Emissions of PAHs, Nitro-PAHs and Quinones (Oxy-PAHs) Associated to PM1.0 and PM2.5 Emitted by a Diesel Engine Fueled with Diesel-Biodiesel-Ethanol Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Fuels and Chemicals
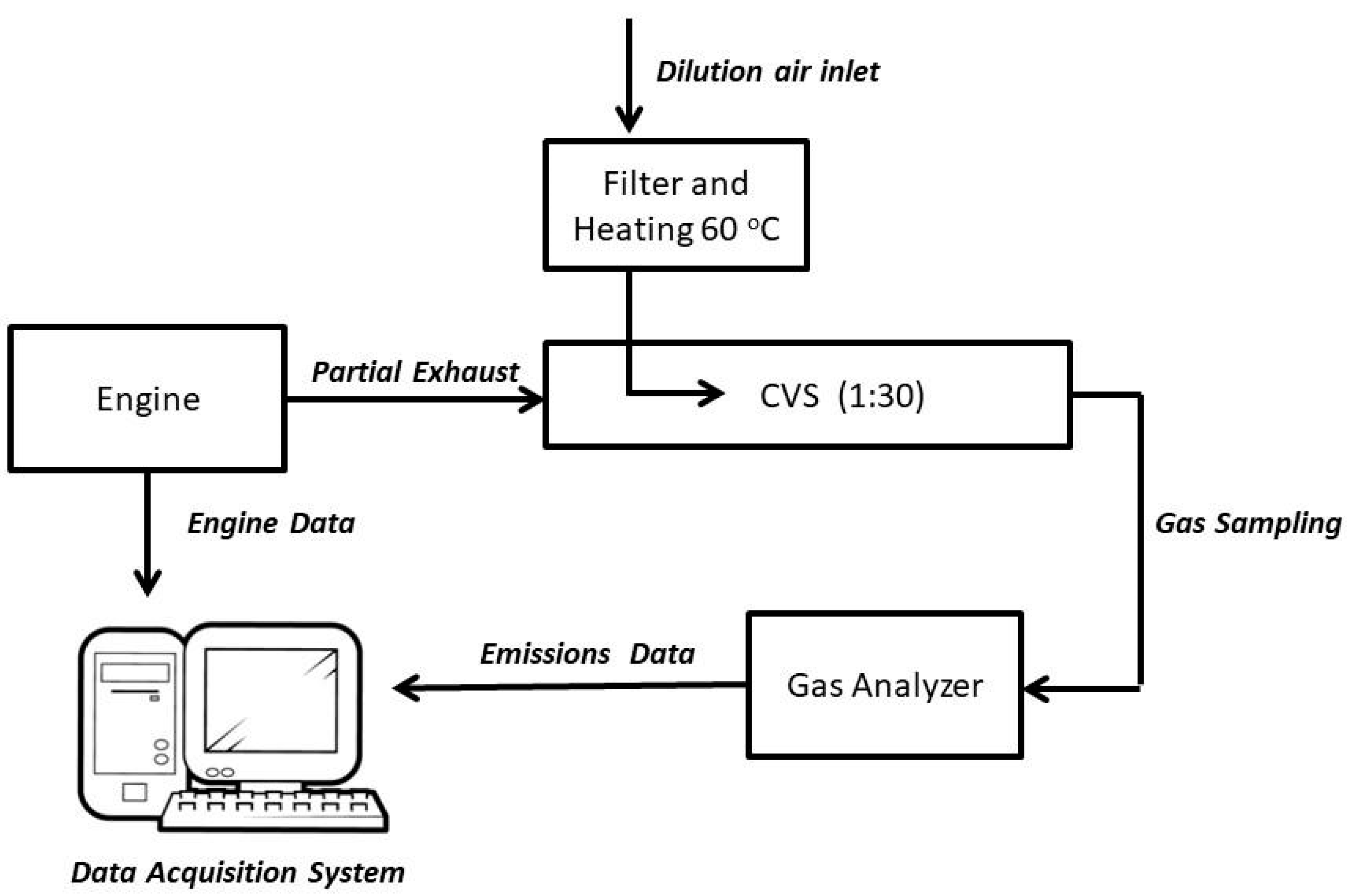
2.2. Sampling: Performance of the Diesel Engine and Particulate Matter Collected
2.3. Instrumentation and Chemical Analysis
2.4. Quality Assurance/Quality Control
2.5. Risk Assessment based on Incremental Lifetime Cancer Risk
3. Results and Discussion
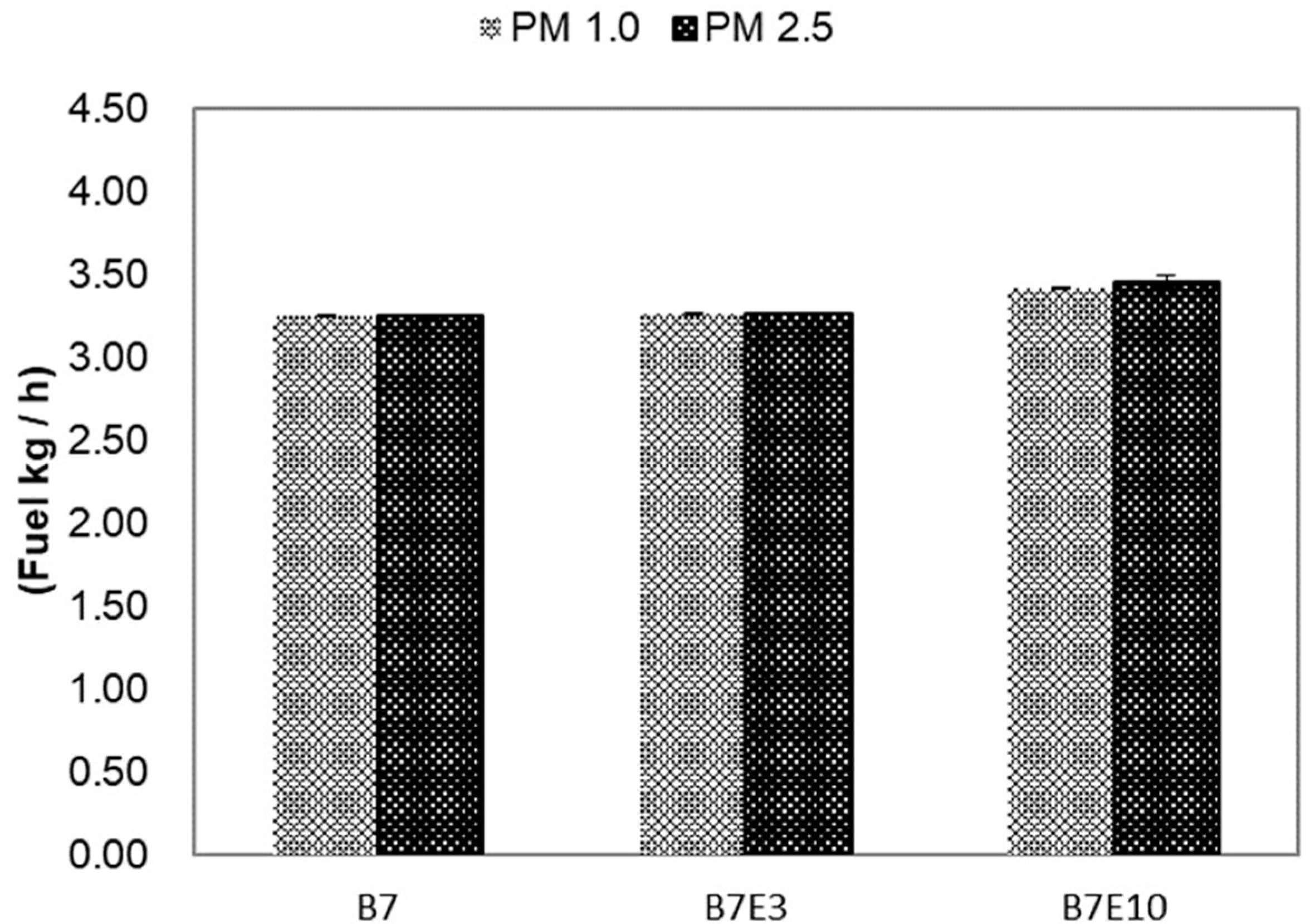
3.1. Specific Fuel Consumption
3.2. Emission Factors for PM1.0 and PM2.5
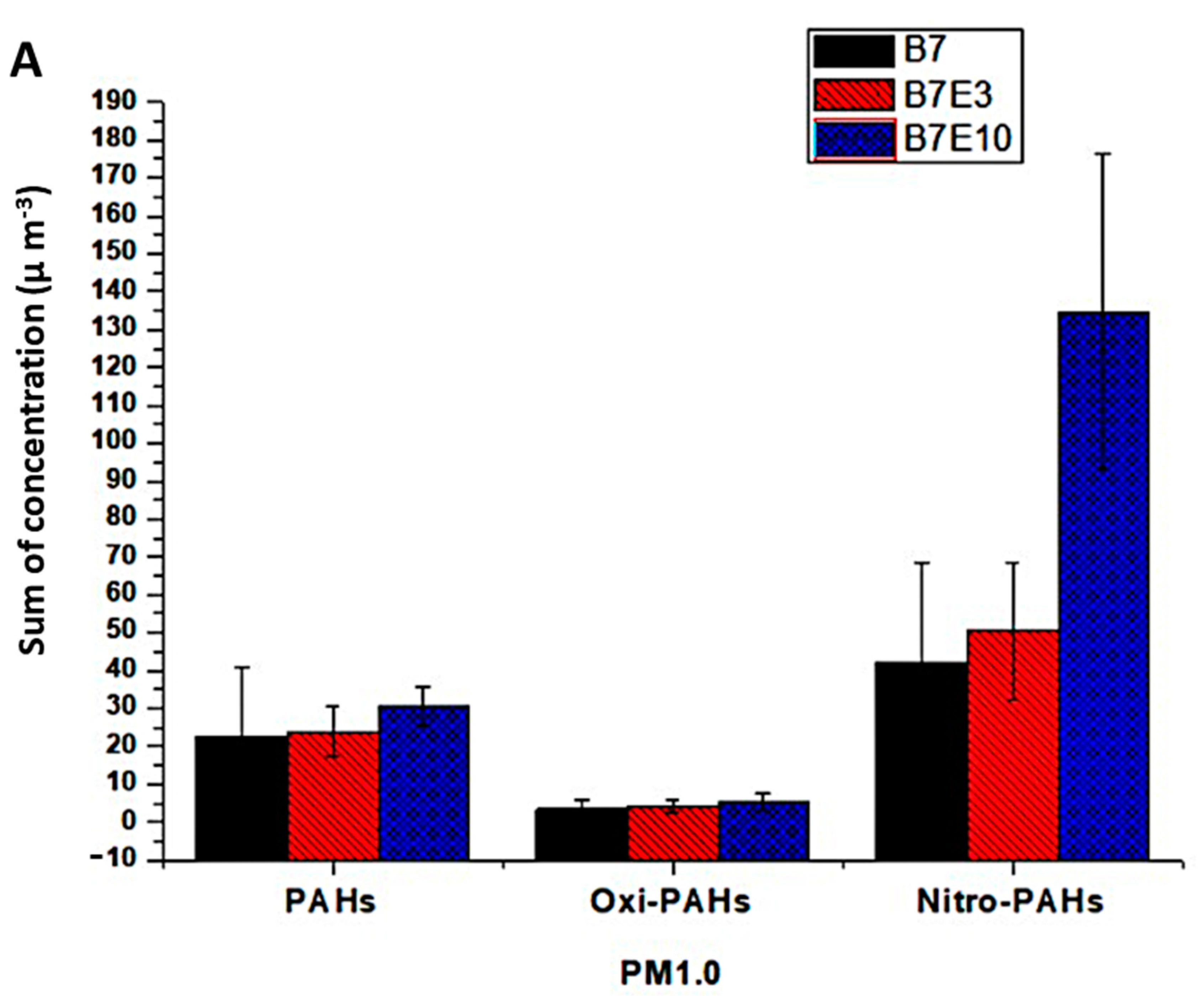
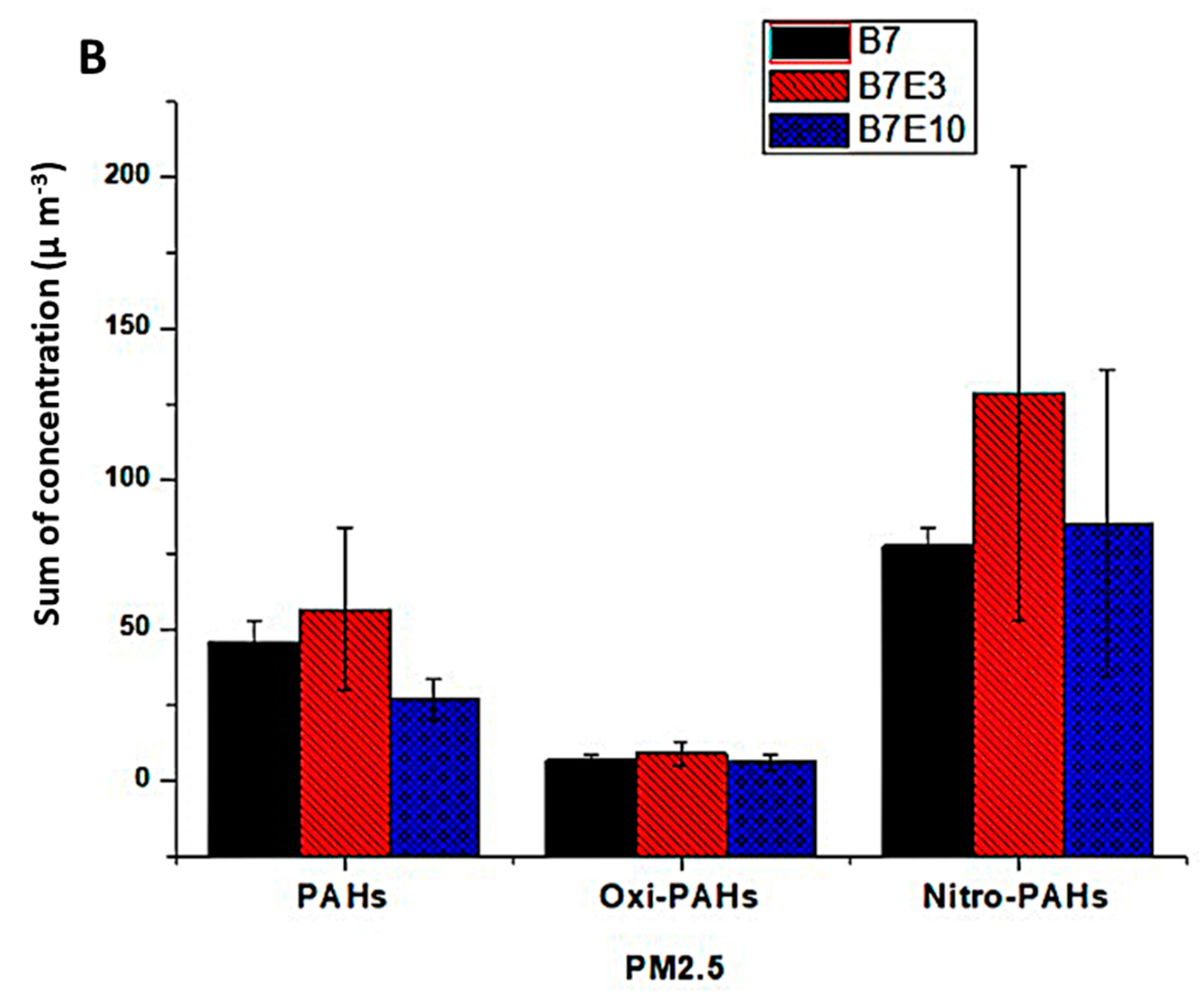
3.3. Concentration of PAH, Nitro-PAH and Quinones Associated with PM1.0 and PM2.5
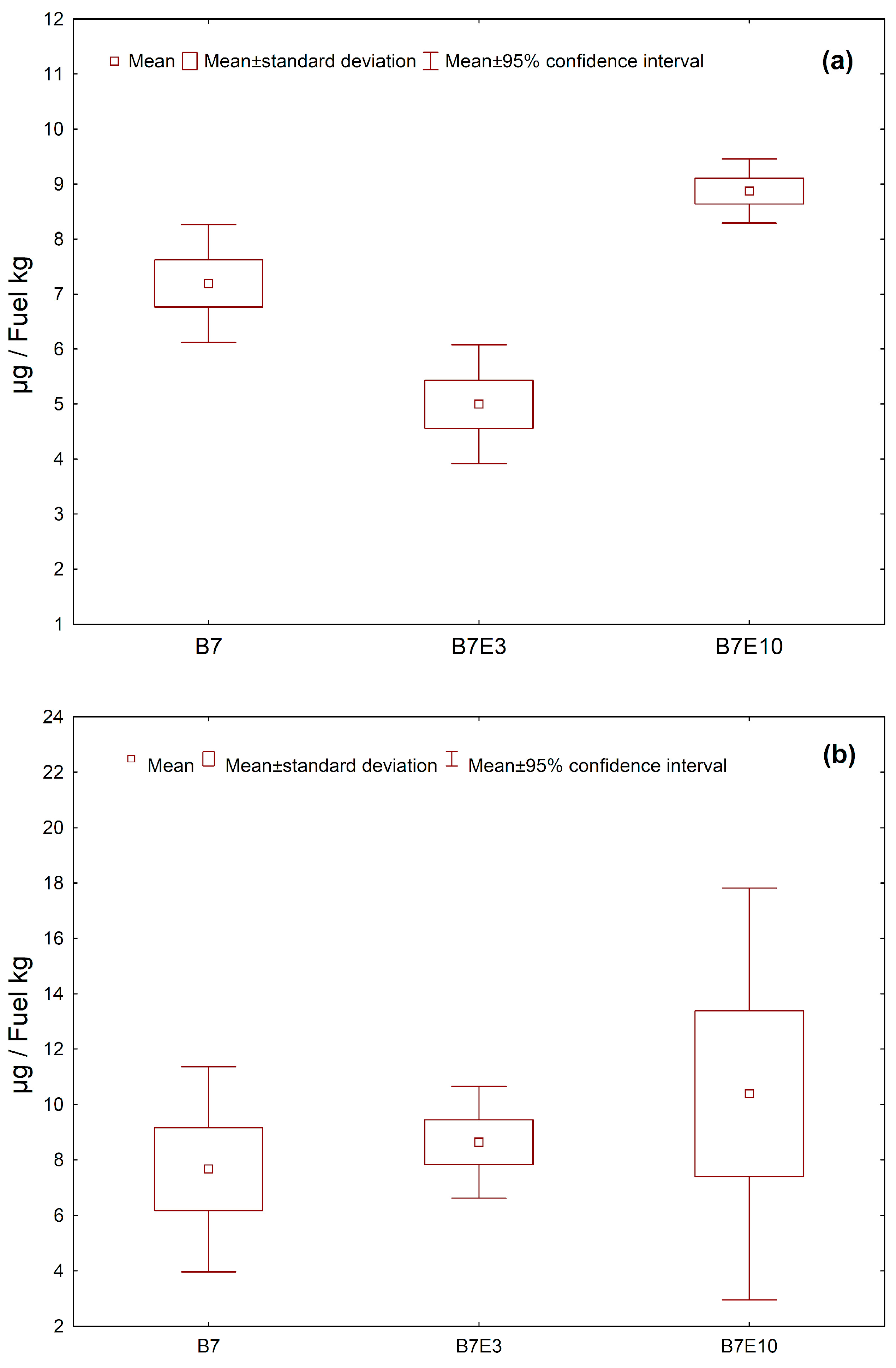
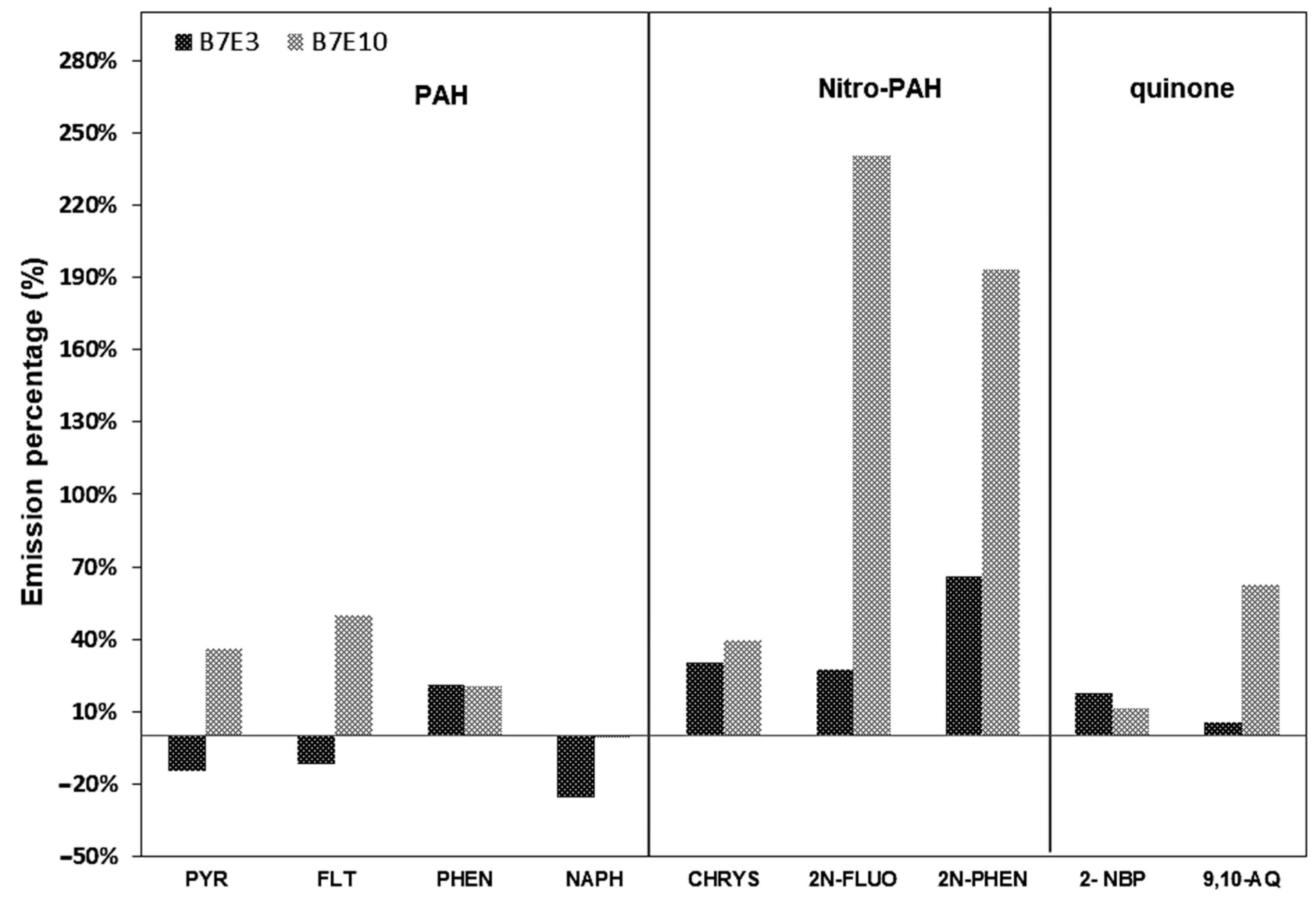
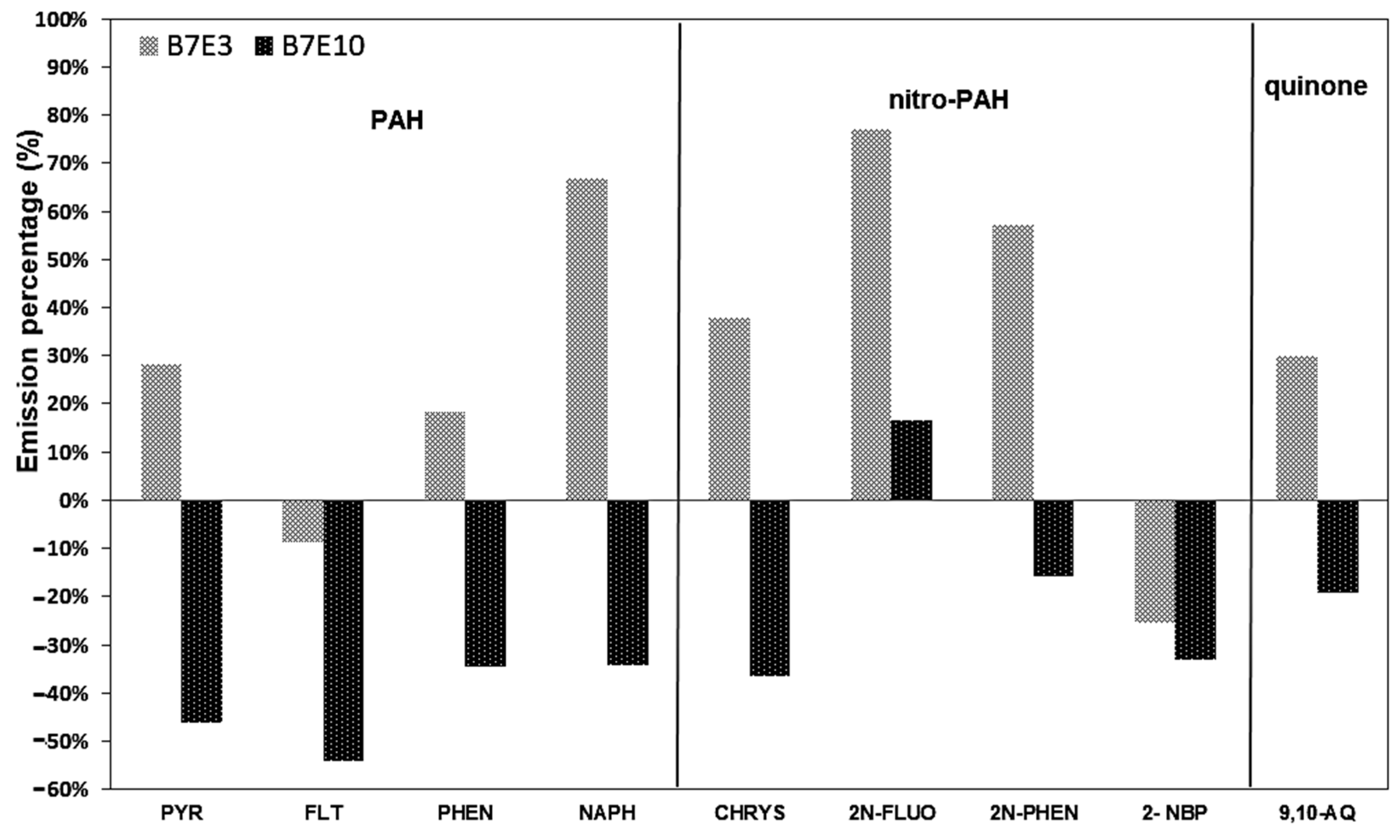
3.4. Emission Factors for PAH, Nitro-PAH, and Quinones in PM1.0 and PM2.5
3.5. Risk Assessment Based on Incremental Lifetime Cancer Risk
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yesilyurt, M.K. A Detailed Investigation on the Performance, Combustion, and Exhaust Emission Characteristics of a Diesel Engine Running on the Blend of Diesel Fuel, Biodiesel and 1-Heptanol (C7 Alcohol) as a next-Generation Higher Alcohol. Fuel 2020, 275, 117893. [Google Scholar] [CrossRef]
- Shah, S.H.; Raja, I.A.; Rizwan, M.; Rashid, N.; Mahmood, Q.; Shah, F.A.; Pervez, A. Potential of microalgal biodiesel production and its sustainability perspectives in Pakistan. Renew. Sustain. Energy Rev. 2018, 81, 76–92. [Google Scholar] [CrossRef]
- Gwalwanshi, M.; Kumar, R.; Kumar Chauhan, M. A Review on Butanol Properties, Production and Its Application in Internal Combustion Engines. Mater. Today Proc. 2022, 62, 6573–6577. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Sarıdemir, S.; Albayrak, S. Experimental Investigation of Combustion, Performance and Emission Characteristics of a Diesel Engine Fuelled with Diesel–Biodiesel–Alcohol Blends. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 389. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of Biodiesel Production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Fattah, I.M.R.; Sanjid, A. Feasibility of Diesel–Biodiesel–Ethanol/Bioethanol Blend as Existing CI Engine Fuel: An Assessment of Properties, Material Compatibility, Safety and Combustion. Renew. Sustain. Energy Rev. 2014, 32, 379–395. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Li, G.-X. On Reliability and Flexibility of Sustainable Energy Application Route for Vehicles in China. Renew. Sustain. Energy Rev. 2015, 51, 830–846. [Google Scholar] [CrossRef]
- Guarieiro, L.L.N.; de Almeida Guerreiro, E.T.; dos Santos Amparo, K.K.; Manera, V.B.; Regis, A.C.D.; Santos, A.G.; Ferreira, V.P.; Leão, D.J.; Torres, E.A.; de Andrade, J.B. Assessment of the Use of Oxygenated Fuels on Emissions and Performance of a Diesel Engine. Microchem. J. 2014, 117, 94–99. [Google Scholar] [CrossRef]
- Guarieiro, L.L.N.; de Souza, A.F.; Torres, E.A.; de Andrade, J.B. Emission Profile of 18 Carbonyl Compounds, CO, CO2, and NO Emitted by a Diesel Engine Fuelled with Diesel and Ternary Blends Containing Diesel, Ethanol and Biodiesel or Vegetable Oils. Atmos. Environ. 2009, 43, 2754–2761. [Google Scholar] [CrossRef]
- Guarieiro, L.L.N.; Guerreiro, E.T.d.A.; Amparo, K.K.d.S.; Leão, D.J.; Torres, E.A.; Andrade, J.B. Estudo Do Perfil De Distribuição De Tamanho E Número De Partículas Emitidas Na Queima De Misturas De Diesel/Biodiesel/Etanol. Blucher Eng. Proc. 2014, 1, 387–393. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Pham, M.; Zhao, D.; Deng, Y.; Le, D.; Zuo, W.; Zhu, H.; Liu, T.; Peng, Q.; Zhang, Z. Effect of Different Technologies on Combustion and Emissions of the Diesel Engine Fueled with Biodiesel: A Review. Renew. Sustain. Energy Rev. 2017, 80, 620–647. [Google Scholar] [CrossRef]
- Mirhashemi, F.S.; Sadrnia, H. NOX Emissions of Compression Ignition Engines Fueled with Various Biodiesel Blends: A Review. J. Energy Inst. 2020, 93, 129–151. [Google Scholar] [CrossRef]
- Palani, Y.; Devarajan, C.; Manickam, D.; Thanikodi, S. Performance and Emission Characteristics of Biodiesel-Blend in Diesel Engine: A Review. Environ. Eng. Res. 2020, 27, 200338. [Google Scholar] [CrossRef]
- Atmanli, A.; Yilmaz, N. An Experimental Assessment on Semi-Low Temperature Combustion Using Waste Oil Biodiesel/C3-C5 Alcohol Blends in a Diesel Engine. Fuel 2020, 260, 116357. [Google Scholar] [CrossRef]
- Bilgili, L. Comparative Assessment of Alternative Marine Fuels in Life Cycle Perspective. Renew. Sustain. Energy Rev. 2021, 144, 110985. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Yusuf, D.A.; Jie, Z.; Bello, T.Y.; Tambaya, M.; Abdullahi, B.; Muhammed-Dabo, I.A.; Yahuza, I.; Dandakouta, H. Influence of Waste Oil-Biodiesel on Toxic Pollutants from Marine Engine Coupled with Emission Reduction Measures at Various Loads. Atmos. Pollut. Res. 2022, 13, 101258. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.B.; Nalla, B.T.; Choubey, G.; Mishra, R.; Vellaiyan, S. Experimental Analysis of Sterculia Foetida Biodiesel and Butanol Blends as a Renewable and Eco-Friendly Fuel. Ind. Crops. Prod. 2022, 178, 114612. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Kalam, M.A.; Masjuki, H.H.; Gul, M.; Soudagar, M.E.M.; Ong, H.C.; Ahmed, W.; Atabani, A.E.; Razzaq, L.; Yusoff, M. Comparative Study of Nanoparticles and Alcoholic Fuel Additives-Biodiesel-Diesel Blend for Performance and Emission Improvements. Fuel 2020, 279, 118434. [Google Scholar] [CrossRef]
- Erdiwansyah; Mamat, R.; Sani, M.S.M.; Sudhakar, K.; Kadarohman, A.; Sardjono, R.E. An Overview of Higher Alcohol and Biodiesel as Alternative Fuels in Engines. Energy Rep. 2019, 5, 467–479. [Google Scholar] [CrossRef]
- Zaharin, M.S.M.; Abdullah, N.R.; Najafi, G.; Sharudin, H.; Yusaf, T. Effects of Physicochemical Properties of Biodiesel Fuel Blends with Alcohol on Diesel Engine Performance and Exhaust Emissions: A Review. Renew. Sustain. Energy Rev. 2017, 79, 475–493. [Google Scholar] [CrossRef]
- Yilmaz, N.; Davis, S.M. Polycyclic Aromatic Hydrocarbon (PAH) Formation in a Diesel Engine Fueled with Diesel, Biodiesel and Biodiesel/n-Butanol Blends. Fuel 2016, 181, 729–740. [Google Scholar] [CrossRef]
- de Oliveira, A.; de Morais, A.M.; Valente, O.S.; Sodré, J.R. Combustion Characteristics, Performance and Emissions from a Diesel Power Generator Fuelled by B7-Ethanol Blends. Fuel Process. Technol. 2015, 139, 67–72. [Google Scholar] [CrossRef]
- Shahir, S.A.; Masjuki, H.H.; Kalam, M.A.; Imran, A.; Ashraful, A.M. Performance and Emission Assessment of Diesel–Biodiesel–Ethanol/Bioethanol Blend as a Fuel in Diesel Engines: A Review. Renew. Sustain. Energy Rev. 2015, 48, 62–78. [Google Scholar] [CrossRef]
- Tsai, J.-H.; Chen, S.-J.; Huang, K.-L.; Lin, W.-Y.; Lee, W.-J.; Lin, C.-C.; Hsieh, L.-T.; Chiu, J.-Y.; Kuo, W.-C. Emissions from a Generator Fueled by Blends of Diesel, Biodiesel, Acetone, and Isopropyl Alcohol: Analyses of Emitted PM, Particulate Carbon, and PAHs. Sci. Total Environ. 2014, 466–467, 195–202. [Google Scholar] [CrossRef]
- Tutak, W. Bioethanol E85 as a Fuel for Dual Fuel Diesel Engine. Energy Convers. Manag. 2014, 86, 39–48. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Burl Donaldson, A.; Darabseh, T. Investigation of CI Engine Emissions in Biodiesel–Ethanol–Diesel Blends as a Function of Ethanol Concentration. Fuel 2014, 115, 790–793. [Google Scholar] [CrossRef]
- How, H.G.; Masjuki, H.H.; Kalam, M.A.; Teoh, Y.H. Engine Performance, Emission and Combustion Characteristics of a Common-Rail Diesel Engine Fuelled with Bioethanol as a Fuel Additive in Coconut Oil Biodiesel Blends. Energy Procedia 2014, 61, 1655–1659. [Google Scholar] [CrossRef]
- Lapuerta, M.; García-Contreras, R.; Campos-Fernández, J.; Dorado, M.P. Stability, Lubricity, Viscosity, and Cold-Flow Properties of Alcohol−Diesel Blends. Energy Fuels 2010, 24, 4497–4502. [Google Scholar] [CrossRef]
- Yilmaz, N.; Davis, S.M. Formation of Polycyclic Aromatic Hydrocarbons and Regulated Emissions from Biodiesel and N-Butanol Blends Containing Water. J. Hazard. Mater. 2022, 437, 129360. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Donaldson, B. Fuel Effects on PAH Formation, Toxicity and Regulated Pollutants: Detailed Comparison of Biodiesel Blends with Propanol, Butanol and Pentanol. Sci. Total Environ. 2022, 849, 157839. [Google Scholar] [CrossRef]
- Nani Guarieiro, L.L.; Nani Guarieiro, A.L. Vehicle Emissions: What Will Change with Use of Biofuel? In Biofuels—Economy, Environment and Sustainability; InTech: London, UK, 2013. [Google Scholar]
- Guarieiro, L.L.N.; Vasconcellos, P.C.; Solci, M.C. Air Pollutants from the Burning of Fossil Fuels and Biofuels: A Brief Review. Rev. Virtual Química 2011, 3, 434–445. [Google Scholar] [CrossRef]
- Vinikoor-Imler, L.C.; Davis, J.A.; Luben, T.J. An Ecologic Analysis of County-Level PM2.5 Concentrations and Lung Cancer Incidence and Mortality. Int. J. Environ. Res. Public Health 2011, 8, 1865–1871. [Google Scholar] [CrossRef]
- Kumar, P.; Robins, A.; Vardoulakis, S.; Britter, R. A Review of the Characteristics of Nanoparticles in the Urban Atmosphere and the Prospects for Developing Regulatory Controls. Atmos. Environ. 2010, 44, 5035–5052. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Lee, C.-F.F. Particulate Matter Emission Characteristics of Diesel Engines with Biodiesel or Biodiesel Blending: A Review. Renew. Sustain. Energy Rev. 2016, 64, 569–581. [Google Scholar] [CrossRef]
- Alves, C. Aerossóis Atmosféricos: Perspectiva Histórica, Fontes, Processos Químicos de Formação e Composição Orgânica. Quim Nova 2005, 28, 859–870. [Google Scholar] [CrossRef]
- Keyte, I.J.; Albinet, A.; Harrison, R.M. On-Road Traffic Emissions of Polycyclic Aromatic Hydrocarbons and Their Oxy- and Nitro- Derivative Compounds Measured in Road Tunnel Environments. Sci. Total Environ. 2016, 566–567, 1131–1142. [Google Scholar] [CrossRef]
- Kooter, I.M.; van Vugt, M.A.T.M.; Jedynska, A.D.; Tromp, P.C.; Houtzager, M.M.G.; Verbeek, R.P.; Kadijk, G.; Mulderij, M.; Krul, C.A.M. Toxicological Characterization of Diesel Engine Emissions Using Biodiesel and a Closed Soot Filter. Atmos. Environ. 2011, 45, 1574–1580. [Google Scholar] [CrossRef]
- Li, Q.; Wyatt, A.; Kamens, R.M. Oxidant Generation and Toxicity Enhancement of Aged-Diesel Exhaust. Atmos. Environ. 2009, 43, 1037–1042. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; Vangrieken, R. Atmospheric Polycyclic Aromatic Hydrocarbons: Source Attribution, Emission Factors and Regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef]
- Jang, E.; Alam, M.S.; Harrison, R.M. Source Apportionment of Polycyclic Aromatic Hydrocarbons in Urban Air Using Positive Matrix Factorization and Spatial Distribution Analysis. Atmos. Environ. 2013, 79, 271–285. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, B.; Park, S.; Kim, E.; Chiaramonti, D. Emission Characteristics of Compression Ignition (CI) Engine Using Diesel Blended with Hydrated Butanol. Fuel 2019, 257, 116037. [Google Scholar] [CrossRef]
- Santos, A.G.; Regis, A.C.D.; da Rocha, G.O.; Bezerra, M.D.A.; de Jesus, R.M.; de Andrade, J.B. A Simple, Comprehensive, and Miniaturized Solvent Extraction Method for Determination of Particulate-Phase Polycyclic Aromatic Compounds in Air. J. Chromatogr. A 2016, 1435, 6–17. [Google Scholar] [CrossRef]
- Pereira, G.M.; Teinilä, K.; Custódio, D.; Gomes Santos, A.; Xian, H.; Hillamo, R.; Alves, C.A.; Bittencourt de Andrade, J.; Olímpio da Rocha, G.; Kumar, P.; et al. Particulate Pollutants in the Brazilian City of São Paulo: 1-Year Investigation for the Chemical Composition and Source Apportionment. Atmos. Chem. Phys. 2017, 17, 11943–11969. [Google Scholar] [CrossRef]
- Santos, A.G.; da Rocha, G.O.; de Andrade, J.B. Occurrence of the Potent Mutagens 2- Nitrobenzanthrone and 3-Nitrobenzanthrone in Fine Airborne Particles. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Tse, H.; Leung, C.W.; Cheung, C.S. Investigation on the Combustion Characteristics and Particulate Emissions from a Diesel Engine Fueled with Diesel-Biodiesel-Ethanol Blends. Energy 2015, 83, 343–350. [Google Scholar] [CrossRef]
- Ghadikolaei, M.A.; Wei, L.; Cheung, C.S.; Yung, K.-F.; Ning, Z. Particulate Emission and Physical Properties of Particulate Matter Emitted from a Diesel Engine Fueled with Ternary Fuel (Diesel-Biodiesel-Ethanol) in Blended and Fumigation Modes. Fuel 2020, 263, 116665. [Google Scholar] [CrossRef]
- Yang, K.; Wei, L.; Cheung, C.S.; Tang, C.; Huang, Z. The Effect of Pentanol Addition on the Particulate Emission Characteristics of a Biodiesel Operated Diesel Engine. Fuel 2017, 209, 132–140. [Google Scholar] [CrossRef]
- Butler, A.D.; Sobotowski, R.A.; Hoffman, G.J.; Machiele, P. Influence of Fuel PM Index and Ethanol Content on Particulate Emissions from Light-Duty Gasoline Vehicles; SAE Mobilus: Warrendale, PA, USA, 2015. [Google Scholar] [CrossRef]
- He, C.; Ge, Y.; Tan, J.; You, K.; Han, X.; Wang, J. Characteristics of Polycyclic Aromatic Hydrocarbons Emissions of Diesel Engine Fueled with Biodiesel and Diesel. Fuel 2010, 89, 2040–2046. [Google Scholar] [CrossRef]
- Storey, J.M.E.; Barone, T.L.; Thomas, J.F.; Huff, S.P. Exhaust Particle Characterization for Lean and Stoichiometric DI Vehicles Operating on Ethanol-Gasoline Blends; SAE Mobilus: Warrendale, PA, USA, 2012. [Google Scholar] [CrossRef]
- Sakai, S.; Rothamer, D. Effect of Ethanol Blending on Particulate Formation from Premixed Combustion in Spark-Ignition Engines. Fuel 2017, 196, 154–168. [Google Scholar] [CrossRef]
- Price, P.; Twiney, B.; Stone, R.; Kar, K.; Walmsley, H. Particulate and Hydrocarbon Emissions from a Spray Guided Direct Injection Spark Ignition Engine with Oxygenate Fuel Blends. SAE Tech. Pap. 2007. [Google Scholar] [CrossRef]
- Lemaire, R.; Therssen, E.; Desgroux, P. Effect of Ethanol Addition in Gasoline and Gasoline–Surrogate on Soot Formation in Turbulent Spray Flames. Fuel 2010, 89, 3952–3959. [Google Scholar] [CrossRef]
- Golea, D.; Rezgui, Y.; Guemini, M.; Hamdane, S. Reduction of PAH and Soot Precursors in Benzene Flames by Addition of Ethanol. J. Phys. Chem. A 2012, 116, 3625–3642. [Google Scholar] [CrossRef]
- Park, S.; Shao, Y.; Liu, J.; Wang, Y. Oxygen Electrocatalysts for Water Electrolyzers and Reversible Fuel Cells: Status and Perspective. Energy Environ. Sci. 2012, 5, 9331. [Google Scholar] [CrossRef]
- Kojima, Y.; Inazu, K.; Hisamatsu, Y.; Okochi, H.; Baba, T.; Nagoya, T. Influence of Secondary Formation on Atmospheric Occurrences of Oxygenated Polycyclic Aromatic Hydrocarbons in Airborne Particles. Atmos. Environ. 2010, 44, 2873–2880. [Google Scholar] [CrossRef]
- Ahmed, T.M.; Ahmed, B.; Aziz, B.K.; Bergvall, C.; Westerholm, R. Native and Oxygenated Polycyclic Aromatic Hydrocarbons in Ambient Air Particulate Matter from the City of Sulaimaniyah in Iraq. Atmos. Environ. 2015, 116, 44–50. [Google Scholar] [CrossRef]
- Cochran, R.E.; Dongari, N.; Jeong, H.; Beránek, J.; Haddadi, S.; Shipp, J.; Kubátová, A. Determination of Polycyclic Aromatic Hydrocarbons and Their Oxy-, Nitro-, and Hydroxy-Oxidation Products. Anal. Chim. Acta 2012, 740, 93–103. [Google Scholar] [CrossRef]
- Lim, H.; Sadiktsis, I.; de Oliveira Galvão, M.F.; Westerholm, R.; Dreij, K. Polycyclic Aromatic Compounds in Particulate Matter and Indoor Dust at Preschools in Stockholm, Sweden: Occurrence, Sources and Genotoxic Potential in Vitro. Sci. Total Environ. 2021, 755, 142709. [Google Scholar] [CrossRef]
- Moussaoui, Y.; Balducci, C.; Cecinato, A.; Meklati, B.Y. Atmospheric Particulate Organic Matter at Urban and Forest Sites of Northern Algeria. Urban Clim. 2013, 4, 85–101. [Google Scholar] [CrossRef]
- Souza, K.F.; Carvalho, L.R.F.; Allen, A.G.; Cardoso, A.A. Diurnal and Nocturnal Measurements of PAH, Nitro-PAH, and Oxy-PAH Compounds in Atmospheric Particulate Matter of a Sugar Cane Burning Region. Atmos. Environ. 2014, 83, 193–201. [Google Scholar] [CrossRef]
- Enya, T.; Suzuki, H.; Watanabe, T.; Hirayama, T.; Hisamatsu, Y. 3-Nitrobenzanthrone, a Powerful Bacterial Mutagen and Suspected Human Carcinogen Found in Diesel Exhaust and Airborne Particulates. Environ. Sci. Technol. 1997, 31, 2772–2776. [Google Scholar] [CrossRef]
- Murahashi, T. Determination of Mutagenic 3-Nitrobenzanthrone in Diesel Exhaust Particulate Matter by Three-Dimensional High-Performance Liquid Chromatography. Analyst 2003, 128, 42–45. [Google Scholar] [CrossRef]
- Arlt, V.M. 3-Nitrobenzanthrone, a Potential Human Cancer Hazard in Diesel Exhaust and Urban Air Pollution: A Review of the Evidence. Mutagenesis 2005, 20, 399–410. [Google Scholar] [CrossRef]
- Phousongphouang, P.T.; Arey, J. Sources of the Atmospheric Contaminants, 2-Nitrobenzanthrone and 3-Nitrobenzanthrone. Atmos. Environ. 2003, 37, 3189–3199. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Meusel, H. Nitrated Polycyclic Aromatic Hydrocarbons (Nitro-PAHs) in the Environment—A Review. Sci. Total Environ. 2017, 581–582, 237–257. [Google Scholar] [CrossRef]
- Guarieiro, A.L.N.; Santos, J.V.d.S.; Eiguren-Fernandez, A.; Torres, E.A.; da Rocha, G.O.; de Andrade, J.B. Redox Activity and PAH Content in Size-Classified Nanoparticles Emitted by a Diesel Engine Fuelled with Biodiesel and Diesel Blends. Fuel 2014, 116, 490–497. [Google Scholar] [CrossRef]
- Ying, W.; Longbao, Z.; Hewu, W. Diesel Emission Improvements by the Use of Oxygenated DME/Diesel Blend Fuels. Atmos. Environ. 2006, 40, 2313–2320. [Google Scholar] [CrossRef]
- Lee, W.-J.; Liu, Y.-C.; Mwangi, F.K.; Chen, W.-H.; Lin, S.-L.; Fukushima, Y.; Liao, C.-N.; Wang, L.-C. Assessment of Energy Performance and Air Pollutant Emissions in a Diesel Engine Generator Fueled with Water-Containing Ethanol–Biodiesel–Diesel Blend of Fuels. Energy 2011, 36, 5591–5599. [Google Scholar] [CrossRef]
- Yilmaz, N.; Donaldson, A. Evidence of PAH Production under Lean Combustion Conditions. Fuel 2007, 86, 2377–2382. [Google Scholar] [CrossRef]
- Karavalakis, G.; Boutsika, V.; Stournas, S.; Bakeas, E. Biodiesel Emissions Profile in Modern Diesel Vehicles. Part 2: Effect of Biodiesel Origin on Carbonyl, PAH, Nitro-PAH and Oxy-PAH Emissions. Sci. Total Environ. 2011, 409, 738–747. [Google Scholar] [CrossRef]
- Bakeas, E.B.; Karavalakis, G. Regulated, Carbonyl and Polycyclic Aromatic Hydrocarbon Emissions from a Light-Duty Vehicle Fueled with Diesel and Biodiesel Blends. Environ. Sci. Process. Impacts 2013, 15, 412–422. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Balasubramanian, R. Influence of Butanol Addition to Diesel–Biodiesel Blend on Engine Performance and Particulate Emissions of a Stationary Diesel Engine. Appl. Energy 2014, 119, 530–536. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Balasubramanian, R. Physicochemical and Toxicological Characteristics of Particulate Matter Emitted from a Non-Road Diesel Engine: Comparative Evaluation of Biodiesel-Diesel and Butanol-Diesel Blends. J. Hazard. Mater. 2014, 264, 395–402. [Google Scholar] [CrossRef]
| Characteristics | Standard | Limits | ||
|---|---|---|---|---|
| S10 a | Biodiesel b | Ethanol c | ||
| Specific mass at 20 °C (kg m−3) | ASTM D4052 | 815.0 and 850.0 | 850.0 and 900.0 | 791.5 |
| Kinematic viscosity at 40 °C (mm2 s−1) | ASTM D445 | 2.0 and 4.5 | 3.0 and 6.0 | - |
| Water content, max. (mg kg−1) | ASTM D6304 | - | - | 200 |
| (a) | |
| Characteristics | Diesel Engine |
| Power (Stand-by—1500RPM—cv/kW/kVA) | 80/59/66 |
| Total Displacement (L) | 3.87 |
| Number of Cylinders | 4, online |
| Combustion System | 4-stroke, Direct Injection |
| Cycle | Turbo powered |
| Compression ratio | 16:1 |
| Cooling | Liquid |
| Operating Temperature (°C) | 77/95 |
| (b) | |
| Characteristics | Dynamometer |
| Product Category | passive dynamometer |
| torque measurement | load cell |
| Power (kW) | 20–500 |
| Torque (Nm) | 25–2.000 |
| Speed (rpm) | 8.000–17.000 |
| Class | Compound | B7 | B7E3 | B7E10 | |||
|---|---|---|---|---|---|---|---|
| µg/m3 | |||||||
| Mean | SD | Mean | SD | Mean | SD | ||
| PAH | Naphthalene | 2.6 | ±1.0 | 2.0 | ±0.8 | 2.7 | ±0.8 |
| Acenaphthylene | 0.4 | ±0.1 | <0.6 ** | - | <0.6 ** | - | |
| Acenaphthene | 0.5 | ±0.5 | 0.6 | ±0.3 | 0.7 | ±0.1 | |
| Fluorene | 0.5 | ±0.5 | 0.6 | ±0.2 | 0.6 | ±0.1 | |
| Phenanthrene | 3.1 | ±2.9 | 3.7 | ±0.9 | 3.9 | ±0.5 | |
| Anthracene | 0.3 | ±0.4 | 0.4 | ±0.2 | 0.3 | ±0.1 | |
| Fluoranthene | 3.4 | ±3.0 | 3.0 | ±0.5 | 5.4 | ±0.8 | |
| Pyrene | 9.4 | ±8.0 | 8.0 | ±1.3 | 13.4 | ±2.0 | |
| Benzo(a)anthracene | 0.5 | ±0.4 | 0.8 | ±0.2 | 0.7 | ±0.2 | |
| Chrysene | 1.3 | ±1.2 | 1.7 | ±0.2 | 1.9 | ±0.4 | |
| Benzo(b)fluoranthene | 0.4 | ±0.3 | 0.7 | ±0.2 | 0.5 | ±0.1 | |
| Benzo(k)fluoranthene | 0.2 | ±0.2 | 0.2 | ±0,1 | 0.3 | ±0.0 | |
| Benzo(a)pyrene | <0.2 ** | - | 1.3 | ±0.3 | <0.2 ** | - | |
| Perylene | <0.6 ** | - | <0.6 ** | - | <0.6 ** | - | |
| Indeno(1,2,3 c,d)pyrene | <0.5 ** | - | 0.2 | ±0.4 | <0.5 ** | - | |
| Dibenzo(a,h)anthracene | <0.8 ** | - | 0.3 | ±0.4 | <0.8 ** | - | |
| Benzo(ghi)perylene | <0.5 ** | - | 0.3 | ±0.5 | <0.5 ** | - | |
| Coronene | <0.5 ** | - | 0.1 | 0.0 | 0.1 | 0.0 | |
| Ʃ | 22.6 | ±18.5 | 23.9 | ±6.5 | 30.5 | ±5.1 | |
| Oxy-PAs | Benzoquinone | <7 ** | - | <7 ** | - | <7 ** | - |
| 1,2-Naphthoquinone | 0.6 | ±0.6 | 1.0 | ±0.2 | 1.0 | ±0.1 | |
| 1,4-Naphthoquinone | 0.5 | ±0.4 | 0.7 | ±0.2 | 0.7 | ±0.1 | |
| 9,10-Phenanthraquinone | <5 ** | - | <5 ** | - | <5 ** | - | |
| 9,10-Anthraquinone | 1.7 | ±1.6 | 1.8 | ±0.7 | 2.9 | ±0.7 | |
| Benzanthrone | 0.5 | ±0.2 | 0.8 | ±0.6 | 0.7 | ±0.5 | |
| Ʃ | 3.3 | ±2.8 | 4.3 | ±1.7 | 5.3 | ±2.4 | |
| Nitro- PAH | 1-Nitronaphthalene | 0.1 | ±0.1 | 0.2 | 0.0 | 0.2 | 0.0 |
| 1-Methyl-4-nitronaphthalene | 0.2 | ±0.2 | 0.2 | ±0.1 | 0.2 | ±0.2 | |
| 2-Nitronaphthalene | 0.5 | ±0.5 | 0.6 | ±0.1 | 0.8 | ±0.1 | |
| 2-Nitrobiphenyl | 1.7 | ±1.5 | 2.0 | ±0.3 | 2.0 | ±0.4 | |
| 1-Methyl-5-nitronaphthalene | 0.5 | ±0.5 | 0.8 | ±0.4 | 1.2 | ±0.4 | |
| 1-Methyl-6-nitronaphthalene | <55 ** | - | <55 ** | - | <55 ** | - | |
| 2-Methyl-4-nitronaphthalene | <62 ** | - | <62 ** | - | <62 ** | - | |
| 3-Nitrobiphenyl | 0.6 | ±0.5 | 0.6 | 0.0 | 0.9 | ±0.2 | |
| 4-Nitrobiphenyl | 0.5 | ±0.4 | 0.4 | ±0.3 | 0.9 | ±0.1 | |
| 5-Nitroacenaphthene | <52 ** | - | <52 ** | - | <52 ** | - | |
| 2-Nitrofluorene | 33.1 | ±28.7 | 42.2 | ±15.9 | 118.1 | ±37.6 | |
| 2-Nitrophenanthrene | <53 ** | - | <53 ** | - | <53 ** | - | |
| 3-Nitrophenanthrene | 1.7 | ±1.5 | 2.8 | ±0.4 | 5.1 | ±1.2 | |
| 9-Nitrophenanthrene | 1.2 | ±1.1 | <35 ** | - | <35 ** | - | |
| 2-Nitroanthracene | <33** | - | <33 ** | - | <33 ** | - | |
| 9-Nitroanthracene | 1.3 | ±1.2 | <15 ** | - | <15 ** | - | |
| 2-Nitrofluoranthene | <3 9** | - | <39 ** | - | <39 ** | - | |
| 3-Nitrofluoranthene | <36 ** | - | <36 ** | - | <36 ** | - | |
| 1-Nitropyrene | <48 ** | - | <48 ** | - | <48 ** | - | |
| 2-Nitropyrene | <27 ** | - | <27 ** | - | <27 ** | - | |
| 4-Nitropyrene | <22 ** | - | <22 ** | - | <22 ** | - | |
| 7-Nitrobenz(a)anthracene | <54 ** | - | <54 ** | - | <54 ** | - | |
| 6-Nitrochrysene | <24 ** | - | <24 ** | - | 5.2 | ±1.4 | |
| 3-Nitrobenzanthrone | 0.7 | ±0.3 | 0.6 | ±0.4 | <4 * | - | |
| 6-Nitrobenzo[a]pyrene | <28 ** | - | <28 ** | - | <28 ** | - | |
| 1-Nitrobenzo[e]pyrene | <11 ** | - | <3 * | - | <3 * | - | |
| 3-Nitrobenzo[e]pyrene | <7 * | - | <7 * | - | <7 * | - | |
| Ʃ | 42.1 | ±26.5 | 50.4 | ±17.9 | 134.6 | ±41.6 | |
| Class | Compounds | B7 | B7E3 | B7E10 | |||
|---|---|---|---|---|---|---|---|
| µg/m3 | |||||||
| Mean | SD | Mean | SD | Mean | SD | ||
| PAH | Naphthalene | 4.3 | ±0.6 | 7.1 | ±3.9 | 2.9 | ±1.0 |
| Acenaphthylene | 0.1 | 0.0 | 0.2 | ±0.1 | 0.1 | ±0.0 | |
| Acenaphthene | 1.3 | ±0.4 | 1.8 | ±0.6 | 0.8 | ±0.1 | |
| Fluorene | 1.3 | ±0.3 | 1.4 | ±0.6 | 0.7 | ±0.2 | |
| Phenanthrene | 10.1 | ±2.7 | 9.3 | ±3.7 | 4.9 | ±0.7 | |
| Anthracene | 1.3 | ±0.5 | 1.0 | ±0.7 | 0.4 | ±0.1 | |
| Fluoranthene | 6.7 | ±0.9 | 7.9 | ±3.2 | 4.6 | ±2.0 | |
| Pyrene | 16.1 | ±1.2 | 20.6 | ±9.4 | 9.1 | ±1.7 | |
| Benzo(a)anthracene | 0.9 | ±0.1 | 1.4 | ±0.9 | 0.6 | ±0.2 | |
| Chrysene | 2.7 | ±0.3 | 3.7 | ±2.2 | 1.8 | ±0.5 | |
| Benzo(b)fluoranthene | 0.5 | ±0.1 | 1.2 | ±0.8 | 0.4 | ±0.1 | |
| Benzo(k)fluoranthene | 0.4 | ±0.2 | 0.8 | ±0.7 | 0.4 | ±0.2 | |
| Benzo(a)pyrene | <0.2 * | - | <0.2 * | - | <0.2 * | - | |
| Perylene | <0.6 * | - | <0.6 * | - | <0.6 * | - | |
| Indeno(1,2,3 c,d)pyrene | <0.5 * | - | <0.5 * | - | <0.5 * | - | |
| Dibenzo(a,h)anthracene | <0.8 * | - | <0.8 * | - | <0.8 * | - | |
| Benzo(ghi)perylene | <0.5 * | - | <0.5 * | - | <0.5 * | - | |
| Coronene | 0.2 | ±0.1 | 0.3 | ±0.1 | 0.1 | 0.0 | |
| Ʃ | 45.9 | ±7.3 | 56.7 | ±26.9 | 26.8 | ±6.8 | |
| Oxy-PAHs | Benzoquinone | <7 * | - | <7 * | - | <7 * | - |
| 1,2-Naphthoquinone | 1.6 | ±0.5 | 2.4 | ±1.2 | 1.5 | ±0.6 | |
| 1,4-Naphthoquinone | 1.1 | ±0.2 | 1.7 | ±0.8 | 0.9 | ±0.1 | |
| 9,10-Phenanthraquinone | <5 * | - | <5 * | - | <5 * | - | |
| 9,10-Anthraquinone | 3.3 | ±0.7 | 4.2 | ±1.7 | 2.8 | ±1.2 | |
| Benzanthrone | 0.9 | ±0.5 | 0.5 | ±0.2 | 0.8 | ±0.7 | |
| Ʃ | 6.9 | ±1.9 | 8.8 | ±3.9 | 6.0 | ±2.6 | |
| Nitro- PAH | 1-Nitronaphthalene | 0.3 | ±0.1 | 0.4 | ±0.1 | 0.3 | ±0.1 |
| 1-Methyl-4-nitronaphthalene | 0.3 | ±0.1 | 0.5 | ±0.1 | 0.2 | ±0.2 | |
| 2-Nitronaphthalene | 1.1 | ±0.4 | 1.3 | ±0.2 | 0.6 | ±0.5 | |
| 2-Nitrobiphenyl | 3.8 | ±0.7 | 2.8 | ±0.8 | 2.7 | ±0.9 | |
| 1-Methyl-5-nitronaphthalene | 1.0 | ±0.3 | 1.2 | ±0.1 | 1.0 | ±0.2 | |
| 1-Methyl-6-nitronaphthalene | <55 * | - | <55 * | - | <55 * | - | |
| 2-Methyl-4-nitronaphthalene | <62 * | - | <62 * | - | <62 * | - | |
| 3-Nitrobiphenyl | 1.1 | ±0.2 | 1.7 | ±0.7 | 0.9 | ±0.1 | |
| 4-Nitrobiphenyl | 1.1 | ±0.3 | 1.3 | ±0.1 | 0.8 | ±0.1 | |
| 5-Nitroacenaphthene | <52 * | - | <52 * | - | <52 * | - | |
| 2-Nitrofluorene | 56.3 | ±0.8 | 99.7 | ±66.2 | 68.8 | ±44.6 | |
| 2-Nitrophenanthrene | <53 * | - | <53 * | - | <53 * | - | |
| 3-Nitrophenanthrene | 5.4 | ±1.0 | 8.5 | ±3.3 | 4.8 | ±1.1 | |
| 9-Nitrophenanthrene | 2.4 | ±0.3 | 3.6 | ±0.9 | 2.1 | ±0.5 | |
| 2-Nitroanthracene | <33 * | - | <33 * | - | <33 * | - | |
| 9-Nitroanthracene | 3.8 | ±1.7 | <15 * | - | <15 * | - | |
| 2-Nitrofluoranthene | <39 * | - | <39 * | - | <39 * | - | |
| 3-Nitrofluoranthene | <36 * | - | <36 * | - | <36 * | - | |
| 1-Nitropyrene | <48 * | - | <48 * | - | <48 * | - | |
| 2-Nitropyrene | <27 * | - | <27 * | - | <27 * | - | |
| 4-Nitropyrene | <22 * | - | <22 * | - | <22 * | - | |
| 7-Nitrobenz(a)anthracene | <54 * | - | 1.4 | ±1.3 | <54 * | - | |
| 6-Nitrochrysene | <24 * | - | 5.2 | ±1.8 | 1.2 | ±2.1 | |
| 3-Nitrobenzanthrone | 1.2 | ±0.4 | 0.8 | ±0.0 | 1.6 | ±0.0 | |
| 6-Nitrobenzo[a]pyrene | <28 * | - | <28 * | - | <28 * | - | |
| 1-Nitrobenzo[e]pyrene | <11 * | - | <11 * | - | <11 * | - | |
| 3-Nitrobenzo[e]pyrene | <22 * | - | <22 * | - | <22 * | - | |
| Ʃ | 77.8 | ±6.3 | 128.4 | ±75.6 | 85.4 | ±50.9 | |
| (a) PM1.0 | |||||||
| B7 | B7E3 | B7E10 | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| PAHs | PYR | 0.69 | ±0.59 | 0.59 | ±0.10 | 0.94 | ±0.14 |
| FLT | 0.25 | ±0.22 | 0.22 | ±0.04 | 0.38 | ±0.05 | |
| PHEN | 0.23 | ±0.22 | 0.27 | ±0.07 | 0.27 | ±0.03 | |
| NAPH | 0.19 | ±0.07 | 0.14 | ±0.06 | 0.19 | ±0.06 | |
| CHRYS | 0.10 | ±0.09 | 0.13 | ±0.02 | 0.14 | ±0.02 | |
| Ʃ | 1.46 | ±0.21 | 1.36 | ±0.17 | 1.92 | ±0.29 | |
| nitro-PAHs | 2N-FLUO | 2.44 | ±2.12 | 3.11 | ±1.17 | 8.30 | ±2.65 |
| 2N-PHEN | 0.12 | ±0.11 | 0.20 | ±0.03 | 0.36 | ±0.09 | |
| 2-NBP | 0.13 | ±0.11 | 0.15 | ±0.02 | 0.14 | ±0.03 | |
| Ʃ | 2.69 | ±1.09 | 3.46 | ±1.38 | 8.81 | ±3.80 | |
| quinones | 9,10-AQ | 0.13 | ±0.12 | 0.13 | ±0.05 | 0.21 | ±0.05 |
| Ʃ | 0.13 | ±0.12 | 0.13 | ±0.05 | 0.21 | ±0.05 | |
| (b) PM2.5 | |||||||
| B7 | B7E3 | B7E10 | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| PAHs | PYR | 1.19 | ±0.09) | 1.52 | (±0.69) | 0.64 | (±0.12) |
| FLT | 0.75 | ±0.20 | 0.68 | ±0.27 | 0.34 | ±0.05 | |
| PHEN | 0.49 | ±0.07 | 0.58 | ±0.23 | 0.32 | ±0.14 | |
| NAPH | 0.40 | ±0.07 | 0.63 | ±0.24 | 0.34 | ±0.08 | |
| CHRYS | 0.20 | ±0.02 | 0.28 | ±0.16 | 0.13 | ±0.04 | |
| Ʃ | 3.03 | ±0.34 | 3.69 | ±0.42 | 1.77 | ±0.16 | |
| nitro PAH | 2N-FLUO | 4.15 | ±0.06 | 7.34 | ±4.87 | 4.84 | ±3.13 |
| 2N-PHEN | 0.31 | ±0.05 | 0.52 | ±0.29 | 0.21 | ±0.07 | |
| 2-NBP | 0.28 | ±0.05 | 0.21 | ±0.06 | 0.19 | ±0.06 | |
| Ʃ | 7.97 | ±1.82 | 12.04 | ±3.29 | 7.13 | ±2.19 | |
| quinones | 9,10-AQ | 0.24 | ±0.05 | 0.31 | ±0.13 | 0.19 | ±0.09 |
| Ʃ | 0.24 | ±0.05 | 0.31 | ±0.13 | 0.19 | ±0.09 | |
| Considering Carcinogenicity Only * | ||||||
| Inhalation Daily Exposure (PM1.0) a | Incremental Lifetime Cancer Risk (PM1.0) | |||||
| Categories | B7 | B7E3 | B7E10 | B7 | B7E3 | B7E10 |
| Adults (>21 years) | 2393 | 30,043 | 3259 | 6.71 × 10−5 | 8.42 × 10−4 | 9.14 × 10−5 |
| Adolescent (11–16 years) | 3195 | 40,119 | 4352 | 1.51 × 10−5 | 1.90 × 10−4 | 2.06 × 10−5 |
| Children (1–11 years) | 1940 | 24,364 | 2643 | 3.61 × 10−5 | 4.54 × 10−4 | 4.92 × 10−5 |
| Infants (<1 year) | 992 | 12,457 | 1351 | 6.54 × 10−5 | 8.22 × 10−5 | 8.91 × 10−5 |
| Inhalation daily exposure (PM2.5) | Incremental lifetime cancer risk (PM2.5) | |||||
| Categories | B7 | B7E3 | B7E10 | B7 | B7E3 | B7E10 |
| Adults (>21 years) | 4262 | 7139 | 3036 | 1.19 × 10−4 | 2.00 × 10−4 | 8.51 × 10−5 |
| Adolescent (11–16 years) | 5692 | 9533 | 4054 | 2.70 × 10−5 | 4.52 × 10−5 | 1.92 × 10−5 |
| Children (1–11 years) | 3457 | 5789 | 2462 | 6.44 × 10−5 | 1.08 × 10−4 | 4.58 × 10−5 |
| Infants (<1 year) | 1767 | 2960 | 1259 | 1.17 × 10−5 | 1.95 × 10−5 | 8.30 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paim, J.N.; Santos, A.G.; Araujo, R.G.O.; Nascimento, M.M.; De Andrade, J.B.; Guarieiro, L.L.N. Emissions of PAHs, Nitro-PAHs and Quinones (Oxy-PAHs) Associated to PM1.0 and PM2.5 Emitted by a Diesel Engine Fueled with Diesel-Biodiesel-Ethanol Blends. Atmosphere 2023, 14, 656. https://doi.org/10.3390/atmos14040656
Paim JN, Santos AG, Araujo RGO, Nascimento MM, De Andrade JB, Guarieiro LLN. Emissions of PAHs, Nitro-PAHs and Quinones (Oxy-PAHs) Associated to PM1.0 and PM2.5 Emitted by a Diesel Engine Fueled with Diesel-Biodiesel-Ethanol Blends. Atmosphere. 2023; 14(4):656. https://doi.org/10.3390/atmos14040656
Chicago/Turabian StylePaim, Joilson Nascimento, Aldenor Gomes Santos, Rennan G. O. Araujo, Madson Moreira Nascimento, Jailson Bittencourt De Andrade, and Lilian Lefol Nani Guarieiro. 2023. "Emissions of PAHs, Nitro-PAHs and Quinones (Oxy-PAHs) Associated to PM1.0 and PM2.5 Emitted by a Diesel Engine Fueled with Diesel-Biodiesel-Ethanol Blends" Atmosphere 14, no. 4: 656. https://doi.org/10.3390/atmos14040656
APA StylePaim, J. N., Santos, A. G., Araujo, R. G. O., Nascimento, M. M., De Andrade, J. B., & Guarieiro, L. L. N. (2023). Emissions of PAHs, Nitro-PAHs and Quinones (Oxy-PAHs) Associated to PM1.0 and PM2.5 Emitted by a Diesel Engine Fueled with Diesel-Biodiesel-Ethanol Blends. Atmosphere, 14(4), 656. https://doi.org/10.3390/atmos14040656






