Characterization of Volatile Organic Compounds (VOCs) from Farms Effluents: Interest of HS-SPME-GC-MS Technique for Laboratory and Field Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Cattle Dung and Slurry for Laboratory Assays
2.2. Poultry Droppings for Laboratory Assays
2.3. Wheat Straw and Litter for Laboratory Assays
2.4. Field Test: Cattle Production Facility and Effluent Treatment
2.5. Volatile Organic Compounds Analysis by HS-SPME-GC-MS
2.6. Post-Acquisition GC-MS Data Processing
3. Results and Discussion
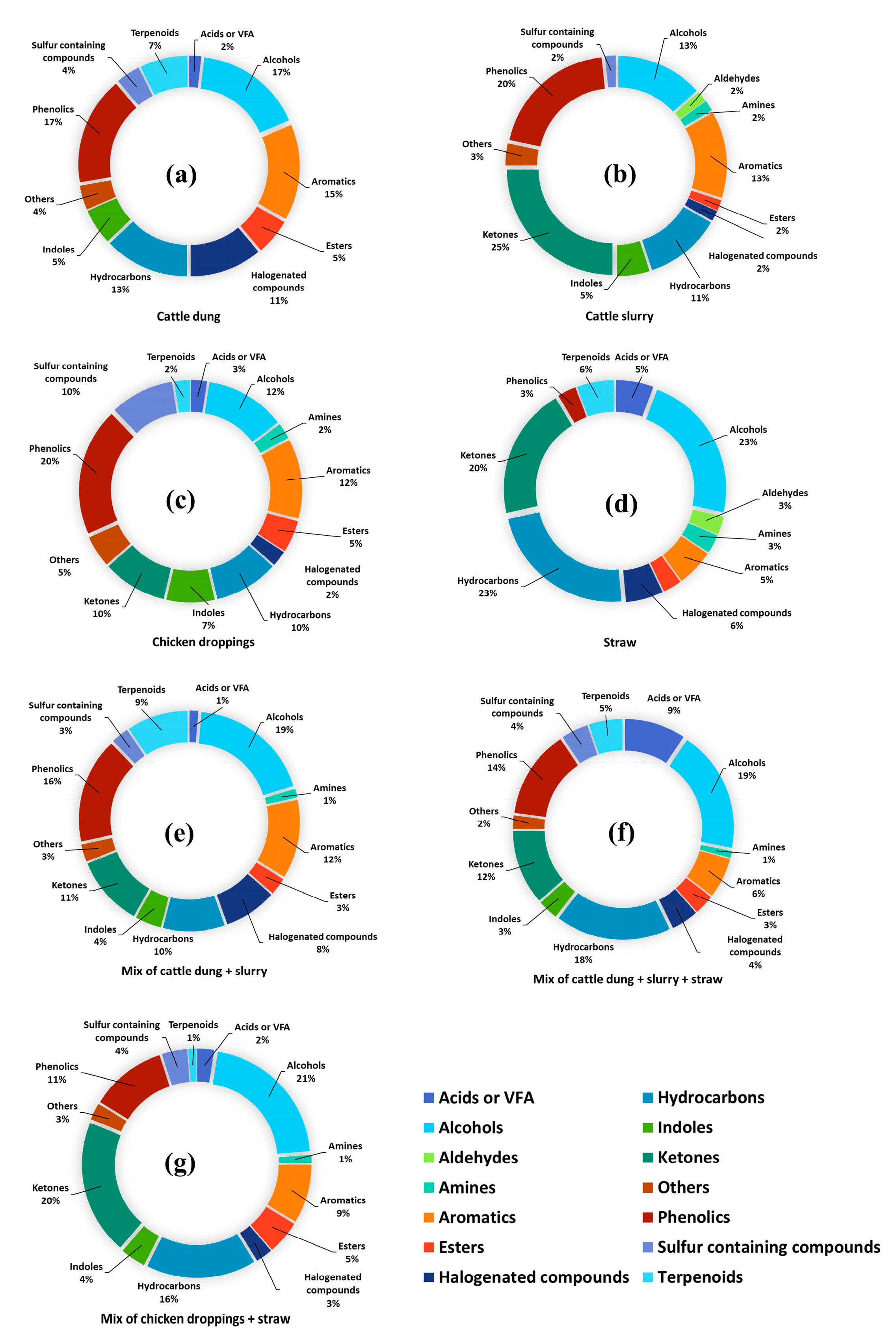
3.1. Laboratory Test: VOC Profiling in Different Effluent Mixtures
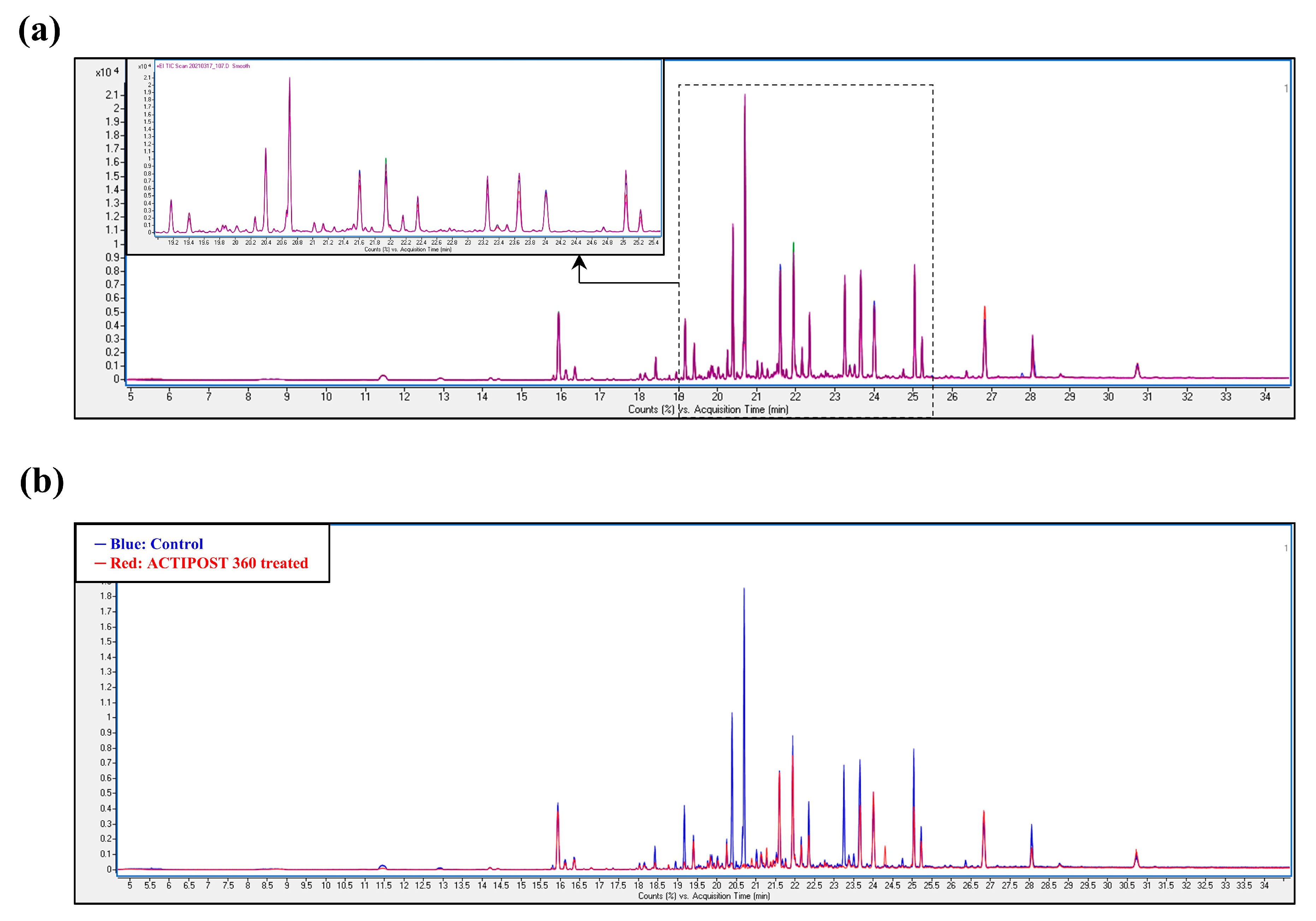
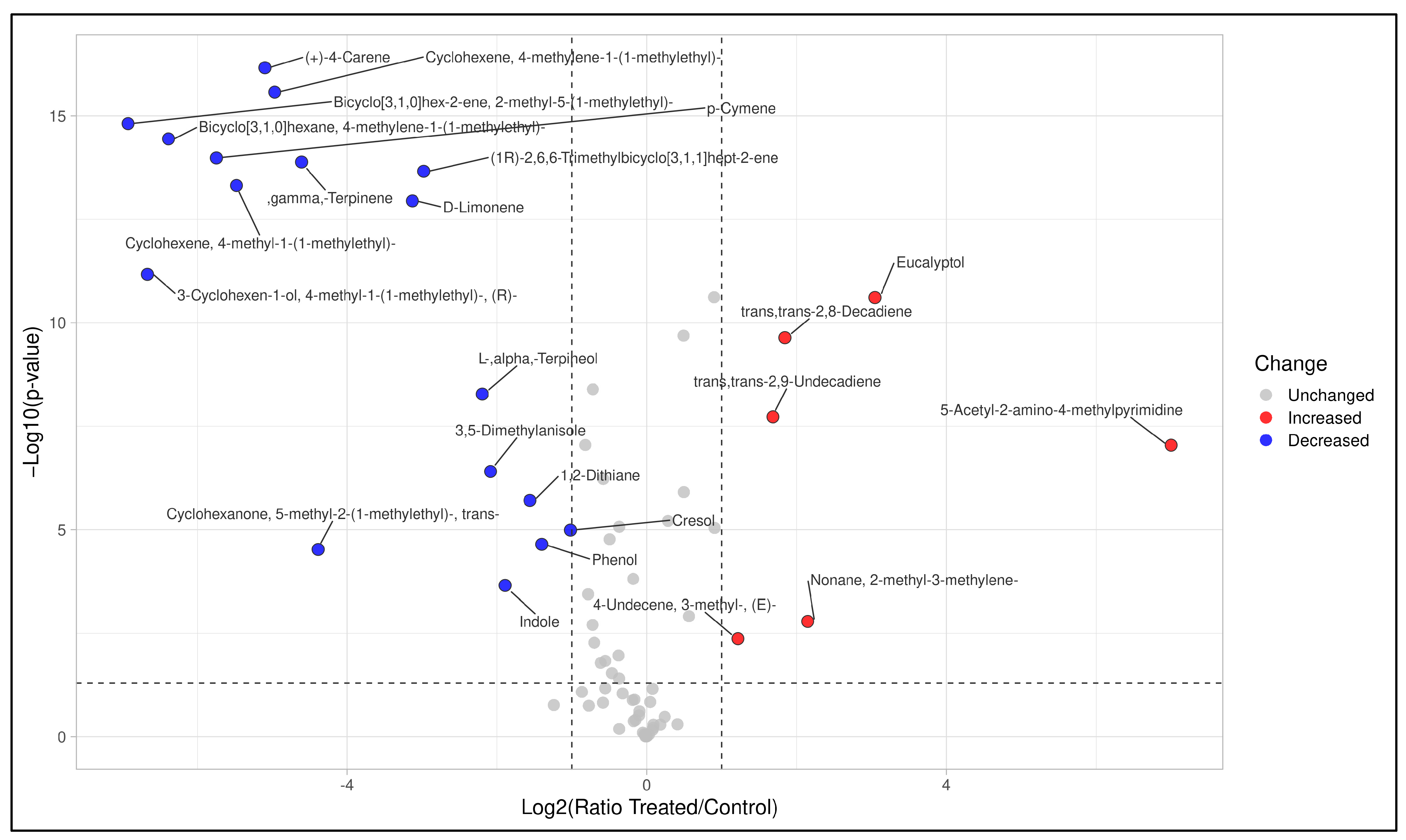
3.2. Field Test: VOC Profiling in the Treated and Untreated Dairy Slurry
3.3. The Interest of HS-SPME-GC-MS for in Farm Effluent Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ch | Chicken droppings |
| Ch + St | Mix of chicken droppings and straw |
| Du | Cattle dung |
| Du + Sl | Mix of cattle dung and slurry |
| Du + Sl + St | Mix of cattle dung, slurry, and straw |
| DVB/CAR/PDMS | Divinylbenzene/carboxen/polydimethylsiloxane |
| EI | Electron impact ionization |
| GC-FID | Gas chromatography-flame ionization detector |
| GC-MS | Gas chromatography-mass spectrometry |
| GC-MS-O | Gas chromatography-mass spectrometry olfactometry |
| HS-SPME | headspace solid-phase microextraction |
| MIMS | Membrane inlet mass spectrometry |
| PTR-Qi-TOF-MS | Proton transfer reaction–quadrupole ion guide–time of flight–mass spectrometry |
| PTR-MS | Proton transfer reaction-mass spectrometry |
| Sl | Cattle slurry |
| SPME | Solid phase microextraction |
| St | Straw |
| TD | Thermal desorption |
| TD-GC-MS | Thermal desorption-gas chromatography-mass spectrometry |
| VOCs | Volatile organic compounds |
References
- United States Environmental Protection Agency, Overview. Sources of Greenhouse Gas Emissions. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 15 September 2022).
- Nie, E.; Zheng, G.; Ma, C. Characterization of Odorous Pollution and Health Risk Assessment of Volatile Organic Compound Emissions in Swine Facilities. Atmos. Environ. 2020, 223, 117233. [Google Scholar] [CrossRef]
- Toro, M.V.; Cremades, L.V.; Calbó, J. Relationship between VOC and NOx Emissions and Chemical Production of Tropospheric Ozone in the Aburrá Valley (Colombia). Chemosphere 2006, 65, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, P.J.; Atkinson, R. Kinetics, Products, and Mechanisms of Secondary Organic Aerosol Formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Koziel, J.A.; Banik, C.; Ma, H.; Lee, M.; Wi, J.; Meiirkhanuly, Z.; O’Brien, S.C.; Li, P.; Andersen, D.S.; et al. Mitigation of Odor, NH3, H2S, GHG, and VOC Emissions With Current Products for Use in Deep-Pit Swine Manure Storage Structures. Front. Environ. Sci. 2020, 8, 613646. [Google Scholar] [CrossRef]
- Blazy, V.; de Guardia, A.; Benoist, J.C.; Daumoin, M.; Guiziou, F.; Lemasle, M.; Wolbert, D.; Barrington, S. Correlation of Chemical Composition and Odor Concentration for Emissions from Pig Slaughterhouse Sludge Composting and Storage. Chem. Eng. J. 2015, 276, 398–409. [Google Scholar] [CrossRef]
- Wattiaux, M.A.; Uddin, M.E.; Letelier, P.; Jackson, R.D.; Larson, R.A. Invited Review: Emission and Mitigation of Greenhouse Gases from Dairy Farms: The Cow, the Manure, and the Field. Appl. Anim. Sci. 2019, 35, 238–254. [Google Scholar] [CrossRef]
- Dunlop, M.W.; Blackall, P.J.; Stuetz, R.M. Odour Emissions from Poultry Litter—A Review Litter Properties, Odour Formation and Odorant Emissions from Porous Materials. J. Environ. Manag. 2016, 177, 306–319. [Google Scholar] [CrossRef]
- McIlroy, J.P.; McGeough, K.L.; Laughlin, R.J.; Carolan, R. Abatement of Ammonia Emissions from Dairy Cow House Concrete Floor Surfaces through Additive Application. Biosyst. Eng. 2019, 188, 320–330. [Google Scholar] [CrossRef]
- Nahm, K.H. Environmental Effects of Chemical Additives Used in Poultry Litter and Swine Manure. Crit. Rev. Environ. Sci. Technol. 2005, 35, 487–513. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Han, M.-F.; Jia, T.-P.; Hu, X.-R.; Zhu, H.-Q.; Tong, Z.; Lin, Y.-T.; Wang, C.; Liu, D.-Z.; Peng, Y.-Z.; et al. Emissions, Measurement, and Control of Odor in Livestock Farms: A Review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef]
- Klenbusch, M.R. Measurement of Gaseous Emission Rates from Land Surfaces Using an Emission Isolation Flux Chamber: User’s Guide; Radian Corp.: Austin, TX, USA, 1986. [Google Scholar]
- Zhou, Z.; Shen, G.; Xu, C.; Song, M.; Chen, X.; Wang, Z.; Fu, K.; Qian, X. Investigation into emission characteristics of odor pollutants in typical livestock and poultry farms in Shanghai. Acta Agric. Zhejiangensis 2019, 31, 790–797. [Google Scholar] [CrossRef]
- Kammer, J.; Décuq, C.; Baisnée, D.; Ciuraru, R.; Lafouge, F.; Buysse, P.; Bsaibes, S.; Henderson, B.; Cristescu, S.M.; Benabdallah, R.; et al. Characterization of Particulate and Gaseous Pollutants from a French Dairy and Sheep Farm. Sci. Total Environ. 2020, 712, 135598. [Google Scholar] [CrossRef] [PubMed]
- Laor, Y.; Koziel, J.A.; Cai, L.; Ravid, U. Chemical-Sensory Characterization of Dairy Manure Odor Using Headspace Solid-Phase Microextraction and Multidimensional Gas Chromatography Mass Spectrometry-Olfactometry. J. Air Waste Manag. Assoc. 2008, 58, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Hough, R.; Archer, D.; Probert, C. A Comparison of Sample Preparation Methods for Extracting Volatile Organic Compounds (VOCs) from Equine Faeces Using HS-SPME. Metabolomics 2018, 14, 19. [Google Scholar] [CrossRef]
- Kaikiti, K.; Stylianou, M.; Agapiou, A. Use of Biochar for the Sorption of Volatile Organic Compounds (VOCs) Emitted from Cattle Manure. Environ. Sci. Pollut. Res. Int. 2021, 28, 59141–59149. [Google Scholar] [CrossRef]
- Begnaud, F.; Pérès, C.; Berdagué, J.-L. Characterization of Volatile Effluents of Livestock Buildings by Solid-Phase Microextraction. Int. J. Environ. Anal. Chem. 2003, 83, 837–849. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Robarge, W.P.; Xiao, C.; Heber, A.J. Volatile Organic Compounds at Swine Facilities: A Critical Review. Chemosphere 2012, 89, 769–788. [Google Scholar] [CrossRef]
- Feilberg, A.; Liu, D.; Adamsen, A.P.S.; Hansen, M.J.; Jonassen, K.E.N. Odorant Emissions from Intensive Pig Production Measured by Online Proton-Transfer-Reaction Mass Spectrometry. Environ. Sci. Technol. 2010, 44, 5894–5900. [Google Scholar] [CrossRef]
- Yuan, B.; Coggon, M.M.; Koss, A.R.; Warneke, C.; Eilerman, S.; Peischl, J.; Aikin, K.C.; Ryerson, T.B.; de Gouw, J.A. Emissions of Volatile Organic Compounds (VOCs) from Concentrated Animal Feeding Operations (CAFOs): Chemical Compositions and Separation of Sources. Atmos. Chem. Phys. 2017, 17, 4945–4956. [Google Scholar] [CrossRef]
- Feilberg, A.; Adamsen, A.P.S.; Lindholst, S.; Lyngbye, M.; Schäfer, A. Evaluation of Biological Air Filters for Livestock Ventilation Air by Membrane Inlet Mass Spectrometry. J. Environ. Qual. 2010, 39, 1085–1096. [Google Scholar] [CrossRef]
- Theodoridis, G.; Koster, E.H.M.; de Jong, G.J. Solid-Phase Microextraction for the Analysis of Biological Samples. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 49–82. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.; Lee, S.-R.; Cho, S.; Ro, K.S.; Spiehs, M.; Woodbury, B.; Silva, P.J.; Han, D.-W.; Choi, H.; Kim, K.-Y.; et al. Efficacy of Different Biochars in Removing Odorous Volatile Organic Compounds (VOCs) Emitted from Swine Manure. ACS Sustain. Chem. Eng. 2018, 6, 14239–14247. [Google Scholar] [CrossRef]
- Laor, Y.; Shabtay, A.; Ravid, U.; Baybikov, R.; Eitam, H. Changes in VOCs Emissions from Fecal Manure throughout the Life Cycle of Beef Cattle. In Proceedings of the 2007 ASABE Annual International Meeting, Minneapolis, Minnesota, 17–20 June 2007; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2007. [Google Scholar]
- Ciganek, M.; Neca, J. Chemical Characterization of Volatile Organic Compounds on Animal Farms. Vet. Med. 2008, 53, 641–651. [Google Scholar] [CrossRef]
- Zhu, F.; Pan, Z.; Hong, C.; Wang, W.; Chen, X.; Xue, Z.; Yao, Y. Analysis of Volatile Organic Compounds in Compost Samples: A Potential Tool to Determine Appropriate Composting Time. Waste Manag. 2016, 58, 98–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Zheng, Y.; Chen, Y.; Yin, F.; Zhang, W.; Dong, H.; Xin, H. Characterization of Volatile Organic Compound (VOC) Emissions from Swine Manure Biogas Digestate Storage. Atmosphere 2019, 10, 411. [Google Scholar] [CrossRef]
- Scheftelowitz, M.; Thrän, D. Unlocking the Energy Potential of Manure—An Assessment of the Biogas Production Potential at the Farm Level in Germany. Agriculture 2016, 6, 20. [Google Scholar] [CrossRef]
- Bhattacharya, A.N.; Taylor, J.C. Recycling Animal Waste as a Feedstuff: A Review. J. Anim. Sci. 1975, 41, 1438–1457. [Google Scholar] [CrossRef]
- van der Hoeven-Hangoor, E.; Rademaker, C.J.; Paton, N.D.; Verstegen, M.W.A.; Hendriks, W.H. Evaluation of Free Water and Water Activity Measurements as Functional Alternatives to Total Moisture Content in Broiler Excreta and Litter Samples. Poult. Sci. 2014, 93, 1782–1792. [Google Scholar] [CrossRef]
- Dunlop, M.W.; Blackall, P.J.; Stuetz, R.M. Water Addition, Evaporation and Water Holding Capacity of Poultry Litter. Sci. Total Environ. 2015, 538, 979–985. [Google Scholar] [CrossRef]
- Miles, D.M.; Rowe, D.E.; Cathcart, T.C. Litter Ammonia Generation: Moisture Content and Organic versus Inorganic Bedding Materials1. Poult. Sci. 2011, 90, 1162–1169. [Google Scholar] [CrossRef]
- Miles, D.M.; Rowe, D.E.; Owens, P.R. Winter Broiler Litter Gases and Nitrogen Compounds: Temporal and Spatial Trends. Atmos. Environ. 2008, 42, 3351–3363. [Google Scholar] [CrossRef]
- Morse, D.; Nordstedt, R.A.; Head, H.H.; Van Horn, H.H. Production and Characteristics of Manure from Lactating Dairy Cows in Florida. Trans. ASAE 1994, 37, 275–279. [Google Scholar] [CrossRef]
- Pereira, J.; Fangueiro, D.; Misselbrook, T.H.; Chadwick, D.R.; Coutinho, J.; Trindade, H. Ammonia and Greenhouse Gas Emissions from Slatted and Solid Floors in Dairy Cattle Houses: A Scale Model Study. Biosyst. Eng. 2011, 109, 148–157. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR Is a Web App for Creating, Exploring, Labeling and Sharing Volcano Plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Meiirkhanuly, Z.; Koziel, J.A.; Chen, B.; Białowiec, A.; Lee, M.; Wi, J.; Banik, C.; Brown, R.C.; Bakshi, S. Mitigation of Gaseous Emissions from Swine Manure with the Surficial Application of Biochars. Atmosphere 2020, 11, 1179. [Google Scholar] [CrossRef]
- Trabue, S.; Scoggin, K.; Tyndall, J.; Sauer, T.; Hernandez-Ramirez, G.; Pfeiffer, R.; Hatfield, J. Odorous Compounds Sources and Transport from a Swine Deep-Pit Finishing Operation: A Case Study. J. Environ. Manag. 2019, 233, 12–23. [Google Scholar] [CrossRef]
- Hernandez, G.; Trabue, S.; Sauer, T.; Pfeiffer, R.; Tyndall, J. Odor Mitigation with Tree Buffers: Swine Production Case Study. Agric. Ecosyst. Environ. 2012, 149, 154–163. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Sánchez-García, M.; Alburquerque, J.A.; Cayuela, M.L. Biochar Reduces Volatile Organic Compounds Generated during Chicken Manure Composting. Bioresour. Technol. 2019, 288, 121584. [Google Scholar] [CrossRef]
- Beck, J.; Heutelbeck, A.; Dunkelberg, H. Volatile Organic Compounds in Dwelling Houses and Stables of Dairy and Cattle Farms in Northern Germany. Sci. Total Environ. 2007, 372, 440–454. [Google Scholar] [CrossRef]
- Hafner, S.D.; Howard, C.; Muck, R.E.; Franco, R.B.; Montes, F.; Green, P.G.; Mitloehner, F.; Trabue, S.L.; Rotz, C.A. Emission of Volatile Organic Compounds from Silage: Compounds, Sources, and Implications. Atmos. Environ. 2013, 77, 827–839. [Google Scholar] [CrossRef]
- Mackie, R.I.; Stroot, P.G.; Varel, V.H. Biochemical Identification and Biological Origin of Key Odor Components in Livestock Waste. J. Anim. Sci. 1998, 76, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, L.J.; Scott, H.D. (Eds.) Air/Soil Exchange Coefficients. In Environmental Exposure From Chemicals; CRC Press: Boca Raton, FL, USA, 1991; ISBN 978-1-351-07178-9. [Google Scholar]
- Zhang, H.; Lindberg, S.E.; Barnett, M.O.; Vette, A.F.; Gustin, M.S. Dynamic Flux Chamber Measurement of Gaseous Mercury Emission Fluxes over Soils. Part 1: Simulation of Gaseous Mercury Emissions from Soils Using a Two-Resistance Exchange Interface Model. Atmos. Environ. 2002, 36, 835–846. [Google Scholar] [CrossRef]
- Hales, K.E.; Parker, D.B.; Cole, N.A. Potential Odorous Volatile Organic Compound Emissions from Feces and Urine from Cattle Fed Corn-Based Diets with Wet Distillers Grains and Solubles. Atmos. Environ. 2012, 60, 292–297. [Google Scholar] [CrossRef]
- Hales, K.; Parker, D.B.; Cole, N.A. Volatile Organic Compound Flux from Manure of Cattle Fed Diets Differing in Grain Processing Method and Co-Product Inclusion. Atmos. Environ. 2015, 100, 20–24. [Google Scholar] [CrossRef]
- de Gouw, J.A.; Howard, C.J.; Custer, T.G.; Baker, B.M.; Fall, R. Proton-Transfer Chemical-Ionization Mass Spectrometry Allows Real-Time Analysis of Volatile Organic Compounds Released from Cutting and Drying of Crops. Environ. Sci. Technol. 2000, 34, 2640–2648. [Google Scholar] [CrossRef]
- Zhu, J. A Review of Microbiology in Swine Manure Odor Control. Agric. Ecosyst. Environ. 2000, 78, 93–106. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joguet, N.; Jing, L.; Jamois, F.; Dumargue, P. Characterization of Volatile Organic Compounds (VOCs) from Farms Effluents: Interest of HS-SPME-GC-MS Technique for Laboratory and Field Test. Atmosphere 2023, 14, 928. https://doi.org/10.3390/atmos14060928
Joguet N, Jing L, Jamois F, Dumargue P. Characterization of Volatile Organic Compounds (VOCs) from Farms Effluents: Interest of HS-SPME-GC-MS Technique for Laboratory and Field Test. Atmosphere. 2023; 14(6):928. https://doi.org/10.3390/atmos14060928
Chicago/Turabian StyleJoguet, Nicolas, Lun Jing, Frank Jamois, and Philippe Dumargue. 2023. "Characterization of Volatile Organic Compounds (VOCs) from Farms Effluents: Interest of HS-SPME-GC-MS Technique for Laboratory and Field Test" Atmosphere 14, no. 6: 928. https://doi.org/10.3390/atmos14060928
APA StyleJoguet, N., Jing, L., Jamois, F., & Dumargue, P. (2023). Characterization of Volatile Organic Compounds (VOCs) from Farms Effluents: Interest of HS-SPME-GC-MS Technique for Laboratory and Field Test. Atmosphere, 14(6), 928. https://doi.org/10.3390/atmos14060928







