Variations in CO2 and CH4 Exchange in Response to Multiple Biophysical Factors from a Mangrove Wetland Park in Southeastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Flux and Meteorological Measurements
2.3. Flux Data Processing and Gap Filling
2.4. Flux Partitioning
3. Results and Discussion
3.1. Temporal Variations in Environmental Conditions
3.2. Seasonal and Interannual Variations in Carbon Fluxes
3.3. Biophysical Controls of Carbon Fluxes
3.4. Carbon Exchange between Natural and Managed Mangroves
| Sites | NEE | GPP | Re | CH4 | GWP100 | References |
|---|---|---|---|---|---|---|
| Natural | ||||||
| Hongkong, China * | −758 to −890 | −2741 to −2827 | 1983 to 1937 | 11.3 to 12.1 | −2812 to −2357 | [24,47] |
| Yunxiao_1, China * | −540 to −857 | −1762 to −1919 | 1238 to 1337 | – | – | [23] |
| Yunxiao_2, China * | −1076 | −2197 | 1121 | 3.1 | −3830 | [13] |
| Gaoqiao, China * | −692 to −738 | −1698 to −1890 | 1027 to 1214 | – | – | [23] |
| Sundarban, India * | −249 | −1271 | 1022 | 56.7 | 1204 | [71,72] |
| Pichavaram, India * | −345 | −2305 | 1072 | – | – | [73] |
| Quintana, Mexico * | −709 | −2473 | 1764 | – | – | [74] |
| Florida, USA * | −1170 | −2233 | 1063 | – | – | [40] |
| Hinchinbrook, Australia ** | −1621 | −3703 | 2082 | – | – | [48] |
| Missionary Bay, Australia ** | −1171 | −2940 | 1769 | – | – | [48] |
| Restored | ||||||
| Leizhou, China * (20-year-old) | −1105 | −2009 | 904 | – | – | [65] |
| Sawi Bay, Thailand ** | −1023 | −4504 | 3481 | – | -- | [48] |
| Nansha, China * (14-year-old) | −195 to 82 | −1195 to −1527 | 1143 to 1703 | 23.2 to 26.3 | 151 to 1283 | This study |
| Disturbed | ||||||
| Matang, Malaysia ** | −745 | −4153 | 3408 | – | – | [48] |
| Beihai, China * | −106 | −341 | 235 | – | – | [75] |
3.5. Present Research Gaps and Future Needs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cabrera, M.A.; Seijo, J.C.; Euan, J.; Pérez, E. Economic values of ecological services from a mangrove ecosystem. Intercoast Netw. 1998, 32, 1–2. [Google Scholar]
- Trégarot, E.; Caillaud, A.; Cornet, C.C.; Taureau, F.; Catry, T.; Cragg, S.M.; Failler, P. Mangrove ecological services at the forefront of coastal change in the French overseas territories. Sci. Total Environ. 2021, 763, 143004. [Google Scholar] [CrossRef] [PubMed]
- Alongi, D.M. Global significance of mangrove blue carbon in climate change mitigation. Sci 2020, 2, 67. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon sequestration in mangrove forests. Carbon Manag. 2012, 3, 313–322. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Erler, D.V.; Murray, R.H.; Eyre, B.D. Methane emissions partially offset “blue carbon” burial in mangroves. Sci. Adv. 2018, 4, eaao4985. [Google Scholar] [CrossRef]
- Wu, L.; Wang, L.; Shi, C.; Yin, D. Detecting mangrove photosynthesis with solar-induced chlorophyll fluorescence. Int. J. Remote Sens. 2022, 43, 1037–1053. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon cycling and storage in mangrove forests. Annu. Rev. Mar. Sci. 2014, 6, 195–219. [Google Scholar] [CrossRef]
- Williams, M.; Rastetter, E.B.; Fernandes, D.N.; Goulden, M.L.; Shaver, G.R.; Johnson, L.C. Predicting gross primary productivity in terrestrial ecosystems. Ecol. Appl. 1997, 7, 882–894. [Google Scholar] [CrossRef]
- Chen, H.; Xu, X.; Fang, C.; Li, B.; Nie, M. Differences in the temperature dependence of wetland CO2 and CH4 emissions vary with water table depth. Nat. Clim. Chang. 2021, 11, 766–771. [Google Scholar] [CrossRef]
- Sanders, C.J.; Maher, D.T.; Tait, D.R.; Williams, D.; Holloway, C.; Sippo, J.Z.; Santos, I.R. Are global mangrove carbon stocks driven by rainfall? J. Geophys. Res. Biogeosci. 2016, 121, 2600–2609. [Google Scholar] [CrossRef]
- Monteith, J.L. Solar radiation and productivity in tropical ecosystems. J. Appl. Ecol. 1972, 9, 747–766. [Google Scholar] [CrossRef]
- Zhu, X.; Qin, Z.; Song, L. How land-sea interaction of tidal and sea breeze activity affect mangrove net ecosystem exchange? J. Geophys. Res. Atmos. 2021, 126, e2020JD034047. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, C.; Qin, Z. Drought-Induced Salinity Enhancement Weakens Mangrove Greenhouse Gas Cycling. J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006416. [Google Scholar] [CrossRef]
- Alongi, D. Impact of Global Change on Nutrient Dynamics in Mangrove Forests. Forests 2018, 9, 596. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Costa, M.D.; Atwood, T.B.; Friess, D.A.; Kelleway, J.J.; Kennedy, H.; Lovelock, C.E.; Serrano, O.; Duarte, C.M. Blue carbon as a natural climate solution. Nat. Rev. Earth Environ. 2021, 2, 826–839. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Z.; Mao, D.; Huang, C.; Lu, C. Spatial-temporal changes of China’s mangrove forests over the past 50 years: An analysis towards the Sustainable Development Goals (SDGs). Chin. Sci. Bull. 2021, 66, 3886–3901. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Z.; Zhang, Y.; Mao, D.; Wang, C. Monitoring loss and recovery of mangrove forests during 42 years: The achievements of mangrove conservation in China. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 535–545. [Google Scholar] [CrossRef]
- Friess, D.; Krauss, K.; Taillardat, P.; Adame, F.; Yando, E.; Cameron, C.; Sasmito, S.; Sillanpää, M. Mangrove Blue Carbon in the Face of Deforestation, Climate Change, and Restoration. Annu. Plant Rev. 2020, 3, 427–456. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Taillardat, P.; Clendenning, J.N.; Cameron, C.; Friess, D.A.; Murdiyarso, D.; Hutley, L.B. Effect of land-use and land-cover change on mangrove blue carbon: A systematic review. Glob. Chang. Biol. 2019, 25, 4291–4302. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, C.; Li, T.; Zhang, C.; Fan, X.; Zhang, Q.; Zhang, Q.; Chen, X.; Zou, X.; Shen, C.; et al. Net CO2 and CH4 emissions from restored mangrove wetland: New insights based on a case study in estuary of the Pearl River, China. Sci. Total Environ. 2022, 811, 151619. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.C.; Tam, N.F.; Ye, Y. Summer fluxes of atmospheric greenhouse gases N2O, CH4 and CO2 from mangrove soil in South China. Sci. Total Environ. 2010, 408, 2761–2767. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, W.; Yan, G.; Yang, S.; Lin, G. Typhoons exert significant but differential impacts on net ecosystem carbon exchange of subtropical mangrove forests in China. Biogeosciences 2014, 11, 5323–5333. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Valach, A.; Shortt, R.; Kasak, K.; Rey-Sanchez, C.; Hemes, K.S.; Baldocchi, D.; Lai, D.Y.F. Methane emissions reduce the radiative cooling effect of a subtropical estuarine mangrove wetland by half. Glob. Chang. Biol. 2020, 26, 4998–5016. [Google Scholar] [CrossRef]
- Liu, J.; Valach, A.; Baldocchi, D.; Lai, D.Y.F. Biophysical controls of ecosystem-scale Methane fluxes from a subtropical estuarine mangrove: Multiscale, nonlinearity, asynchrony and causality. Glob. Biogeochem. Cycles 2022, 36, e2021GB007179. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Yan, B. Blue carbon sink function and carbon neutrality potential of mangroves. Sci. Total Environ. 2022, 822, 153438. [Google Scholar] [CrossRef]
- Qiu, P.; Xu, S.; Fu, Y.; Xie, G. A primary study on plant community of Wanqingsha Semi-constructed wetland in Nansha District of Guangzhou City. Ecol. Sci. 2011, 30, 43–50. [Google Scholar]
- Savitzky, A.; Golay, M.J. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Kljun, N.; Calanca, P.; Rotach, M.; Schmid, H.P. A simple two-dimensional parameterisation for Flux Footprint Prediction (FFP). Geosci. Model Dev. 2015, 8, 3695–3713. [Google Scholar] [CrossRef]
- Vickers, D.; Mahrt, L. Quality control and flux sampling problems for tower and aircraft data. J. Atmos. Ocean Technol. 1997, 14, 512–526. [Google Scholar] [CrossRef]
- Nakai, T.; Shimoyama, K. Ultrasonic anemometer angle of attack errors under turbulent conditions. Agric. For. Meteorol. 2012, 162, 14–26. [Google Scholar] [CrossRef]
- Wilczak, J.M.; Oncley, S.P.; Stage, S.A. Sonic anemometer tilt correction algorithms. Bound. Layer Meteorol. 2001, 99, 127–150. [Google Scholar] [CrossRef]
- Fan, H.; Liang, S. Status quo of mangrove conservation and management in China. In Mangrove Research and Management in China; He, H.F.H., Ed.; Science Press: Beijing, China, 1995; pp. 173–202. [Google Scholar]
- Schotanus, P.; Nieuwstadt, F.; De Bruin, H. Temperature measurement with a sonic anemometer and its application to heat and moisture fluxes. Bound. Layer Meteorol. 1983, 26, 81–93. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapour transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Goulden, M.L.; McMillan, A.; Winston, G.; Rocha, A.; Manies, K.; Harden, J.W.; Bond-Lamberty, B. Patterns of NPP, GPP, respiration, and NEP during boreal forest succession. Glob. Chang. Biol. 2011, 17, 855–871. [Google Scholar] [CrossRef]
- Mauder, M.; Foken, T. Documentation and Instruction Manual of the Eddy-Covariance Software Package TK3 (Update). In Micrometeorology, Technical Report Arbeitsergebnisse Nr; University of Bayreuth: Bayreuth, Germany, 2015; p. 62. [Google Scholar]
- Wutzler, T.; Lucas-Moffat, A.; Migliavacca, M.; Knauer, J.; Sickel, K.; Šigut, L.; Menzer, O.; Reichstein, M. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 2018, 15, 5015–5030. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Barr, J.G.; Engel, V.; Fuentes, J.D.; Zieman, J.C.; O’Halloran, T.L.; Smith, T.J., III; Anderson, G.H. Controls on mangrove forest-atmosphere carbon dioxide exchanges in western Everglades National Park. J. Geophys. Res. Biogeosci. 2010, 115, G02020. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Paramanik, S.; Varghese, R.; Behera, M.D.; Barnwal, S.; Behera, S.K.; Bhattyacharya, B.K. Photosynthetic Variables Estimation in a Mangrove Forest. In Advances in Remote Sensing for Forest Monitoring; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 126–149. [Google Scholar]
- Delgado, R.C.; Pereira, M.G.; Teodoro, P.E.; dos Santos, G.L.; de Carvalho, D.C.; Magistrali, I.C.; Vilanova, R.S. Seasonality of gross primary production in the Atlantic Forest of Brazil. Glob. Ecol. Conserv. 2018, 14, e00392. [Google Scholar] [CrossRef]
- Feagin, R.; Forbrich, I.; Huff, T.; Barr, J.; Ruiz-Plancarte, J.; Fuentes, J.; Najjar, R.; Vargas, R.; Vázquez-Lule, A.; Windham-Myers, L. Tidal Wetland Gross Primary Production across the Continental United States: 2000–2019. Glob. Biogeochem. Cycles 2020, 34, e2019GB006349. [Google Scholar] [CrossRef]
- Suwa, R.; Khan, M.N.I.; Hagihara, A. Canopy photosynthesis, canopy respiration and surplus production in a subtropical mangrove Kandelia candel forest, Okinawa Island, Japan. Mar. Ecol. Prog. Ser. 2006, 320, 131–139. [Google Scholar] [CrossRef]
- Leopold, A.; Marchand, C.; Renchon, A.; Deborde, J.; Quiniou, T.; Allenbach, M. Net ecosystem CO2 exchange in the “Coeur de Voh” mangrove, New Caledonia: Effects of water stress on mangrove productivity in a semi-arid climate. Agric. For. Meteorol. 2016, 223, 217–232. [Google Scholar] [CrossRef]
- Liu, J.; Lai, D.Y.F. Subtropical mangrove wetland is a stronger carbon dioxide sink in the dry than wet seasons. Agric. For. Meteorol. 2019, 278, 107644. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon payments for mangrove conservation: Ecosystem constraints and uncertainties of sequestration potential. Environ. Sci. Policy 2011, 14, 462–470. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis. J. Mar. Sci. Eng. 2020, 8, 767. [Google Scholar] [CrossRef]
- Wang, Q.; He, N.; Yu, G.; Gao, Y.; Wen, X.; Wang, R.; Koerner, S.E.; Yu, Q. Soil microbial respiration rate and temperature sensitivity along a north-south forest transect in eastern China: Patterns and influencing factors. J. Geophys. Res. Biogeosci. 2016, 121, 399–410. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A.; Luo, Y. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Aspinwall, M.J.; Vårhammar, A.; Blackman, C.J.; Tjoelker, M.G.; Ahrens, C.; Byrne, M.; Tissue, D.T.; Rymer, P.D. Adaptation and acclimation both influence photosynthetic and respiratory temperature responses in Corymbia calophylla. Tree Physiol. 2017, 37, 1095–1112. [Google Scholar] [CrossRef]
- Sturchio, M.A.; Chieppa, J.; Chapman, S.K.; Canas, G.; Aspinwall, M.J. Temperature acclimation of leaf respiration differs between marsh and mangrove vegetation in a coastal wetland ecotone. Glob. Chang. Biol. 2022, 28, 612–629. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Xu, X.; Chen, H.; Zhu, T.; Xu, J.; Xu, X.; Li, J.; Liang, C.; Li, B. Temperature fluctuation promotes the thermal adaptation of soil microbial respiration. Nat. Ecol. Evol. 2023, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, Y.; Zhang, W.; Song, C. CH4MODwetland: A biogeophysical model for simulating methane emissions from natural wetlands. Ecol. Model. 2010, 221, 666–680. [Google Scholar] [CrossRef]
- Daulat, W.E.; Clymo, R.S. Effects of temperature and watertable on the efflux of methane from peatland surface cores. Atmos. Environ. 1998, 32, 3207–3218. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, T.; Li, B.; Fang, C.; Nie, M. The thermal response of soil microbial methanogenesis decreases in magnitude with changing temperature. Nat. Commun. 2020, 11, 5733. [Google Scholar] [CrossRef] [PubMed]
- Kip, N.; van Winden, J.F.; Pan, Y.; Bodrossy, L.; Reichart, G.-J.; Smolders, A.J.; Jetten, M.S.; Damsté, J.S.S.; den Camp, H.J.O. Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nat. Geosci. 2010, 3, 617–621. [Google Scholar] [CrossRef]
- Mitsch, W. Wetlands and climate change. Natl. Wetl. Newsl. 2016, 38, 5–11. [Google Scholar]
- Poffenbarger, H.J.; Needelman, B.A.; Megonigal, J.P. Salinity Influence on Methane Emissions from Tidal Marshes. Wetlands 2011, 31, 831–842. [Google Scholar] [CrossRef]
- DeLaune, R.D.; Smith, C.J.; Patrick, W.H., Jr. Methane release from Gulf coast wetlands. Tellus B Chem. Phys. Meteorol. 1983, 35, 8–15. [Google Scholar] [CrossRef]
- Bartlett, K.B.; Bartlett, D.S.; Harriss, R.C.; Sebacher, D.I. Methane emissions along a salt marsh salinity gradient. Biogeochemistry 1987, 4, 183–202. [Google Scholar] [CrossRef]
- O’Connor, J.J.; Fest, B.J.; Sievers, M.; Swearer, S.E. Impacts of land management practices on blue carbon stocks and greenhouse gas fluxes in coastal ecosystems—A meta-analysis. Glob. Chang. Biol. 2020, 26, 1354–1366. [Google Scholar] [CrossRef]
- Poungparn, S.; Komiyama, A. Net ecosystem productivity studies in mangrove forests. Rev. Agric. Sci. 2013, 1, 61–64. [Google Scholar] [CrossRef]
- Cui, X.; Liang, J.; Lu, W.; Chen, H.; Liu, F.; Lin, G.; Xu, F.; Luo, Y.; Lin, G. Stronger ecosystem carbon sequestration potential of mangrove wetlands with respect to terrestrial forests in subtropical China. Agric. For. Meteorol. 2018, 249, 71–80. [Google Scholar] [CrossRef]
- Deemer, B.R.; Harrison, J.A.; Li, S.; Beaulieu, J.J.; DelSontro, T.; Barros, N.; Bezerra-Neto, J.F.; Powers, S.M.; dos Santos, M.A.; Vonk, J.A. Greenhouse Gas Emissions from Reservoir Water Surfaces: A New Global Synthesis. BioScience 2016, 66, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Chanton, J.P.; Bauer, J.E.; Glaser, P.A.; Siegel, D.I.; Kelley, C.A.; Tyler, S.C.; Romanowicz, E.H.; Lazrus, A. Radiocarbon evidence for the substrates supporting methane formation within northern Minnesota peatlands. Geochim. Cosmochim. Acta 1995, 59, 3663–3668. [Google Scholar] [CrossRef]
- Cui, M.; Ma, A.; Qi, H.; Zhuang, X.; Zhuang, G.; Zhao, G. Warmer temperature accelerates methane emissions from the Zoige wetland on the Tibetan Plateau without changing methanogenic community composition. Sci. Rep. 2015, 5, 11616. [Google Scholar] [CrossRef]
- Dunfield, P.; Dumont, R.; Moore, T.R. Methane production and consumption in temperate and subarctic peat soils: Response to temperature and pH. Soil Biol. Biochem. 1993, 25, 321–326. [Google Scholar] [CrossRef]
- Li, T.; Xie, B.; Wang, G.; Zhang, W.; Zhang, Q.; Vesala, T.; Raivonen, M. Field-scale simulation of methane emissions from coastal wetlands in China using an improved version of CH4MODwetland. Sci. Total Environ. 2016, 559, 256–267. [Google Scholar] [CrossRef]
- Jha, C.S.; Rodda, S.R.; Thumaty, K.C.; Raha, A.; Dadhwal, V. Eddy covariance based methane flux in Sundarbans mangroves, India. J. Earth Syst. Sci. 2014, 123, 1089–1096. [Google Scholar] [CrossRef]
- Biswas, H.; Mukhopadhyay, S.K.; Sen, S.; Jana, T.K. Spatial and temporal patterns of methane dynamics in the tropical mangrove dominated estuary, NE coast of Bay of Bengal, India. J. Mar. Syst. 2007, 68, 55–64. [Google Scholar] [CrossRef]
- Gnanamoorthy, P.; Selvam, V.; Chakraborty, S.; Pramit, D.; Karipot, A. Eddy covariance measurements of carbon dioxide (CO2) exchange in Pichavaram Mangrove Ecosystem, Southeast Coast of India. In Proceedings of the International Forestry and Environment Symposium, Session VI—Climate Change and Disaster Management, Trabzon, Turkey, 7—10 November 2017; p. 72. [Google Scholar]
- Alvarado Barrientos, M.S.; López Adame, H.; Lazcano Hernández, H.E.; Arellano Verdejo, J.; Hernández Arana, H.A. Ecosystem-atmosphere exchange of CO2, water, and energy in a basin mangrove of the northeastern coast of the Yucatan Peninsula. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005811. [Google Scholar] [CrossRef]
- Sun, M.; Mo, W.; Xie, M.; Chen, Y.; Pan, L. Characteristics of Net Ecosystem Carbon Exchange and Its Influence Factors over the Mangrove in Guangxi. J. Ecol. Environ. 2021, 37, 909–916, (in Chinese with abstract). [Google Scholar]
- Cameron, C.; Hutley, L.B.; Munksgaard, N.C.; Phan, S.; Aung, T.; Thinn, T.; Aye, W.M.; Lovelock, C.E. Impact of an extreme monsoon on CO2 and CH4 fluxes from mangrove soils of the Ayeyarwady Delta, Myanmar. Sci. Total Environ. 2021, 760, 143422. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liao, B.; Jiang, Z.; Xin, K.; Xiong, Y.; Guan, W. Emission of Greenhouse Gases (CH4 and CO2) into the Atmosphere from Restored Mangrove Soil in South China. J. Coastal Res. 2020, 37, 52–58. [Google Scholar] [CrossRef]
- Chuang, P.-C.; Young, M.B.; Dale, A.W.; Miller, L.G.; Herrera-Silveira, J.A.; Paytan, A. Methane fluxes from tropical coastal lagoons surrounded by mangroves, Yucatán, Mexico. J. Geophys. Res. Biogeosci. 2017, 122, 1156–1174. [Google Scholar] [CrossRef]
- Adame, M.F.; Lovelock, C.E. Carbon and nutrient exchange of mangrove forests with the coastal ocean. Hydrobiologia 2011, 663, 23–50. [Google Scholar] [CrossRef]
- Adame, M.F.; Brown, C.J.; Bejarano, M.; Herrera-Silveira, J.A.; Ezcurra, P.; Kauffman, J.B.; Birdsey, R. The undervalued contribution of mangrove protection in Mexico to carbon emission targets. Conserv. Lett. 2018, 11, e12445. [Google Scholar] [CrossRef]
- Murdiyarso, D.; Purbopuspito, J.; Kauffman, J.B.; Warren, M.W.; Sasmito, S.D.; Donato, D.C.; Manuri, S.; Krisnawati, H.; Taberima, S.; Kurnianto, S. The potential of Indonesian mangrove forests for global climate change mitigation. Nat. Clim. Chang. 2015, 5, 1089–1092. [Google Scholar] [CrossRef]
- Howard, J.; Sutton-Grier, A.; Herr, D.; Kleypas, J.; Landis, E.; McLeod, E.; Pidgeon, E.; Simpson, S. Clarifying the role of coastal and marine systems in climate mitigation. Front. Ecol. Environ. 2017, 15, 42–50. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; McKee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Qin, Z.; Deng, X.; Griscom, B.; Huang, Y.; Li, T.; Smith, P.; Yuan, W.; Zhang, W. Natural climate solutions for China: The last mile to carbon neutrality. Adv. Atmos. Sci. 2021, 38, 889–895. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, W.; Qin, Z.; Zhang, W.; Yu, Y.; Li, T.; Zhang, Q.; Wang, G.; Yu, L.; Wang, Y.; et al. The role of China’s terrestrial carbon sequestration 2010–2060 in offsetting energy-related CO2 emissions. Natl. Sci. Rev. 2022, 9, nwac057. [Google Scholar] [CrossRef] [PubMed]
- Osland, M.J.; Spivak, A.C.; Nestlerode, J.A.; Lessmann, J.M.; Almario, A.E.; Heitmuller, P.T.; Russell, M.J.; Krauss, K.W.; Alvarez, F.; Dantin, D.D. Ecosystem development after mangrove wetland creation: Plant-soil change across a 20-year chronosequence. Ecosystems 2012, 15, 848–866. [Google Scholar] [CrossRef]
- Phan, S.M.; Nguyen, H.T.T.; Nguyen, T.K.; Lovelock, C. Modelling above ground biomass accumulation of mangrove plantations in Vietnam. For. Ecol. Manag. 2019, 432, 376–386. [Google Scholar] [CrossRef]
- Schile, L.M.; Kauffman, J.B.; Crooks, S.; Fourqurean, J.W.; Glavan, J.; Megonigal, J.P. Limits on carbon sequestration in arid blue carbon ecosystems. Ecol. Appl. 2017, 27, 859–874. [Google Scholar] [CrossRef] [PubMed]

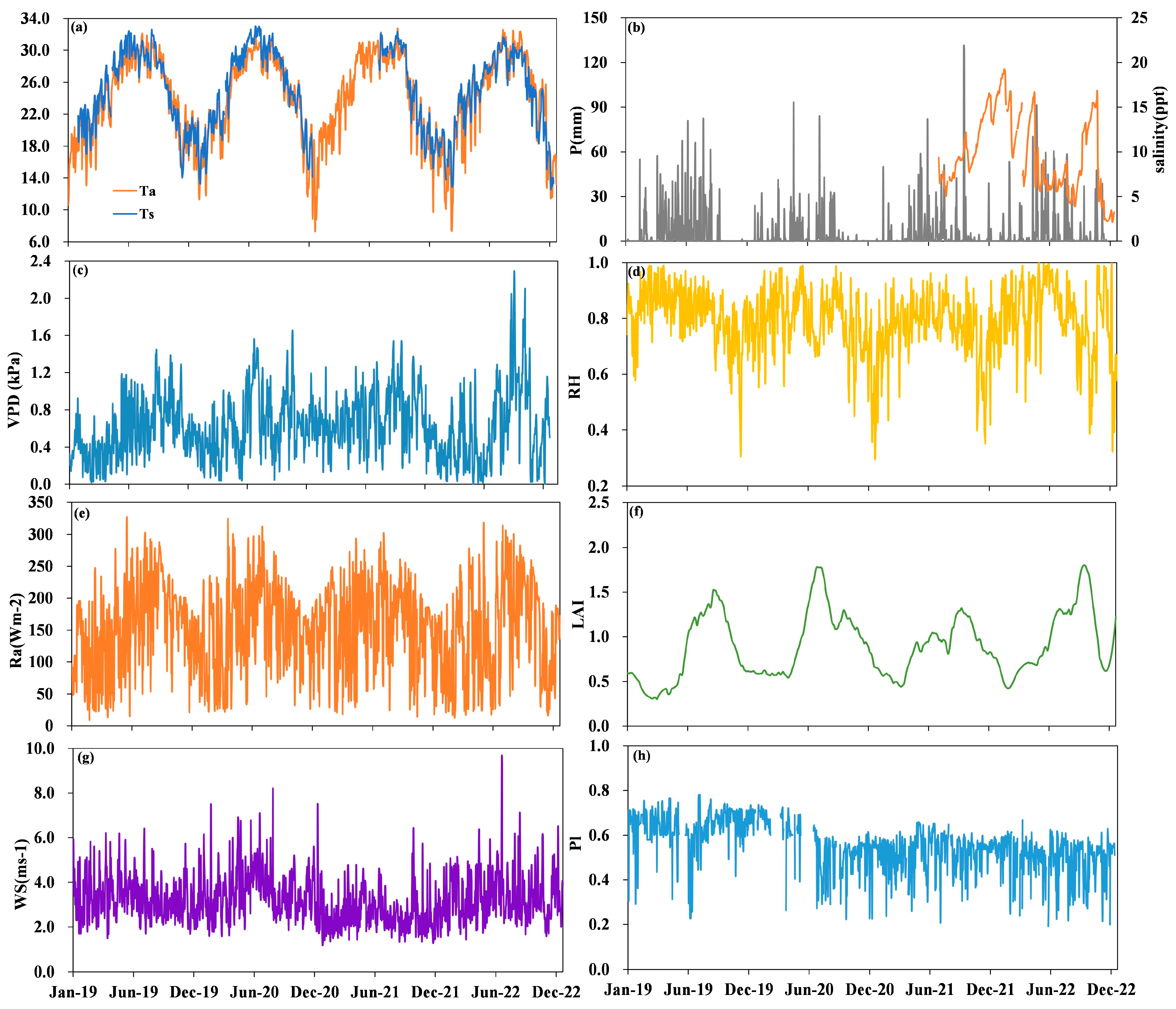
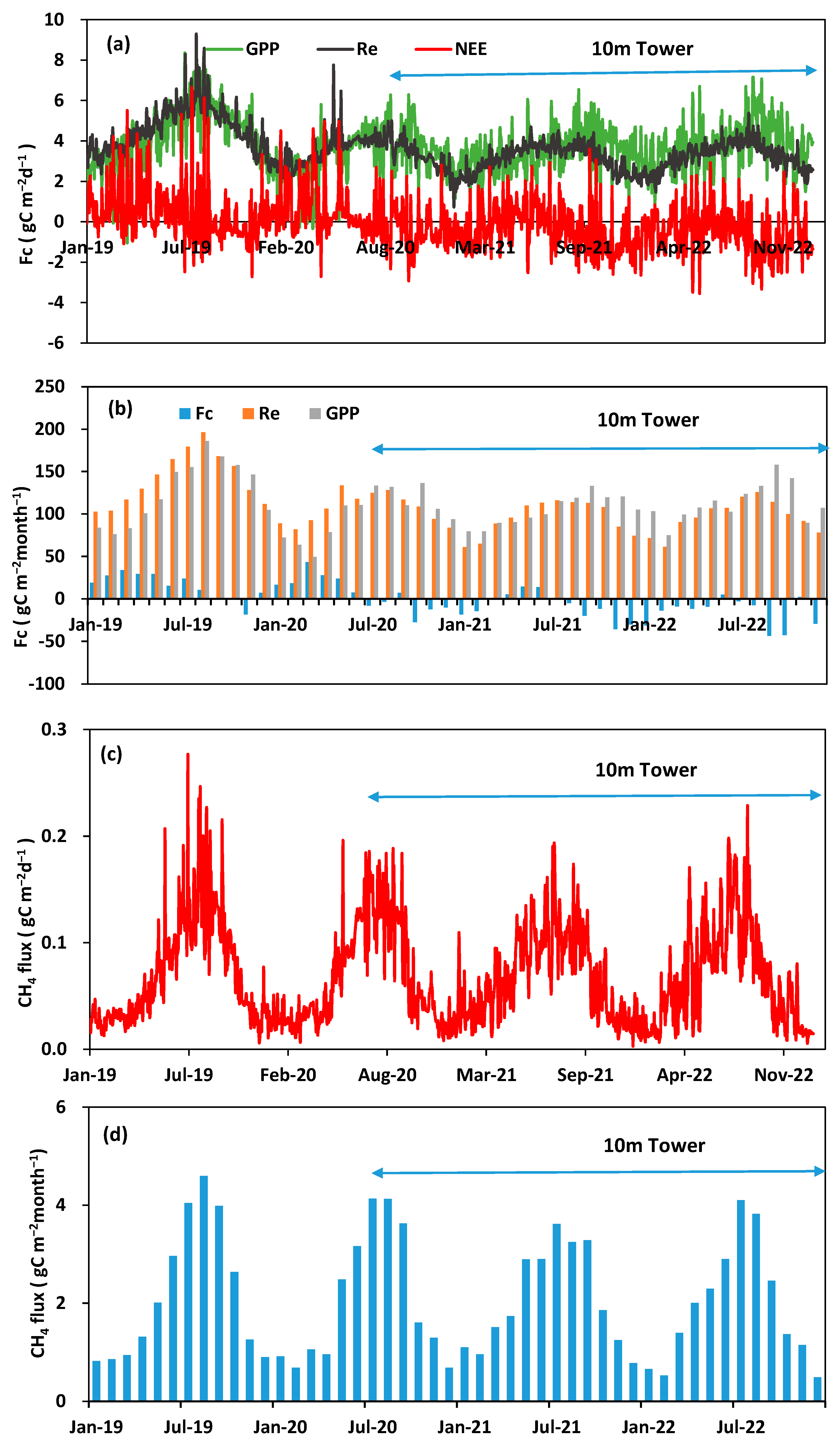
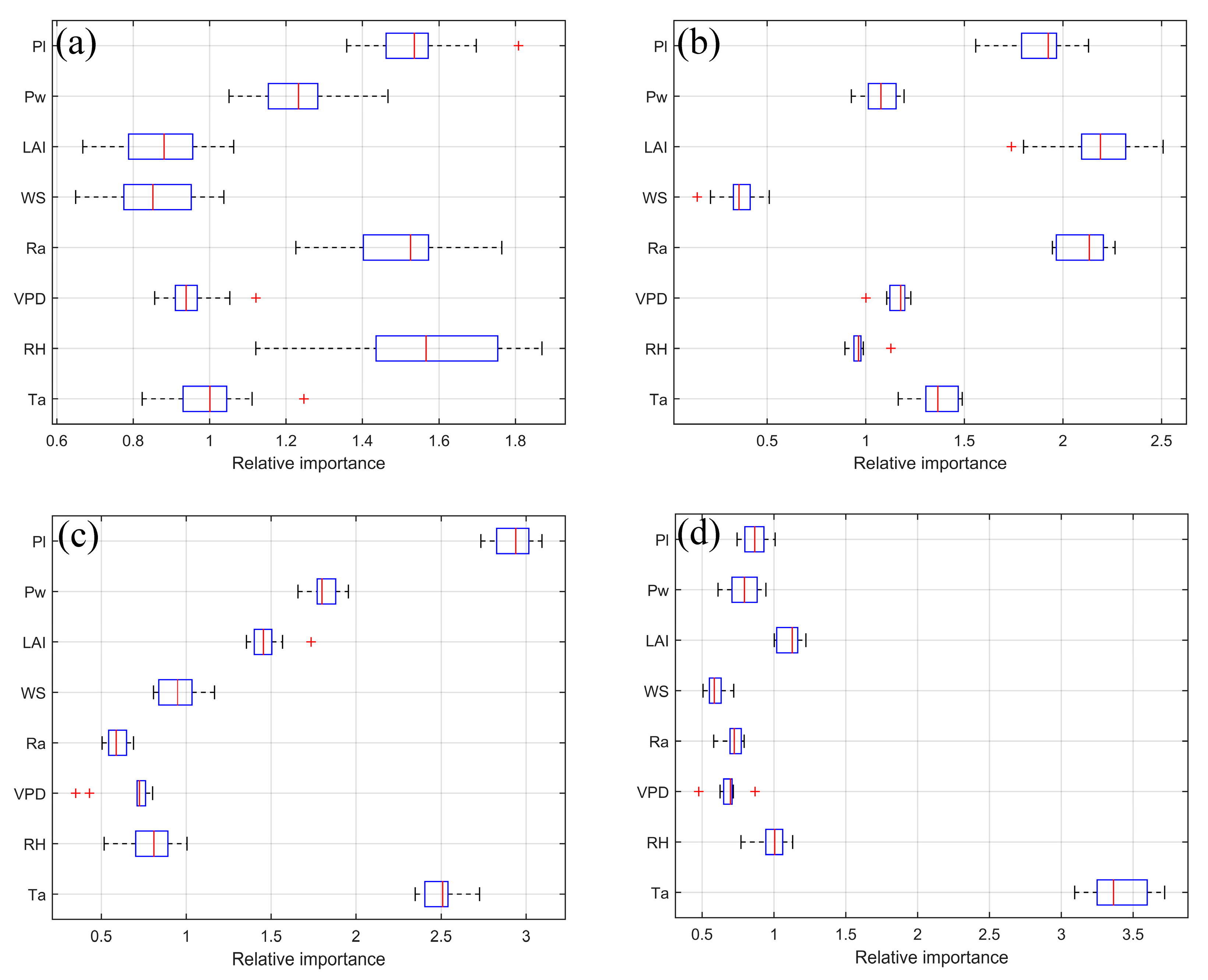

| Drivers and Fluxes | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|
| Ta (℃) * | 24.22 | 23.99 | 24.37 | 23.36 |
| P (mm) ** | 2085.70 | 1356.60 | 1594.10 | 1921.20 |
| s (ppt) * | – | – | 10.21 | 9.41 |
| VPD (kPa) * | 0.56 | 0.62 | 0.73 | 0.59 |
| RH * | 0.82 | 0.79 | 0.75 | 0.80 |
| Ra (W m−2) * | 153.04 | 151.49 | 159.05 | 143.94 |
| Ws (m s−1) * | 3.29 | 3.58 | 2.58 | 3.35 |
| LAI * | 0.79 | 0.99 | 0.86 | 0.96 |
| Pl * | 0.64 | 0.56 | 0.52 | 0.49 |
| NEE (g C m−2 a−1) ** | 175.70 | 81.92 | −102.89 | −194.53 |
| Re (g C m−2 a−1) ** | 1702.90 | 1276.42 | 1142.92 | 1161.56 |
| GPP (g C m−2 a−1) ** | −1527.20 | −1194.50 | −1245.81 | −1356.09 |
| CH4 (g C m−2 a−1) ** | 26.34 | 24.76 | 25.14 | 23.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhao, X.; Chen, X.; Xiao, C.; Fan, X.; Shen, C.; Sun, M.; Shen, Z.; Zhang, Q. Variations in CO2 and CH4 Exchange in Response to Multiple Biophysical Factors from a Mangrove Wetland Park in Southeastern China. Atmosphere 2023, 14, 805. https://doi.org/10.3390/atmos14050805
Wang C, Zhao X, Chen X, Xiao C, Fan X, Shen C, Sun M, Shen Z, Zhang Q. Variations in CO2 and CH4 Exchange in Response to Multiple Biophysical Factors from a Mangrove Wetland Park in Southeastern China. Atmosphere. 2023; 14(5):805. https://doi.org/10.3390/atmos14050805
Chicago/Turabian StyleWang, Chunlin, Xiaosong Zhao, Xianyan Chen, Chan Xiao, Xingwang Fan, Chong Shen, Ming Sun, Ziqi Shen, and Qiang Zhang. 2023. "Variations in CO2 and CH4 Exchange in Response to Multiple Biophysical Factors from a Mangrove Wetland Park in Southeastern China" Atmosphere 14, no. 5: 805. https://doi.org/10.3390/atmos14050805
APA StyleWang, C., Zhao, X., Chen, X., Xiao, C., Fan, X., Shen, C., Sun, M., Shen, Z., & Zhang, Q. (2023). Variations in CO2 and CH4 Exchange in Response to Multiple Biophysical Factors from a Mangrove Wetland Park in Southeastern China. Atmosphere, 14(5), 805. https://doi.org/10.3390/atmos14050805






