Ozone Formation at a Suburban Site in the Pearl River Delta Region, China: Role of Biogenic Volatile Organic Compounds
Abstract
1. Introduction
2. Methodology
2.1. Site Description
2.2. Sample Collection and Chemical Analysis
2.3. AtChem2-MCM Model
2.3.1. Model Description and Input
2.3.2. Model Performance
2.3.3. Relative Incremental Reactivity (RIR)
2.3.4. Empirical Kinetic Modelling Approach (EKMA)
3. Results and Discussion
3.1. Overviews
3.1.1. Levels of Meteorological Parameters and Measured Species
3.1.2. Diurnal Variation
3.2. O3-Precursor Relationships
3.2.1. O3–VOCs–NOx Sensitivity
3.2.2. Relative Importance of VOCs in O3 Formation
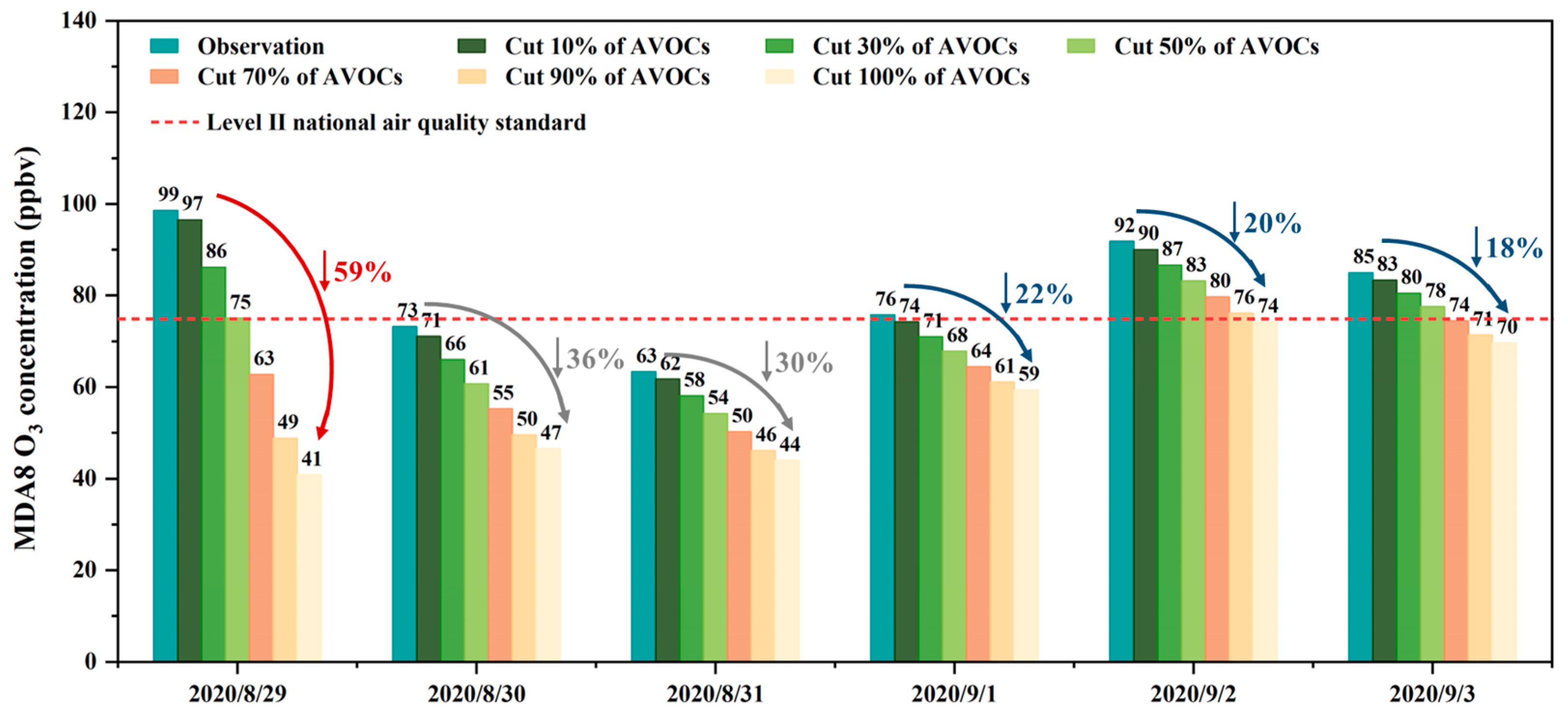
3.3. AVOCs Control Strategies under the Influence of High BVOC Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oltmans, S.J.; Johnson, B.J.; Harris, J.M.; Thompson, A.M.; Liu, H.Y.; Chan, C.Y.; Vomel, H.; Fujimoto, T.; Brackett, V.G.; Chang, W.L.; et al. Tropospheric ozone over the North Pacific from ozonesonde observations. J. Geophys. Res. Atmos. 2004, 109, D15S01. [Google Scholar] [CrossRef]
- Monks, P.S.; Archibald, A.T.; Colette, A.; Cooper, O.; Coyle, M.; Derwent, R.; Fowler, D.; Granier, C.; Law, K.S.; Mills, G.E.; et al. Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmos. Chem. Phys. 2015, 15, 8889–8973. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Zhu, J.; Shah, V.; Shen, L.; Bates, K.H.; Zhang, Q.; Zhai, S.X. A two-pollutant strategy for improving ozone and particulate air quality in China. Nat. Geosci. 2019, 12, 906–910. [Google Scholar] [CrossRef]
- Benish, S.E.; He, H.; Ren, X.R.; Roberts, S.J.; Salawitch, R.J.; Li, Z.Q.; Wang, F.; Wang, Y.Y.; Zhang, F.; Shao, M.; et al. Measurement report: Aircraft observations of ozone, nitrogen oxides, and volatile organic compounds over Hebei Province, China. Atmos. Chem. Phys. 2020, 20, 14523–14545. [Google Scholar] [CrossRef]
- Carter, W.P.L.; Pierce, J.A.; Luo, D.M.; Malkina, I.L. Environmental chamber study of maximum incremental reactivities of volatile organic-compounds. Atmos. Environ. 1995, 29, 2499–2511. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Clemitshaw, K.C. Ozone and other secondary photochemical pollutants: Chemical processes governing their formation in the planetary boundary layer. Atmos. Environ. 2000, 34, 2499–2527. [Google Scholar] [CrossRef]
- Unger, N.; Bond, T.C.; Wang, J.S.; Koch, D.M.; Menon, S.; Shindell, D.T.; Bauer, S. Attribution of climate forcing to economic sectors. Proc. Natl. Acad. Sci. USA 2010, 107, 3382–3387. [Google Scholar] [CrossRef]
- Wilkinson, S.; Mills, G.; Illidge, R.; Davies, W.J. How is ozone pollution reducing our food supply? J. Exp. Bot. 2012, 63, 527–536. [Google Scholar] [CrossRef]
- Yue, X.; Unger, N.; Harper, K.; Xia, X.G.; Liao, H.; Zhu, T.; Xiao, J.F.; Feng, Z.Z.; Li, J. Ozone and haze pollution weakens net primary productivity in China. Atmos. Chem. Phys. 2017, 17, 6073–6089. [Google Scholar] [CrossRef]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Broberg, M.; Uddling, J.; Jaramillo, F.; Davies, W.J.; Dentener, F.; Van den Berg, M.; et al. Ozone pollution will compromise efforts to increase global wheat production. Glob. Chang. Biol. 2018, 24, 3560–3574. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Malley, C.S.; Henze, D.K.; Kuylenstierna, J.C.I.; Vallack, H.W.; Davila, Y.; Anenberg, S.C.; Turner, M.C.; Ashmore, M.R. Updated global estimates of respiratory mortality in adults ≥30 years of age attributable to long-term ozone exposure. Environ. Health Perspect. 2017, 125, 087021. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, Y.Q.; Niu, T.; Zhang, X.C.; Gong, S.L.; Zhang, Y.M.; Sun, J.Y. Atmospheric aerosol compositions in China: Spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols. Atmos. Chem. Phys. 2012, 12, 779–799. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Zamora, M.L.; Peng, J.F.; Shang, D.J.; Zheng, J.; Du, Z.F.; Wu, Z.; Shao, M.; Zeng, L.M.; et al. Elucidating severe urban haze formation in China. Proc. Natl. Acad. Sci. USA 2014, 111, 17373–17378. [Google Scholar] [CrossRef]
- Hu, J.L.; Chen, J.J.; Ying, Q.; Zhang, H.L. One-year simulation of ozone and particulate matter in China using WRF/CMAQ modeling system. Atmos. Chem. Phys. 2016, 16, 10333–10350. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Brimblecombe, P.; Lam, Y.F.; Li, L.; Zhang, L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 2017, 575, 1582–1596. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hong, J.Y.; Zhang, L.; Cooper, O.R.; Schultz, M.G.; Xu, X.B.; Wang, T.; Gao, M.; Zhao, Y.H.; Zhang, Y.H. Severe surface ozone pollution in China: A global perspective. Environ. Sci. Technol. Lett. 2018, 5, 487–494. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013-2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Shen, L.; Lu, X.; De Smedt, I.; Liao, H. Increases in surface ozone pollution in China from 2013 to 2019: Anthropogenic and meteorological influences. Atmos. Chem. Phys. 2020, 20, 11423–11433. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, Y.H.; Zeng, L.M.; Tang, X.Y.; Zhang, J.; Zhong, L.J.; Wang, B.G. Ground-level ozone in the Pearl River Delta and the roles of VOC and NOx in its production. J. Environ. Manag. 2009, 90, 512–518. [Google Scholar] [CrossRef]
- An, J.L.; Zou, J.N.; Wang, J.X.; Lin, X.; Zhu, B. Differences in ozone photochemical characteristics between the megacity Nanjing and its suburban surroundings, Yangtze River Delta, China. Environ. Sci. Pollut. Res. 2015, 22, 19607–19617. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.M.; Yuan, Z.B.; Zheng, J.Y.; Huang, Z.J.; Shao, M.; Li, Z.K.; Huang, X.B.; Guo, H.; Louie, P.K.K. Ambient ozone control in a photochemically active region: Short term despiking or long-term attainment? Environ. Sci. Technol. 2016, 50, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhu, L.Y.; Wang, S.L.; Meng, X.Y.; Zhang, M.G.; Hu, J. Modeling study of impacts on surface ozone of regional transport and emissions reductions over North China Plain in summer 2015. Atmos. Chem. Phys. 2018, 18, 12207–12221. [Google Scholar] [CrossRef]
- Xing, J.; Ding, D.; Wang, S.X.; Zhao, B.; Jang, C.; Wu, W.J.; Zhang, F.F.; Zhu, Y.; Hao, J.M. Quantification of the enhanced effectiveness of NOx control from simultaneous reductions of VOC and NH3 for reducing air pollution in the Beijing-Tianjin-Hebei region, China. Atmos. Chem. Phys. 2018, 18, 7799–7814. [Google Scholar] [CrossRef]
- Tan, Z.F.; Lu, K.D.; Jiang, M.Q.; Su, R.; Dong, H.B.; Zeng, L.M.; Xie, S.D.; Tan, Q.W.; Zhang, Y.H. Exploring ozone pollution in Chengdu, southwestern China: A case study from radical chemistry to O3-VOC-NOx sensitivity. Sci. Total Environ. 2018, 636, 775–786. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Fu, J.S.; Zheng, S.; Wang, W. Process analysis of typical summertime ozone episodes over the Beijing area. Sci. Total Environ. 2008, 399, 147–157. [Google Scholar] [CrossRef]
- Pan, X.; Kanaya, Y.; Tanimoto, H.; Inomata, S.; Wang, Z.; Kudo, S.; Uno, I. Examining the major contributors of ozone pollution in a rural area of the Yangtze River Delta region during harvest season. Atmos. Chem. Phys. 2015, 15, 6101–6111. [Google Scholar] [CrossRef]
- Su, R.; Lu, K.; Yu, J.; Tan, Z.; Jiang, M.; Li, J.; Xie, S.; Wu, Y.; Zeng, L.; Zhai, C.; et al. Exploration of the formation mechanism and source attribution of ambient ozone in Chongqing with an observation-based model. Sci. China Earth Sci. 2018, 61, 23–32. [Google Scholar] [CrossRef]
- Ran, L.; Zhao, C.S.; Xu, W.Y.; Han, M.; Lu, X.Q.; Han, S.Q.; Lin, W.L.; Xu, X.B.; Gao, W.; Yu, Q.; et al. Ozone production in summer in the megacities of Tianjin and Shanghai, China: A comparative study. Atmos. Chem. Phys. 2012, 12, 7531–7542. [Google Scholar] [CrossRef]
- Tan, Z.F.; Lu, K.D.; Jiang, M.Q.; Su, R.; Wang, H.L.; Lou, S.R.; Fu, Q.Y.; Zhai, C.Z.; Tan, Q.W.; Yue, D.L.; et al. Daytime atmospheric oxidation capacity in four Chinese megacities during the photochemically polluted season: A case study based on box model simulation. Atmos. Chem. Phys. 2019, 19, 3493–3513. [Google Scholar] [CrossRef]
- Xue, L.K.; Wang, T.; Gao, J.; Ding, A.J.; Zhou, X.H.; Blake, D.R.; Wang, X.F.; Saunders, S.M.; Fan, S.J.; Zuo, H.C.; et al. Ground-level ozone in four Chinese cities: Precursors, regional transport and heterogeneous processes. Atmos. Chem. Phys. 2014, 14, 13175–13188. [Google Scholar] [CrossRef]
- Li, K.; Chen, L.H.; Ying, F.; White, S.J.; Jang, C.; Wu, X.C.; Gao, X.; Hong, S.M.; Shen, J.D.; Azzi, M.; et al. Meteorological and chemical impacts on ozone formation: A case study in Hangzhou, China. Atmos. Res. 2017, 196, 40–52. [Google Scholar] [CrossRef]
- Lin, H.T.; Wang, M.; Duan, Y.S.; Fu, Q.Y.; Ji, W.H.; Cui, H.X.; Jin, D.; Lin, Y.F.; Hu, K. O3 sensitivity and contributions of different NMHC sources in O3 formation at urban and suburban sites in Shanghai. Atmosphere 2020, 11, 295. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Li, K.; Han, L.; Zhang, X.; Wu, X.; Gao, X.; Azzi, M.; Cen, K. Atmospheric ozone chemistry and control strategies in Hangzhou, China: Application of a 0-D box model. Atmos. Res. 2020, 246, 105109. [Google Scholar] [CrossRef]
- Ling, Z.H.; Guo, H. Contribution of VOC sources to photochemical ozone formation and its control policy implication in Hong Kong. Environ. Sci. Policy 2014, 38, 180–191. [Google Scholar] [CrossRef]
- He, Z.R.; Wang, X.M.; Ling, Z.H.; Zhao, J.; Guo, H.; Shao, M.; Wang, Z. Contributions of different anthropogenic volatile organic compound sources to ozone formation at a receptor site in the Pearl River Delta region and its policy implications. Atmos. Chem. Phys. 2019, 19, 8801–8816. [Google Scholar] [CrossRef]
- Yu, D.; Tan, Z.F.; Lu, K.D.; Ma, X.F.; Li, X.; Chen, S.Y.; Zhu, B.; Lin, L.L.; Li, Y.T.; Qiu, P.P.; et al. An explicit study of local ozone budget and NOx-VOCs sensitivity in Shenzhen China. Atmos. Environ. 2020, 224, 117304. [Google Scholar] [CrossRef]
- Shen, H.Q.; Liu, Y.H.; Zhao, M.; Li, J.; Zhang, Y.N.; Yang, J.; Jiang, Y.; Chen, T.S.; Chen, M.; Huang, X.B.; et al. Significance of carbonyl compounds to photochemical ozone formation in a coastal city (Shantou) in eastern China. Sci. Total Environ. 2021, 764, 144031. [Google Scholar] [CrossRef]
- Zou, Y.; Deng, X.J.; Deng, T.; Yin, C.Q.; Li, F. One-year characterization and reactivity of isoprene and its impact on surface ozone formation at a suburban site in Guangzhou, China. Atmosphere 2019, 10, 201. [Google Scholar] [CrossRef]
- Liu, X.; Wang, N.; Lyu, X.; Zeren, Y.; Jiang, F.; Wang, X.; Zou, S.; Ling, Z.; Guo, H. Photochemistry of ozone pollution in autumn in Pearl River Estuary, South China. Sci. Total Environ. 2021, 754, 141812. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.L.; Wu, Z.F.; Luo, S.L.; Song, W.; Wang, X.M. Ozone episodes during and after the 2018 Chinese National Day holidays in Guangzhou: Implications for the control of precursor VOCs. J. Environ. Sci. 2022, 114, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.W.; Cheng, H.R.; Peng, J.; Jiang, H.M.; Lyu, X.P.; Zeng, P.; Wang, Z.W.; Guo, H. Impact of long-range atmospheric transport on volatile organic compounds and ozone photochemistry at a regional background site in central China. Atmos. Environ. 2021, 246, 118093. [Google Scholar] [CrossRef]
- Xie, Y.T.; Cheng, C.L.; Wang, Z.H.; Wang, K.; Wang, Y.; Zhang, X.C.; Li, X.H.; Ren, L.J.; Liu, M.; Li, M. Exploration of O3-precursor relationship and observation-oriented O3 control strategies in a non-provincial capital city, southwestern China. Sci. Total Environ. 2021, 800, 149422. [Google Scholar] [CrossRef] [PubMed]
- Chameides, W.L.; Lindsay, R.W.; Richardson, J.; Kiang, C.S. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science 1988, 241, 1473–1475. [Google Scholar] [CrossRef]
- Ryerson, T.B.; Trainer, M.; Holloway, J.S.; Parrish, D.D.; Huey, L.G.; Sueper, D.T.; Frost, G.J.; Donnelly, S.G.; Schauffler, S.; Atlas, E.L.; et al. Observations of ozone formation in power plant plumes and implications for ozone control strategies. Science 2001, 292, 719–723. [Google Scholar] [CrossRef]
- Chen, W.H.; Guenther, A.B.; Wang, X.M.; Chen, Y.H.; Gu, D.S.; Chang, M.; Zhou, S.Z.; Wu, L.L.; Zhang, Y.Q. Regional to global biogenic isoprene emission responses to changes in vegetation from 2000 to 2015. J. Geophys. Res. Atmos. 2018, 123, 3757–3771. [Google Scholar] [CrossRef]
- Tie, X.X.; Li, G.H.; Ying, Z.M.; Guenther, A.; Madronich, S. Biogenic emissions of isoprenoids and NO in China and comparison to anthropogenic emissions. Sci. Total Environ. 2006, 371, 238–251. [Google Scholar] [CrossRef]
- Chan, C.K.; Yao, X. Air pollution in mega cities in China. Atmos. Environ. 2008, 42, 1–42. [Google Scholar] [CrossRef]
- Zou, Y.; Deng, X.J.; Zhu, D.; Gong, D.C.; Wang, H.; Li, F.; Tan, H.B.; Deng, T.; Mai, B.R.; Liu, X.T.; et al. Characteristics of 1 year of observational data of VOCs, NOx and O3 at a suburban site in Guangzhou, China. Atmos. Chem. Phys. 2015, 15, 6625–6636. [Google Scholar] [CrossRef]
- Guenther, A.; Zimmerman, P.; Wildermuth, M. Natural volatile organic compound emission rate emissions for U.S. woodland landscapes. Atmos. Environ. 1994, 28, 1197–1210. [Google Scholar] [CrossRef]
- Klinger, L.F.; Li, Q.J.; Guenther, A.B.; Greenberg, J.P.; Baker, B.; Bai, J.H. Assessment of volatile organic compound emissions from ecosystems of China. J. Geophys. Res. Atmos. 2002, 107, 4603. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wong, P.; Cheung, B.K.H.; Guenther, A. Improved land cover and emission factors for modeling biogenic volatile organic compounds emissions from Hong Kong. Atmos. Environ. 2010, 44, 1456–1468. [Google Scholar] [CrossRef]
- Zheng, J.; Zheng, Z.; Yu, Y.; Zhong, L. Temporal, spatial characteristics and uncertainty of biogenic VOC emissions in the Pearl River Delta region, China. Atmos. Environ. 2010, 44, 1960–1969. [Google Scholar] [CrossRef]
- Wang, X.M.; Situ, S.P.; Guenther, A.; Chen, F.; Wu, Z.Y.; Xia, B.C.; Wang, T.J. Spatiotemporal variability of biogenic terpenoid emissions in Pearl River Delta, China, with high-resolution land-cover and meteorological data. Tellus Ser. B-Chem. Phys. Meteorol. 2011, 63, 241–254. [Google Scholar] [CrossRef]
- Situ, S.; Guenther, A.; Wang, X.; Jiang, X.; Turnipseed, A.; Wu, Z.; Bai, J.; Wang, X. Impacts of seasonal and regional variability in biogenic VOC emissions on surface ozone in the Pearl River delta region, China. Atmos. Chem. Phys. 2013, 13, 11803–11817. [Google Scholar] [CrossRef]
- Wu, K.; Yang, X.Y.; Chen, D.; Gu, S.; Lu, Y.Q.; Jiang, Q.; Wang, K.; Ou, Y.H.; Qian, Y.; Shao, P.; et al. Estimation of biogenic VOC emissions and their corresponding impact on ozone and secondary organic aerosol formation in China. Atmos. Res. 2020, 231, 104656. [Google Scholar] [CrossRef]
- Wang, H.L.; Wu, K.; Liu, Y.M.; Sheng, B.S.; Lu, X.; He, Y.P.; Xie, J.L.; Wang, H.C.; Fan, S.J. Role of heat wave-induced biogenic VOC enhancements in persistent ozone episodes formation in Pearl River Delta. J. Geophys. Res. Atmos. 2021, 126, e2020JD034317. [Google Scholar] [CrossRef]
- Li, L.Y.; Chen, Y.; Xie, S.D. Spatio-temporal variation of biogenic volatile organic compounds emissions in China. Environ. Pollut. 2013, 182, 157–168. [Google Scholar] [CrossRef]
- Zeng, J.Q.; Song, W.; Zhang, Y.L.; Mu, Z.B.; Pang, W.H.; Zhang, H.N.; Wang, X.M. Emissions of isoprenoids from dominant tree species in subtropical China. Front. For. Glob. Chang. 2022, 5, 1089676. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, X.M.; Blake, D.R.; Li, L.F.; Zhang, Z.; Wang, S.Y.; Guo, H.; Lee, F.S.C.; Gao, B.; Chan, L.Y.; et al. Aromatic hydrocarbons as ozone precursors before and after outbreak of the 2008 financial crisis in the Pearl River Delta region, south China. J. Geophys. Res. Atmos. 2012, 117, D15306. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, X.M.; Barletta, B.; Simpson, I.J.; Blake, D.R.; Fu, X.X.; Zhang, Z.; He, Q.F.; Liu, T.Y.; Zhao, X.Y.; et al. Source attributions of hazardous aromatic hydrocarbons in urban, suburban and rural areas in the Pearl River Delta (PRD) region. J. Hazard. Mater. 2013, 250, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wang, X.M.; Zhang, Z.; Lu, S.J.; Huang, Z.H.; Li, L.F. Sources of C2-C4 alkenes, the most important ozone nonmethane hydrocarbon precursors in the Pearl River Delta region. Sci. Total Environ. 2015, 502, 236–245. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Wang, X.; Li, S.; Zhu, M.; Yu, Q.; Li, G.; Huang, Z.; Zhang, H.; Wu, Z.; et al. Volatile organic compounds at a rural site in Beijing: Influence of temporary emission control and wintertime heating. Atmos. Chem. Phys. 2018, 18, 12663–12682. [Google Scholar] [CrossRef]
- Wu, Z.F.; Zhang, Y.L.; He, J.J.; Chen, H.Z.; Huang, X.L.; Wang, Y.J.; Yu, X.; Yang, W.Q.; Zhang, R.Q.; Zhu, M.; et al. Dramatic increase in reactive volatile organic compound (VOC) emissions from ships at berth after implementing the fuel switch policy in the Pearl River Delta Emission Control Area. Atmos. Chem. Phys. 2020, 20, 1887–1900. [Google Scholar] [CrossRef]
- Wu, Z.F.; Zhang, Y.L.; Pei, C.L.; Huang, Z.Z.; Wang, Y.J.; Chen, Y.N.; Yan, J.H.; Huang, X.Q.; Xiao, S.X.; Luo, S.L.; et al. Real-world emissions of carbonyls from vehicles in an urban tunnel in south China. Atmos. Environ. 2021, 258, 118491. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Saunders, S.M.; Wagner, V.; Pilling, M.J. Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): Tropospheric degradation of aromatic volatile organic compounds. Atmos. Chem. Phys. 2003, 3, 181–193. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Young, J.C.; Rickard, A.R. The MCM v3.3.1 degradation scheme for isoprene. Atmos. Chem. Phys. 2015, 15, 11433–11459. [Google Scholar] [CrossRef]
- Saunders, S.M.; Jenkin, M.E.; Derwent, R.G.; Pilling, M.J. Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): Tropospheric degradation of non-aromatic volatile organic compounds. Atmos. Chem. Phys. 2003, 3, 161–180. [Google Scholar] [CrossRef]
- Sommariva, R.; Cox, S.; Martin, C.; Boronska, K.; Young, J.; Jimack, P.K.; Pilling, M.J.; Matthaios, V.N.; Nelson, B.S.; Newland, M.J.; et al. AtChem (version 1), an open-source box model for the Master Chemical Mechanism. Geosci. Model Dev. 2020, 13, 169–183. [Google Scholar] [CrossRef]
- Huang, J.P.; Fung, J.C.H.; Lau, A.K.H.; Qin, Y. Numerical simulation and process analysis of typhoon-related ozone episodes in Hong Kong. J. Geophys. Res. Atmos. 2005, 110, D05301. [Google Scholar] [CrossRef]
- Cardelino, C.A.; Chameides, W.L. An observation-based model for analyzing ozone precursor relationships in the urban atmosphere. J. Air Waste Manag. Assoc. 1995, 45, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.P.; Guo, H.; Simpson, I.J.; Meinardi, S.; Louie, P.K.K.; Ling, Z.H.; Wang, Y.; Liu, M.; Luk, C.W.Y.; Wang, N.; et al. Effectiveness of replacing catalytic converters in LPG-fueled vehicles in Hong Kong. Atmos. Chem. Phys. 2016, 16, 6609–6626. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, Q.; Liu, Q.; Chen, F.; He, Z.; Guo, H.; Ling, Z. Assessment of atmospheric photochemical reactivity in the Yangtze River Delta using a photochemical box model. Atmos. Res. 2020, 245, 105088. [Google Scholar] [CrossRef]
- Hui, L.R.; Liu, X.G.; Tan, Q.W.; Feng, M.; An, J.L.; Qu, Y.; Zhang, Y.H.; Jiang, M.Q. Characteristics, source apportionment and contribution of VOCs to ozone formation in Wuhan, Central China. Atmos. Environ. 2018, 192, 55–71. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Chen, Y.F.; Zhou, M.; Zheng, B.; Li, K.; Liu, Y.M.; Lin, J.T.; Fu, T.M.; Zhang, Q. Exploring 2016–2017 surface ozone pollution over China: Source contributions and meteorological influences. Atmos. Chem. Phys. 2019, 19, 8339–8361. [Google Scholar] [CrossRef]
- Ren, J.; Hao, Y.F.; Simayi, M.; Shi, Y.Q.; Xie, S.D. Spatiotemporal variation of surface ozone and its causes in Beijing, China since 2014. Atmos. Environ. 2021, 260, 118556. [Google Scholar] [CrossRef]
- Deng, C.; Tian, S.; Li, Z.; Li, K. Spatiotemporal characteristics of PM2.5 and ozone concentrations in Chinese urban clusters. Chemosphere 2022, 295, 133813. [Google Scholar] [CrossRef]
- Yang, Y.D.; Shao, M.; Kessel, S.; Li, Y.; Lu, K.D.; Lu, S.H.; Williams, J.; Zhang, Y.H.; Zeng, L.M.; Noelscher, A.C.; et al. How the OH reactivity affects the ozone production efficiency: Case studies in Beijing and Heshan, China. Atmos. Chem. Phys. 2017, 17, 7127–7142. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Wang, H.; Jing, S.; Lou, S.; Saiz-Lopez, A.; Zhou, B. Observationally constrained modeling of atmospheric oxidation capacity and photochemical reactivity in Shanghai, China. Atmos. Chem. Phys. 2020, 20, 1217–1232. [Google Scholar] [CrossRef]
- Yang, Y.C.; Liu, X.G.; Zheng, J.; Tan, Q.W.; Feng, M.; Qu, Y.; An, J.L.; Cheng, N.L. Characteristics of one-year observation of VOCs, NOx, and O3 at an urban site in Wuhan, China. J. Environ. Sci. 2019, 79, 297–310. [Google Scholar] [CrossRef]
- Pei, C.L.; Yang, W.Q.; Zhang, Y.L.; Song, W.; Xiao, S.X.; Wang, J.; Zhang, J.P.; Zhang, T.; Chen, D.H.; Wang, Y.J.; et al. Decrease in ambient volatile organic compounds during the COVID-19 lockdown period in the Pearl River Delta region, south China. Sci. Total Environ. 2022, 823, 153720. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lyu, X.; Wang, Y.; Jiang, F.; Guo, H. Intercomparison of O3 formation and radical chemistry in the past decade at a suburban site in Hong Kong. Atmos. Chem. Phys. 2019, 19, 5127–5145. [Google Scholar] [CrossRef]
- Meng, Y.; Song, J.; Zeng, L.; Zhang, Y.; Zhao, Y.; Liu, X.; Guo, H.; Zhong, L.; Ou, Y.; Zhou, Y.; et al. Ambient volatile organic compounds at a receptor site in the Pearl River Delta region: Variations, source apportionment and effects on ozone formation. J. Environ. Sci. 2022, 111, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Barletta, B.; Meinardi, S.; Simpson, I.J.; Zou, S.; Rowland, F.S.; Blake, D.R. Ambient mixing ratios of nonmethane hydrocarbons (NMHCs) in two major urban centers of the Pearl River Delta (PRD) region: Guangzhou and Dongguan. Atmos. Environ. 2008, 42, 4393–4408. [Google Scholar] [CrossRef]
- Tang, J.H.; Chu, K.W.; Chan, L.Y.; Chen, Y.J. Non-methane hydrocarbon emission profiles from printing and electronic industrial processes and its implications on the ambient atmosphere in the Pearl River Delta, South China. Atmos. Pollut. Res. 2014, 5, 151–160. [Google Scholar] [CrossRef]
- Han, D.M.; Wang, Z.; Cheng, J.P.; Wang, Q.; Chen, X.J.; Wang, H.L. Volatile organic compounds (VOCs) during non-haze and haze days in Shanghai: Characterization and secondary organic aerosol (SOA) formation. Environ. Sci. Pollut. Res. 2017, 24, 18619–18629. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, F.; Cheng, H.R.; Simpson, I.J.; Wang, X.M.; Ding, A.J.; Wang, T.J.; Saunders, S.M.; Wang, T.; Lam, S.H.M.; et al. Concurrent observations of air pollutants at two sites in the Pearl River Delta and the implication of regional transport. Atmos. Chem. Phys. 2009, 9, 7343–7360. [Google Scholar] [CrossRef]
- Chou, C.C.K.; Tsai, C.Y.; Chang, C.C.; Lin, P.H.; Liu, S.C.; Zhu, T. Photochemical production of ozone in Beijing during the 2008 Olympic Games. Atmos. Chem. Phys. 2011, 11, 9825–9837. [Google Scholar] [CrossRef]
- Zong, R.H.; Yang, X.; Wen, L.; Xu, C.H.; Zhu, Y.H.; Chen, T.S.; Yao, L.; Wang, L.W.; Zhang, J.M.; Yang, L.X.; et al. Strong ozone production at a rural site in the North China Plain: Mixed effects of urban plumes and biogenic emissions. J. Environ. Sci. 2018, 71, 261–270. [Google Scholar] [CrossRef]
- Fu, T.M.; Zheng, Y.Q.; Paulot, F.; Mao, J.Q.; Yantosca, R.M. Positive but variable sensitivity of August surface ozone to large-scale warming in the southeast United States. Nat. Clim. Chang. 2015, 5, 454–458. [Google Scholar] [CrossRef]
- Guidolotti, G.; Pallozzi, E.; Gavrichkova, O.; Scartazza, A.; Mattioni, M.; Loreto, F.; Calfapietra, C. Emission of constitutive isoprene, induced monoterpenes, and other volatiles under high temperatures in Eucalyptus camaldulensis: A C-13 labelling study. Plant Cell Environ. 2019, 42, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Serrano, A.M.; Bourtsoukidis, E.; Alves, E.G.; Bauwens, M.; Stavrakou, T.; Llusia, J.; Filella, I.; Guenther, A.; Williams, J.; Artaxo, P.; et al. Amazonian biogenic volatile organic compounds under global change. Glob. Chang. Biol. 2020, 26, 4722–4751. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.R.; Guo, H.; Wang, X.M.; Saunders, S.M.; Lam, S.H.M.; Jiang, F.; Wang, T.J.; Ding, A.J.; Lee, S.C.; Ho, K.F. On the relationship between ozone and its precursors in the Pearl River Delta: Application of an observation-based model (OBM). Environ. Sci. Pollut. Res. 2010, 17, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Guo, H.; Lyu, X.P.; Cheng, H.R.; Ling, Z.H.; Louie, P.K.K.; Simpson, I.J.; Meinardi, S.; Blake, D.R. Long-term O3-precursor relationships in Hong Kong: Field observation and model simulation. Atmos. Chem. Phys. 2017, 17, 10919–10935. [Google Scholar] [CrossRef]
- Zhu, J.X.; Cheng, H.R.; Peng, J.; Zeng, P.; Wang, Z.W.; Lyu, X.P.; Guo, H. O3 photochemistry on O3 episode days and non-O3 episode days in Wuhan, Central China. Atmos. Environ. 2020, 223, 117236. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, S.X.; Wang, X.M.; Zhang, Y.L.; Pei, C.L.; Chen, D.H.; Jiang, M.; Liao, T. Volatile organic compounds monitored online at three photochemical assessment monitoring stations in the Pearl River Delta (PRD) region during summer 2016: Sources and emission areas. Atmosphere 2021, 12, 327. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Yu, Y.F.; Mo, Z.W.; Zhang, Z.; Wang, X.M.; Yin, S.S.; Peng, K.; Yang, Y.; Feng, X.Q.; Cai, H.H. Industrial sector-based volatile organic compound (VOC) source profiles measured in manufacturing facilities in the Pearl River Delta, China. Sci. Total Environ. 2013, 456, 127–136. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.C.; Goldstein, A.H.; Harley, R.A. Long-term trends in California mobile source emissions and ambient concentrations of black carbon and organic aerosol. Environ. Sci. Technol. 2015, 49, 5178–5188. [Google Scholar] [CrossRef]
- Mo, Z.W.; Shao, M.; Lu, S.H. Compilation of a source profile database for hydrocarbon and OVOC emissions in China. Atmos. Environ. 2016, 143, 209–217. [Google Scholar] [CrossRef]
- Sha, Q.E.; Zhu, M.N.; Huang, H.W.; Wang, Y.Z.; Huang, Z.J.; Zhang, X.C.; Tang, M.S.; Lu, M.H.; Chen, C.; Shi, B.W.; et al. A newly integrated dataset of volatile organic compounds (VOCs) source profiles and implications for the future development of VOCs profiles in China. Sci. Total Environ. 2021, 793, 148348. [Google Scholar] [CrossRef]
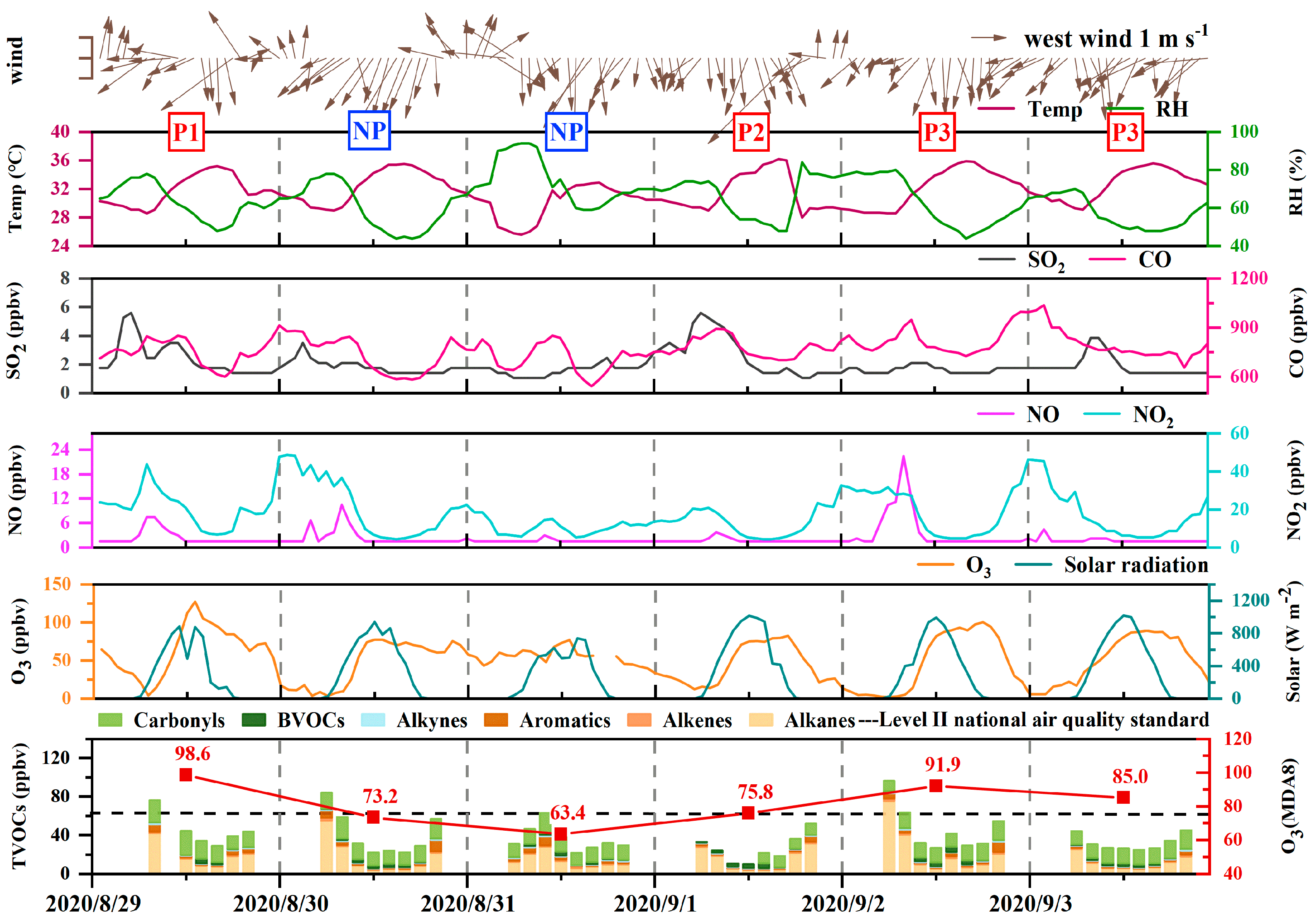

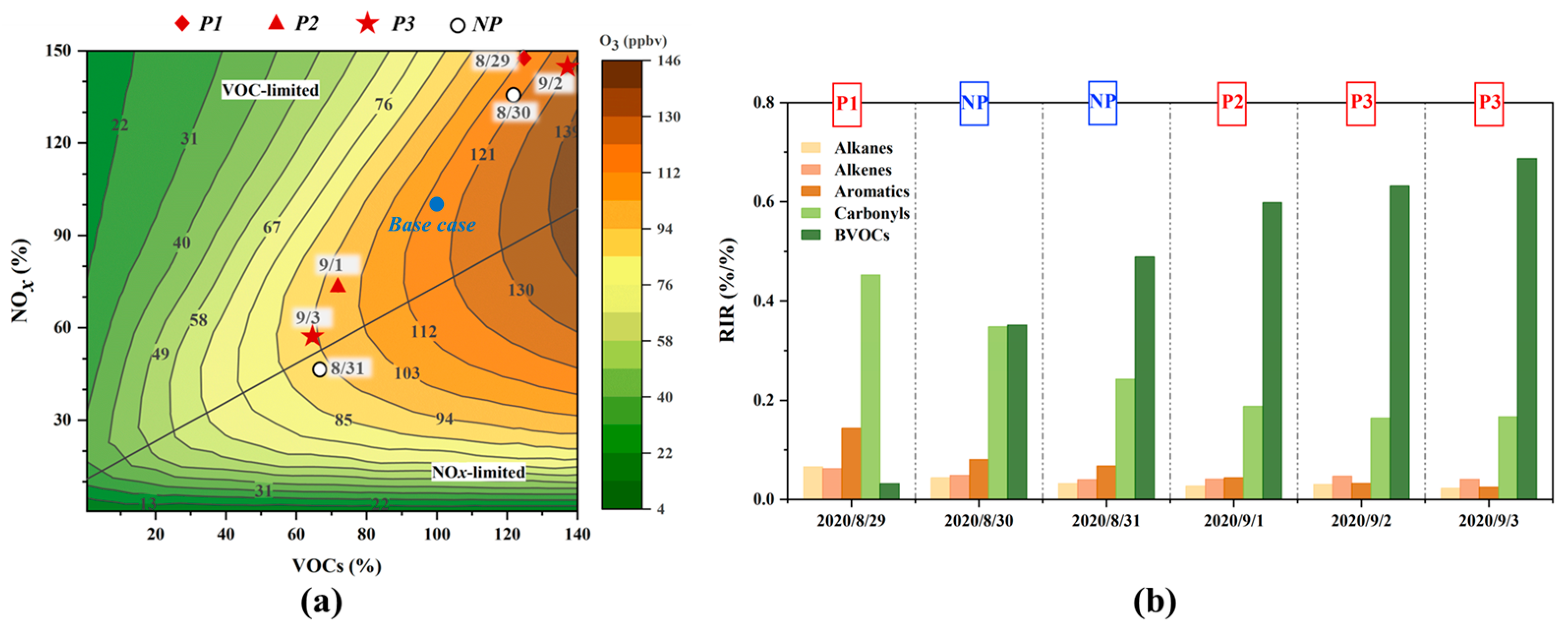
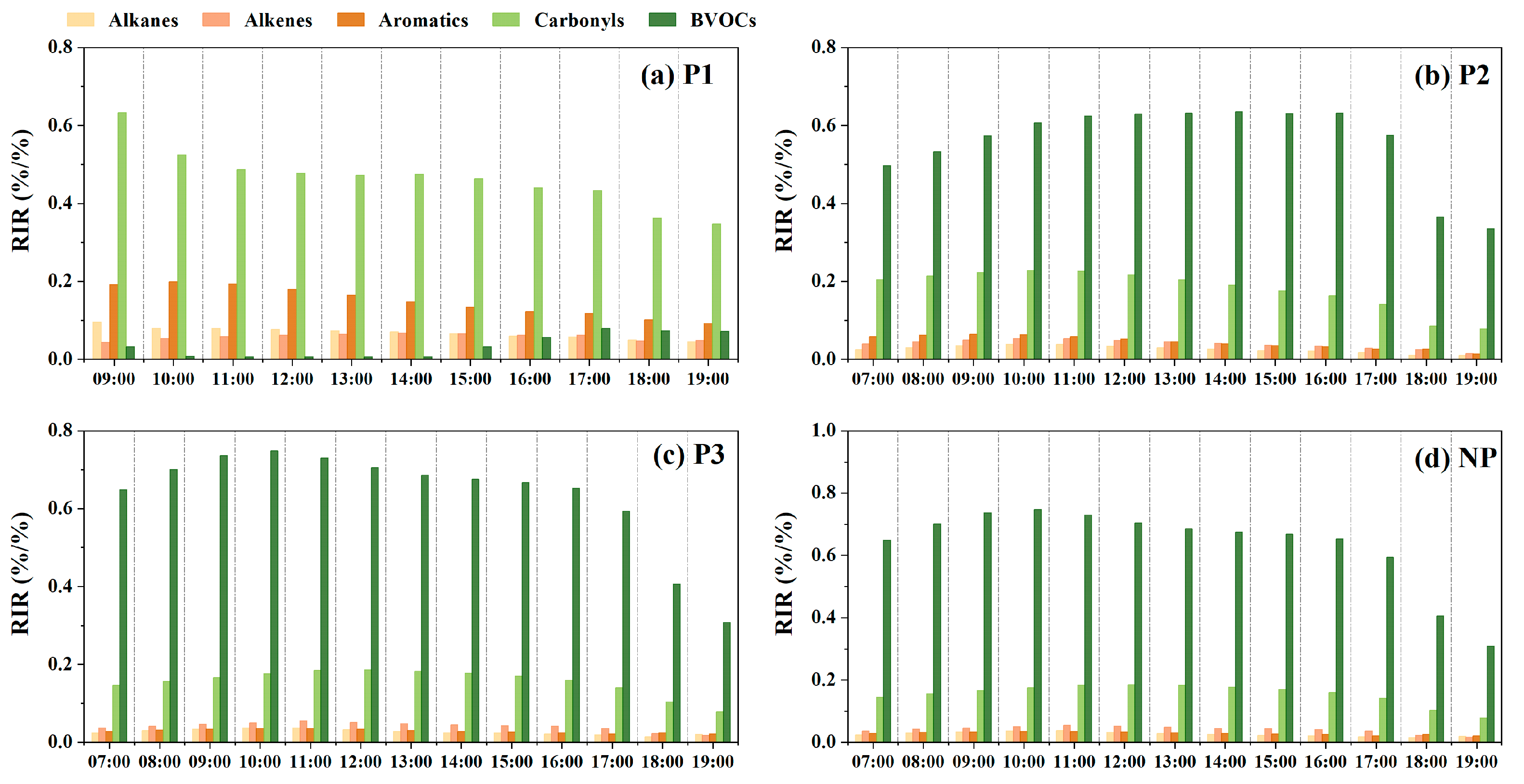
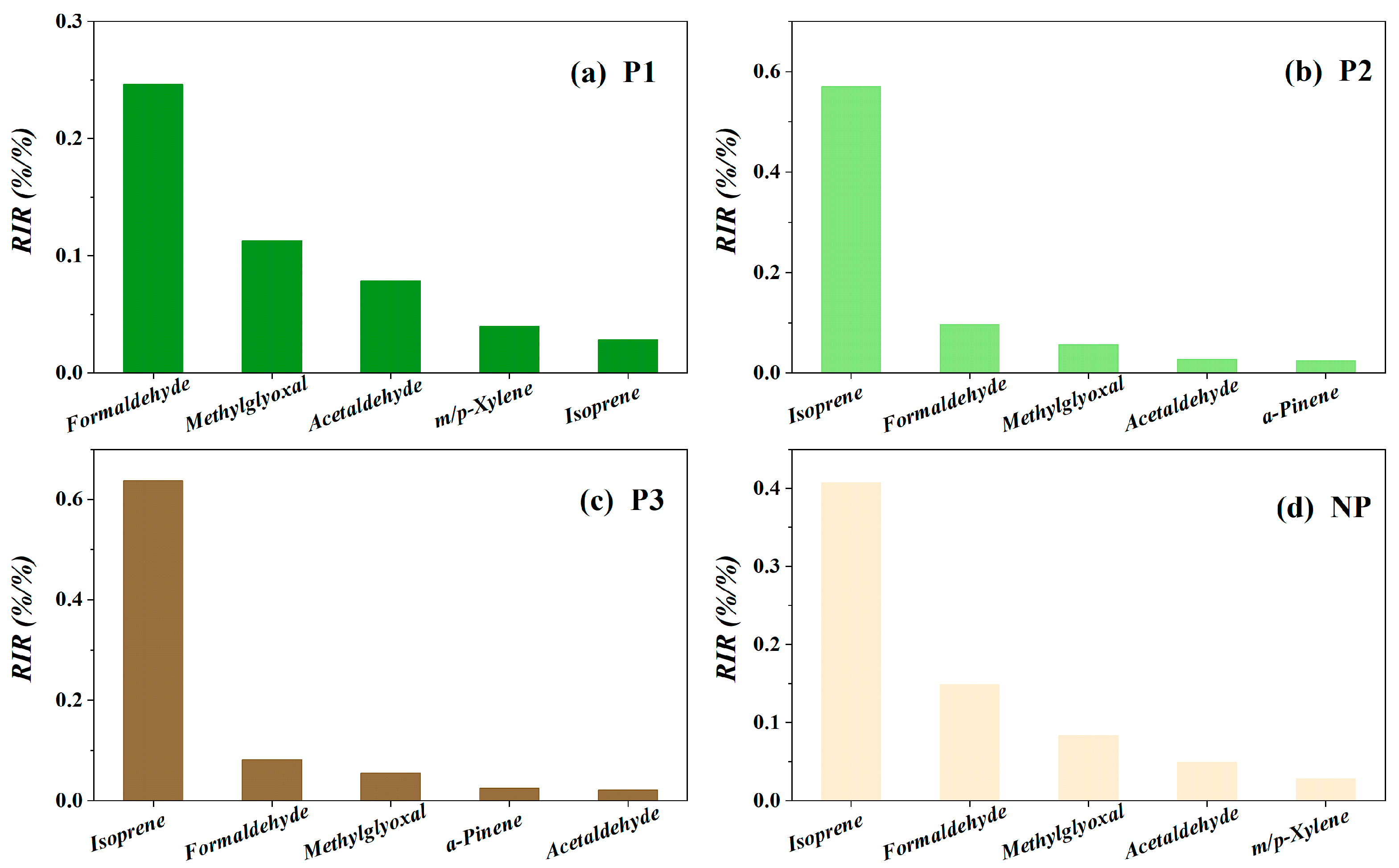

| P1 | P2 | P3 | NP | |||||
|---|---|---|---|---|---|---|---|---|
| Average ± S.D. | Maximum | Average ± S.D. | Maximum | Average ± S.D. | Maximum | Average ± S.D. | Maximum | |
| Temperature (°C) | 32.5 ± 2.13 | 35.2 | 31.5 ± 2.66 | 36.2 | 32.4 ± 2.43 | 35.9 | 31.2 ± 2.61 | 35.5 |
| Solar radiation (W m−2) | 459 ± 330 | 886 | 544 ± 378 | 1016 | 511 ± 348 | 1018 | 434 ± 293 | 939 |
| Relative humidity (%) | 60.7 ± 8.91 | 78.0 | 66.4 ± 11.1 | 84.0 | 60.1 ± 11.0 | 80.0 | 67.3 ± 13.7 | 94.0 |
| Wind speed (m s−1) | 0.928 ± 0.351 | 1.90 | 0.900 ± 0.411 | 2.60 | 0.898 ± 0.283 | 1.40 | 1.01 ± 0.318 | 1.70 |
| O3 (ppbv) | 69.2 ± 33.1 | 127 | 43.3 ± 25.9 | 82.6 | 48.6 ± 35.0 | 100 | 50.6 ± 22.6 | 77.5 |
| SO2 (ppbv) | 2.06 ± 1.21 | 5.60 | 2.65 ± 1.50 | 5.60 | 1.76 ± 0.565 | 3.85 | 1.75 ± 0.473 | 3.50 |
| CO (ppbv) | 759 ± 80.5 | 913 | 779 ± 59.9 | 893 | 810 ± 87.0 | 1038 | 725 ± 96.8 | 879 |
| NO (ppbv) | 2.57 ± 1.82 | 7.47 | 1.71 ± 0.560 | 3.73 | 2.84 ± 3.82 | 22.4 | 2.05 ± 1.63 | 10.5 |
| NO2 (ppbv) | 21.1 ± 10.4 | 47.7 | 13.8 ± 7.56 | 32.6 | 18.6 ± 12.3 | 46.3 | 16.1 ± 12.3 | 48.7 |
| Alkanes (ppbv) | 18.6 ± 12.6 | 41.8 | 14.5 ± 11.4 | 31.2 | 17.0 ± 18.1 | 75.1 | 15.1 ± 13.2 | 54.8 |
| Alkenes (ppbv) | 1.42 ± 0.441 | 1.97 | 1.25 ± 0.474 | 1.96 | 1.43 ± 0.624 | 2.92 | 1.39 ± 0.605 | 3.09 |
| Aromatics (ppbv) | 3.31 ± 2.48 | 7.42 | 1.66 ± 1.88 | 5.97 | 2.45 ± 2.58 | 10.6 | 3.45 ± 3.37 | 11.9 |
| Alkynes (ppbv) | 1.08 ± 0.475 | 1.87 | 0.868 ± 0.228 | 1.07 | 1.07 ± 0.480 | 1.94 | 1.09 ± 0.444 | 1.74 |
| Carbonyls (ppbv) | 18.8 ± 4.57 | 25.3 | 11.0 ± 0.773 | 11.8 | 15.6 ± 2.17 | 19.6 | 16.0 ± 2.86 | 22.3 |
| BVOCs (ppbv) | 1.24 ± 1.90 | 4.70 | 2.11 ± 2.12 | 5.55 | 2.14 ± 1.76 | 5.39 | 1.68 ± 1.41 | 4.41 |
| TVOCs (ppbv) | 44.5 ± 16.7 | 76.2 | 25.9 ± 14.1 | 52.0 | 39.7 ± 18.8 | 96.5 | 38.7 ± 17.6 | 84.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Y.; Xiao, S.; Wu, Z.; Wang, X. Ozone Formation at a Suburban Site in the Pearl River Delta Region, China: Role of Biogenic Volatile Organic Compounds. Atmosphere 2023, 14, 609. https://doi.org/10.3390/atmos14040609
Wang J, Zhang Y, Xiao S, Wu Z, Wang X. Ozone Formation at a Suburban Site in the Pearl River Delta Region, China: Role of Biogenic Volatile Organic Compounds. Atmosphere. 2023; 14(4):609. https://doi.org/10.3390/atmos14040609
Chicago/Turabian StyleWang, Jun, Yanli Zhang, Shaoxuan Xiao, Zhenfeng Wu, and Xinming Wang. 2023. "Ozone Formation at a Suburban Site in the Pearl River Delta Region, China: Role of Biogenic Volatile Organic Compounds" Atmosphere 14, no. 4: 609. https://doi.org/10.3390/atmos14040609
APA StyleWang, J., Zhang, Y., Xiao, S., Wu, Z., & Wang, X. (2023). Ozone Formation at a Suburban Site in the Pearl River Delta Region, China: Role of Biogenic Volatile Organic Compounds. Atmosphere, 14(4), 609. https://doi.org/10.3390/atmos14040609









