Environmental Analysis, Monitoring, and Process Control Strategy for Reduction of Greenhouse Gaseous Emissions in Thermochemical Reactions
Abstract
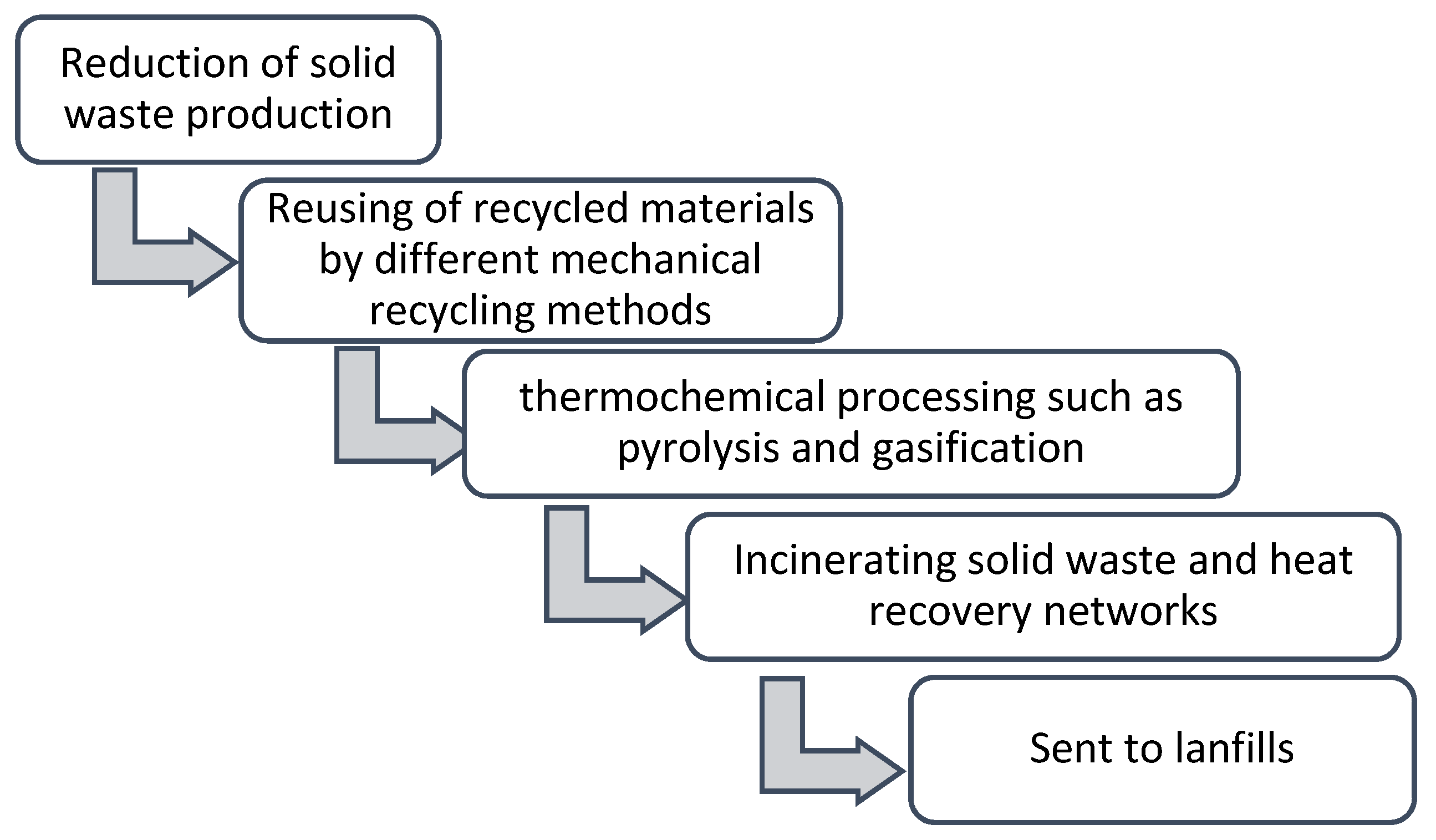
1. Introduction
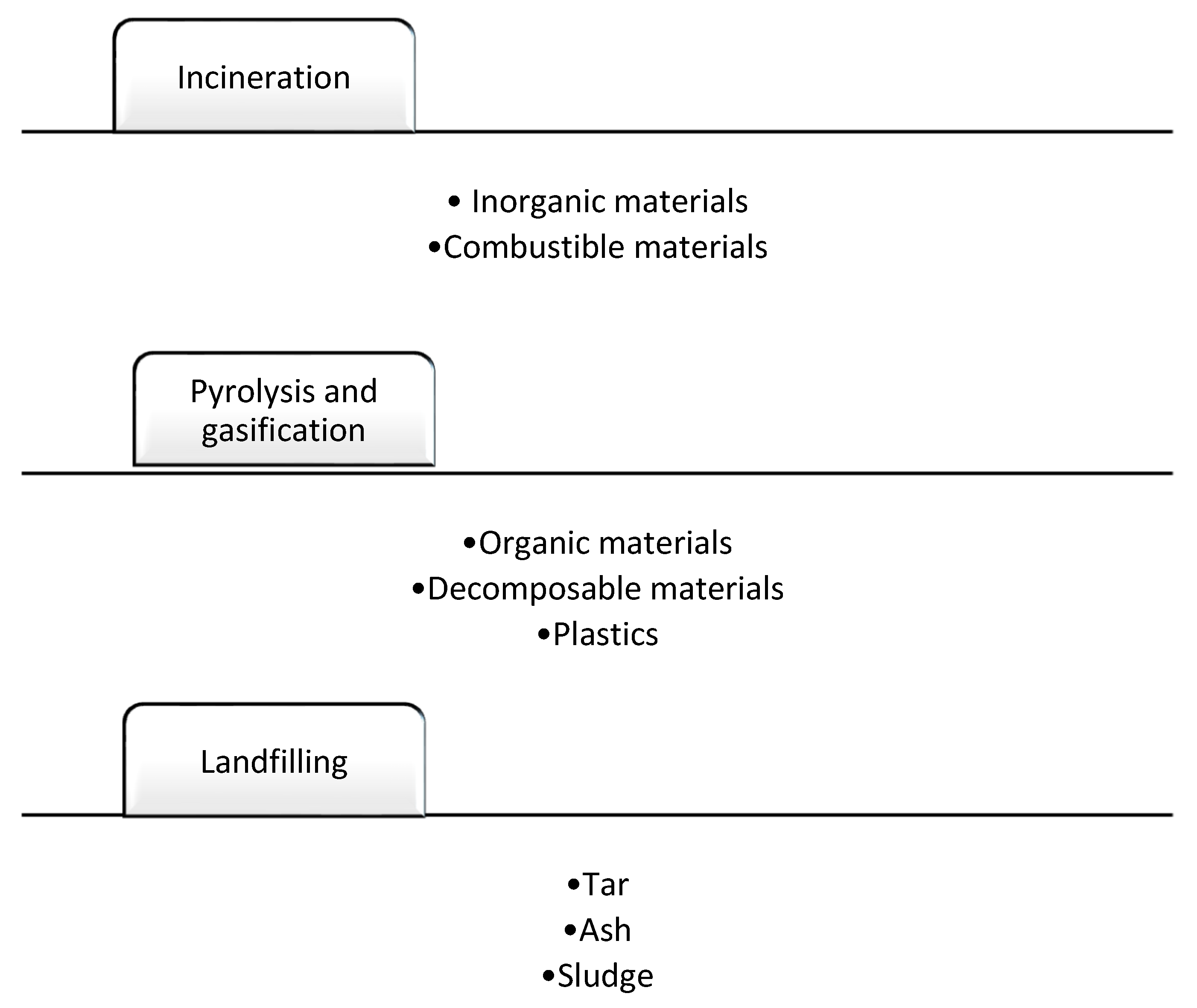
Incineration and Landfilling Industrial Stages
- Processing of municipal solid waste.
- The collection system of unprocessed deposits, such as metals, wood, polymers, and organic materials, using magnetic separators.
- High-temperature incinerators with excess oxygen supply.
- Ash removal system and separators.
- Steam and electricity generation.
- Ash disposal systems.
2. Environmental Assessment of Greenhouse Gas Emissions from Thermochemical Reactions
- Municipal solid waste processing.
- A gas separation and processing unit.
- An environmental control and monitoring unit.
- A gas and steam combustion unit.
- A steam generation unit.
- A waste to energy process system.
- A heat integration unit.
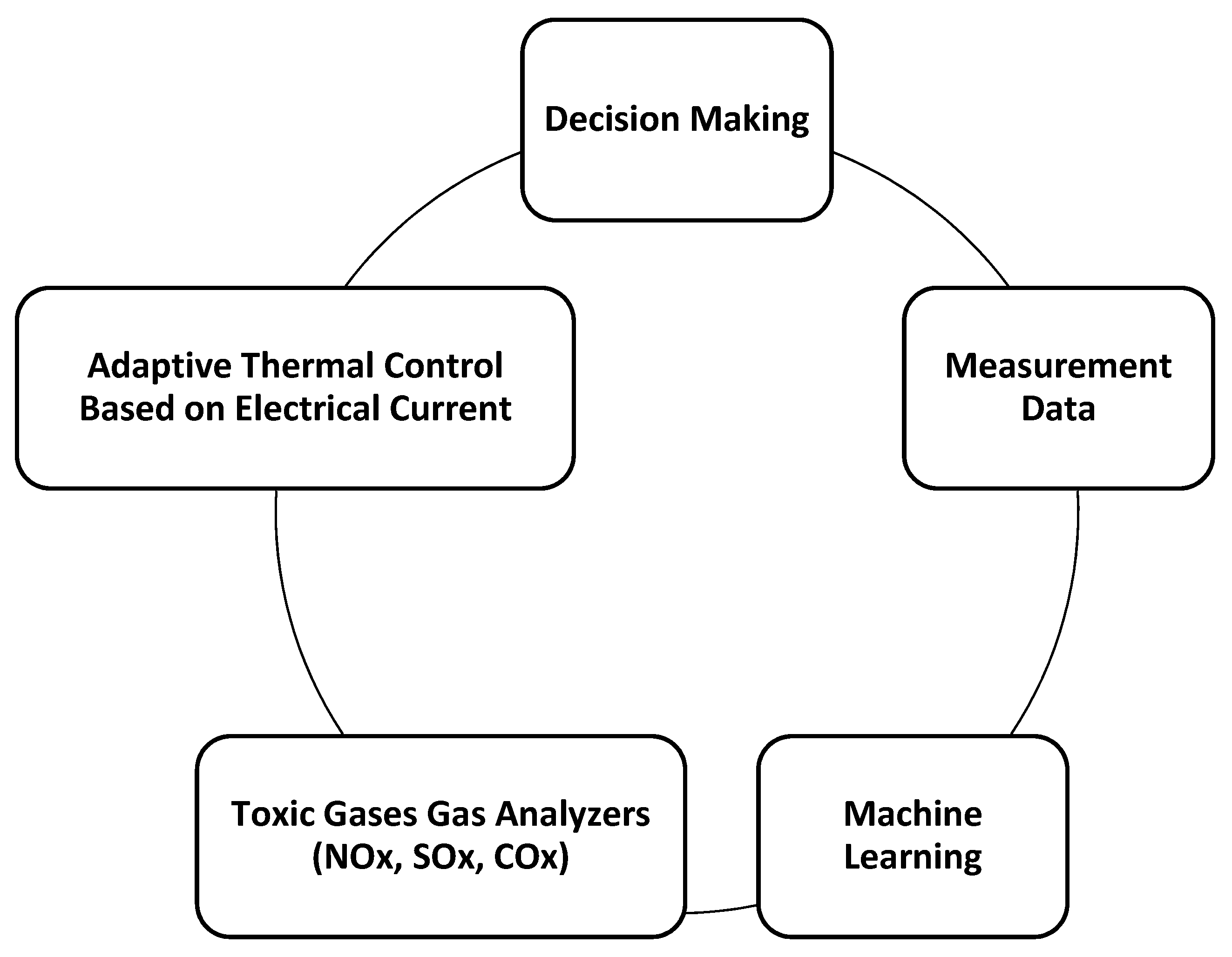
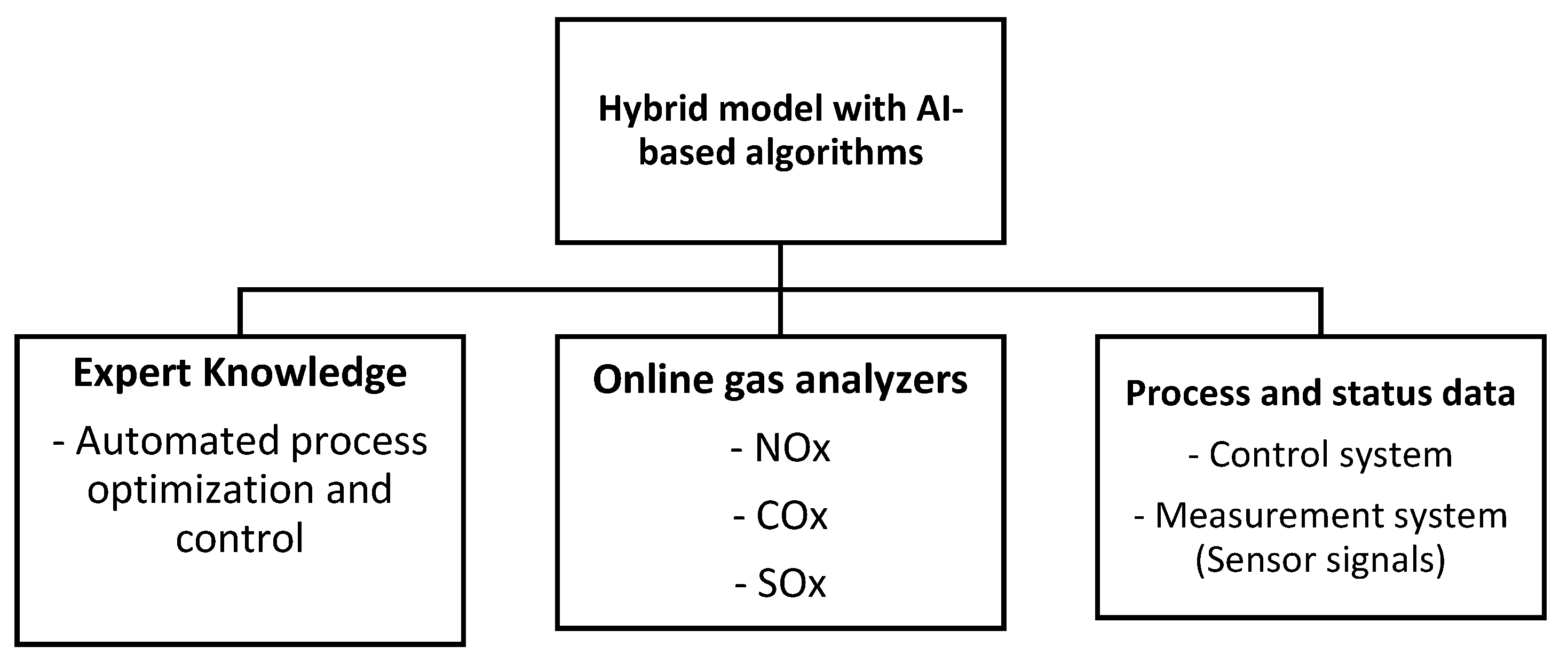
3. Recommended Monitoring and Process Control Techniques for Thermochemical Reactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHP | combined heat and power cycle |
| GWP | global warming potential |
| LHV | lower heating value MJ/nm3 |
| HTP | human toxicity potential |
| MSW | municipal solid waste |
| MSWM | municipal solid waste management |
| PSD | particle size diameter |
| RDF | refused derived fuel (i.e., treated MSW feedstock) |
| VOC | volatile organic compound |
| Nomenclature | |
| energy value (KJ/Kg) | |
| K | Kelvin |
| Mg/Rm3 | milligram per dry cubic meter of flue gas |
References
- Di Gianfilippo, M.; Costa, G.; Pantini, S.; Allegrini, E.; Lombardi, F.; Astrup, T.F. LCA of management strategies for RDF incineration and gasification bottom ash based on experimental leaching data. Waste Manag. 2016, 47, 285–298. [Google Scholar] [CrossRef]
- Jardine, C.; Hrudey, S.; Shortreed, J.; Craig, L.; Krewski, D.; Furgal, C.; McColl, S. Risk Management Frameworks for Human Health and Environmental Risks. J. Toxicol. Environ. Health B Crit. Rev. 2003, 6, 569–720. [Google Scholar] [CrossRef] [PubMed]
- Ćetković, J.; Lakić, S.; Živković, A.; Žarković, M.; Vujadinović, R. Economic analysis of measures for GHG emission reduction. Sustainability 2021, 13, 1712. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Sundarakannan, R.; Arumugaprabu, V.; Manikandan, V.; Vigneshwaran, S. Mechanical property analysis of biochar derived from cashew nut shell waste reinforced polymer matrix. Mater. Res. Express 2019, 6, 125349. [Google Scholar] [CrossRef]
- Yang, R.X.; Jan, K.; Chen, C.T.; Chen, W.T.; Wu, K.C.W. Thermochemical Conversion of Plastic Waste into Fuels, Chemicals, and Value-Added Materials: A Critical Review and Outlooks. ChemSusChem 2022, 15, e202200171. [Google Scholar] [CrossRef] [PubMed]
- Farulla, G.A.; Cellura, M.; Guarino, F.; Ferraro, M. A review of thermochemical energy storage systems for power grid support. Appl. Sci. 2020, 10, 3142. [Google Scholar] [CrossRef]
- Kane, S.; van Roijen, E.; Ryan, C.; Miller, S. Reducing the environmental impacts of plastics while increasing strength: Biochar fillers in biodegradable, recycled, and fossil-fuel derived plastics. Compos. Part C Open Access 2022, 8, 100253. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Ramanathan, A.; Begum, K.M.M.S.; Pereira, A.O.; Cohen, C. Pyrolysis of waste biomass: Toward sustainable development. In A Thermo-Economic Approach to Energy from Waste; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chiueh, P.T.; Lo, S.L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Sabogal, O.S.; Valin, S.; Thiery, S.; Salvador, S. Pyrolysis of solid waste and its components in a lab scale induction-heating reactor. Detritus 2021, 15, 107–112. [Google Scholar] [CrossRef]
- Xu, X.; Chen, R.; Pan, R.; Zhang, D. Pyrolysis Kinetics, Thermodynamics, and Volatiles of Representative Pine Wood with Thermogravimetry-Fourier Transform Infrared Analysis. Energy Fuels 2020, 34, 1859–1869. [Google Scholar] [CrossRef]
- Haeldermans, T.; Campion, L.; Kuppens, T.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. A comparative techno-economic assessment of biochar production from different residue streams using conventional and microwave pyrolysis. Bioresour. Technol. 2020, 318, 124083. [Google Scholar] [CrossRef]
- Mandviwala, C.; Vilches, T.B.; Seemann, M.; Faust, R.; Thunman, H. Thermochemical conversion of polyethylene in a fluidized bed: Impact of transition metal-induced oxygen transport on product distribution. J. Anal. Appl. Pyrolysis 2022, 163, 105476. [Google Scholar] [CrossRef]
- Khodaei, H.; Gonzalez, L.; Chapela, S.; Porteiro, J.; Nikrityuk, P.; Olson, C. CFD-based coupled multiphase modeling of biochar production using a large-scale pyrolysis plant. Energy 2021, 217, 119325. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Ma, D.; Chen, C.; Wang, Y.; Wang, W.; Li, A. Exergy and energy analysis of pyrolysis of plastic wastes in rotary kiln with heat carrier. Process Saf. Environ. Prot. 2020, 142, 203–211. [Google Scholar] [CrossRef]
- Wijayanti, W.; Wijayanti, W.; Musyaroh; Sasongko, M.N.; Kusumastuti, R.S. Modelling analysis of pyrolysis process with thermal effects by using Comsol Multiphysics. Case Stud. Therm. Eng. 2021, 28, 101625. [Google Scholar] [CrossRef]
- Bach, Q.V.; Chen, W.H.; Eng, C.F.; Wang, C.W.; Liang, K.C.; Kuo, J.Y. Pyrolysis characteristics and non-isothermal torrefaction kinetics of industrial solid wastes. Fuel 2019, 251, 118–125. [Google Scholar] [CrossRef]
- Nam, N.H.; Anh, K.D.; Truc, L.G.T.; Ha, T.A.; Ha, V.T.T. Pyrolysis of cashew nut shell: A parametric study. Vietnam J. Chem. 2020, 58, 506–511. [Google Scholar] [CrossRef]
- Kirtania, K. Thermochemical conversion processes for waste biorefinery. In Waste Biorefinery: Potential and Perspectives; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X. The thermochemical conversion of biomass into biofuels. In Biomass, Biopolymer-Based Materials, and Bioenergy: Construction, Biomedical, and Other Industrial Applications; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Kundu, K.; Chatterjee, A.; Bhattacharyya, T.; Roy, M.; Kaur, A. Thermochemical Conversion of Biomass to Bioenergy: A Review. In Prospects of Alternative Transportation Fuels; Energy, Environment, and Sustainability; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Casini, D.; Rizzo, A.M. Thermochemical Conversion of Microalgae: Challenges and Opportunities. Energy Procedia 2015, 75, 819–826. [Google Scholar] [CrossRef]
- Guragain, Y.N.; Vadlani, P.V. Renewable Biomass Utilization: A Way Forward to Establish Sustainable Chemical and Processing Industries. Clean Technol. 2021, 3, 243–259. [Google Scholar] [CrossRef]
- Nzeribe, B.N.; Crimi, M.; Thagard, S.M.; Holsen, T.M. Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Wijakmatee, T.; Hemra, N.; Wongsakulphasatch, S.; Narataruksa, P.; Cheenkachorn, K.; Prapainainar, C. Process intensification of biodiesel production with integrated microscale reactor and separator. Chem. Eng. Process. Process Intensif. 2021, 164, 108422. [Google Scholar] [CrossRef]
- Baliban, R.C.; Elia, J.A.; Floudas, C.A. Optimization framework for the simultaneous process synthesis, heat and power integration of a thermochemical hybrid biomass, coal, and natural gas facility. Comput. Chem. Eng. 2011, 35, 1647–1690. [Google Scholar] [CrossRef]
- Ghimire, N.; Bakke, R.; Bergland, W.H. Liquefaction of lignocellulosic biomass for methane production: A review. Bioresour. Technol. 2021, 332, 125068. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, S.C.; Carrere, H.; Maciel Filho, R.; Costa, A.C. Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour. Technol. 2011, 102, 7887–7895. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. A novel approach in organic waste utilization through biochar addition in wood/polypropylene composites. Waste Manag. 2015, 38, 132–140. [Google Scholar] [CrossRef]
- Dalai, A.K.; Batta, N.; Eswaramoorthi, I.; Schoenau, G.J. Gasification of refuse derived fuel in a fixed bed reactor for syngas production. Waste Manag. 2009, 29, 252–258. [Google Scholar] [CrossRef]
- Anastasovski, A.; Rasković, P.; Guzović, Z. A review of heat integration approaches for organic rankine cycle with waste heat in production processes. Energy Convers. Manag. 2020, 221, 113175. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Aboughaly, M.; Ayoub, N. Comparative study of MSW heat treatment processes and electricity generation. J. Energy Inst. 2018, 91, 481–488. [Google Scholar] [CrossRef]
- Moya, D.; Aldás, C.; Jaramillo, D.; Játiva, E.; Kaparaju, P. Waste-To-Energy Technologies: An opportunity of energy recovery from Municipal Solid Waste, using Quito-Ecuador as case study. Energy Procedia 2017, 134, 327–336. [Google Scholar] [CrossRef]
- Bishoge, O.K.; Huang, X.; Zhang, L.; Ma, H.; Danyo, C. The adaptation of waste-to-energy technologies: Towards the conversion of municipal solid waste into a renewable energy resource. Environ. Rev. 2019, 27, 435–446. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, X.; Peng, X.; Yu, Z.; Fang, S.; Lin, Y.; Fan, Y. Combustion, pyrolysis and char CO2-gasification characteristics of hydrothermal carbonization solid fuel from municipal solid wastes. Fuel 2016, 181, 905–915. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Li, Q.; Faramarzi, A.; Zhang, S.; Wang, Y.; Hu, X.; Gholizadeh, M. Progress in catalytic pyrolysis of municipal solid waste. Energy Convers. Manag. 2020, 226, 113525. [Google Scholar] [CrossRef]
- EVenturini, E.; Vassura, I.; Passarini, F.; Bernardi, E.; Ciacci, L.; Ferroni, L. The environmental impact of a municipal solid waste incinerator: 15 years of monitoring. WIT Trans. Ecol. Environ. 2014, 180, 305–316. [Google Scholar] [CrossRef]
- Gutierrez-Gomez, A.C.; Gallego, A.G.; Palacios-Bereche, R.; de Campos Leite, J.T.; Neto, A.M. Energy recovery potential from Brazilian municipal solid waste via combustion process based on its thermochemical characterization. J. Clean. Prod. 2021, 293, 126145. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Bunsan, S.; Chen, W.Y.; Chen, H.W.; Chuang, Y.H.; Grisdanurak, N. Modeling the dioxin emission of a municipal solid waste incinerator using neural networks. Chemosphere 2013, 92, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Burra, K.G.; Wang, Z.; Liu, X.; Kerdsuwan, S.; Gupta, A.K. Energy recovery from composite acetate polymer-biomass wastes via pyrolysis and CO2-assisted gasification. J. Energy Resour. Technol. Trans. ASME 2021, 143, 042305. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, R.; Wang, J.; Chen, X.; Ge, X.; Chen, M. Waste-to-energy: Dehalogenation of plastic-containing wastes. Waste Manag. 2016, 49, 287–303. [Google Scholar] [CrossRef]
- Gwenzi, W. Wastewater, waste, and water-based epidemiology (WWW-BE): A novel hypothesis and decision-support tool to unravel COVID-19 in low-income settings? Sci. Total Environ. 2022, 806, 150680. [Google Scholar] [CrossRef] [PubMed]
- Monni, S. From landfilling to waste incineration: Implications on GHG emissions of different actors. Int. J. Greenh. Gas Control 2012, 8, 82–89. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, Z.; Zhu, S.; Lou, Z.; Zhu, N.; Feng, L. The identification and health risk assessment of odor emissions from waste landfilling and composting. Sci. Total Environ. 2019, 649, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Demirgian, J.C. Development of calibration standards for fourier transform infrared spectrometer in continuous monitoring of incinerator emissions. Waste Manag. 1995, 15, 233–241. [Google Scholar] [CrossRef]
- Tan, R.R.; Aviso, K.B.; Foo, D.C.Y. Carbon emissions pinch analysis of economic systems. J. Clean. Prod. 2018, 182, 863–871. [Google Scholar] [CrossRef]
- Priya, G.S.K.; Bandyopadhyay, S. Multiple objectives Pinch Analysis. Resour. Conserv. Recycl. 2017, 119, 128–141. [Google Scholar] [CrossRef]
- Pacheco-López, A.; Lechtenberg, F.; Somoza-Tornos, A.; Graells, M.; Espuña, A. Economic and Environmental Assessment of Plastic Waste Pyrolysis Products and Biofuels as Substitutes for Fossil-Based Fuels. Front. Energy Res. 2021, 9, 676233. [Google Scholar] [CrossRef]
- Gamisch, B.; Gaderer, M.; Dawoud, B. On the development of thermochemical hydrogen storage: An experimental study of the kinetics of the redox reactions under different operating conditions. Appl. Sci. 2021, 11, 1623. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Aboughaly, M.; Damideh, V.; Hassen, I. RF-ICP thermal plasma for thermoplastic waste pyrolysis process with high conversion yield and tar elimination. Processes 2020, 8, 281. [Google Scholar] [CrossRef]
- Gupta, A.; Armatis, P.D.; Sabharwall, P.; Fronk, B.M.; Utgikar, V. Kinetics of Ca(OH)2 decomposition in pure Ca(OH)2 and Ca(OH)2-CaTiO3 composite pellets for application in thermochemical energy storage system. Chem. Eng. Sci. 2021, 246, 116986. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger reactors for pyrolysis of biomass and wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Jagtap, A.; Kalbande, S.R. Investigation on pyrolysis kinetics and thermodynamic parameters of soybean straw: A comparative study using model-free methods. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Risthaus, K.; Bürger, I.; Lutz, M.; Funayama, S.; Kato, Y.; Linder, M.; Schmidt, M. Experimental and Numerical Investigation of the Dehydration of Ca(OH)2 at Low Steam Pressures. Processes 2022, 10, 325. [Google Scholar] [CrossRef]
- Takeuchi, S.; Mori, T.; Sato, D.; Inubushi, T.; Ougiya, N.; Kanda, M. Development of substructure real-time online testing system for seismically isolated building with a small response delay using existing experimental equipment. AIJ J. Technol. Des. 2022, 28, 121–126. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, F.; Yu, Z.; Shi, D.; Liu, S.; Chen, M. Highly sensitive folic acid colorimetric sensor enabled by free-standing molecularly imprinted photonic hydrogels. Polym. Bull. 2022, 79, 1857–1871. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Fernández, A.; Bernalte, E.; Fernández-Ramos, C.; Moise, S.; Estrela, P.; Di Lorenzo, M. An impedimetric immunosensor for the selective detection of CD34+ T-cells in human serum. Sens. Actuators B Chem. 2022, 356, 131306. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, X.; Ma, H.; Liu, C.; Zhou, C.; Qi, F. Real-time monitoring biomass pyrolysis via on-line photoionization ultrahigh-resolution mass spectrometry. Fuel 2019, 235, 962–971. [Google Scholar] [CrossRef]
- Dhahak, A.; Grimmer, C.; Neumann, A.; Rüger, C.; Sklorz, M.; Streibel, T.; Zimmermann, R.; Mauviel, G.; Burkle-Vitzthum, V. Real time monitoring of slow pyrolysis of polyethylene terephthalate (PET) by different mass spectrometric techniques. Waste Manag. 2020, 106, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Kong, C.; Ren, L.; Sun, Y.; Chang, Z. Real-time monitoring of the oil shale pyrolysis process using a bionic electronic nose. Fuel 2022, 313, 122672. [Google Scholar] [CrossRef]
- Pipkin, W.; Belganeh, R.; Robberson, W.; Allen, H.L.; Cook, A.M.; Watanabe, A. Identification of microplastics in environmental monitoring using pyrolysis–GC–MS analysis. LC-GC N. Am. 2021, 39, 179–186. [Google Scholar]
- Navarra, A.; Wilson, R.; Parra, R.; Toro, N.; Ross, A.; Nave, J.-C.; Mackey, P.J. Quantitative methods to support data acquisition modernization within copper smelters. Processes 2020, 8, 1478. [Google Scholar] [CrossRef]
- Zhu, W.; Zhan, Y.; Romagnoli, J.A. A deep learning approach on industrial pyrolysis reactor monitoring. Chem. Eng. Trans. 2019, 74, 691–696. [Google Scholar] [CrossRef]
- Özsin, G.; Apaydın-Varol, E.; Kılıç, M.; Pütün, A.E.; Pütün, E. Pyrolysis of petroleum sludge under non-isothermal conditions: Thermal decomposition behavior, kinetics, thermodynamics, and evolved gas analysis. Fuel 2021, 300, 120980. [Google Scholar] [CrossRef]
- Troup, G.M.; Georgakis, C. Process systems engineering tools in the pharmaceutical industry. Comput. Chem. Eng. 2013, 51, 157–171. [Google Scholar] [CrossRef]
- Brown, R.C. Process Intensification through Directly Coupled Autothermal Operation of Chemical Reactors. Joule 2020, 4, 2268–2289. [Google Scholar] [CrossRef]
- Rizkin, B.A.; Popovich, K.; Hartman, R.L. Artificial Neural Network control of thermoelectrically-cooled microfluidics using computer vision based on IR thermography. Comput. Chem. Eng. 2019, 121, 584–593. [Google Scholar] [CrossRef]
- Labutin, A.N.; Vaško, M.; Kuric, I.; Nevinitsyn, V.Y.; Sága, M.; Zagarinskaya, Y.N.; Volkova, G.V. Analytical synthesis of non-linear control algorithms of a chemical reactor thermal mode. Processes 2021, 9, 644. [Google Scholar] [CrossRef]
- Cukor, P.; Persiani, C. Evaluation of two pyrolyzers for the analysis of insoluble polymers. J. Macromol. Sci. Part A Chem. 1974, 8, 105–117. [Google Scholar] [CrossRef]
- Risoluti, R.; Fabiano, M.A.; Gullifa, G.; Ciprioti, S.V.; Materazzi, S. FTIR-evolved gas analysis in recent thermoanalytical investigations. Appl. Spectrosc. Rev. 2017, 52, 39–72. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Forloni, F.; Otura, M.; Engström, C.; Öberg, P. Experimental Comparison of Hub- and Roller-Type Chassis Dynamometers for Vehicle Exhaust Emissions. Energies 2022, 15, 2402. [Google Scholar] [CrossRef]
- Fraser, A. Basslines, brains, bits, bytes, and burgers: Working with, and within the limits to, Marxism. Hum. Geogr. 2022, 15, 147–153. [Google Scholar] [CrossRef]
- Martínez-Castillo, J.I.; Saldaña-Robles, A.; Ozuna, C. Arsenic stress in plants: A metabolomic perspective. Plant Stress 2022, 3, 100055. [Google Scholar] [CrossRef]
- Nerín, C.; Bourdoux, S.; Faust, B.; Gude, T.; Lesueur, C.; Simat, T.; Stoermer, A.; Van Hoek, E.; Oldring, P. Guidance in selecting analytical techniques for identification and quantification of non-intentionally added substances (NIAS) in food contact materials (FCMS). Food Addit. Contam. Part A 2022, 39, 620–643. [Google Scholar] [CrossRef] [PubMed]
- Mandelis, A. Focus on test, measurement, and analytical equipment. Phys. Today 2020, 73, 58. [Google Scholar] [CrossRef]
- Vlasov, A.I.; Echeistov, V.V.; Krivoshein, A.I.; Shakhnov, V.A.; Filin, S.S.; Migalin, V.S. An information system of predictive maintenance analytical support of industrial equipment. J. Appl. Eng. Sci. 2018, 16, 515–522. [Google Scholar] [CrossRef]
- Mandelis, A. Focus on test, measurement, analytical equipment, and instrumentation. Phys. Today 2021, 74, 55–58. [Google Scholar] [CrossRef]
- Uraikul, V.; Chan, C.W.; Tontiwachwuthikul, P. Artificial intelligence for monitoring and supervisory control of process systems. Eng. Appl. Artif. Intell. 2007, 20, 115–131. [Google Scholar] [CrossRef]
- Qin, M.; Guo, H.; Dai, Z.; Yan, X.; Ning, X. Advances in flexible and wearable pH sensors for wound healing monitoring. J. Semicond. 2019, 40, 111607. [Google Scholar] [CrossRef]
| Chemical Process | Hydrocarbon Oils | Char (Solid) | Product Gas | Conversion Efficiency (%) | Electrical Efficiency (%) |
|---|---|---|---|---|---|
| Fast pyrolysis | 60–70% | 10–15% | 10–25% | 25–45 | 15 |
| Gasification | ≈20% | ≈20% | ≈85% | 26–40 | 34 |
| Pollutant Gas | Operational Methods | Absorption Efficiency (%) |
|---|---|---|
| SO2 | Wet scrubbing or dry cyclone | 55–95 |
| HCl | Wet or semi-dry scrubbing | 70–90 |
| NO2 | Catalytic reduction | 15–63 |
| Heavy metals | Dry scrubbing and electrostatic precipitators | 75–95 |
| Ash | Carbon filters and optimum operating conditions | >95 |
| Dioxins and furans | Activated carbon beds and filters | 50–90 |
| Pollutant Gas | Maximum Allowable at the Exhaust (mg/Nm3) | Required Removal Efficiency (%) |
|---|---|---|
| Ash | 10 | 99.9 |
| HCl | 10 | >99 |
| SO2 | 5 | 99.5 |
| NOx | 70 | 86 |
| HF | 1 | 96 |
| Hg | 0.01 | 99 |
| Dioxins | 0.1 | 98 |
| Gaseous Emission | Pyrolysis (g/Tonne) | Gasification of Non-RDF (g/Tonne) | Gasification of RDF (g/Tonne) |
|---|---|---|---|
| Nitrogen oxide | 196 | 781 | 453 |
| Sulphur dioxide | 28 | 19.5 | 10.5 |
| Carbon monoxide | 28.1 | 195 | 116 |
| Hydrogen chloride | 5.62 | 3.91 | 17.2 |
| Hydrogen fluoride | 0.562 | 3.91 | 0.116 |
| Dust | 8.43 | 39.1 | 6.97 |
| MSW (%) | Mass wt.% per ton of MSW (%) |
|---|---|
| Char ash | 26–45 |
| Flying ash | 1–5 |
| Syngas with acidic components | 1.5–4 |
| Syngas with semi-dry acid | 1–6 |
| Gaseous Component | Incineration (g/T) | Landfilling (g/T) |
|---|---|---|
| Nitrogen oxide | 1600 | 680 |
| Particulates | 39 | 5.3 |
| SO2 | 42 | 53 |
| HCl | 58 | 3 |
| HF | 8 | 3 |
| VOCs | 8 | 6.4 |
| Cadmium | 0.005 | 0.071 |
| Nickel | 0.05 | 0.0095 |
| Arsenic | 0.005 | 0.0012 |
| Mercury | 0.05 | 0.0012 |
| Dioxins and furans | 4 × 10−7 | 1.4 × 10−7 |
| Carbon dioxide | 1,000,000 | 300,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboughaly, M.; Fattah, I.M.R. Environmental Analysis, Monitoring, and Process Control Strategy for Reduction of Greenhouse Gaseous Emissions in Thermochemical Reactions. Atmosphere 2023, 14, 655. https://doi.org/10.3390/atmos14040655
Aboughaly M, Fattah IMR. Environmental Analysis, Monitoring, and Process Control Strategy for Reduction of Greenhouse Gaseous Emissions in Thermochemical Reactions. Atmosphere. 2023; 14(4):655. https://doi.org/10.3390/atmos14040655
Chicago/Turabian StyleAboughaly, Mohamed, and I. M. Rizwanul Fattah. 2023. "Environmental Analysis, Monitoring, and Process Control Strategy for Reduction of Greenhouse Gaseous Emissions in Thermochemical Reactions" Atmosphere 14, no. 4: 655. https://doi.org/10.3390/atmos14040655
APA StyleAboughaly, M., & Fattah, I. M. R. (2023). Environmental Analysis, Monitoring, and Process Control Strategy for Reduction of Greenhouse Gaseous Emissions in Thermochemical Reactions. Atmosphere, 14(4), 655. https://doi.org/10.3390/atmos14040655









