, NY, USA, 1959. [Google Scholar]
- Curtis, J.T. The Vegetation of Wisconsin, an Ordination of Plant Community; University Wisconsin Press: Madison, WI, USA, 1959. [Google Scholar]
- Magurran, A.E.; Magurran, A.E. Diversity Indices and Species Abundance Models. In Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; pp. 7–45. [Google Scholar]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Knigt, D.H. A Distance Method for Constructing Forest Profile Diagram and Obtaining Structural Data. Trop. Ecol. 1963, 1, 89–94. [Google Scholar]
- FSI. Volume Equations for Forests of India, Nepal and Bhutan; Forest Survey of India, Ministry of Environment and Forests, Govt. of India: Dehradun, India, 1996.
- Chaturvedi, A.N. General Standard Volume Tables for Semal (Bombax ceiba L.). In Dehradun: Manager of Publications; Records, I., Ed.; Forest Research Institute: Mumbai, India, 1973; Volume 12, pp. 1–8. [Google Scholar]
- Brown, S.L.; Schroeder, P.; Kern, J.S. Spatial Distribution of Biomass in Forests of the Eastern USA. For. Ecol. Manag. 1999, 123, 81–90. [Google Scholar] [CrossRef]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root Biomass Allocation in the World’s Upland Forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef]
- IPCC. Land Use, Land-Use Change and Forestry: A Special Report of the Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK, 2000; p. 375. [Google Scholar]
- Nowak, D.J.; Hoehn, R.; Crane, D.E. Oxygen Production by Urban Trees in the United States. Arboricult. Urban For. 2007, 33, 220–226. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Ed.; Cambridge University Press: New York, NY, USA, 2007; p. 996. [Google Scholar]
- Mukherjee, S.; Ghosh, P.B. Estimation of Carbon Credit and Direct Carbon Footprint by Solar Photovoltaic Cells in West Bengal, India. Int. J. Low-Carbon Technol. 2014, 9, 52–55. [Google Scholar] [CrossRef]
- Mishra, R. Ecology Work Book Oxford and IBH Publishing Company Calcutta; Oxford and IBH Publishing Co.: Calcutta, India, 1968; 242p. [Google Scholar]
- Walkley, A.J.; Black, I.A. Estimation of Soil Organic Carbon by the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Brown, S. Exploration of the Carbon Sequestration Potential of Classified Forest in the Republic of Guinea. In Report Submitted to the United States Agency for International Development; Winrock International: Pasig, Philippines, 2004. [Google Scholar]
- Rana, D.; Kapoor, K.S.; Samant, S.S.; Bhatt, A. Plant Species Conservation Priority Index for Preparing Management Strategies: A Case Study from the Western Himalayas of India. Small-Scale For. 2020, 19, 461–481. [Google Scholar] [CrossRef]
- Ahmad, M.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R.; Lone, G.R.; Kaur, S.; Jaryan, V.; Kohli, R.K. Niche Width Analyses Facilitate Identification of High-Risk Endemic Species at High Altitudes in Western Himalayas. Ecol. Eng. 2021, 126, 107653. [Google Scholar] [CrossRef]
- Koerner, S.E.; Smith, M.D.; Burkepile, D.E.; Hanan, N.P.; Avolio, M.L.; Collins, S.L.; Knapp, A.K.; Lemoine, N.P.; Forrestel, E.J.; Eby, S.; et al. Change in Dominance Determines Herbivore Effects on Plant Biodiversity. Nat. Ecol. Evol. 2018, 2, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, K.; Patzelt, A.; Dickhoefer, U.; Schlecht, E.; Buerkert, A. Vegetation Patterns and Diversity Along an Altitudinal and a Grazing Gradient in the Jabal al Akhdar Mountain Range of Northern Oman. J. Arid Environ. 2009, 73, 1035–1045. [Google Scholar] [CrossRef]
- Zhang, J.T.; Dong, Y. Factors Affecting Species Diversity of Plant Communities and the Restoration Process in the Loess Area of China. Ecol. Eng. 2010, 36, 345–350. [Google Scholar] [CrossRef]
- Ballabha, R.; Kuniyal, A.; Tiwari, P.; Tiwari, J.K. Forest Structure and Composition in the Vicinity of Srinagar Hydroelectric Power Project in Alaknanda Valley, Garhwal Himalaya, India. J. For. Environ. Sci. 2020, 36, 78–90. [Google Scholar]
- Gairola, S.; Sharma, C.M.; Suyal, S.; Ghildiya, S.K. Species Composition and Diversity in Mid-altitudinal Moist Temperate Forests of the Western Himalaya. J. For. Environ. Sci. 2011, 27, 1–15. [Google Scholar]
- Malik, Z.A.; Bhatt, A.B. Phytosociological Analysis of Woody Species in Kedarnath Wildlife Sanctuary and Its Adjoining Areas in Western Himalaya, India. J. For. Environ. Sci. 2015, 31, 149–163. [Google Scholar] [CrossRef]
- Uniyal, P.; Pokhriyal, P.; Dasgupta, S.; Bhatt, D.; Todaria, N.P. Plant Diversity in Two Forest Types Along the Disturbance Gradient in Dewalgarh Watershed, Garhwal Himalaya. Curr. Sci. 2010, 98, 938–943. [Google Scholar]
- McCain, C.M. Global Analysis of Bird Elevational Diversity. Glob. Ecol. Biogeogr. 2009, 18, 346–360. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.A. Elevational Gradients in Species Richness; Wiley online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Sinha, S.; Badola, H.K.; Chhetri, B.; Gaira, K.S.; Lepcha, J.; Dhyani, P.P. Effect of Altitude and Climate in Shaping the Forest Compositions of Singalila National Park in Khangchendzonga Landscape, Eastern Himalaya, India. J. Asia-Pac. Biodivers. 2018, 11, 267–275. [Google Scholar] [CrossRef]
- Rana, S.K.; Gross, K.; Price, T.D. Drivers of Elevational Richness Peaks, Evaluated for Trees in the East Himalaya. Ecology 2019, 100, e02548. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Wang, X.; Sun, H.; Fan, Y.; Wu, Y.; Lin, X.; Chang, J. Forest Type and Height Are Important in Shaping the Altitudinal Change of Radial Growth Response to Climate Change. Sci. Rep. 2019, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Van, Y.T.; Cochard, R. Tree Species Diversity and Utilities in a Contracting Lowland Hillside Rainforest Fragment in Central Vietnam. For. Ecosyst. 2017, 4, 9. [Google Scholar] [CrossRef]
- Thakur, U.; Bisht, N.S.; Kumar, M.; Kumar, A. Influence of Altitude on Diversity and Distribution Pattern of Trees in Himalayan Temperate Forests of Churdhar Wildlife Sanctuary, India. Water Air Soil Pollut. 2021, 232, 205. [Google Scholar] [CrossRef]
- Gairola, S.; Ghildiyal, S.K.; Sharma, C.M.; Suyal, S. Species Richness and Diversity Along an Altitudinal Gradient in Moist Temperate Forest of Garhwal Himalaya. Am. J. Sci. 2009, 5, 119–128. [Google Scholar]
- Wani, Z.A.; Akash, S.; Pant, S. Tree Diversity and Regeneration Dynamics in Gulmarg Wildlife Sanctuary, Kashmir Himalaya. Acta Ecol. Sin. 2023, 43, 375–381. [Google Scholar] [CrossRef]
- Negi, V.; Bhardwaj, D.R.; Sharma, P.; Pala, N.A. Tree Species Composition and Diversity in Natural Temperate Forests of the North-Western Himalayas. Acta Ecol. Sin. 2022, 42, 653–660. [Google Scholar] [CrossRef]
- Sharma, C.M.; Gairola, S.; Ghildiyal, S.K.; Suyal, S. Physical Properties of Soils in Relation to Forest Composition in Moist Temperate Valley Slopes of the Central Western Himalaya. J. For. Environ. Sci. 2010, 26, 117–129. [Google Scholar]
- Pant, S.; Sammant, S.S. Diversity and Regeneration Status of Tree Species in Khokhan Wildlife Sanctuary, North-Western Himalaya. Trop. Ecol. 2012, 53, 317–331. [Google Scholar]
- Thakur, N.S.; Gupta, N.K.; Gupta, B.; Nauni, S.H. Phytosociological Analysis of Woody and Nonwoody Components Under Some Agroforestry Systems in Western Himalaya-A Case Study. Indian J. Agrofor. 2004, 6, 65–71. [Google Scholar]
- Whittaker, R.H. Dominance and Diversity in Land Plant Communities: Numerical Relations of Species Express the Importance of Competition in Community Function and Evolution. Science 1965, 147, 250–260. [Google Scholar] [CrossRef]
- Raturi, G.P. Forest Community Structure Along an Altitudinal Gradient of District Rudraprayag of Garhwal Himalaya, India. Ecologia 2012, 2, 76–84. [Google Scholar] [CrossRef]
- Shaheen, H.; Ullah, Z.; Khan, S.M.; Harper, D.M. Species Composition and Community Structure of Western Himalayan Moist Temperate Forests in Kashmir. For. Ecol. Manag. 2012, 278, 138–145. [Google Scholar] [CrossRef]
- Zhang, J.; Presley, G.N.; Hammel, K.E.; Ryu, J.S.; Menke, J.R.; Figueroa, M.; Hu, D.; Orr, G.; Schilling, J.S. Localizing Gene Regulation Reveals a Staggered Wood Decay Mechanism for the Brown Rot Fungus Postia Placenta. Proc. Natl. Acad. Sci. USA 2016, 113, 10968–10973. [Google Scholar] [CrossRef] [PubMed]
- Mustaqeem Ahmad, S.K.U.; Singh, R.D. Patterns of Alien Plant Species Richness Across Gradients of Altitude: Analyses from the Himalayan State of Himachal Pradesh. Trop. Ecol. 2018, 59, 35–43. [Google Scholar]
- Rawat, D.S.; Dash, S.S.; Sinha, B.K.; Kumar, V.; Banerjee, A.; Singh, P. Community Structure and Regeneration Status of Tree Species in Eastern Himalaya: A Case Study from Neora Valley National Park, West Bengal, India. Taiwania 2018, 63, 16–24. [Google Scholar]
- Newbold, T.; Hudson, L.N.; Hill, S.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Uniyal, S.K.; Batish, D.R.; Singh, H.P.; Jaryan, V.; Rathee, S.; Sharma, P.; Kohli, R.K. Patterns of Plant Communities Along Vertical Gradient in Dhauladhar Mountains in Lesser Himalayas in North-Western India. Sci. Total Environ. 2020, 716, 136919. [Google Scholar] [CrossRef]
- Bala, G.; Caldeira, K.; Wickett, M.; Phillips, T.J.; Lobell, D.B.; Delire, C.; Mirin, A. Combined Climate and Carbon-Cycle Effects of Large-Scale Deforestation. Proc. Natl. Acad. Sci. USA 2007, 104, 6550–6555. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Noble, I.R.; Bolin, B.; Ravindranath, N.H.; Verardo, D.J.; Dokken, D.J. Land Use, Land-Use Change, and Forestry A Special Report of IPCC; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Canadell, J.G.; Raupach, M.R. Managing Forests for Climate Change Mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef]
- Luyssaert, S.; Schulze, E.D.; Börner, A.; Knohl, A.; Hessenmöller, D.; Law, B.E.; Ciais, P.; Grace, J. Old-Growth Forests as Global Carbon Sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.A.; Kumar, M.; Todaria, N.P.; Pandey, R. Biomass and Soil Carbon Along Latitudinal Gradients in Temperate Cedrus deodara Forests in Central Himalaya, India: Implications for Climate Change Mitigation. Ecol. Indic. 2020, 111, 06025. [Google Scholar] [CrossRef]
- Spracklen, D.V.; Righelato, R. Tropical Montane Forests Are a Larger than Expected Global Carbon Store. Biogeosciences 2014, 11, 2741–2754. [Google Scholar] [CrossRef]
- Negi, V.S.; Joshi, B.C.; Pathak, R.; Rawal, R.S.; Sekar, K.C. Assessment of Fuelwood Diversity and Consumption Patterns in Cold Desert Part of Indian Himalaya: Implication for Conservation and Quality of Life. J. Clean. Prod. 2018, 196, 23–31. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The Vertical Distribution of Soil Carbon and Its Relation to Climate and Vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Yang, Y.; Mohammat, A.; Feng, J.; Zhou, R.; Fang, J. Storage, Patterns and Environmental Controls of Soil Organic Carbon in China. Biogeochemistry 2007, 84, 131–141. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Fang, J.; Piao, S.; Shen, H.; Zhao, S.; Peng, C. Altitudinal Changes in Carbon Storage of Temperate Forests on MT Changbai, Northeast China. J. Plant Res. 2010, 123, 439–452. [Google Scholar] [CrossRef]
- Charan, G.; Bharti, V.K.; Jadhav, S.E.; Kumar, S.; Angchok, D.; Acharya, S.; Kumar, P.; Srivastava, R.B. Altitudinal Variations in Soil Carbon Storage and Distribution Patterns in Cold Desert High Altitude Microclimate of India. Afr. J. Agric. Res. 2012, 7, 6313–6319. [Google Scholar] [CrossRef]
- Gupta, M.K.; Sharma, S.D. Sequestered Organic Carbon Status in the Soils Under Grassland in Uttarakhand State, India. Appl. Ecol. Environ. Sci. 2013, 1, 7–9. [Google Scholar] [CrossRef]
- Wei, Y.; Li, M.; Chen, H.; Lewis, B.J.; Yu, D.; Zhou, W.; Fang, X.; Zhao, W.; Dai, L. Variation in Carbon Storage and Its Distribution by Stand Age and Forest Type in Boreal and Temperate Forests in Northeast China. PLoS ONE 2013, 8, e72201. [Google Scholar]
- Zhang, Y.; Ai, J.; Sun, Q.; Li, Z.; Hou, L.; Song, L.; Tang, G.; Li, L.; Shao, G. Soil Organic Carbon and Total Nitrogen Stocks as Affected by Vegetation Types and Altitude Across the Mountainous Regions in the Yunnan Province, South-Western China. Catena 2021, 196, 104872. [Google Scholar] [CrossRef]
- Dar, J.A.; Somaiah, S. Soil Organic Carbon Stock Assessment in Two Temperate Forest Types of Western Himalaya of Jammu And Kashmir, India. J. For. Res. 2014, 3, 114. [Google Scholar] [CrossRef]
- Paul, E.A.; Follet, R.F.; Leavitt, S.W.; Halvorson, A.; Peterson, G.A.; Lyon, D.J. Radiocarbon Dating for Determination of Soil Organic Pool Sizes and Dynamics. Am. J. Soil Sci. Soc. 1997, 61, 1058–1067. [Google Scholar] [CrossRef]
- Trumbore, S.E. Age of Soil Organic Matter and Soil Respiration: Radiocarbon Constraints on Belowground Carbon Dynamics. Ecol. Appl. 2000, 10, 399–411. [Google Scholar] [CrossRef]
- Schulp, C.J.E.; Nabuurs, G.-J.; Verburg, P.H.; de Waal, R.W. Effect of Tree Species on Carbon Stocks in Forest Floor and Mineral Soil and Implications for Soil Carbon Inventories. For. Ecol. Manag. 2008, 256, 482–490. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Kumar, M.; Bussmann, R.W. Altitude Variation in Soil Organic Carbon Stock in Coniferous Subtropical and Broad Leaf Temperate Forests in Garhwal Himalaya. Carbon Balance Manag. 2009, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Wang, X.J.; Xu, M.G.; Huang, S.M.; Liu, H.; Peng, C. Soil Organic Carbon Dynamics Under Long-Term Fertilization in Arable Land of Northern China. Biogeosciences 2010, 7, 409–425. [Google Scholar] [CrossRef]
- Townsend, A.R.; Vitousek, P.M.; Amundson, R.R. Soil Organic Matter Dynamics Along Gradients in Temperature and Land Use on the Island of Hawaii. Ecology 1996, 76, 721–733. [Google Scholar] [CrossRef]
- Conant, R.T.; Klopatek, J.M.; Malin, R.C.; Klopatek, C.C. Carbon Pools and Fluxes Along an Environmental Gradient in Northern Arizona. Biogeochemistry 1998, 43, 43–61. [Google Scholar] [CrossRef]
- Babu, S.; Singh, R.; Avasthe, R.K.; Yadav, G.S.; Mohapatra, K.P.; Selvan, T.; Das, A.; Singh, V.K.; Valente, D.; Petrosillo, I. Soil Carbon Dynamics in Indian Himalayan Intensified Organic Rice-Based Cropping Sequences. Ecol. Indic. 2020, 114, 106292. [Google Scholar] [CrossRef]
- Rawat, S.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Thakur, T.K.; Kumar, M.; Cabral-Pinto, M.M. Variation in carbon stock and soil properties in different Quercus leucotrichophora forests of Garhwal Himalaya. Catena 2022, 213, 106210. [Google Scholar] [CrossRef]
- Rawat, D.; Mukhopadhyay, D.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Sati, S.P. Harnessing Soil Ecosystem Services for Achieving Soil-Based SDGs in Indian Himalaya. In Soil Carbon Dynamics in Indian Himalayan Region; Mishra, G., Giri, K., Nath, A.J., Francaviglia, R., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Chauhan, M.; Kumar, M.; Kumar, A. Impact of Carbon Stocks of Anogeissus latifolia on Climate Change and Socioeconomic Development: A Case Study of Garhwal Himalaya, India. Water Air Soil Pollut. 2020, 231, 436. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Konsam, B.; Sheikh, M.A.; Pandey, R. Above and Below-Ground Biomass Production in Pinus roxburghii Forests Along Altitudes in Garhwal Himalaya, India. Curr. Sci. 2019, 116, 1506–1514. [Google Scholar] [CrossRef]
- Singh, A.P.; Bijalwan, A.; Bisht, T.P.; Singh, B.; Kumar, S.; Tariyal, N. Evaluation of growth, yield, economics and soil properties of agri-horticulture systems in mid-hill situations of Himalayas. Agrofor. Syst. 2023, 97, 1113–1130. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, A.; Kumar, R.; Konsam, B.; Nazir, A.; Pala, B.J.A. Carbon Stock Potential in Pinus roxburghii Forests of Indian Himalayan Regions. Environ. Dev. Sustain. 2021, 23, 12463–12478. [Google Scholar] [CrossRef]
- Keerthika, A.; Chavan, S.B. Oxygen Production Potential of Trees in India. Curr. Sci. 2022, 122, 850. [Google Scholar] [CrossRef]
- Mokashi, M.V.; Ghatanatti, S.M.; Mutanal, S.M. Oxygen Productivity in Different Tree Species. Indian J. Ecol. 2021, 48, 373–375. [Google Scholar]
- Vikrant, K.K.; Chauhan, D.S.; Rizvi, R.H. CO2 Mitigation Potential of Traditional Agroforestry Systems along Elevations in Tehri District of North West Himalaya, India, 2023. Available online: https://www.preprints.org/manuscript/202310.1119/v1 (accessed on 2 February 2024).
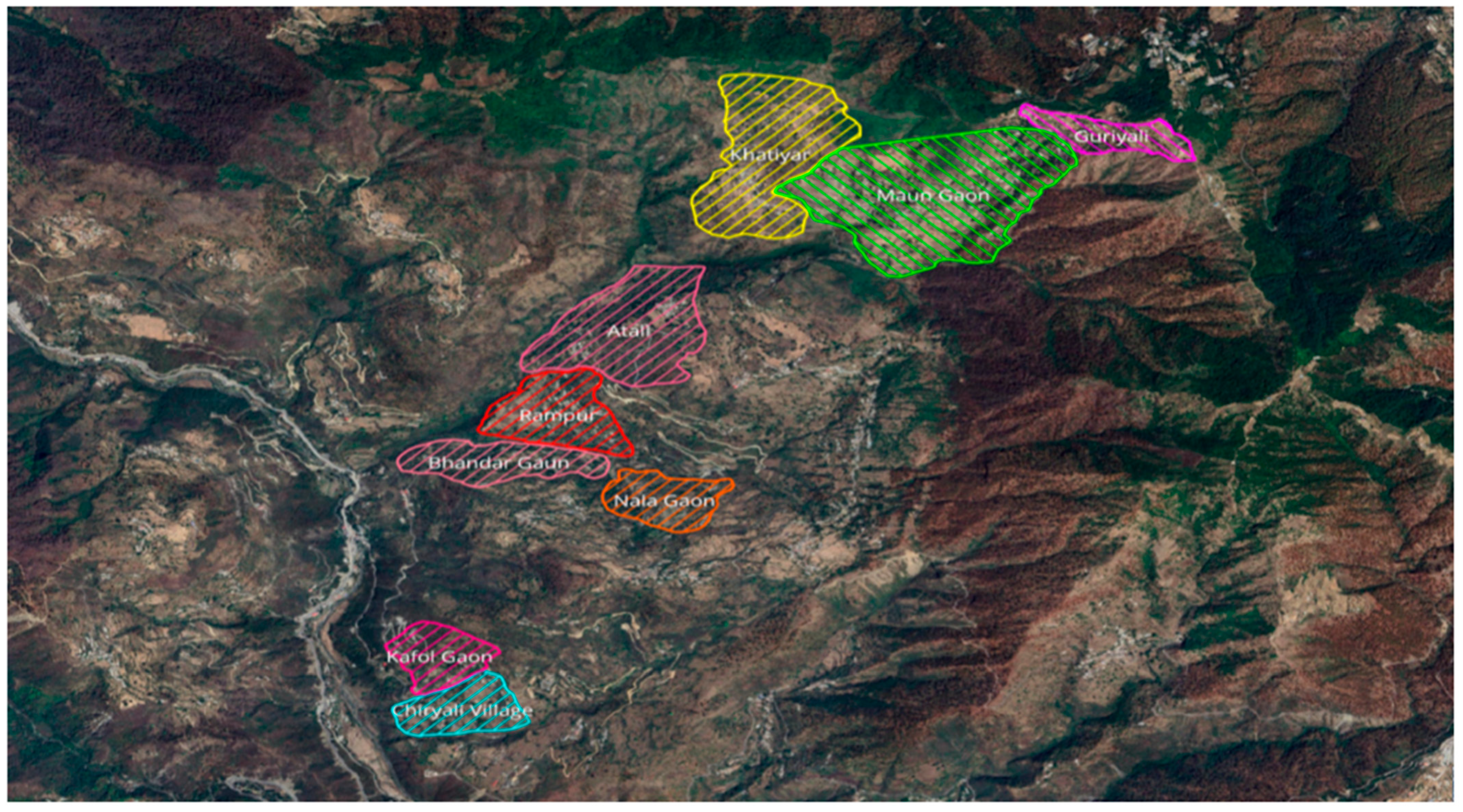
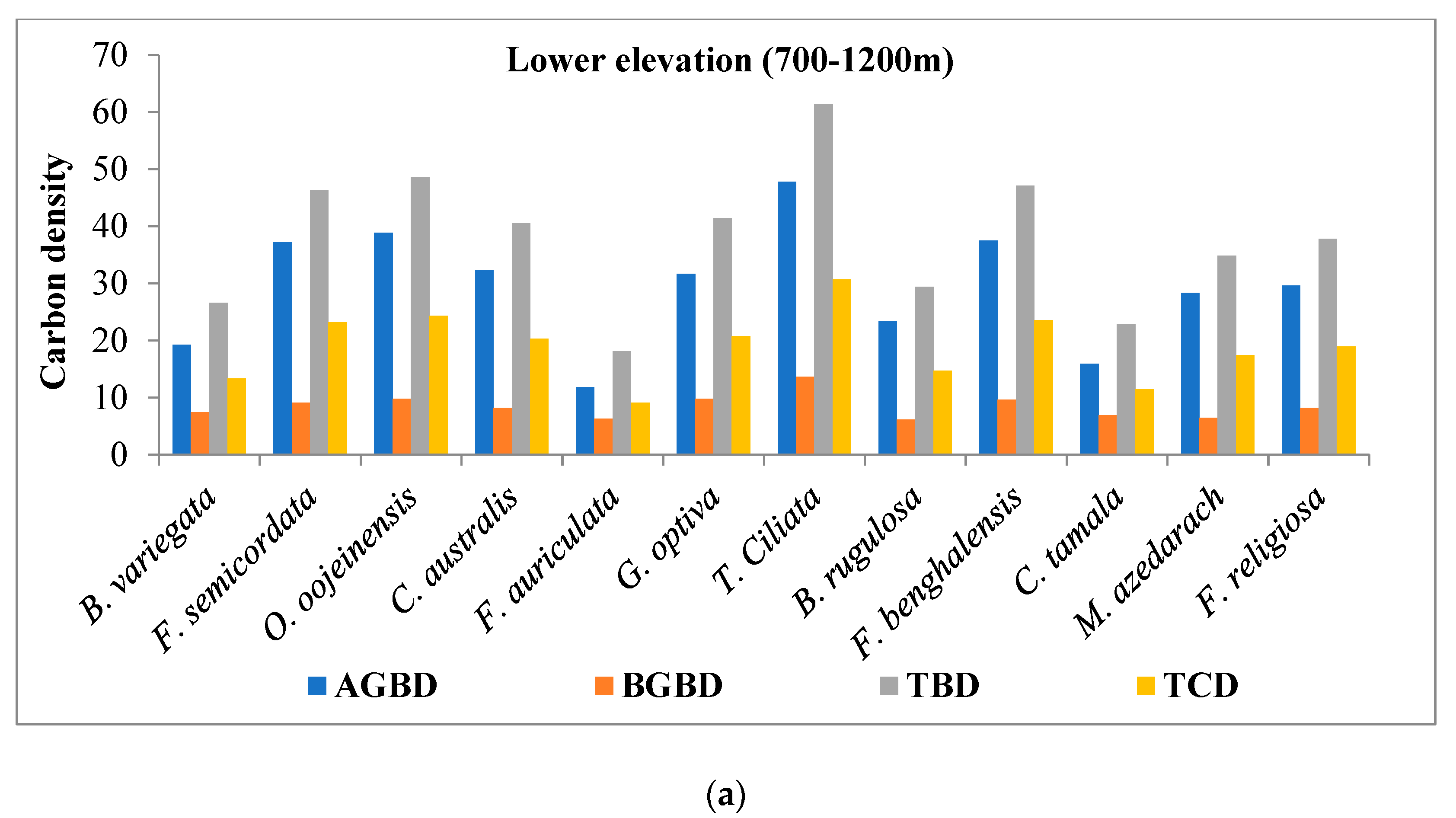
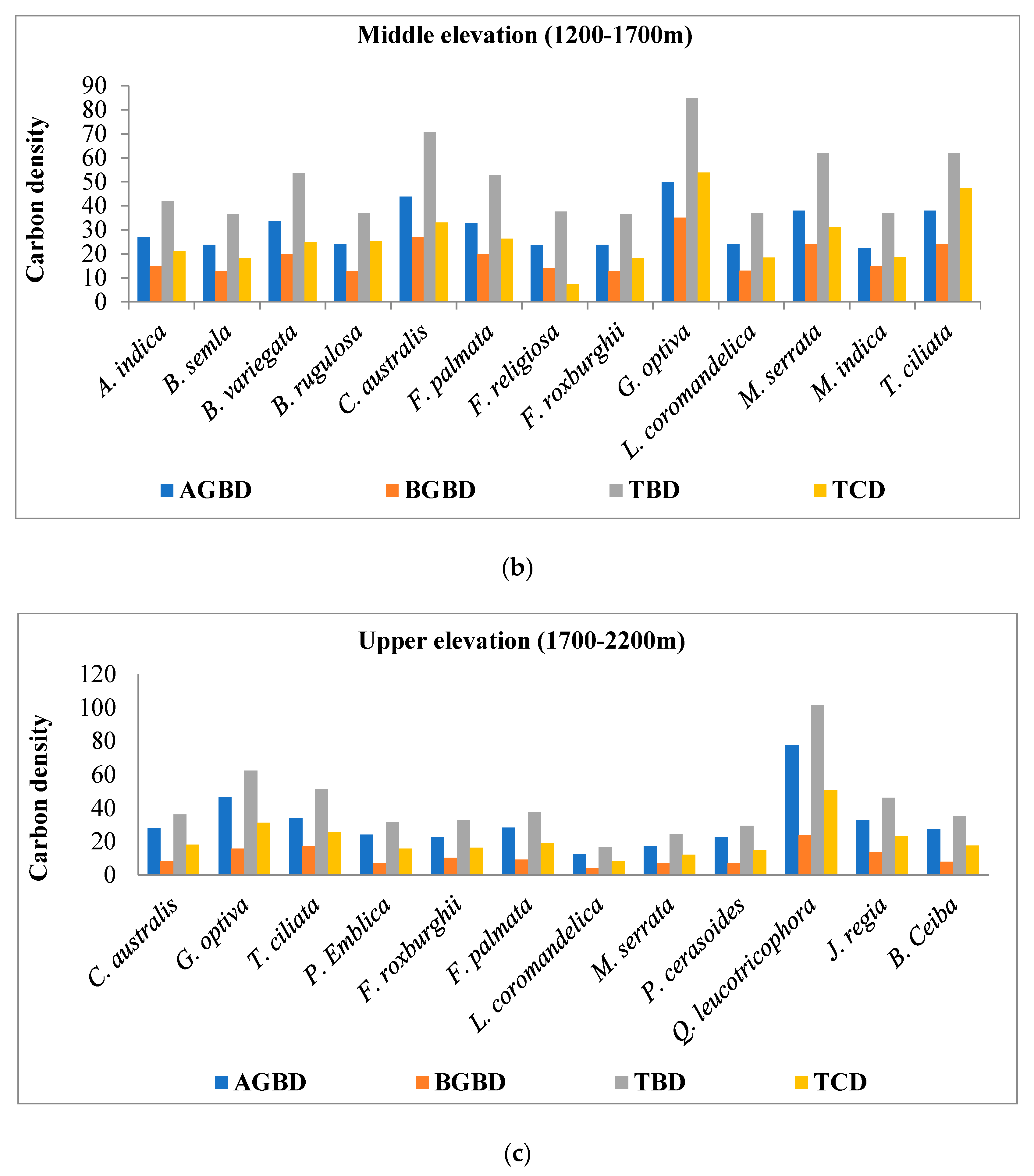
| Elevation | Village | Elevation | Latitude | Longitude |
|---|---|---|---|---|
| Lower 700–1200 | Chiryali | 824 | 30°16′52″ N | 78°22′7″ E |
| Kafol Gaon | 1086 | 30°16′27″ N | 78°22′1″ E | |
| Bhandar Gaon | 1108 | 30°17′41″ N | 78°22′43″ E | |
| Middle 1200–1700 | Rampur | 1253 | 30°17′91″ N | 78°22′93″ E |
| Nala | 1302 | 30°16′28″ N | 78°22′49″ E | |
| Atali | 1443 | 30°18′18″ N | 78°23′21″ E | |
| Upper 1700–2200 | Khatiyar | 1703 | 30°18′0″ N | 78°23′33″ E |
| Moun | 1837 | 30°30′21″ N | 78°39′44″ E | |
| Guriyal | 2040 | 30°30′9″ N | 78°40′05″ E |
| Common Name | Botanical Name | Volume Equation |
|---|---|---|
| Banj | Quercus leucotrichophora | V/D2 = 0.085356/D2 − 1.258189/D + 7.702984 |
| Bhimal | Grewia optiva | V = −0.44075 + 7.49221D − 36.09962D2 + 71.91238D3 |
| Khadik | Celtis australis | V = 0.23781 − 2.09431 × D + 7.78268 × D2 |
| Timla | Ficus auriculata | √V = 0.03629 + 3.95389D − 0.84421√D |
| Bedu | Ficus palmate | √V = 0.03629 + 3.95389D − 0.84421√D |
| Buransh | Rhododendron arboreum | V = 0.06007 − 0.21874√D + 3.63428D2 |
| Khair | Acacia catechu | √ V = 0.02384 − 0.72161D + 7.46888D2 (L) |
| Harad | Terminalia chebula | √V = −0.2264 + 2.93587D (L) |
| Chir pine | Pinus roxburghii | √V = 0.05131 + 3.9859D − 1.0245√D |
| Melu | Pyrus pashia | V = 0.01284 + 0.2138D2H (G) |
| Guriyal | Bauhinia variegate | V = −0.04262 + 6.09491D2 |
| - | Lannea coromandelica | V = 0.19381 − 0.83928√D + 10.32053D2 (L) |
| Dainkan | Melia azedarach | V = −0.03510 + 5.32981D2 |
| Rest of species | -- | V = 0.00855 + 0.4432D2 + 0.28813D2H |
| Trees | Lower Elevation (700–1200) | Middle Elevation (1200–1700) | Upper Elevation (1700–2200) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density (ha−1) | Frequency (%) | A/F Ratio | IVI | Density (ha−1) | Frequency (%) | A/F Ratio | IVI | Density (ha−1) | Frequency (%) | A/F Ratio | IVI | |
| Bauhinia variegata | 45.0 | 70.0 | 0.04 | 32.07 | 25.0 | 60.0 | 0.03 | 24.78 | - | - | - | - |
| Ficus semicordata | 40.0 | 90.0 | 0.02 | 43.05 | - | - | - | - | - | - | - | - |
| Ougeinia oojeinensis | 40.0 | 80.0 | 0.03 | 40.35 | - | - | - | - | - | - | - | - |
| Celtis australis | 27.5 | 60.0 | 0.03 | 28.69 | 27.5 | 60.0 | 0.03 | 32.95 | 20.0 | 60.0 | 0.02 | 38.73 |
| Ficus auriculata | 10.0 | 20.0 | 0.10 | 8.14 | - | - | - | - | - | - | ||
| Grewia optiva | 30.0 | 70.0 | 0.02 | 41.17 | 42.5 | 60.0 | 0.02 | 53.82 | 35.0 | 60 | 0.04 | 59.33 |
| Toona ciliata | 17.5 | 50.0 | 0.03 | 38.01 | 10.0 | 30.0 | 0.04 | 47.47 | 5.0 | 20.0 | 0.05 | 12.96 |
| Boehmeria rugulosa | 17.5 | 30.0 | 0.06 | 12.42 | 17.5 | 60.0 | 0.02 | 25.23 | - | - | - | - |
| Ficus benghalensis | 7.5 | 40.0 | 0.02 | 26.15 | - | - | - | - | - | - | - | - |
| Cinnamomum tamala | 7.5 | 30.0 | 0.03 | 8.74 | - | - | - | - | - | - | - | - |
| Melia azedarach | 7.5 | 30.0 | 0.03 | 10.40 | - | - | - | - | - | - | - | - |
| Ficus religiosa | 2.5 | 50.0 | 0.00 | 10.81 | 7.5 | 60.0 | 0.03 | 7.32 | - | - | - | - |
| Phyllanthus emblica | - | - | - | - | - | - | - | - | 2.5 | 10. | 0.10 | 4.23 |
| Azadirachta indica | - | - | - | - | 10.0 | 40.0 | 0.03 | 12.60 | - | - | - | - |
| Ficus roxburghii | - | - | - | - | 15.0 | 40.0 | 0.04 | 15.70 | 10.0 | 30.0 | 0.04 | 15.42 |
| Bauhinia semla | - | - | - | - | 5.0 | 60.0 | 0.03 | 7.98 | - | - | - | - |
| Mangifera indica | - | - | - | - | 12.5 | 60.0 | 0.01 | 21.73 | - | - | - | - |
| Ficus palmata | - | - | - | - | 7.5 | 30.0 | 0.03 | 9.48 | 7.5 | 20.0 | 0.08 | 12.93 |
| Lannea coromandelica | - | - | - | - | 5.0 | 30.0 | 0.05 | 7.33 | 7.5 | 30. | 0.03 | 13.92 |
| Morus serrata | - | - | - | - | 7.5 | 30.0 | 0.03 | 22.47 | 12.5 | 40.0 | 0.02 | 22.56 |
| Prunus cerasoides | - | - | - | - | - | - | - | - | 2.5 | 10.0 | 0.10 | 6.25 |
| Quercus leucotricophora | - | - | - | - | - | - | - | - | 42.5 | 60.0 | 0.05 | 48.18 |
| Juglans regia | - | - | - | - | - | - | - | - | 7.5 | 10.0 | 0.10 | 14.27 |
| Bombex ceiba | - | - | - | - | - | - | - | - | 12.5 | 50.0 | 0.02 | 44.74 |
| Trees | Lower Elevation (700–1200m) | Middle Elevation (1200–1700m) | Upper Elevation (1700–2200m) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density (ha−1) | Frequency (%) | A/Fratio | IVI | Density (ha−1) | Frequency (%) | A/Fratio | IVI | Density (ha−1) | Frequency (%) | A/Fratio | IVI | |
| Ougeiniaoojeinensis | 37.5 | 30.0 | 0.17 | 30.64 | - | - | - | - | - | - | - | - |
| Anogeissus latifolia | 37.5 | 60.0 | 0.04 | 40.66 | - | - | - | - | - | - | - | - |
| Dalbergia sissoo | 47.5 | 70.0 | 0.04 | 71.10 | - | - | - | - | - | - | - | - |
| Acacia catechu | 40.0 | 40.0 | 0.10 | 37.14 | - | - | - | - | - | - | - | - |
| Phyllanthus emblica | 37.5 | 50.0 | 0.06 | 30.56 | - | - | - | - | - | - | - | - |
| Terminalia chebula | 35.0 | 30.0 | 0.16 | 28.44 | - | - | - | - | - | - | - | - |
| Terminalia bellirica | 47.5 | 80.0 | 0.03 | 61.45 | - | - | - | - | - | - | - | - |
| Ficus religiosa | - | - | - | - | 10.0 | 40.0 | 0.03 | 52.47 | - | - | - | - |
| Lannea coromandelica | - | - | - | - | 22.5 | 60.0 | 0.03 | 72.22 | - | - | - | - |
| Pyrus pashia | - | - | - | - | 27.5 | 60.0 | 0.03 | 76.78 | 17.5 | 60.0 | 0.02 | 28.13 |
| Punica granatum | - | - | - | - | 12.5 | 50.0 | 0.02 | 38.39 | - | - | - | - |
| Prunus cerasoides | - | - | - | - | 27.5 | 60.0 | 0.02 | 60.15 | - | - | - | - |
| Quercus leucotricophora | - | - | - | - | 72.5 | 80.0 | 0.05 | 64.21 | ||||
| Pinus roxburghii | - | - | - | - | - | - | - | - | 50.0 | 60.0 | 0.05 | 79.69 |
| Myrica esculenta | - | - | - | - | - | - | - | - | 35.0 | 60.0 | 0.04 | 38.13 |
| Rhododendron arboreum | - | - | - | - | - | - | - | - | 27.5 | 70.0 | 0.02 | 36.48 |
| Lyonia ovalifolia | - | - | - | - | - | - | - | - | 25.0 | 70.0 | 0.02 | 26.35 |
| Cornus capitata | - | - | - | - | - | - | - | - | 52.0 | 70.0 | 0.02 | 26.32 |
| Altitude (m asl) | Total O2 Production (Mg ha−1) | Net O2 Production (Mg ha−1 yr−1) | Carbon Credits (Indian Rupees) | |||
|---|---|---|---|---|---|---|
| Agroforestry | Forest | Agroforestry | Forest | Agroforestry | Forest | |
| 700–1200 | 50.56 | 240.21 | 3.16 | 11.57 | 12,316.10 | 45,077.81 |
| 1200–1700 | 63.44 | 58.18 | 4.02 | 2.32 | 15,659.64 | 9038.43 |
| 1700–2200 | 72.10 | 264.10 | 3.00 | 9.97 | 11,709.82 | 38,843.61 |
| Altitude (masl) | Land Use | Marglef Index (MI) | Menheink Index (MeI) | Simpson’s, Diversity Index (D) | Shannon-Weiner Index (H) | Pielo Equitability (J) |
|---|---|---|---|---|---|---|
| 700–1200 | Agroforestry | 2.39 | 0.25 | 0.88 | 2.23 | 0.91 |
| Forest | 1.27 | 0.24 | 0.61 | 2.36 | 0.66 | |
| 1200–1700 | Agroforestry | 2.76 | 0.29 | 0.88 | 2.34 | 0.94 |
| Forest | 1.12 | 0.42 | 0.70 | 1.62 | 0.47 | |
| 1700–2200 | Agroforestry | 2.85 | 0.31 | 0.86 | 2.19 | 0.96 |
| Forest | 1.30 | 0.25 | 0.72 | 2.02 | 0.66 |
| Source of Variation | DF | F Ratio | |||
|---|---|---|---|---|---|
| AGBD | BGBD | TBD | TCD | ||
| Altitude | 2 | 66.56 * | 25.50 * | 54.77 * | 54.79 * |
| Land use | 1 | 387.58 * | 157.62 * | 324.41 * | 324.51 * |
| Altitude × Land use | 2 | 65.32 * | 28.39 * | 55.23 * | 55.25 * |
| Altitude (masl) | Land Use | Soil Bulk Density (g cm−3) | Soil Organic Carbon (%) | Soil Organic Carbon Stock (Mg C ha−1) | |||
|---|---|---|---|---|---|---|---|
| 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | 0–15 cm | 15–30 cm | ||
| Lower (700–1200) | Agroforestry | 1.18 | 1.22 | 2.63 | 2.41 | 46.55 | 44.10 |
| Forest | 0.98 | 1.06 | 4.31 | 3.78 | 63.36 | 60.10 | |
| Middle (1200–1700) | Agroforestry | 0.92 | 1.08 | 4.29 | 3.62 | 59.20 | 58.64 |
| Forest | 1.21 | 1.27 | 2.38 | 2.21 | 43.20 | 42.10 | |
| Upper (1700–2200) | Agroforestry | 1.28 | 1.36 | 2.68 | 2.49 | 51.46 | 50.80 |
| Forest | 1.09 | 1.12 | 4.38 | 4.2 | 71.61 | 70.56 | |
| Source of Variation | DF | F Ratio | ||
|---|---|---|---|---|
| BD | SOC | SOCS | ||
| Altitude | 2 | 34.81 * | 100.52 * | 77.91 |
| Land Use | 1 | 74.62 * | 210.39 * | 175.64 |
| Depth | 1 | 0.002 | 0.95 | 0.0001 |
| Altitude × Land use | 2 | 1.00 | 36.58 * | 18.13 * |
| Altitude × Depth | 2 | 4.80 * | 0.31 | 1.49 |
| Land use × Depth | 1 | 9.92 * | 0.04 | 6.66 * |
| Altitude × Land use × Depth | 2 | 8.17 * | 0.17 | 5.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, N.; Riyal, M.K.; Singh, B.; Khanduri, V.P.; Rawat, D.; Singh, C.; Pinto, M.M.S.C.; Kumar, M. Carbon Sequestration Potential of Agroforestry versus Adjoining Forests at Different Altitudes in the Garhwal Himalayas. Atmosphere 2024, 15, 313. https://doi.org/10.3390/atmos15030313
Singh N, Riyal MK, Singh B, Khanduri VP, Rawat D, Singh C, Pinto MMSC, Kumar M. Carbon Sequestration Potential of Agroforestry versus Adjoining Forests at Different Altitudes in the Garhwal Himalayas. Atmosphere. 2024; 15(3):313. https://doi.org/10.3390/atmos15030313
Chicago/Turabian StyleSingh, Naresh, Manoj Kumar Riyal, Bhupendra Singh, Vinod Prasad Khanduri, Deepa Rawat, Chandramohan Singh, Marina M. S. Cabral Pinto, and Munesh Kumar. 2024. "Carbon Sequestration Potential of Agroforestry versus Adjoining Forests at Different Altitudes in the Garhwal Himalayas" Atmosphere 15, no. 3: 313. https://doi.org/10.3390/atmos15030313
APA StyleSingh, N., Riyal, M. K., Singh, B., Khanduri, V. P., Rawat, D., Singh, C., Pinto, M. M. S. C., & Kumar, M. (2024). Carbon Sequestration Potential of Agroforestry versus Adjoining Forests at Different Altitudes in the Garhwal Himalayas. Atmosphere, 15(3), 313. https://doi.org/10.3390/atmos15030313







