Abstract
(1) Background: The increase in cardiovascular risk related to air pollution has been a matter of interest in recent years. The role of particulate matter 2.5 (PM2.5) has been postulated as a possible factor for premature death, including cardiovascular death. The role of long-term exposure to PM10 is less known. The aim of the study was to assess the individual relationship between air pollution in habitation and the development of coronary artery disease. (2) Methods: Out of 227 patients who underwent coronary angiography, 63 (38 men and 25 women) with a mean age of 69 (63–74) years, with nonsignificant atherosclerotic changes at the initial examination, were included in the study. The baseline and repeated coronary angiography were compared to reveal patients with atherosclerotic progression and its relation to demographic and clinical factors and exposure to air pollution in the habitation place. (3) Results: In the performed analysis, we found a significant correlation between Syntax score in de novo lesions and BMI (Spearman’s rho −0.334, p = 0.008). The significant and strong correlation between median annual PM10 values of 20 µg/m3 and at least 25 µg/m3 in air pollution and the risk of de novo coronary disease was noticed (Spearman’s rho = 0.319, p = 0.011 and Spearman’s rho = 0.809, p < 0.001, respectively). (4) Conclusions: There is a positive correlation between long-term exposure to PM10 air pollution and coronary artery disease progression, demonstrated by the increase in Syntax score. The presented analysis revealed increased morbidity at lower PM10 concentrations than generally recommended thresholds. Therefore, further investigations concerning air pollution’s influence on cardiovascular risk should be accompanied by promoting lifestyle changes in the population and revisiting the needs for environmental guidelines.
1. Introduction
Air pollutants are composed of particulate matter (PM), including coarse particles, characterized by being 10 micrometers or less to 2.5 micrones in size (PM10); fine particles, defined as being 2.5 micrones or less in diameter (PM2.5); and ultrafine particles and gaseous pollutants, such as nitrogen dioxide and ozone. The inhalation of PM2.5 generated by the combustion of fossil fuels is considered as one of the non-traditional risk factors for coronary artery disease [1].
In their analysis, Zhang et al. [2] pointed out the possible role of PM2.5 air pollution in the early stages of cardiovascular disease progression. Inflammatory activation induced by air pollutants is triggered either by the particles themselves or by reactive oxygen species generated in the course of tissue damage [3]. They contribute to cardiovascular disease in various pathways, including thrombus formation and platelet activation induction [4].
In his review, Bhatnagar indicated that exposure to PM2.5 increased blood pressure, thrombosis, and insulin resistance [5]. The relation between long-term exposure to PM2.5 and increased cardiovascular mortality was presented in epidemiological studies [6]. PM2.5 is mostly postulated as a triggering factor, which initiates oxidative stress and inflammation in the lungs [7]. Long-term exposure to an increased air concentration of PM2.5 is related to increased mortality [8,9], while the clinical effect of PM10 is less clear. In their meta-analysis, Wu et al. [10] pointed out the significance of increased risk of cardiorespiratory diseases within a few hours after exposure to air pollution including PM10. The exact mechanism of the ultrafine particles’ adverse effect on human organisms is not clearly defined but is related to oxidative stress anticipation [11]. The inflammatory activation related to intracellular calcium influx in macrophages secondary to air pollutants was presented [12]. In their experimental study, Brow et al. [13] revealed increased pro-inflammatory cytokines such as tumor necrosis factors alpha (TNF-alpha) and interleukin 1 (IL-1) by macrophages exposed to PM10 particles.
The traditional risk factors for coronary artery disease progression, such as arterial hypertension, hyperlipidemia, diabetes mellitus, or gender, are believed to be the primary aspects that play crucial roles in atherosclerosis progression. The non-classical factors, such as inflammatory activation, air pollution, or environmental factors, are claimed to play more significant roles in combination with the aforementioned factors.
Previous studies sµggested PM acts as an environmental endocrine disruptor for blood glucose, including insulin resistance and lipid hemostasis, by increased adipokine secretion [14]. In their analysis, Marin-Palma et al. [15] noticed the increased production of pro-inflammatory cytokines, such as IL-1β and IL-6, with PM10 exposure. The cardiotoxic effect of PM10 was presented in Chen et al.’s [16] experimental study. However, data on the influence of PM on the progression of coronary artery disease are lacking. Previous studies were based on populational epidemiological analyses, while the influence of air pollution exposure in individuals is less known. The aim of the study was to assess the individual relationship between air pollution in a habitation and the evolution of coronary artery disease.
2. Materials and Methods
2.1. Patients’ Study Group
There were 227 patients (149 men and 178 women) with a mean age of 70 (64–74) years presenting anginal symptoms of median Canadian Cardiovascular Society (CCS) grade 2 (1.2–2.5) who underwent diagnostic coronary angiographies. Out of the whole group, 146 presented significant atherosclerotic coronary artery disease. The remaining 63 subjects with nonsignificant coronary culprit lesions estimated to have a Syntax score of zero upon initial examination were taken into account in further analyses, as presented in the study flow chart (Figure 1).
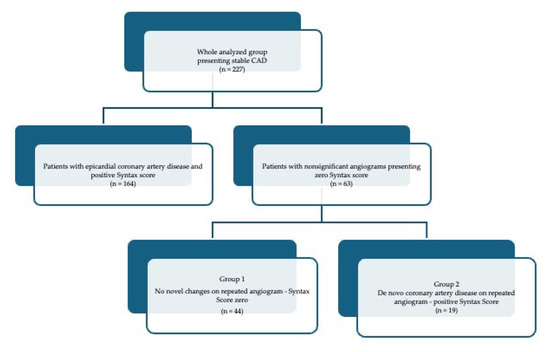
Figure 1.
Flow chart of patients enrolled into the analysis.
A Syntax score of I was calculated based on the calculator available at https://syntaxscore.org/calculator/syntaxscore/frameset.htm (accessed on 1 December 2023) (Syntax Score calculator 1, version 2.28, 2022).
All these patients presented stable chronic coronary syndrome and underwent repeated coronary angiography due to the re-occurrence of clinical symptoms, such as easy fatigue and shortness of breath on exertion. The repeated coronary angiographies were performed after 1686 (1310–1968) days and revealed de novo epicardial coronary artery disease in 19 patients (Group 2). The median (Q1–Q3) Syntax score upon the repeated examination was 7 (4–9.5) in Group 2 and remained zero in Group 1.
Patients with repetitive hospitalization due to the reappearance of stable coronary syndrome were enrolled into the analysis. All information was collected retrospectively from the hospital data, including first and second hospitalization. Only stable coronary disease patients were taken into account in the analysis, as acute coronary syndromes were reported to be linked to short-lasting air pollution derangements [17]. Demographic and clinical data were collected. Body mass index (BMI) was calculated. Blood samples were obtained at each admission, and their results were analyzed.
All the patients were treated according to contemporary guidelines on chronic coronary syndromes, including antiplatelets, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, beta-blockers and statins, or statin/ezetimibe combination.
All the angiographic findings were evaluated by Syntax score to estimate anatomic culprit lesions’ locations in the coronary bed [18]. Those with a Syntax score of zero were further analyzed in terms of de novo changes (positive Syntax Score at the repeated angiogram) or no novel changes. The median (Q1–Q3) Syntax score at the repeated examination was 7 (4–9.5) in Group 2 and remained zero in Group 1. The demographical and clinical characteristics are presented in Table 1.

Table 1.
Demographical and clinical characteristics of Group 1 (no coronary disease on repetitive angiograms) vs. Group 2 (presenting positive Syntax score) upon initial admission.
2.2. Air Pollution Exposure
The concept of assessing patients’ individual exposure to air pollution was the use of all available air quality data.
For this purpose, we used data developed within the State Environmental Monitoring (SEM) in Poland. SEM is the system of measurements, assessments, and outlooks of the state of the environment as well as the system of collecting, processing, and disseminating information about the environment. SEM was established pursuant to the Act of Inspection of Environmental Protection to provide reliable data on the state of the environment.
The basis for assessing the level of individual exposure to air pollution of particulate matter (PM) with diameters ≤ 2.5 μm (PM2.5) and above 2.5 to ≤10 μm (PM10) and nitrogen dioxides (NO2) of each of the patients was air quality measurement data and spatial distributions of air concentration maps provided by the Chief Inspectorate of Environmental Protection [19].
The maps were from the national air quality modeling system elaborated upon by Institute of Environmental Protection—National Research Institute in Poland (IEP-NRI) in accordance with the legal obligation set out in the Environmental Protection Act in Poland.
This system was based on yearly high-resolution, bottom-up emission inventory maps stored in the Central Emission Database, elaborated upon based on Standard Nomenclature for Air Pollution (SNAP) categories including main air pollutant emission sectors in Poland, like residential emissions, energy production, industry, transport, or agriculture [20]. Air emission maps are implemented as input data to GEM-AQ models, among others, and operate in the Copernicus Atmosphere Monitoring Service—Regional Production (CAMS2_40) [20,21]. GEM-AQ is a semi-Lagrangian chemical weather model developed at Environment Canada in which air quality processes and tropospheric chemistry are implemented in a weather prediction model, the Global Environmental Multiscale (GEM) [22]. In the GEM-AQ, the air concentration fields are performed using a 0.025-degree resolution grid.
To improve modeling results, air pollutant concentrations from air quality monitoring measurement stations operating within the State Environmental Monitoring System were used in the assimilation data process. In order to increase the resolution of the grid of the corrected results of the GEM-AQ model (to more precisely reflect the variability in concentrations in the patients’ place of living), we used downscaling statistical methods based on the Expert-in-the-loop Stepwise Regression procedure elaborated upon in the Neurosmog project [23]. This is experimentally validated for real-life data from various sources aiming at predicting air pollution [24].
2.3. Statistical Analysis
Continuous variables were reported as medians and interquartile ranges (Q1–Q3) since data did not follow normal distribution. Categorical data were presented as numbers and percentages. The comparison of interval parameters between both groups was performed by the Mann–Whitney test. Categorical data were compared by Fisher’s exact test. Spearman correlation analysis was used to describe the correlation between the variables. Statistical analysis was performed using JASP statistical software (JASP Team; 2023. Version 0.18.1). p < 0.05 was considered statistically significant.
3. Results
3.1. Angiographic and Laboratory Results on Readmission
Our final study group included 63 patients (38 men and 25 women) with a mean age of 69 (63–74) years with a baseline Syntax score of zero, who were divided into two subgroups based on the Syntax score at repeated coronary angiography (see Figure 1). The subgroups were significantly different regarding gender differences (p = 0.047) but not age (p = 0.505) and co-morbidities (Table 1). The laboratory results on readmission did not reveal any statistical differences in laboratory results (Table 2).

Table 2.
Laboratory results on readmission.
3.2. Angiographic Results
The repeated coronary angiographies were performed after 1699 (1310–1863) and 1881 (1407–2210) days in group 1 and 2, respectively (p = 0.087). The coronary lesions detected in repeated examinations are presented in Table 3.

Table 3.
Angiographic results of repeated examination.
3.3. Air Pollution Exposure
During the time gap interval of 1686 (1310–1968) days for the whole group, patients were exposed to air pollutants in their habitation places, and the individual annual exposure was calculated. The median values of PM2.5, PM10, and NO2 are presented in Table 4.

Table 4.
Air pollution annual concentrations.
A geo-map was made, which presents the relation between long-term PM10 air pollution exposure in each of the analyzed patients and their habitation place in Figure 2.
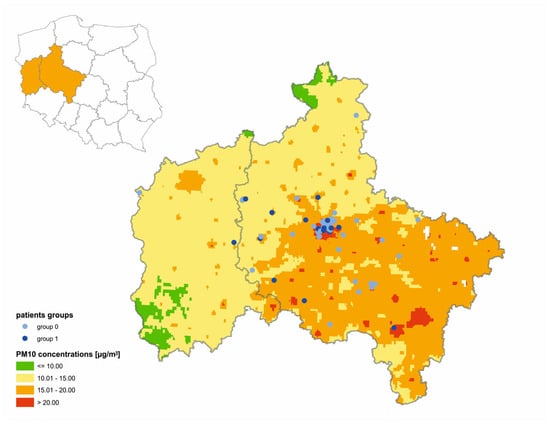
Figure 2.
Individuals’ PM10 long-term exposure in relation to their habitation place in analyzed group.
The significant difference between patients with zero and positive Syntax scores in the de novo coronary lesions was presented, in terms of PM10 air pollution exposure (p < 0.001), in overall and yearly median values. PM10 was the only ambient pollutant that differentiated the presented groups. The characteristics of patients with no coronary changes vs. de novo coronary lesions were analyzed in relation to recommended values of air pollutants presented by European Union (EU) authorities [25] and the World Health Organization (WHO) [26], as presented in Table 5.

Table 5.
Comparison of long-term exposure to PM10 at various thresholds between both groups.
3.4. Correlations
3.4.1. Demographical and Clinical Factors Significant for Atherosclerotic Lesion Development
In the performed analysis, we found a significant correlation between Syntax score in de novo lesions and BMI (Spearman’s rho −0.334, p = 0.008).
3.4.2. PM10 Air Pollution Exposure as a Potential Risk Factor for Atherosclerotic Lesion Development
A strong positive correlation between PM10 air pollution and a positive Syntax score in de novo lesions was estimated (Spearman’s rho 0.757, p < 0.001).
There was no correlation between the median annual PM10 values within 15 µg/m3 in air pollution and the risk of de novo coronary disease (Spearman’s rho 0.083, p = 0.516).
The significant correlation between the median annual PM10 values of 20 µg/m3 in air pollution and the risk of de novo coronary disease (Spearman’s rho = 0.319, p = 0.011) was noticed in the presented analysis.
When the analysis was performed for a median annual PM10 values of at least 25 µg/m3 in air pollution and the risk of de novo coronary disease, a strong correlation was observed (Spearman’s rho = 0.809, p < 0.001).
4. Discussion
The results of our retrospective analysis point out the significant strong correlation between long-term PM10 exposure and increased risk for de novo coronary artery disease development. These results not only indicate the relation between inhaled particulate matter (PM10) and coronary atherosclerosis, but also reveal increased morbidity at lower PM10 concentrations than at thresholds widely accepted in authorities’ recommendations [27].
Our analysis points out the significance of PM10 in de novo coronary artery disease developments estimated by Syntax score, while previous reports focused mainly on PM2.5 particles [28,29]. Among air pollutant particles, mainly PM2.5 was found to be associated with cardiovascular morbidity [30]. Several analyses indicated the influence of exposure to PM2.5 particles on human health, including increased cardiovascular risk [31], pulmonary diseases [32], diabetic risk [33], or endocrinal disturbances [34]. The relation between ventricular arrythmias and exposure to PM2.5 air pollution was sµggested [35]. In their meta-analysis, Chen et al. [36] pointed out the association between PM2.5 and PM10 and increased all-cause mortality. Han et al. [37] found the association between ambient air pollution and metabolic dysfunction-associated fatty liver disease. Montone et al. [38], in their analysis of 126 patients with acute coronary syndrome, showed the relation between air pollution exposure related to patients’ home address and vulnerable culprit lesions estimated by their morphology in optical coherence tomography (OCT). In an epidemiological study by Luo et al. [39], the potential association between PM10 and PM2.5 and coronary artery stenosis was examined. Our correlation sµggests the novel and important effect of PM10 on cardiovascular pathology. We showed that changes in the degree of exposure to PM10 may result in progression in an individual patient.
The possible explanations for these phenomena have been extensively studied. It was shown in animal and human models that the inhalation of air pollutants causes phagocytosis of the PM in the lung tissue, which promotes inflammatory induction by activated neutrophils, which release cytokines and stimulate monocyte release from bone marrow [40,41,42]. Histologically, this inflammatory response may result in plaque cell turnover, the occurrence of extracellular lipid pools in coronary and aortic tissues, and changes in the total amount of lipids in aortic lesions [43]. Our and other previous studies in patients with coronary artery disease proved the association between inflammatory response and the occurrence of coronary artery disease and worse outcomes [44,45]. Cytokines contribute to diseases with chronic inflammatory process, such as atherosclerosis. Exposure to PM2.5 may increase metalloproteinase levels [46].
Our study results may be explained by the described observations. Most probably, simple insignificant coronary plaques, assessed as not significant changes in baseline coronary angiography, could have changed into substantial stenosis after exposure to PM10 and the activation of exaggerated inflammatory processes.
The coronary plaque progression evaluated by its composition characteristics was found to be a possible target factor for future event reduction [47]. Optical coherence tomography provided significant impacts on culprit lesion arrangement, allowing for further analysis of the future risk of rupture/erosion [48]. The noninvasive evaluation of quantitative plaque changes as predictors of acute coronary events was sµggested based on serial coronary computed tomography angiography (CCTA) [49]. The PARADIGM registry data [50] results presented the relation between patient-specific phenotypes of coronary atherosclerosis lesions, yielding distinct patterns of disease progression and outcomes. Not only lesion structure but also perivascular adipose tissue solidity may indicate vessel inflammation as an independent element of atherosclerosis progression [51].
In addition to atherosclerotic lesion composition, its possible modification has gained attention in recent times. The possible influences of therapies like statin use were claimed to be possible modifiable features [52]. In the CARDIOPREV study, the relation between diet and coronary disease progression risk was presented [53]. In addition to traditional risk factors that are claimed to be responsible for the majority of cardiovascular events [54], externals like climatic changes are correspondingly postulated [55].
In our previous analysis [56], we found a significant relation between NO2 and increased risk for coronary lesion progression in larger agglomerations in contrast to small villages, which could be influenced by road transport and the industrial combustion and processes sectors. In our current study, long-term exposure to PM10 was positively correlated with chronic coronary atherosclerosis progression, estimated by de novo Syntax Score development.
Our analysis was based on Syntax score evaluation as a reliable evaluation of atherosclerotic lesions for clinical decision making [57,58]. Syntax score is a useful tool for coronary artery disease evaluation [18]. Its established value with regard to the objective complexity of coronary artery disease enabled us to estimate the progression of atherosclerotic lesions in coronary trees. Since only patients with initial Syntax scores of zero were enrolled into the analysis, the factors that may influence atherosclerotic plaque development and progression were taken into account in the analysis.
Our finding regarding the significance of sex differences between groups is consistent with previous reports [59,60,61,62]. The significant difference was noticed regarding hyperlipidemia, but we may conclude that patients with initially normal angiograms who were diagnosed with lipid disturbances were treated more scrupulously as the lipidograms revealed lower low-density lipoprotein (LDL) concentrations. Importantly, even thoµgh the intensification of therapy led to an LDL decrease, the progression of coronary artery disease was observed. Therefore, the identification of other factors influencing disease development is crucial. Based on our results, we can conclude that environmental pollution is one of the important causes.
Sram [63] underlined that 70% of PM10 pollution comes not from power plants but from local heating. In Shen et al.’s [64] analysis, PM10 as a carbonaceous air pollution component was found to be primarily affected by coal burning, gasoline, and diesel vehicle exhausts. According to our analysis, inhaled particles such as PM10 can be considered as potential triggers for further processes, inducing patho-mechanisms involved in atherosclerotic plaque formation. Moreover, the recommended thresholds for PM10 air concentrations that are considered safe for human health according to current recommendations should be revised and changed to lower levels based on our analysis results.
Study limitations:
The retrospective analysis was based on patients with repeated hospitalizations due to chronic coronary syndrome, who represented one district in the country, covering 3.5 million habitants, which is limited to one university hospital. The study was performed on consecutive patients requiring repeated angiography within 1 year in a center that is focused on chronic coronary artery diagnosis by angiographic examination from the region. The study was performed on a relatively low number of patients but in whom the personalized individual air pollution exposure risk was calculated. The study was based on chronic coronary syndrome patients as acute syndromes were not taken into consideration, but these are sµggested to be related to acute air pollution/temperature disturbances which was not examined in the performed analysis.
The Syntax score results were estimated by the same team of interventional cardiologists from a single but highly skilled reference center, and the potential impact of this on the obtained results due to operators’ qualifications should be regarded as non-significant.
5. Conclusions
There is a positive correlation between long-term exposure to PM10 air pollution and coronary artery disease progression, demonstrated by the increase in Syntax score. The presented analysis revealed increased morbidity at lower PM10 concentrations than generally recommended thresholds. Therefore, further investigations concerning air pollution’s influence on cardiovascular risk should be accompanied by promoting lifestyle changes in the population and revisiting the needs for environmental guidelines.
Author Contributions
Conceptualization, T.U. and K.S.; methodology, T.U. and K.S.; software, K.S.; validation, T.U., A.O.-W., J.B., M.W. and K.S.; formal analysis, T.U., J.B. and M.W.; investigation, T.U., A.O.-W., K.S., P.T. and J.S.; resources, K.S., A.O.-W., J.B., M.W., P.T. and J.S.; data curation, T.U., K.S. and M.W.; writing—original draft preparation, T.U.; writing—review and editing, T.U., A.O.-W., K.S., B.K., Z.K., M.J., A.T. and K.J.F.; visualization, K.S.; supervision, K.J.F., A.T. and M.J.; project administration, T.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Poznan University of Medical Sciences, Poznan, Poland (protocol code 55/20 from 16 January 2020), for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data will be available for 3 years following the publication after reasonable request is presented in e-mail correspondence to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Y.; Qiu, X.; Wei, Y.; Schwartz, J.D. Long-Term Exposure to Ambient PM2.5 and Hospitalizations for Myocardial Infarction Among US Residents: A Difference-in-Differences Analysis. J. Am. Heart Assoc. 2023, 2023, e029428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qian, Z.M.; Chen, L.; Zhao, X.; Cai, M.; Wang, C.; Zou, H.; Wu, Y.; Zhang, Z.; Li, H.; et al. Exposure to Air Pollution during Pre-Hypertension and Subsequent Hypertension, Cardiovascular Disease, and Death: A Trajectory Analysis of the UK Biobank Cohort. Environ. Health Perspect. 2023, 131, 17008–17018. [Google Scholar] [CrossRef] [PubMed]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Nogueira, J.B. Air pollution and cardiovascular disease. Rev. Port. Cardiol. 2009, 40, 715–733. [Google Scholar]
- Bhatnagar, A. Cardiovascular Effects of Particulate Air Pollution. Annu. Rev. Med. 2022, 73, 393–406. [Google Scholar] [CrossRef]
- Bourdrel, T.; Bind, M.A.; Béjot, Y.; Morel, O.; Argacha, J.F. Cardiovascular effects of air pollution. Arch. Cardiovasc. Dis. 2017, 110, 634–642. [Google Scholar] [CrossRef]
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214. [Google Scholar] [CrossRef]
- Hayes, R.B.; Lim, C.; Zhang, Y.; Cromar, K.; Shao, Y.; Reynolds, H.R.; Silverman, D.T.; Jones, R.R.; Park, Y.; Jerrett, M.; et al. PM2.5 air pollution and cause-specific cardiovascular disease mortality. Int. J. Epidemiol. 2020, 49, 25–35. [Google Scholar] [CrossRef]
- Krittanawong, C.; Qadeer, Y.K.; Hayes, R.B.; Wang, Z.; Virani, S.; Thurston, G.D.; Lavie, C.J. PM2.5 and Cardiovascular Health Risks. Curr. Probl. Cardiol. 2023, 48, 101670. [Google Scholar] [CrossRef]
- Wu, K.; Ho, H.C.; Su, H.; Huang, C.; Zheng, H.; Zhang, W.; Tao, J.; Hossain, M.Z.; Zhang, Y.; Hu, K.; et al. A systematic review and meta-analysis of intraday effects of ambient air pollution and temperature on cardiorespiratory morbidities: First few hours of exposure matters to life. EBioMedicine 2022, 86, 104327–104340. [Google Scholar] [CrossRef]
- Donaldson, K.; Stone, V.; Seaton, A.; MacNee, W. Ambient particle inhalation and the cardiovascular system: Potential mechanisms. Environ. Health Perspect. 2001, 109, 523–527. [Google Scholar]
- Stone, V.; Brown, D.M.; Watt, N.; Wilson, M.; Donaldson, K.; Ritchie, H.; MacNee, W. Ultrafine Particle-Mediated Activation of Macrophages: Intracellular Calcium Signaling and Oxidative Stress. Inhal. Toxicol. 2000, 12, 345–351. [Google Scholar] [CrossRef]
- Brown, D.M.; Donaldson, K.; Stone, V. Effects of PM10 in human peripheral blood monocytes and J774 macrophages. Respir. Res. 2004, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Gaio, V.; Roquette, R.; Monteiro, A.; Ferreira, J.; Lopes, D.; Dias, C.M.; Nunes, B. PM10 exposure interacts with abdominal obesity to increase blood triglycerides: A cross-sectional linkage study. Eur. J. Public Health 2022, 32, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Marín-Palma, D.; Tabares-Guevara, J.H.; Zapata-Cardona, M.I.; Zapata-Builes, W.; Taborda, N.; Rµgeles, M.T.; Hernandez, J.C. PM10 promotes an inflammatory cytokine response that may impact SARS-CoV-2 replication in vitro. Front. Immunol. 2023, 14, 1161135–1161147. [Google Scholar] [CrossRef] [PubMed]
- Cen, J.; Jia, Z.L.; Zhu, C.Y.; Wang, X.F.; Zhang, F.; Chen, W.Y.; Liu, K.C.; Li, S.Y.; Zhang, Y. Particulate matter (PM10) induces cardiovascular developmental toxicity in zebrafish embryos and larvae via the ERS, Nrf2 and Wnt pathways. Chemosphere 2020, 250, 126288–126299. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jiang, Y.; Hu, J.; Chen, H.; Li, H.; Meng, X.; Ji, J.S.; Gao, Y.; Wang, W.; Liu, C.; et al. Hourly Air Pollutants and Acute Coronary Syndrome Onset in 1.29 Million Patients. Circulation 2022, 145, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Onuma, Y.; Garg, S.; Sarno, G.; Van den Brand, M.; Kappetein, A.P.; Van Dyck, N.; Mack, M.; Holmes, D.; Feldman, T.; et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention 2009, 5, 50–56. [Google Scholar] [CrossRef]
- Mapa Wykonana w Oparciu o Modelowanie Matematyczne Przygotowane Przez Instytut Ochrony Środowiska—Państwowy Instytut Badawczy Oraz Metodę Obiektywnego Szacowania. PM10 (Śr. Roczna)—Rozkład Przestrzenny Średniego Rocznego Stężenia PM10 [µg/m3]. Available online: https://powietrze.gios.gov.pl/pjp/maps/modeling (accessed on 10 December 2023).
- Tagaris, E.; Sotiropoulou, R.E.P.; Gounaris, N.; Andronopoulos, S.; Vlachogiannis, D. Effect of the Standard Nomenclature for Air Pollution (SNAP) categories on air quality over Europe. Atmosphere 2015, 6, 1119–1128. [Google Scholar] [CrossRef]
- European Air Quality, Copernicus, Atmosphere Monitoring Service. Available online: https://www.regional.atmosphere.copernicus.eu/ (accessed on 27 December 2022).
- Kaminski, J.W.; Neary, L.; Struzewska, J.; McConnell, J.C.; Lupu, A.; Jarosz, J.; Toyota, K.; Gong, S.L.; Côté, J.; Liu, X. GEM-AQ, an online global multiscale chemical weather modelling system: Model description and evaluation of gas phase chemistry processes. Atmos. Chem. Phys. 2008, 8, 3255–3281. [Google Scholar] [CrossRef]
- Markevych, I.; Orlov, N.; Grellier, J.; Kaczmarek-Majer, K.; Lipowska, M.; Sitnik-Warchulska, K.; Mysak, Y.; Baumbach, C.; Wierzba-Łukaszyk, M.; Hussain Soomro, M.; et al. NeuroSmog: Determining the impact of air pollution on the developing brain: Project protocol. Int. J. Environ. Res. Public Health 2022, 19, 310. [Google Scholar] [CrossRef]
- Fraszczyk, M.; Kaczmarek-Majer, K.; Hryniewicz, O.; Skotak, K.; Degórska, A. Expert-in-the-loop Stepwise Regression and its Application in Air Pollution Modeling. In Proceedings of the 2022 IEEE 11th International Conference on Intelligent Systems (IS), Warsaw, Poland, 12–14 October 2022; pp. 1–7. [Google Scholar]
- The European Union (EU) has Developed an Extensive Body of Legislation Which Establishes Standards and Objectives for a Number of Pollutants in Air. Available online: https://www.eea.europa.eu/themes/air/air-quality-concentrations/air-quality-standards (accessed on 27 December 2022).
- WHO Air Quality Guidelines. Available online: https://www.c40knowledgehub.org/s/article/WHO-Air-Quality-Guidelines?language=en_US (accessed on 27 December 2022).
- Iriti, M.; Piscitelli, P.; Missoni, E.; Miani, A. Air Pollution and Health: The Need for a Medical Reading of Environmental Monitoring Data. Int. J. Environ. Res. Public. Health 2020, 17, 2174. [Google Scholar] [CrossRef]
- Shamsa, E.H.; Song, Z.; Kim, H.; Shamsa, F.; Hazlett, L.D.; Zhang, K. The links of fine airborne particulate matter exposure to occurrence of cardiovascular and metabolic diseases in Michigan, USA. PLoS Glob. Public Health 2022, 2, e0000707. [Google Scholar] [CrossRef]
- Gao, Y.; Sheng, W.; Yang, Y. Air pollution and coronary heart disease-related hospital visits in Beijing, China: Time-series analysis using a generalized additive model. Environ. Sci. Pollut. Res. Int. 2023, 30, 36938–36951. [Google Scholar] [CrossRef] [PubMed]
- Macchi, C.; Sirtori, C.R.; Corsini, A.; Mannuccio Mannucci, P.; Ruscica, M. Pollution from fine particulate matter and atherosclerosis: A narrative review. Environ. Int. 2023, 175, 107923. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Cao, X.; Guo, Y.; Wang, D.; Qiu, W.; Zhou, C.; Zhou, M.; Chen, W.; Zhang, X. Associations between short-term PM2.5 exposure and daily hospital admissions for circulatory system diseases in Ganzhou, China: A time series study. Front. Public Health 2023, 11, 1134516–1134527. [Google Scholar] [CrossRef] [PubMed]
- Soleimanifar, N.; Assadiasl, S.; Kalateh, E.; Hassanvand, M.S.; Sadr, M.; Mojtahedi, H.; Nadafi, K.; Nicknam, M.H.; Edalatifard, M. Circulating Exosomes and Ambient Air Pollution Exposure in COPD. Chronic Obstr. Pulm. Dis. 2023, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Feizi, A.; Shahraki, P.K.; Najafabadi, A.M.; Iraj, B.; Abyar, M.; Amini, M.; Meamar, R.; Aminorroaya, A. The association of exposure to air pollution with changes in plasma glucose indices, and incidence of diabetes and prediabetes: A prospective cohort of first-degree relatives of patients with type 2 diabetes. J. Res. Med. Sci. 2023, 28, 21–30. [Google Scholar] [PubMed]
- Liu, J.; Zhao, K.; Qian, T.; Li, X.; Yi, W.; Pan, R.; Huang, Y.; Ji, Y.; Su, H. Association between ambient air pollution and thyroid hormones levels: A systematic review and meta-analysis. Sci. Total Environ. 2023, 904, 166780–166796. [Google Scholar] [CrossRef] [PubMed]
- Pallikadavath, S.; Vali, Z.; Patel, R.; Mavilakandy, A.; Peckham, N.; Clegg, M.; Sandilands, A.J.; Ng, G.A. The Influence of Environmental Air Pollution on Ventricular Arrhythmias: A Scoping Review. Curr. Cardiol. Rev. 2022, 18, e160422203685. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974–105997. [Google Scholar] [CrossRef]
- Han, X.; Guo, B.; Wang, L.; Chen, K.; Zhou, H.; Huang, S.; Xu, H.; Pan, X.; Chen, J.; Gao, X.; et al. The mediation role of blood lipids on the path from air pollution exposure to MAFLD: A longitudinal cohort study. Sci. Total Environ. 2023, 904, 166347–166351. [Google Scholar] [CrossRef]
- Montone, R.A.; Camilli, M.; Russo, M.; Termite, C.; La Vecchia, G.; Iannaccone, G.; Rinaldi, R.; Gurgoglione, F.; Del Buono, M.G.; Sanna, T.; et al. Air Pollution and Coronary Plaque Vulnerability and Instability: An Optical Coherence Tomography Study. JACC Cardiovasc. Imaging 2022, 15, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Xie, X.; Wu, J.; Zhang, L.; Zheng, X.; Xie, M.; Lin, N.; Xiao, H.; Zeng, J.; Lan, G.; et al. Association of ambient PM10 and PM2.5 with coronary stenosis measured using selective coronary angiography. Ecotoxicol. Environ. Saf. 2023, 262, 115338. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Wiggs, B.; English, D.; Hogg, J.C.; Van Eeden, S.F. Phagocytosis of small carbon particles (PM10) by alveolar macrophages stimulates the release of polymorphonuclear leukocytes from bone marrow. Am. J. Respir. Crit. Care Med. 1997, 155, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ishii, H.; Hogg, J.C.; Shih, C.H.; Yatera, K.; Vincent, R.; Van Eeden, S.F. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am. J. Respir. Crit. Care Med. 2004, 170, 891–897. [Google Scholar] [CrossRef]
- Valderrama, A.; Ortiz-Hernández, P.; Agraz-Cibrián, J.M.; Tabares-Guevara, J.H.; Gómez, D.M.; Zambrano-Zaragoza, J.F.; Taborda, N.A.; Hernandez, J.C. Particulate matter (PM10) induces in vitro activation of human neutrophils, and lung histopathological alterations in a mouse model. Sci. Rep. 2022, 12, 7581–7597. [Google Scholar] [CrossRef]
- Suwa, T.; Hogg, J.C.; Quinlan, K.B.; Ohgami, A.; Vincent, R.; Van Eeden, S.F. Particulate air pollution induces progression of atherosclerosis. J. Am. Coll. Cardiol. 2002, 39, 935–942. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Michalak, M.; Komosa, A.; Olasińska-Wiśniewska, A.; Filipiak, K.J.; Tykarski, A.; Jemielity, M. Predictive value of systemic inflammatory response index (SIRI) for complex coronary artery disease occurrence in patients presenting with angina equivalent symptoms. Cardiol. J. 2023, 6, 1–13. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Michalak, M.; Olasińska-Wiśniewska, A.; Rodzki, M.; Witkowska, A.; Gąsecka, A.; Buczkowski, P.; Perek, B.; Jemielity, M. Neutrophil Counts, Neutrophil-to-Lymphocyte Ratio, and Systemic Inflammatory Response Index (SIRI) Predict Mortality after Off-Pump Coronary Artery Bypass Surgery. Cells 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Shen, T.J.; Chen, P.Y.; Chen, T.C.; Yeh, J.H.; Tsou, S.C.; Lai, C.Y.; Chen, C.H.; Chang, Y.Y. Particulate matter 2.5 exposure induces epithelial-mesenchymal transition via PI3K/AKT/mTOR pathway in human retinal pigment epithelial ARPE-19 cells. Biochem. Biophys. Res. Commun. 2022, 617, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.P.; Lum, M.; Nerleker, N.; Nicholls, S.J.; Layland, J. Coronary Atherosclerotic Plaque Regression: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, T.; Allard-Ratick, M.P.; Thondapu, V.; Sµgiyama, T.; Raffel, O.C.; Barlis, P.; Poon, E.K.W.; Araki, M.; Nakajima, A.; Minami, Y.; et al. Optical Coherence Tomography of Coronary Plaque Progression and Destabilization: JACC Focus Seminar Part 3/3. J. Am. Coll. Cardiol. 2021, 78, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Van Driest, F.Y.; Bijns, C.M.; Van der Geest, R.J.; Broersen, A.; Dijkstra, J.; Scholte, A.J.H.A.; Jukema, J.W. Utilizing (serial) coronary computed tomography angiography (CCTA) to predict plaque progression and major adverse cardiac events (MACE): Results, merits and challenges. Eur. Radiol. 2022, 32, 3408–3422. [Google Scholar] [CrossRef]
- Yoon, Y.E.; Baskaran, L.; Lee, B.C.; Pandey, M.K.; Goebel, B.; Lee, S.E.; Sung, J.M.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; et al. Differential progression of coronary atherosclerosis according to plaque composition: A cluster analysis of PARADIGM registry data. Sci. Rep. 2021, 11, 17121–17122. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Sung, J.M.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; Cademartiri, F.; Chinnaiyan, K.; Choi, J.H.; Chun, E.J.; Conte, E.; et al. Association Between Changes in Perivascular Adipose Tissue Density and Plaque Progression. JACC Cardiovasc. Imaging 2022, 15, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Van Rosendael, A.R.; Van den Hoogen, I.J.; Gianni, U.; Ma, X.; Tantawy, S.W.; Bax, A.M.; Lu, Y.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; et al. Association of Statin Treatment With Progression of Coronary Atherosclerotic Plaque Composition. JAMA Cardiol. 2021, 6, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Torres, J.; Alcalá-Diaz, J.F.; Torres-Peña, J.D.; Gutierrez-Mariscal, F.M.; Leon-Acuña, A.; Gómez-Luna, P.; Fernández-Gandara, C.; Quintana-Navarro, G.M.; Fernandez-Garcia, J.C.; Perez-Martinez, P.; et al. Mediterranean Diet Reduces Atherosclerosis Progression in Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. Stroke 2021, 52, 3440–3449. [Google Scholar] [CrossRef]
- Teo, K.K.; Rafiq, T. Cardiovascular Risk Factors and Prevention: A Perspective From Developing Countries. Can. J. Cardiol. 2021, 37, 733–743. [Google Scholar] [CrossRef]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef]
- Urbanowicz, T.; Skotak, K.; Filipiak, K.J.; Olasińska-Wiśniewska, A.; Szczepański, K.; Wyrwa, M.; Sikora, J.; Tykarski, A.; Jemielity, M. Long-Term Exposure of Nitrogen Oxides Air Pollution (NO2) Impact for Coronary Artery Lesion Progression—Pilot Study. J. Pers. Med. 2023, 13, 1376. [Google Scholar] [CrossRef] [PubMed]
- Chaus, A.; Uretsky, B.F. SYNTAX Score for Clinical Decision-Making: Necessity, Nicety, or Neither? Cardiovasc. Revasc. Med. 2022, 37, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Banning, A.P.; Serruys, P.; De Maria, G.L.; Ryan, N.; Walsh, S.; Gonzalo, N.; Jan van Geuns, R.; Onuma, Y.; Sabate, M.; Davies, J.; et al. Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: Final results of the SYNTAX II study. Eur. Heart J. 2022, 43, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Waheed, N.; Elias-Smale, S.; Malas, W.; Maas, A.H.; Sedlak, T.L.; Tremmel, J.; Mehta, P.K. Sex differences in non-obstructive coronary artery disease. Cardiovasc. Res. 2020, 116, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Asleh, R. Persistent sex differences in outcomes after coronary heart disease: Time to move from observation to action. Heart 2022, 108, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kawakami, R.; Sakamoto, A.; Cornelissen, A.; Mori, M.; Kawai, K.; Ghosh, S.; Romero, M.E.; Kolodgie, F.D.; Finn, A.V.; et al. Sex Differences in Coronary Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, T.; Michalak, M.; Olasińska-Wiśniewska, A.; Haneya, A.; Straburzyńska-Migaj, E.; Bociański, M.; Jemielity, M. Gender differences in coronary artery diameters and survival results after off-pump coronary artery bypass (OPCAB) procedures. J. Thorac. Dis. 2021, 13, 2867–2873. [Google Scholar] [CrossRef]
- Sram, R.J. Impact of Air Pollution on the Health of the Population in Parts of the Czech Republic. Int. J. Environ. Res. Public Health 2020, 17, 6454. [Google Scholar] [CrossRef]
- Shen, L.J.; Wang, H.L.; Sun, J.J.; Liu, S.Y.; Liu, H.W.; Zhao, T.L. Pollution Characteristics of Carbonaceous Components in PM10 and PM2.5 of Road Dust Fall and Soil Dust in Xi’an. Huan Jing Ke Xue 2023, 44, 4843–4852. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).