Characteristics of Airborne Pollutants in the Area of an Agricultural–Industrial Complex near a Petrochemical Industry Facility
Abstract
1. Introduction
2. Experimental
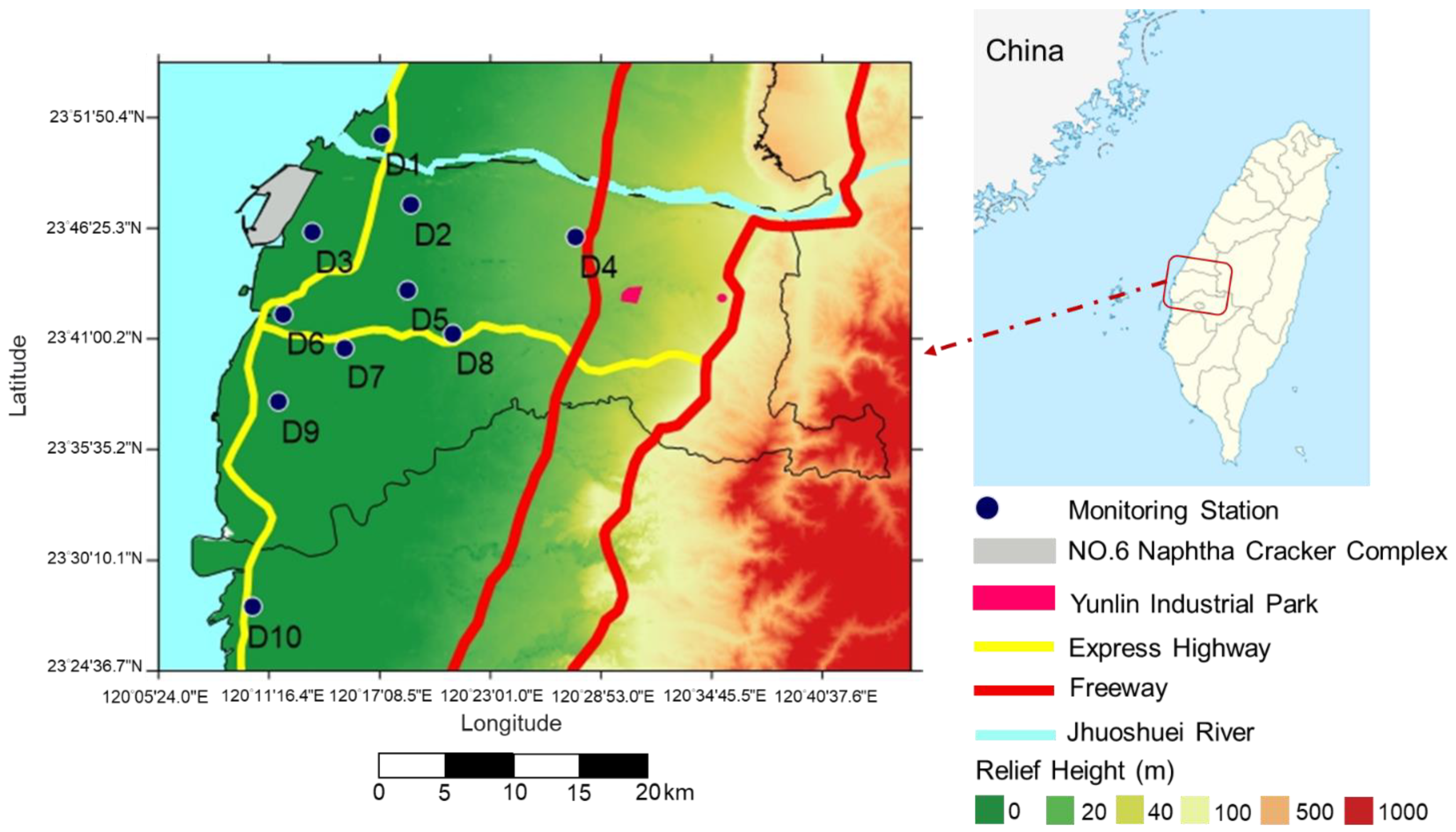
2.1. Study Area
2.2. Emission Characteristics
2.3. Ambient Air Monitoring Stations
2.4. VOC Analysis
2.5. Ozone Formation Potential (OFP) of VOC Species
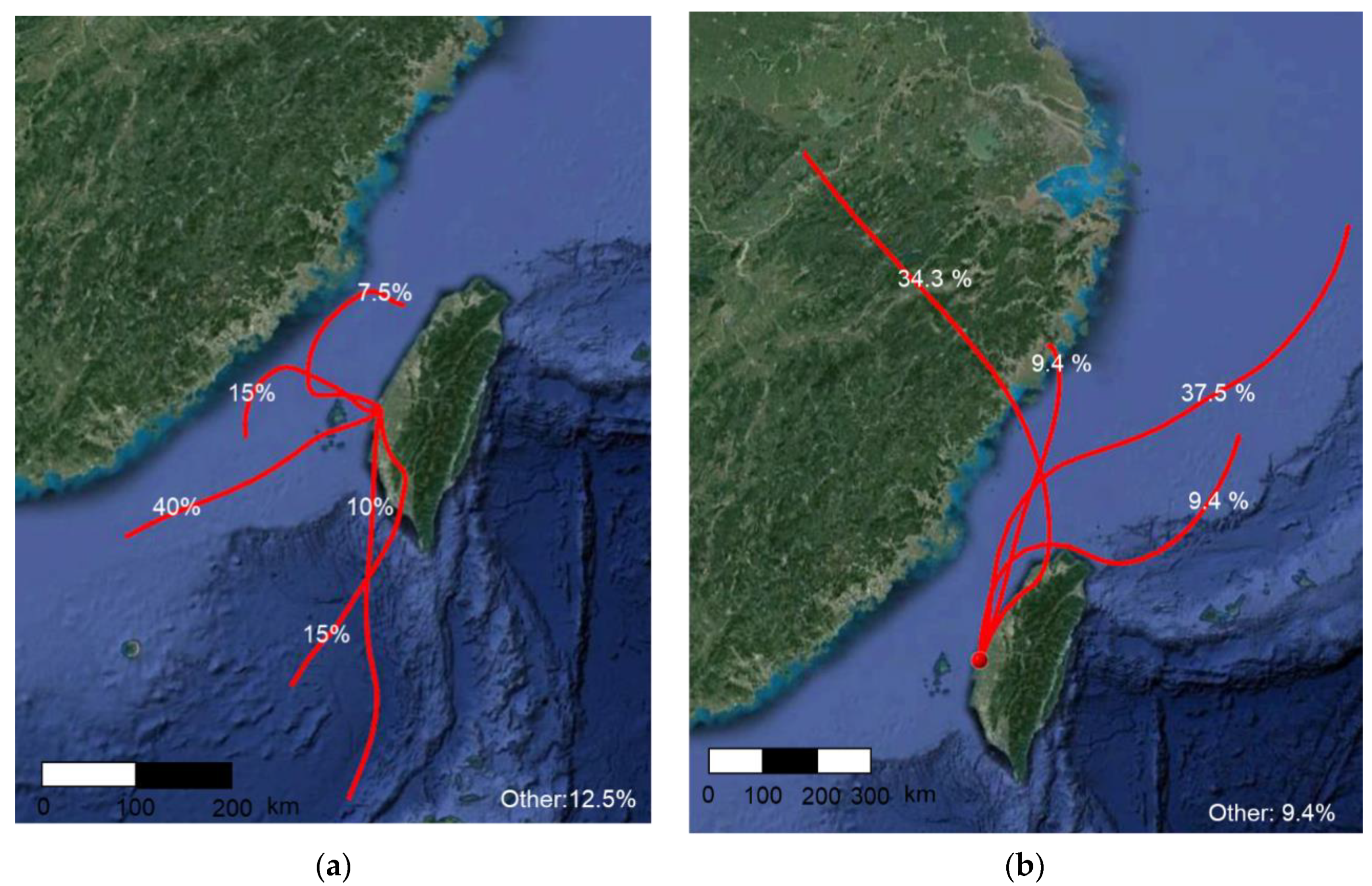
2.6. Back Trajectory Analysis
2.7. Positive Matrix Factorization (PMF)
2.8. Statistical Analysis
3. Results and Discussion
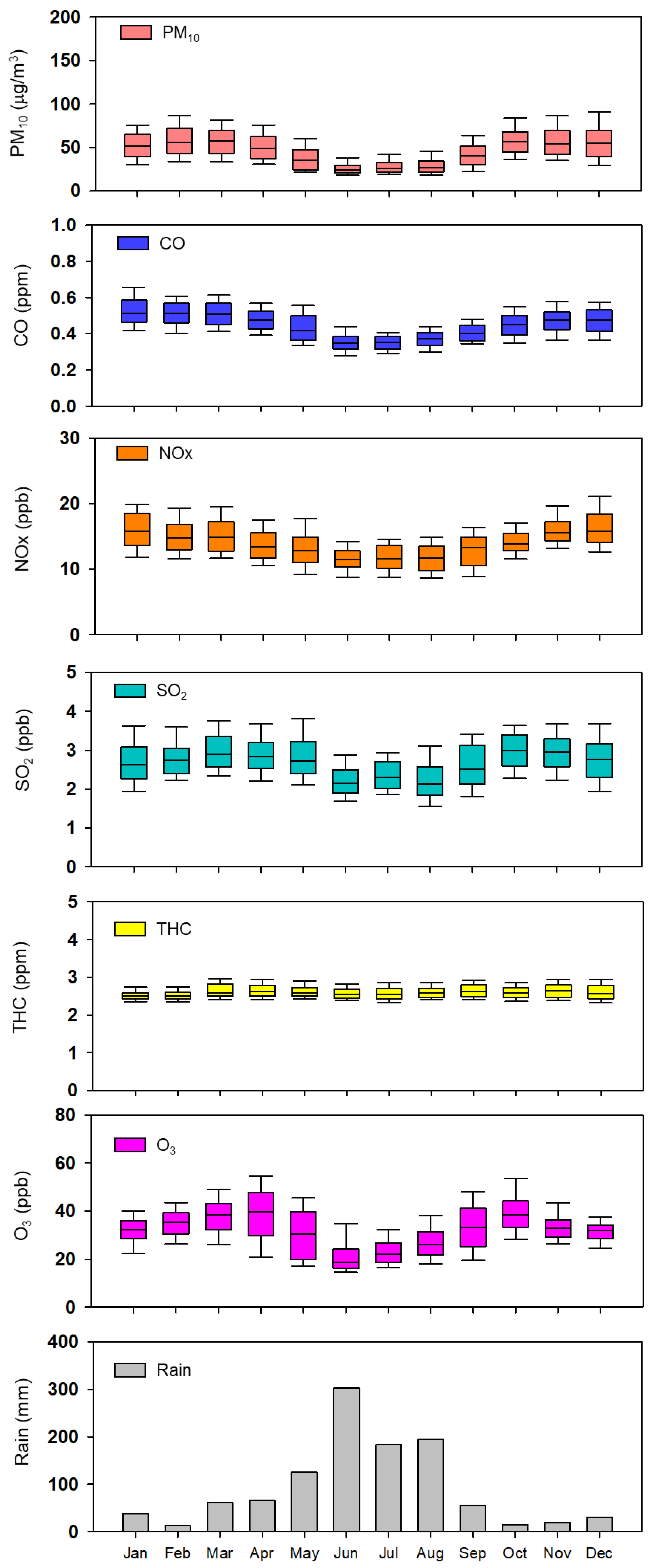
3.1. Criteria Air Pollutant Concentrations
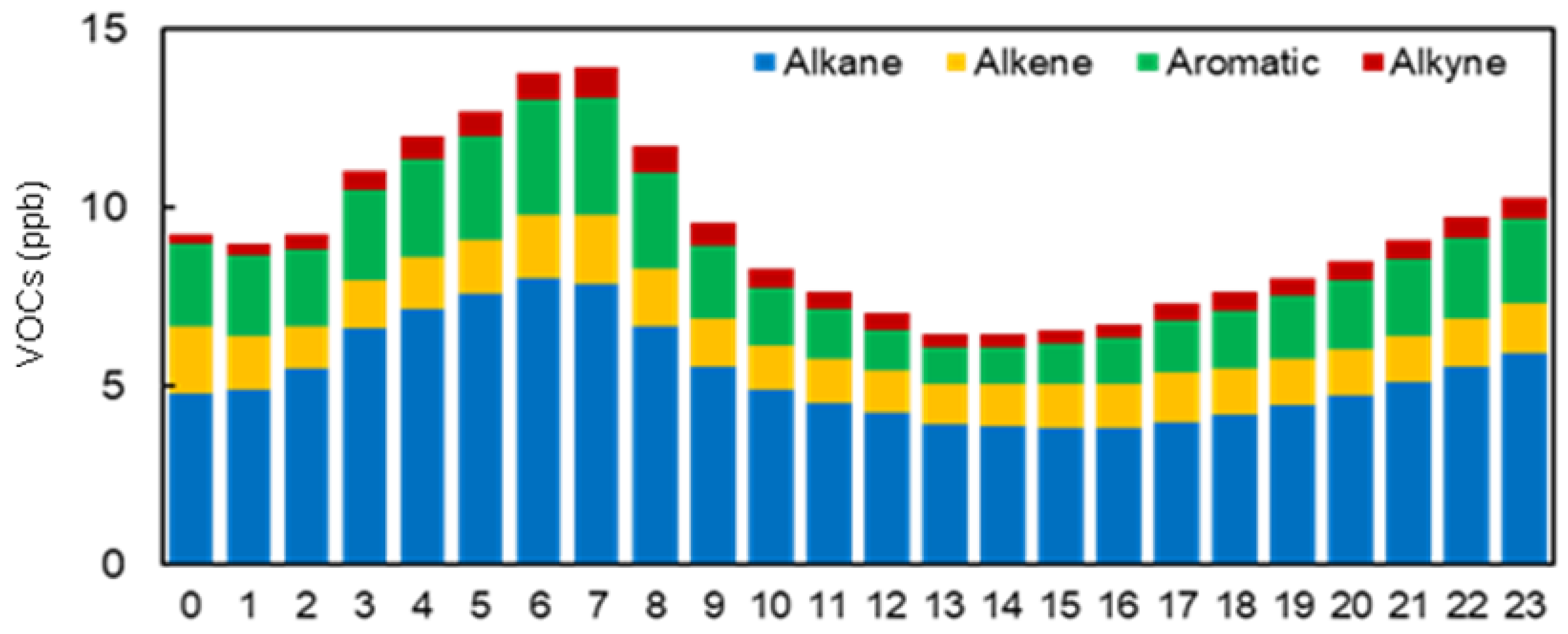
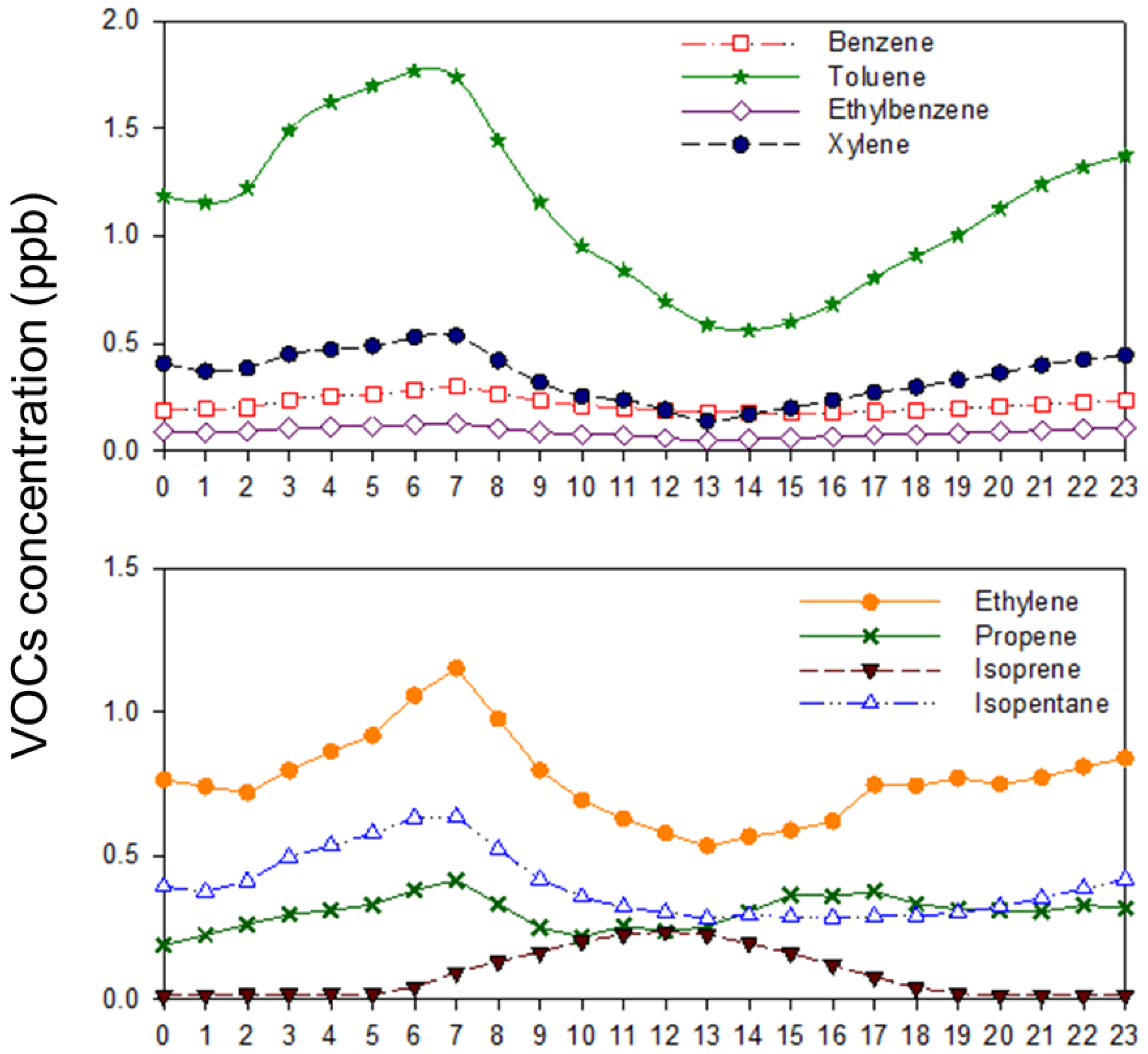
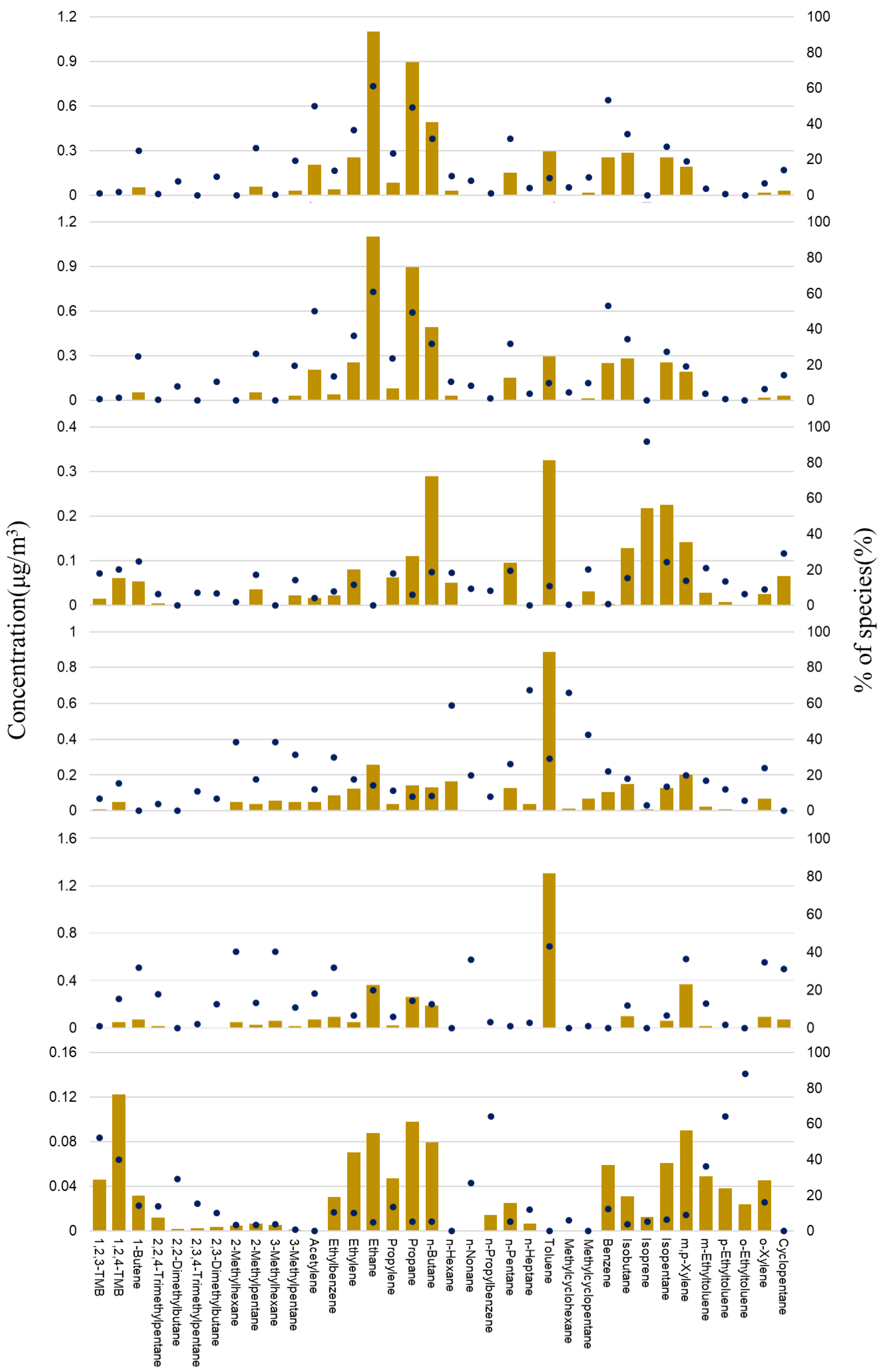
3.2. VOC Concentrations
3.3. Ozone Formation Potential (Hourly Variations)
3.3.1. Air Mass Transport—Back Trajectory Analysis
3.3.2. Contribution of Emission Sources
3.3.3. VOC Sources for PMF Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USEPA. Health Effects Notebook for Hazardous Air Pollutants. 2013. Available online: http://www.epa.gov/ttn/atw/hlthef/hapindex.html (accessed on 1 June 2022).
- Guo, H.; Lee, S.C.; Chan, L.Y.; Li, W.M. Risk assessment of exposure to volatile organic compounds in different indoor environments. Environ. Res. 2004, 94, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Wang, W.C.; Zeng, L.X.; Chiang, H.L. Health risk assessment of exposure to selected volatile organic compounds emitted from an integrated iron and steel plant. Inhal. Toxicol. 2010, 22, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.B.; Chen, W.H.; Yuan, C.S.; Yang, J.C.; Zhao, Q.L. Comparative assessments of VOC emission rates and associated health risks from wastewater treatment processes. J. Environ. Monit. 2012, 14, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, T.B.; Trainer, M.; Angevine, W.M.; Brock, C.A.; Dissly, R.W.; Fehsenfeld, F.C.; Frost, G.J.; Goldan, P.D.; Holloway, J.S.; Hubler, G.; et al. Effect of petrochemical industrial emissions of reactive alkenes and NOx on tropospheric ozone formation in Houston, Texas. J. Geophys. Res.-Atmos. 2003, 108, 4249. [Google Scholar] [CrossRef]
- Elbir, T.; Cetin, B.; Cetin, E.; Bayram, A.; Odabasi, M. Characterization of Volatile Organic Compounds (VOCs) and Their Sources in the Air of Izmir, Turkey. Environ. Monit. Assess. 2007, 133, 149–160. [Google Scholar] [CrossRef]
- Wang, H.; Lou, S.; Huang, C.; Qiao, L.; Tang, X.; Chen, C.; Zeng, L.; Wang, Q.; Zhou, M.; Lu, S.; et al. Source Profiles of Volatile Organic Compounds from Biomass Burning in Yangtze River Delta, China. Aerosol Air Qual. Res. 2014, 14, 818–828. [Google Scholar] [CrossRef]
- Drozd, G.T.; Zhao, Y.; Saliba, G.; Frodin, B.; Maddox, C.; Weber, R.J.; Chang, M.C.O.; Maldonado, H.; Sardar, S.; Robinson, A.L.; et al. Time Resolved Measurements of Speciated Tailpipe Emissions from Motor Vehicles: Trends with Emission Control Technology, Cold Start Effects, and Speciation. Environ. Sci. Technol. 2016, 50, 13592–13599. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, Y.; Wang, T.; Xu, H.; Norris, P.; Pan, W.P. Emission of volatile organic compounds (VOCs) during coal combustion at different heating rates. Fuel 2018, 225, 554–562. [Google Scholar] [CrossRef]
- Liu, H.; Man, H.; Tschantz, M.; Wu, Y.; He, K.; Hao, J. VOC from vehicular evaporation emissions: Status and control strategy. Environ. Sci. Technol. 2015, 49, 14424–14431. [Google Scholar] [CrossRef]
- Yuan, B.; Shao, M.; Lu, S.H.; Wang, B. Source profiles of volatile organic compounds associated with solvent use in Beijing, China. Atmos. Environ. 2010, 44, 1919–1926. [Google Scholar] [CrossRef]
- Fuentes, J.D.; Lerdau, M.; Atkinson, R.; Baldocchi, D.; Bottenheim, J.W.; Ciccioli, P.; Lamb, B.; Geron, C.; Gu, L.; Guenther, A.; et al. Biogenic hydrocarbons in the atmospheric boundary layer: A review. Bull. Am. Meteorol. Soc. 2000, 81, 1537–1575. [Google Scholar] [CrossRef]
- Mo, Z.; Shao, M.; Lu, S.; Niu, H.; Zhou, M.; Sun, J. Characterization of nonmethane hydrocarbons and their sources in an industrialized coastal city, Yangtze River Delta, China. Sci. Total Environ. 2017, 593–594, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Lu, S.; Shao, M. Volatile organic compound (VOC) emissions and health risk assessment in paint and coatings industry in the Yangtze River Delta, China. Environ. Pollut. 2021, 269, 115740. [Google Scholar] [CrossRef] [PubMed]
- de Gouw, J.A.; Middlebrook, A.M.; Warneke, C.; Goldan, P.D.; Kuster, W.C.; Roberts, J.M.; Fehsenfeld, F.C.; Worsnop, D.R.; Canagaratna, M.R.; Pszenny, A.A.P.; et al. Budget of organic carbon in a polluted atmosphere: Results from the New England Air Quality Study in 2002. J. Geophys. Res. 2005, 110, D16305. [Google Scholar] [CrossRef]
- Wang, M.; Qin, W.; Chen, W.; Zhang, L.; Zhang, Y.; Zhang, X.; Xie, X. Seasonal variability of VOCs in Nanjing, Yangtze River delta: Implications for emission sources and photochemistry. Atmos. Environ. 2020, 223, 117254. [Google Scholar] [CrossRef]
- Liu, P.W.G.; Yao, Y.C.; Tsai, J.H.; Hsu, Y.C.; Chang, L.P.; Chang, K.H. Source impacts by volatile organic compounds in an industrial city of southern. Taiwan Sci. Total Environ. 2008, 398, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Chen, W.H.; Yuan, C.S.; Lin, C.S. Multivariate analysis of effects of diurnal temperature and seasonal humidity variations by tropical savanna climate on the emissions of anthropogenic volatile organic compounds. Sci. Total Environ. 2014, 470, 311–323. [Google Scholar] [CrossRef]
- Kalabokas, P.D.; Hatzianestis, J.; Bartzis, J.G.; Papagiannakopoulos, P. Atmospheric concentrations of saturated and aromatic hydrocarbons around a Greek oil refinery. Atmos. Environ. 2001, 35, 2545–2555. [Google Scholar] [CrossRef]
- Guenther, A.; Karl, T.; Harley, P.; Wiedinmyer, C.; Palmer, P.I.; Geron, C. Estimates of global terrestrial isoprene emissions using MEGAN (model of emissions of gases and aerosols from nature). Atmos. Chem. Phys. 2006, 6, 3181–3210. [Google Scholar] [CrossRef]
- Cetin, E.; Odabasi, M.; Seyfioglu, R. Ambient volatile organic compound (VOC) concentrations around a petrochemical complex and a petroleum refinery. Sci. Total. Environ. 2003, 312, 103–112. [Google Scholar] [CrossRef]
- Chang, K.H.; Chen, T.F.; Huang, H.C. Estimation of biogenic volatile organic compounds emissions in subtropical island e Taiwan. Sci. Total Environ. 2005, 346, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Hanai, Y.; Masunaga, S. Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Qual. Atmos. Health 2010, 3, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.-S.; Ansari, M.-F.; Gavane, A.-G.; Pandit, V.-I.; Nema, P.; Devotta, S. Seasonal variation of toxic benzene emissions in petroleum refinery. Environ. Monit. Assess. 2007, 28, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pandya, G.H.; Gavane, A.G.; Bhanarkar, A.D.; Kondawar, V.K. Concentrations of volatile organic compounds (VOCs) at an oil refinery. Int. J. Environ. Stud. 2006, 63, 337–351. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Baltrėnaitė, E.; Šerevičienė, V.; Pereira, P. Atmospheric BTEX concentrations in the vicinity of the crude oil refinery of the Baltic region. Environ. Monit. Assess. 2011, 182, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Dumanoglu, Y.; Kara, M.; Altiok, H.; Odabasi, M.; Elbir, T.; Bayram, A. Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized region. Atmos. Environ. 2014, 98, 168–178. [Google Scholar] [CrossRef]
- Song, Y.; Dai, W.; Shao, M.; Liu, Y.; Lu, S.; Kuster, W.; Goldan, P. Comparison of receptor models for source apportionment of volatile organic compounds in Beijing, China. Environ. Pollut. 2008, 156, 174–183. [Google Scholar] [CrossRef]
- Volkamer, R.; Jimenez, J.L.; San Martini, F.; Dzepina, K.; Zhang, Q.; Salcedo, D.; Molina, L.T.; Worsnop, D.R.; Molina, M.J. Secondary organic aerosol formation from anthropogenic air pollution: Rapid and higher than expected. Geophys. Res. Lett. 2006, 33, L17811. [Google Scholar] [CrossRef]
- Gariazzo, C.; Pelliccioni, A.; Di Filippo, P.; Sallusti, F.; Cecinato, A. Monitoring and analysis of volatile organic compounds around an oil refinery. Water Air Soil Pollut. 2005, 167, 17–38. [Google Scholar] [CrossRef]
- Hoyt, D.; Raun, L.H. Measured and estimated benzene and volatile organic carbon (VOC) emissions at a major U.S. refinery/chemical plant: Comparison and prioritization. J. Air Waste Manag. Assoc. 2015, 65, 1020–1031. [Google Scholar] [CrossRef]
- Lin, T.Y.; Sree, U.; Tseng, S.H.; Chiu, K.H.; Wu, C.H.; Lo, J.G. Volatile organic compound concentrations in ambient air of Kaohsiung petroleum refinery in Taiwan. Atmos. Environ. 2004, 38, 4111–4122. [Google Scholar] [CrossRef]
- Sonibare, J.A.; Akeredolu, F.A.; Obanijesu, E.O.O.; Adebiyi, F.M. Contribution of volatile organic compounds to Nigeria’s Airshed by petroleum refineries. Pet. Sci. Technol. 2007, 25, 503–516. [Google Scholar] [CrossRef]
- Wei, W.; Lv, Z.; Yang, G.; Cheng, S.; Li, Y.; Wang, L. VOCs emission rate estimate for complicated industrial area source using an inverse-dispersion calculation method: A case study on a petroleum refinery in Northern China. Environ. Pollut. 2016, 218, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, Y.; Teiri, H.; Nazmara, S.; Parseh, I. Environmental and biological monitoring of exposures to VOCs in a petrochemical complex in Iran. Environ. Sci. Pollut. Res. 2018, 25, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gao, S.; Fu, Q.; Cheng, J.; Chen, X.; Xu, H.; Liang, S.; Zhou, Y.; Ma, Y. Do volatile organic compounds (VOCs) emitted from petrochemical industries affect regional PM2.5? Atmos. Res. 2018, 209, 123–130. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhong, L.; Lau, A.K.H.; Yu, J.Z.; Louie, P.K.K. Volatile organic compounds in the Pearl River Delta: Identification of source regions and recommendations for emission-oriented monitoring strategies. Atmos. Environ. 2013, 76, 162–172. [Google Scholar] [CrossRef]
- Mo, Z.; Shao, M.; Lu, S.; Qu, H.; Zhou, M.; Sun, J.; Gou, B. Process-specific emission characteristics of volatile organic compounds (VOCs) from petrochemical facilities in the Yangtze River Delta, China. Sci. Total Environ. 2015, 533, 422–431. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Dong, M.; Li, W.; Gao, X.; Ye, R.; Zhang, D. VOCs emission characteristics and priority control analysis based on VOCs emission inventories and ozone formation potentials in Zhoushan. Atmos. Environ. 2018, 182, 234–241. [Google Scholar] [CrossRef]
- Feng, Y.; Xiao, A.; Jia, R.; Zhu, S.; Gao, S.; Li, B.; Shi, N.; Zou, B. Emission characteristics and associated assessment of volatile organic compounds from process units in a refinery. Environ. Pollut. 2020, 265, 115026. [Google Scholar] [CrossRef]
- Lyu, X.; Guo, H.; Wang, Y.; Zhang, F.; Nie, K.; Dang, J.; Liang, Z.; Dong, S.; Zeren, Y.; Zhou, B.; et al. Hazardous volatile organic compounds in ambient air of China. Chemosphere 2020, 246, 125731. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Arevalo, A.P.; Sotomayor, S.; Bailon-Moscoso, N. Contamination by oil crude extraction-refinement and their effects on human health. Environ. Pollut. 2017, 231, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Meng, J.; Zhao, X.; Li, Q.; Shi, B.; Wu, M.; Zhang, Q.; Su, G.; Hu, J.; Shu, X. High-spatial-resolution VOCs emission from the petrochemical industries and its differential regional effect on soil in typical economic zones of China. Sci. Total Environ. 2022, 827, 154318. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Identification of Carcinogenic Hazards to Human, World Health Organization. Available online: https://monographs.iarc.who.int/ (accessed on 7 September 2022).
- Liang, Y.; Liu, X.; Wu, F.; Guo, Y.; Fan, X.; Xiao, H. The year-round variations of VOC mixing ratios and their sources in Kuytun City (northwestern China), near oilfields. Atmos. Pollut. Res. 2020, 11, 1513–1523. [Google Scholar] [CrossRef]
- Taiwan Environmental Protection Agency (TEPA). Taiwan Air Pollutants Emission Data System. 2023. Available online: https://teds.epa.gov.tw/Introduction.aspx (accessed on 1 April 2023).
- Russell, A.; Milford, J.; Bergin, M.S.; McBride, S.; McNair, L.; Yang, Y.; Stockwell, W.; Croes, B. Urban Ozone Control and Atmospheric Reactivity of Organic Gases. Science 1995, 269, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.P.L. Updated Maximum Incremental Reactivity Scale and Hydrocarbon in Reactivities for Regulatory Applications; Prepared for California Air Resources Board Contract 07-339; University of California: Riverside, CA, USA, 2009. [Google Scholar]
- Fang, X.; Saito, T.; Park, S.; Li, S.; Yokouchi, Y.; Prinn, R.G. Performance of Back-Trajectory Statistical Methods and Inverse Modeling Method in Locating Emission Sources. ACS Earth Space Chem. 2018, 2, 843–851. [Google Scholar] [CrossRef]
- Leuchner, M.; Rappenglück, B. VOC source–receptor relationships in Houston during TexAQS-II. Atmos. Environ. 2010, 44, 4056–4067. [Google Scholar] [CrossRef]
- Paatero, P. Least Squares Formulation of Robust, Non-Negative Factor Analysis. Chemom. Intell. Lab. Syst. 1997, 37, 23–35. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Kuo, C.P.; Liao, H.T.; Chou, C.C.; Wu, C.F. Source apportionment of particulate matter and selected volatile organic compounds with multiple time resolution data. Sci. Total Environ. 2014, 472, 880–887. [Google Scholar] [CrossRef]
- Reff, A.; Eberly, S.I.; Bhave, P.V. Receptor modeling of ambient particulate matter data using positive matrix factorization: Review of existing methods. J. Air Waste Manag. 2007, 57, 146–154. [Google Scholar] [CrossRef]
- Poirot, R.L.; Wishinski, P.R.; Hopke, P.K.; Polisar, A.V. Comparative Application of Multiple Receptor Methods to Identify Aerosol Sources in Northern Vermont. Environ. Sci. Technol. 2001, 35, 4622–4636. [Google Scholar] [CrossRef] [PubMed]
- Buzcu, B.; Fraser, M.P. Source identification and apportionment of volatile organic compounds in Houston, TX. Atmos. Environ. 2006, 40, 2385–2400. [Google Scholar] [CrossRef]
- Helmig, D.; Rossabi, S.; Hueber, J.; Tans, P.; Montzka, S.A.; Masarie, K.; Thoning, K.; Plass-Duelmer, C.; Claude, A.; Carpenter, L.J.; et al. Reversal of global atmospheric ethane and propane trends largely due to US oil and natural gas production. Nat. Geosci. 2016, 9, 490–495. [Google Scholar] [CrossRef]
- Angot, H.; Davel, C.; Wiedinmyer, C.; Pétron, G.; Chopra, J.; Hueber, J.; Blanchard, B.; Bourgeois, I.; Vimont, I.; Montzka, S.A.; et al. Temporary pause in the growth of atmospheric ethane and propane in 2015–2018. Atmos. Chem. Phys. 2021, 21, 15153–15170. [Google Scholar] [CrossRef]
- Benchaita, T. Greenhouse Gas Emissions from New Petrochemical Plants; No. IDB-TN-562; Inter-American Development Bank: Washington, DC, USA, 2013. [Google Scholar]
- Zhang, M.; Ge, Y.; Li, J.; Wang, X.; Tan, J.; Hao, L.; Xu, H.; Hao, C.; Wang, J.; Qian, L. Effects of ethanol and aromatic contents of fuel on the non-regulated exhaust emissions and their ozone forming potential of E10-fueled China-6 compliant vehicles. Atmos. Environ. 2021, 264, 118688. [Google Scholar] [CrossRef]
- Li, C.; Cui, M.; Zheng, J.; Chen, Y.; Liu, J.; Ou, J.; Tang, M.; Sha, Q.; Yu, F.; Liao, S.; et al. Variability in real-world emissions and fuel consumption by diesel construction vehicles and policy implications. Sci. Total Environ. 2021, 786, 147256. [Google Scholar] [CrossRef]
- NAEI. UK National Atmospheric Emissions Inventory. 2020. Available online: http://naei.beis.gov.uk/ (accessed on 20 December 2022).
- Miller, L.; Lemke, L.D.; Xu, X.; Molaroni, S.M.; You, H.; Wheeler, A.J.; Booza, J.; Grgicak-Mannion, A.; Krajenta, R.; Graniero, P.; et al. Intra-urban correlation and spatial variability of air toxics across an international airshed in Detroit, Michigan (USA) and Windsor, Ontario (Canada). Atmos. Environ. 2010, 44, 1162–1174. [Google Scholar] [CrossRef]
- Hoque, R.R.; Khillare, P.S.; Agarwal, T.; Shridhar, V.; Balachandran, S. Spatial and temporal variation of BTEX in the urban atmosphere of Delhi, India. Sci. Total Environ. 2008, 392, 30–40. [Google Scholar] [CrossRef]
- Barletta, B.; Meinardi, S.; Rowland, F.S.; Chan, C.Y.; Wang, X.; Zou, S.; Chan, L.Y.; Blake, D.R. Volatile organic compounds in 43 Chinese cities. Atmos. Environ. 2005, 39, 5979–5990. [Google Scholar] [CrossRef]
- Buczynska, A.J.; Krata, A.; Stranger, M.; Godoi, A.F.L.; Kontozova-Deutsch, V.; Bencs, L.; Naveau, I.; Roekens, E.; Grieken, R.V. Atmospheric BTEX-concentrations in an area with intensive street traffic. Atmos. Environ. 2009, 43, 311–318. [Google Scholar] [CrossRef]
- Gelencser, A.; Siszler, K.; Hlavay, J. Toluene-benzene concentration ratio as a tool for characterizing the distance from vehicular emission sources. Environ. Sci. Technol. 1997, 31, 2869–2872. [Google Scholar] [CrossRef]
- Chiang, P.C.; Chiang, Y.C.; Chang, E.E.; Chang, S.C. Characterizations of hazardous air pollutants emitted from motor vehicles. Environ. Toxicol. Chem. 1996, 56, 85–104. [Google Scholar] [CrossRef]
- Liu, J.; Mu, Y.; Zhang, Y.; Zhang, Z.; Wang, X.; Liu, Y.; Sun, Z. Atmospheric levels of BTEX compounds during the 2008 Olympic Games in the urban area of Beijing. Sci. Total Environ. 2009, 408, 109–116. [Google Scholar] [CrossRef]
- Nelson, P.F.; Quigley, S.M. The m,p-xylenes: Ethylbenzene ratio. A technique for estimating hydrocarbon age in ambient atmospheres. Atmos. Environ. 1983, 17, 659–662. [Google Scholar] [CrossRef]
- Prinn, R.; Cunnold, D.; Rasmussen, R.; Simmonds, P.; Alyea, F.; Crawford, A.; Fraser, P.; Rosen, R. Atmospheric trends in methylchloroform and the global average for the hydroxyl radical. Science 1987, 238, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Kim, Y.P. Seasonal characteristics of ambient volatile organic compounds in Seoul, Korea. Atmos. Environ. 2001, 35, 2603–2614. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Chameides, W.L.; Cardelino, C.; Blake, D.R.; Streets, D.G. Source characteristics of volatile organic compounds during high ozone episodes in Hong Kong, Southern China. Atmos. Chem. Phys. 2008, 8, 4983–4996. [Google Scholar] [CrossRef]
- Abbasi, F.; Pasalari, H.; Delgado-Saborit, J.M.; Rafiee, A.; Abbasi, A.; Hoseini, M. Characterization and risk assessment of BTEX in ambient air of a Middle Eastern City. Process Saf. Environ. Prot. 2020, 139, 98–105. [Google Scholar] [CrossRef]
- Roumeliotis, T.; Guenther, A.; Durward, D.; Pourbafrani, H. Relative Impact of Volatile Organic Compound Emissions from Agriculture on Air Quality of Urban Centres. In Food and Fisheries; Governments of Canada and British Columbia, British Columbia Ministry of Agriculture: Abbotsford, BC, Canada, 2021. [Google Scholar]
- König, G.; Brunda, M.; Puxbaum, H.; Hewitt, C.N.; Duckham, S.C.; Rudolph, J. Relative contribution of oxygenated hydrocarbons to the total biogenic VOC emissions of selected mid-European agricultural and natural plant species. Atmos. Environ. 1995, 29, 861–874. [Google Scholar] [CrossRef]
- Li, M.; Pozzer, A.; Lelieveld, J.; Williams, J. Global atmospheric ethane, propane and methane trends(2006–2016). Earth Syst. Sci. Data 2021, 2021, 1–30. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, V.; Shabin, M.; Hakkim, H.; Bonsang, B.; Gros, V. Non-methane hydrocarbon (NMHC) fingerprints of major urban and agricultural emission sources for use in source apportionment studies. Atmos. Chem. Phys. 2020, 20, 12133–12152. [Google Scholar] [CrossRef]
- Gentner, D.R.; Harley, R.A.; Miller, A.M.; Goldstein, A.H. Diurnal and Seasonal Variability of Gasoline-Related Volatile Organic Compound Emissions in Riverside, California. Environ. Sci. Technol. 2009, 43, 4247–4252. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Y.; Sun, L.; Zhang, Q.; Mao, H. Emissions of volatile organic compounds (VOCs) from gasoline- and liquified natural gas (LNG)-fueled vehicles in tunnel studies. Atmos. Environ. 2020, 234, 117626. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Barletta, B.; Simpson, I.J.; Blake, D.R.; Fu, X.; Zhang, Z.; He, Q.; Liu, T.; Zhao, X.; et al. Source attributions of hazardous aromatic hydrocarbons in urban, suburban and rural areas in the Pearl River Delta (PRD) region. J. Hazard. Mater. 2013, 250–251, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ho, S.S.H.; Xue, Y.; Huang, Y.; Wang, L.; Cheng, Y.; Dai, W.; Zhong, H.; Cao, J.; Lee, S. Characterizations of volatile organic compounds (VOCs) from vehicular emissions at roadside environment: The first comprehensive study in Northwestern China. Atmos. Environ. 2017, 161, 1–12. [Google Scholar] [CrossRef]
- Sun, J.; Wu, F.; Hu, B.; Tang, G.; Zhang, J.; Wang, Y. VOC characteristics, emissions and contributions to SOA formation during hazy episodes. Atmos. Environ. 2016, 141, 560–570. [Google Scholar] [CrossRef]
- Dalsøren, S.B.; Myhre, G.; Hodnebrog, Ø.; Myhre, C.L.; Stohl, A.; Pisso, I.; Schwietzke, S.; Höglund-Isaksson, L.; Helmig, D.; Reimann, S.; et al. Discrepancy between simulated and observed ethane and propane levels explained by underestimated fossil emissions. Nat. Geosci. 2018, 11, 178–184. [Google Scholar] [CrossRef]
- Ghanta, M.; Fahey, D.; Subramaniam, B. Environmental impacts of ethylene production from diverse feedstocks and energy sources. Appl. Petrochem. Res. 2014, 4, 167–179. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Chen, D.; Li, Q.; Thai, P.; Gong, D.; Li, Y.; Zhang, C.; Gu, Y.; Zhou, L.; et al. Emission characteristics of volatile organic compounds and their secondary organic aerosol formation potentials from a petroleum refinery in Pearl River Delta, China. Sci. Total Environ. 2017, 584–585, 1162–1174. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R. Measurement of emissions from air pollution sources, 2. C1 through C30 organic compounds from medium duty diesel trucks. Environ. Sci. Technol. 1999, 33, 1578–1587. [Google Scholar] [CrossRef]
- Reiter, M.S.; Kockelman, K.M. The problem of cold starts: A closer look at mobile source emissions levels. Transp. Res. D Transp. Environ. 2016, 43, 123–132. [Google Scholar] [CrossRef]
- Chen, T.F.; Tsai, C.Y.; Chen, C.H.; Chang, K.H. Effect of long-range transport from changing emission on ozone-NOx-VOC sensitivity: Implication of control strategy to improve ozone. J. Innov. Technol. 2021, 3, 39–49. [Google Scholar] [CrossRef]


| Compounds | PM10 | SO2 | NOx | O3 | THC | CO |
|---|---|---|---|---|---|---|
| μg m−3 | ppb | ppb | ppb | ppm | ppm | |
| 2016 (n = 366) | 44.53 ± 5.84 | 3.02 ± 0.38 | 13.53 ± 1.93 | 30.23 ± 1.43 | 2.53 ± 0.11 | 0.44 ± 0.05 |
| 2017 (n = 365) | 51.54 ± 8.47 | 2.77 ± 0.24 | 15.77 ± 1.24 | 32.11 ± 1.99 | 2.72 ± 0.11 | 0.44 ± 0.05 |
| 2018 (n = 365) | 49.53 ± 8.00 | 2.63 ± 0.23 | 12.31 ± 1.72 | 33.35 ± 2.49 | 2.61 ± 0.38 | 0.43 ± 0.02 |
| 2019 (n = 365) | 45.82 ± 7.85 | 2.71 ± 0.11 | 14.12 ± 0.91 | 33.03 ± 2.70 | 2.64 ± 0.40 | 0.48 ± 0.01 |
| 2020 (n = 366) | 39.96 ± 6.27 | 2.39 ± 0.11 | 14.14 ± 1.45 | 31.39 ± 2.24 | 2.51 ± 0.31 | 0.40 ± 0.01 |
| D1(n = 1827) | 51.79 ± 4.40 | 2.80 ± 0.23 | 13.74 ± 1.34 | 31.31 ± 2.17 | 2.72 ± 0.16 | 0.44 ± 0.04 |
| D2 (n = 1827) | 60.98 ± 9.34 | 2.78 ± 0.20 | 13.69 ± 1.30 | 30.06 ± 0.85 | 2.80 ± 0.15 | 0.45 ± 0.02 |
| D3 (n = 1827) | 50.49 ± 5.24 | 2.79 ± 0.30 | 13.69 ± 1.31 | 33.50 ± 2.69 | 2.40 ± 0.18 | 0.42 ± 0.04 |
| D4 (n = 1827) | 47.70 ± 5.72 | 2.35 ± 0.20 | 17.50 ± 1.29 | 30.27 ± 1.14 | 2.69 ± 0.26 | 0.40 ± 0.05 |
| D5 (n = 1827) | 42.78 ± 5.12 | 2.83 ± 0.35 | 14.24 ± 1.34 | 30.54 ± 1.24 | 2.93 ± 0.18 | 0.43 ± 0.03 |
| D6 (n = 1827) | 39.76 ± 4.55 | 2.83 ± 0.46 | 12.77 ± 1.50 | 35.17 ± 0.84 | 2.29 ± 0.26 | 0.41 ± 0.04 |
| D7 (n = 1827) | 39.99 ± 8.16 | 2.70 ± 0.29 | 14.09 ± 1.30 | 30.70 ± 0.88 | 2.40 ± 0.32 | 0.46 ± 0.04 |
| D8 (n = 1827) | 43.78 ± 2.86 | 2.78 ± 0.38 | 14.62 ± 1.09 | 31.23 ± 1.76 | 2.73 ± 0.23 | 0.47 ± 0.04 |
| D9 (n = 1827) | 42.19 ± 3.15 | 2.64 ± 0.28 | 12.65 ± 1.73 | 33.35 ± 2.69 | 2.36 ± 0.24 | 0.47 ± 0.05 |
| D10 (n = 1827) | 43.31 ± 4.93 | 2.52 ± 0.22 | 12.79 ± 1.34 | 34.10 ± 2.47 | 2.69 ± 0.15 | 0.45 ± 0.03 |
| Compounds | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| n = 366 | n = 365 | n = 365 | n = 365 | n = 366 | |
| Ethane | 1.56 ± 0.95 | 1.56 ± 0.93 | 1.53 ± 0.88 | 1.64 ± 0.93 | 1.51 ± 0.90 |
| Toluene | 1.34 ± 0.87 | 1.22 ± 0.77 | 1.02 ± 0.64 | 0.98 ± 0.60 | 0.85 ± 0.53 |
| Propane | 1.26 ± 0.65 | 1.24 ± 0.81 | 1.12 ± 0.58 | 1.12 ± 0.60 | 1.06 ± 0.59 |
| n-Butane | 0.81 ± 0.34 | 0.77 ± 0.31 | 0.72 ± 0.30 | 0.71 ± 0.30 | 0.67 ± 0.27 |
| Ethylene | 0.80 ± 0.55 | 0.76 ± 0.35 | 0.70 ± 0.30 | 0.73 ± 0.38 | 0.66 ± 0.33 |
| Acetylene | 0.67 ± 0.32 | 0.56 ± 0.34 | 0.50 ± 0.28 | 0.50 ± 0.24 | 0.41 ± 0.23 |
| Isobutane | 0.49 ± 0.20 | 0.42 ± 0.18 | 0.36 ± 0.15 | 0.38 ± 0.17 | 0.36 ± 0.15 |
| Isopentane | 0.43 ± 0.16 | 0.43 ± 0.17 | 0.37 ± 0.15 | 0.35 ± 0.13 | 0.32 ± 0.13 |
| Propene | 0.37 ± 0.37 | 0.37 ± 0.30 | 0.28 ± 0.35 | 0.23 ± 0.18 | 0.26 ± 0.23 |
| m,p-Xylene | 0.25 ± 0.12 | 0.28 ± 0.14 | 0.24 ± 0.12 | 0.23 ± 0.12 | 0.25 ± 0.18 |
| Benzene | 0.25 ± 0.14 | 0.25 ± 0.15 | 0.20 ± 0.12 | 0.18 ± 0.12 | 0.16 ± 0.10 |
| Pentane | 0.24 ± 0.09 | 0.22 ± 0.09 | 0.18 ± 0.08 | 0.17 ± 0.08 | 0.17 ± 0.07 |
| n-Hexane | 0.16 ± 0.10 | 0.15 ± 0.09 | 0.12 ± 0.06 | 0.11 ± 0.09 | 0.09 ± 0.08 |
| 1-Butene | 0.16 ± 0.05 | 0.17 ± 0.04 | 0.14 ± 0.09 | 0.10 ± 0.04 | 0.11 ± 0.04 |
| 2-Methylpentane | 0.12 ± 0.05 | 0.10 ± 0.04 | 0.07 ± 0.03 | 0.07 ± 0.03 | 0.06 ± 0.03 |
| Isoprene | 0.10 ± 0.11 | 0.08 ± 0.08 | 0.09 ± 0.09 | 0.08 ± 0.07 | 0.09 ± 0.08 |
| 1,2,4-Trimethylbenzene | 0.09 ± 0.04 | 0.10 ± 0.05 | 0.09 ± 0.04 | 0.08 ± 0.04 | 0.06 ± 0.04 |
| 3-Methylpentane | 0.09 ± 0.04 | 0.07 ± 0.03 | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.05 ± 0.02 |
| o-Xylene | 0.08 ± 0.05 | 0.09 ± 0.05 | 0.07 ± 0.04 | 0.07 ± 0.04 | 0.07 ± 0.07 |
| Ethylbenzene | 0.08 ± 0.05 | 0.10 ± 0.05 | 0.08 ± 0.05 | 0.08 ± 0.04 | 0.07 ± 0.05 |
| Others | 0.70 | 0.72 | 0.56 | 0.51 | 0.45 |
| Alkanes | 5.66 ± 2.45 | 5.44 ± 2.33 | 4.91 ± 2.18 | 4.96 ± 2.24 | 4.63 ± 2.05 |
| Alkenes | 1.49 ± 0.86 | 1.46 ± 0.62 | 1.23 ± 0.48 | 1.16 ± 0.55 | 1.45 ± 0.50 |
| Aromatics | 2.24 ± 1.25 | 2.19 ± 1.19 | 1.84 ± 0.99 | 1.72 ± 0.92 | 1.55 ± 0.88 |
| Alkynes | 0.67 ± 0.32 | 0.56 ± 0.34 | 0.50 ± 0.28 | 0.50 ± 0.24 | 0.41 ± 0.23 |
| Sum | 10.06 | 9.68 | 8.52 | 8.36 | 7.73 |
| Compound | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|
| n = 366 | n = 365 | n = 365 | n = 365 | n = 366 | |
| Toluene | 24.26 | 22.12 | 18.48 | 17.83 | 15.36 |
| m,p-Xylene | 8.51 | 9.46 | 8.27 | 7.81 | 8.43 |
| Ethylene | 8.30 | 7.89 | 7.20 | 7.52 | 6.79 |
| Propene | 7.37 | 7.54 | 5.64 | 4.71 | 5.28 |
| 1,2,4-Trimethylbenzene | 4.04 | 4.49 | 3.93 | 3.32 | 2.73 |
| Isoprene | 3.09 | 2.48 | 2.72 | 2.33 | 2.72 |
| 1-Butene | 3.51 | 3.88 | 3.22 | 2.21 | 2.43 |
| o-Xylene | 2.81 | 2.93 | 2.39 | 2.29 | 2.38 |
| n-Butane | 2.22 | 2.12 | 1.98 | 1.95 | 1.85 |
| Isopentane | 1.86 | 1.82 | 1.59 | 1.50 | 1.39 |
| 1,2,3-Trimethylbenzene | 1.79 | 1.77 | 1.68 | 1.39 | 1.23 |
| Isobutane | 1.43 | 1.22 | 1.07 | 1.11 | 1.05 |
| 3-Ethyltoluene | 1.62 | 1.62 | 1.40 | 1.21 | 1.00 |
| Ethylbenzene | 1.11 | 1.27 | 1.07 | 1.01 | 0.96 |
| Propane | 1.11 | 1.10 | 0.99 | 0.99 | 0.93 |
| Pentane | 0.92 | 0.86 | 0.70 | 0.67 | 0.66 |
| Cyclopentane | 0.47 | 0.46 | 0.41 | 0.45 | 0.70 |
| Ethane | 0.54 | 0.54 | 0.53 | 0.56 | 0.52 |
| trans-2-Butene | 1.13 | 1.02 | 0.67 | 0.50 | 0.44 |
| 1,3,5-Trimethylbenzene | 0.67 | 0.87 | 0.66 | 0.56 | 0.41 |
| Acetylene | 0.68 | 0.57 | 0.51 | 0.51 | 0.41 |
| Benzene | 0.57 | 0.58 | 0.47 | 0.41 | 0.37 |
| Others | 6.28 | 6.35 | 4.85 | 4.18 | 3.33 |
| Alkanes | 12.85 | 12.16 | 10.40 | 10.17 | 9.41 |
| Alkenes | 24.31 | 23.90 | 20.11 | 17.69 | 18.08 |
| Aromatics | 46.45 | 46.34 | 39.39 | 36.63 | 33.47 |
| Alkynes | 0.68 | 0.57 | 0.51 | 0.51 | 0.41 |
| Sum | 84.29 | 82.97 | 70.41 | 65.00 | 61.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, J.-H.; How, V.; Wang, W.-C.; Chiang, H.-L. Characteristics of Airborne Pollutants in the Area of an Agricultural–Industrial Complex near a Petrochemical Industry Facility. Atmosphere 2023, 14, 803. https://doi.org/10.3390/atmos14050803
Tsai J-H, How V, Wang W-C, Chiang H-L. Characteristics of Airborne Pollutants in the Area of an Agricultural–Industrial Complex near a Petrochemical Industry Facility. Atmosphere. 2023; 14(5):803. https://doi.org/10.3390/atmos14050803
Chicago/Turabian StyleTsai, Jiun-Horng, Vivien How, Wei-Chi Wang, and Hung-Lung Chiang. 2023. "Characteristics of Airborne Pollutants in the Area of an Agricultural–Industrial Complex near a Petrochemical Industry Facility" Atmosphere 14, no. 5: 803. https://doi.org/10.3390/atmos14050803
APA StyleTsai, J.-H., How, V., Wang, W.-C., & Chiang, H.-L. (2023). Characteristics of Airborne Pollutants in the Area of an Agricultural–Industrial Complex near a Petrochemical Industry Facility. Atmosphere, 14(5), 803. https://doi.org/10.3390/atmos14050803






