Bioaerosol Sensor for In Situ Measurement: Real-Time Measurement of Bioaerosol Particles in a Real Environment and Demonstration of the Effectiveness of Air Purifiers to Reduce Bioaerosol Particle Concentrations at Hot Spots
Abstract
:1. Introduction
2. Materials and Methods
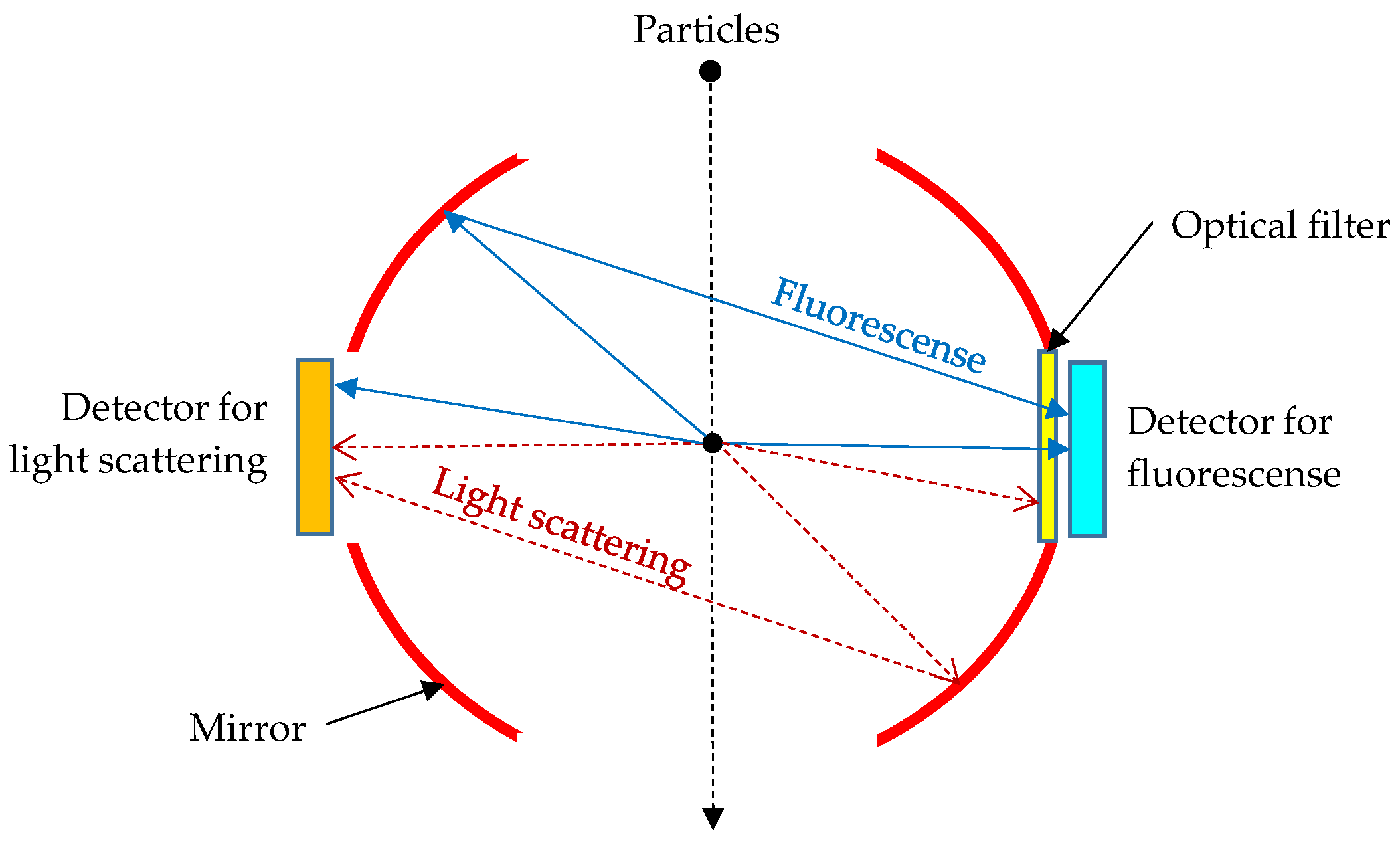
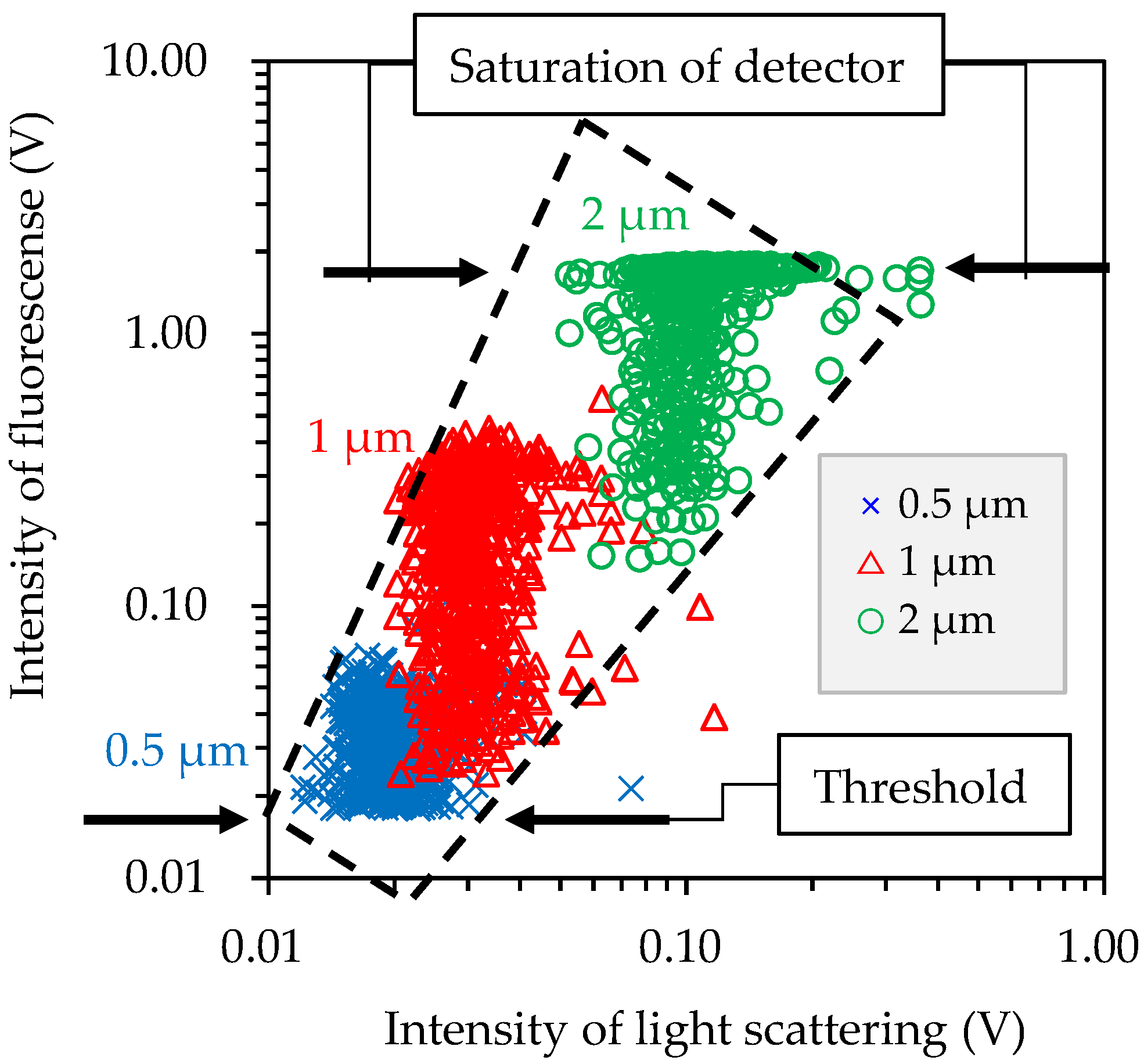
2.1. Bioaerosol Sensor and Its Calibration Method
2.2. In Situ Measurement
2.2.1. Site Description: A Shared Student Space at Kogakuin University
2.2.2. Measurement Items and Methods
2.2.3. Methods to Detect Hot Spots and Validate Mitigation Measures
- C = suspended or bioaerosol particle concentration (p/m3);
- Co = initial indoor concentration or concentration immediately after bioaerosol particle generation is stopped (p/m3);
- M = amount of suspended or bioaerosol particles generated (p/h);
- Q = equivalent clean airflow rate (m3/h);
- V = room volume (m3);
- t = elapsed time (h).
3. Results
3.1. Bioaerosol Sensor Performance Test
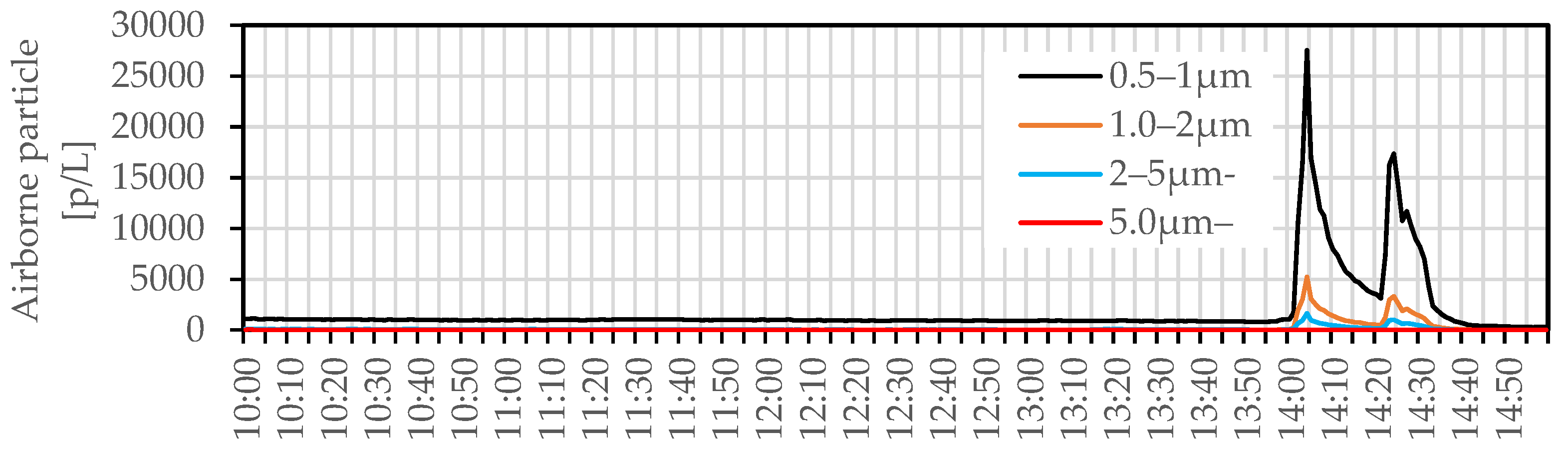
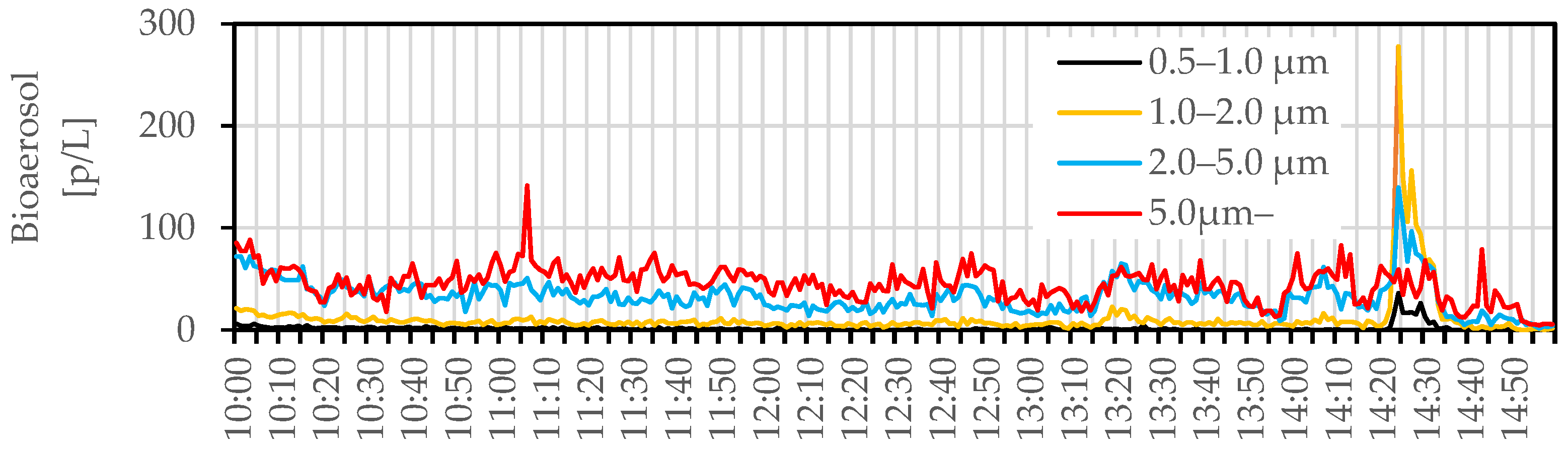
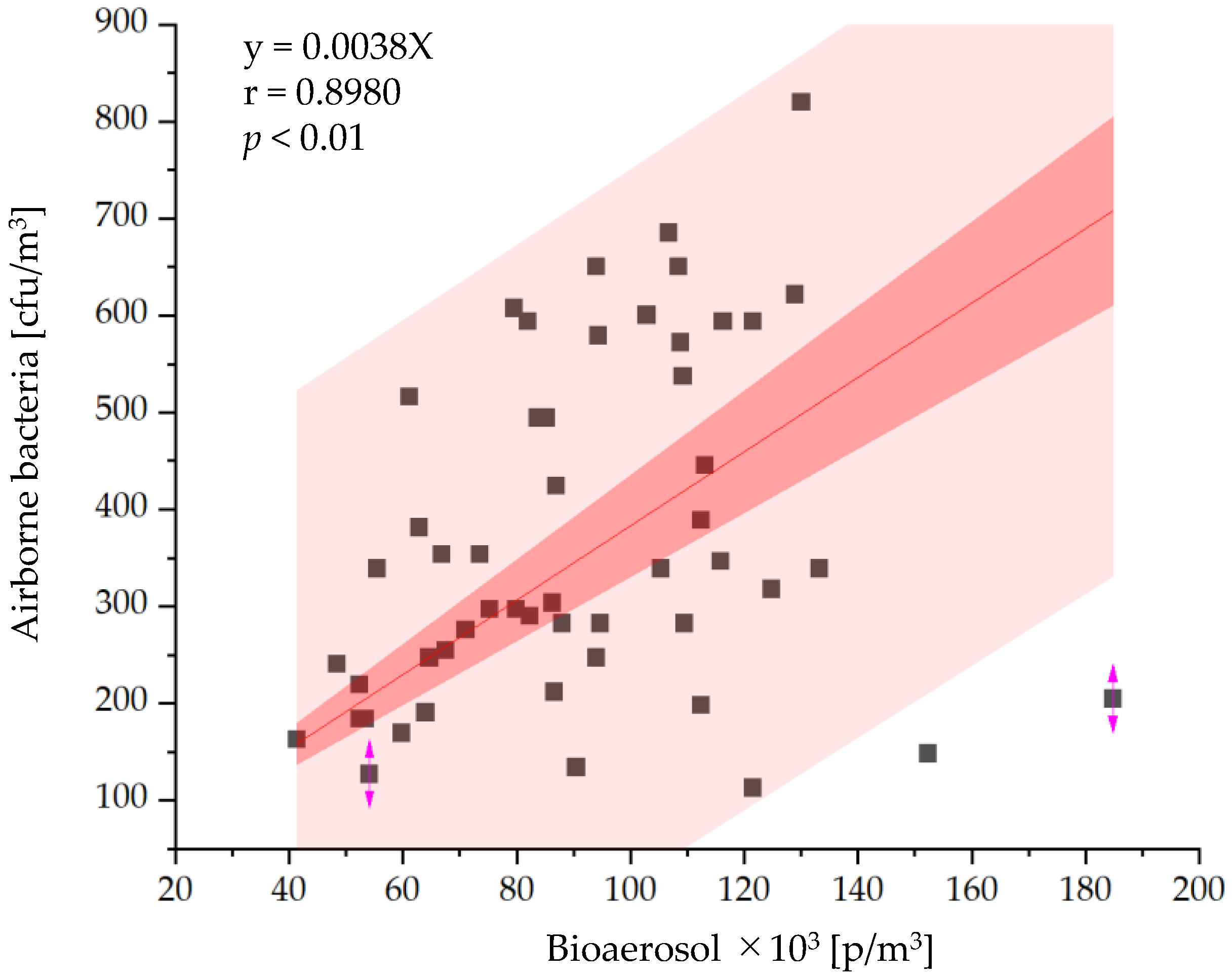
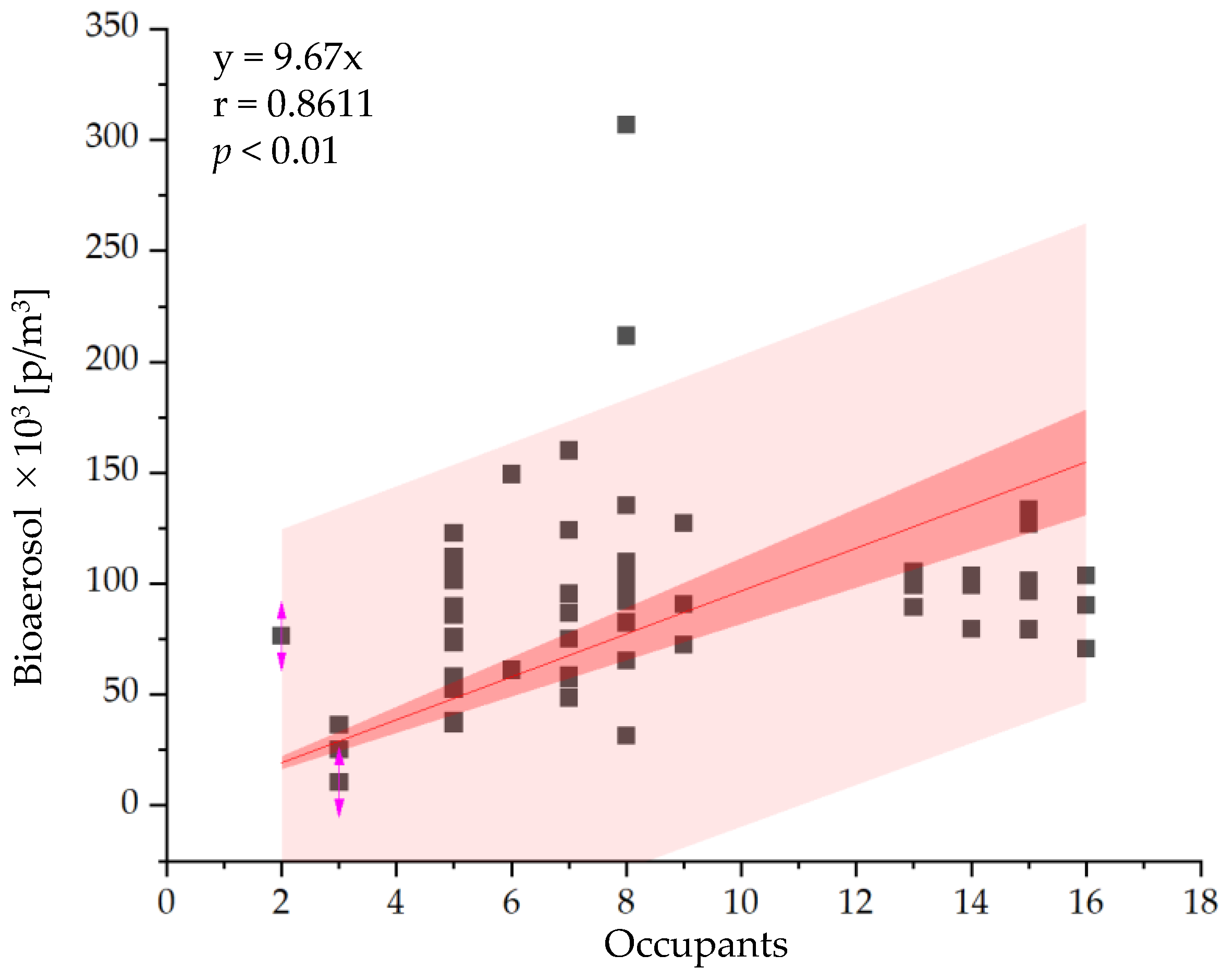
3.2. In Situ Measurement
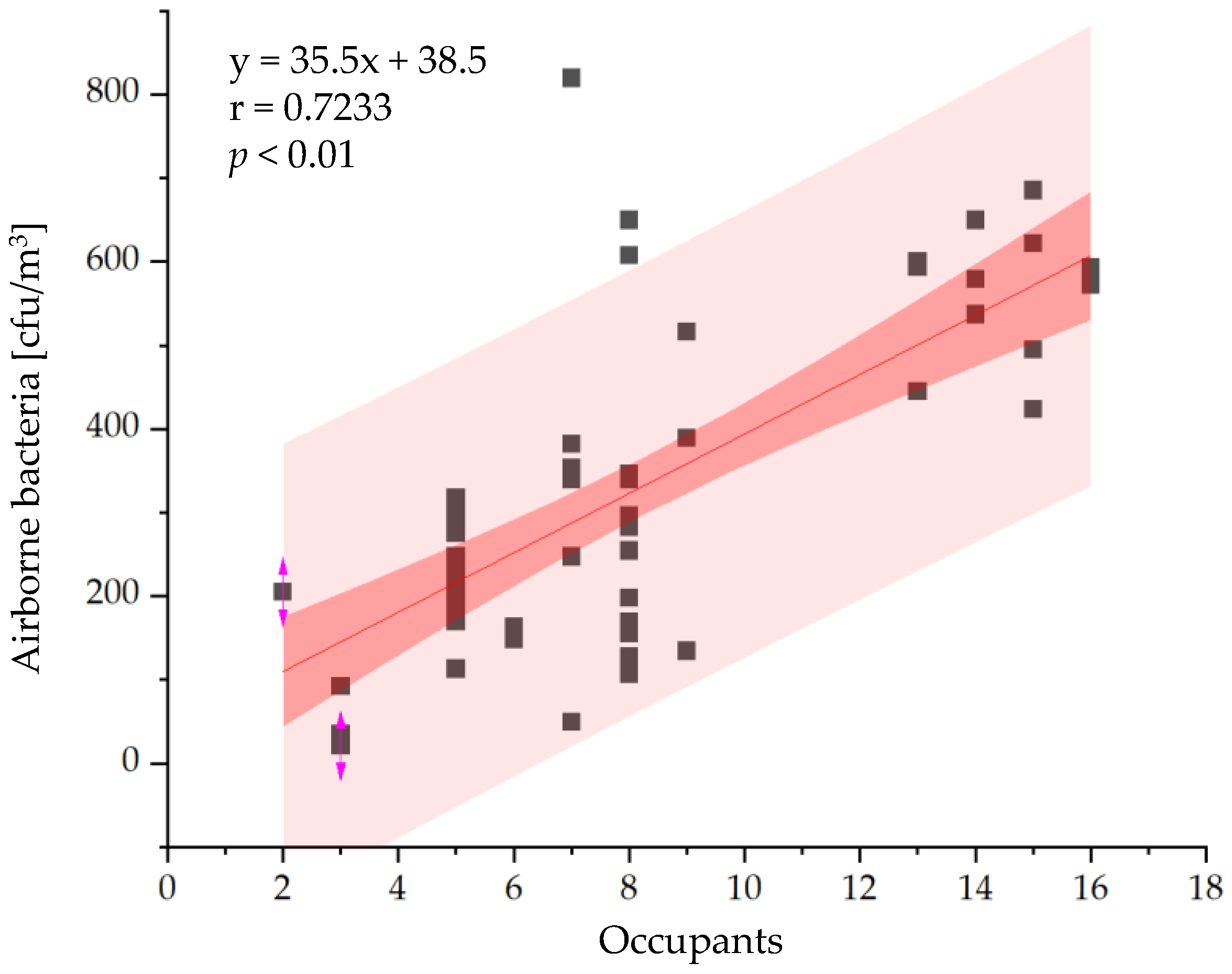
3.2.1. Bioaerosol Particles and Suspended Bacteria
3.2.2. Demonstration of the Effect of Air Purifiers on Bioaerosol Particle Concentration Reduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, T.A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Anal. Env. Epid. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- Adams, R.I.; Bhangar, S.; Dannemiller, K.C.; Eisen, J.A.; Fierer, N.; Gilbert, J.A.; Green, J.L.; Miller, S.L.; Siegel, J.A.; Stephens, B.; et al. Ten questions concerning the microbiomes of buildings. Build. Environ. 2016, 109, 224–234. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Stephens, B. Microbiology of the built environment. Nat. Rev. Microbiol. 2018, 16, 661–670. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol Health Effects and Exposure Assessment: Progress and Prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef]
- Verreault, D.; Moineau, S.; Duchaine, C. Methods for Sampling of Airborne Viruses. Microbiol. Mol. Biol. Rev. 2008, 72, 413–444. [Google Scholar] [CrossRef]
- Haig, C.W.; Mackay, W.G.; Walker, J.T.; Williams, C. Bioaerosol sampling: Sampling mechanisms, bioefficiency and field studies. J. Hosp. Infect. 2016, 93, 242–255. [Google Scholar] [CrossRef]
- ISO 16000-16; Indoor Air–Part 16: Detection and Enumeration of Moulds–Sampling by Filtration. ISO Copyright Office Publisher: Geneva, Switzerland, 2008.
- ISO 16000-18; Indoor Air–Part 18: Detection and Enumeration of Moulds–Sampling by Impaction. ISO Copyright Office Publisher: Geneva, Switzerland, 2011.
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic Identification and In Situ Detection of Individual Microbial Cells without Cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Ferguson, R.; Buckley, E.N.; Palumbo, A. Response of marine bacterioplankton to differential filtration and confinement. Appl. Environ. Microbiol. 1984, 47, 49–55. [Google Scholar] [CrossRef]
- Kogure, K.; Simidu, U.; Taga, N. A tentative direct microscopic method for counting living marine bacteria. Can. J. Microbiol. 1979, 25, 415–420. [Google Scholar] [CrossRef]
- Kogure, K.; Simidu, U.; Taga, N. Distribution of viable marine bacteria in neritic seawater around Japan. Can. J. Microbiol. 1980, 26, 318–323. [Google Scholar] [CrossRef]
- Jones, J.G. The effect of environmental factors on estimated viable and total populations of planktonic bacteria in lakes and experimental enclosures. Freshwater Biol. 1977, 7, 67–91. [Google Scholar] [CrossRef]
- Staley, J.T.; Konopka, A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu. Rev. Microbiol. 1985, 39, 321–346. [Google Scholar] [CrossRef]
- Wagner, M.; Amann, R.; Lemmer, H.; Schleifer, K.H. Probing activated sludge with proteobacteria-specific oligonucleotides: Inadequacy of culture-dependent methods for describing microbial community structure. Appl. Environ. Microbiol. 1993, 59, 1520–1525. [Google Scholar] [CrossRef]
- Wagner, M.; Erhart, R.; Manz, W.; Amann, R.; Lemmer, H.; Wedi, D.; Schleifer, K.H. Development of an rRNA-targeted oligonucleotide probe specific for the genus Acinetobacter and its application for in situ monitoring in activated sludge. Appl. Environ. Microbiol. 1994, 60, 792–800. [Google Scholar] [CrossRef]
- Torsvik, V.; Goksoyr, J.; Daae, F.L. High diversity of DNA of soil bacteria. Appl. Environ. Microbiol. 1990, 56, 782–787. [Google Scholar] [CrossRef]
- Kembel, S.W.; Jones, E.; Kline, J.; Northcutt, D.; Stenson, J.; Womack, A.M.; Bohannan, B.J.; Brown, G.Z.; Green, J.L. Architectural design influences the diversity and structure of the built environment microbiome. ISME J. 2012, 6, 1469–1479. [Google Scholar] [CrossRef]
- Kim, H.Y.; Estes, C.R.; Duncan, A.G.; Wade, B.D.; Cleary, F.C.; Lloyd, C.R.; Ellis, W., Jr.; Powers, L.S. Real-Time Detection of Microbial Contamination. IEEE Eng. Med. Biol. Mag. 2004, 23, 122–129. [Google Scholar] [CrossRef]
- Pan, Y.L. Detection and characterization of biological and other organic-carbon aerosol particles in atmosphere using fluorescence. J. Quant. Spectrosc. Radiat. Transf. 2015, 150, 12–35. [Google Scholar] [CrossRef]
- Li, J.K.; Asali, E.C.; Humphrey, A.E. Monitoring Cell Concentration and Activity by Multiple Excitation Fluorometry. Biotechnol. Prog. 1991, 7, 21–27. [Google Scholar] [CrossRef]
- Dalterio, R.A.; Nelson, W.H.; Britt, D.; Sperry, J.F.; Tanguay, J.F.; Suib, S.L. The Steady-State and Decay Characteristics of Primary Fluorescence from Live Bacteria. Appl. Spectrosc. 1987, 41, 234–241. [Google Scholar] [CrossRef]
- Mason, H.Y.; Lloyd, C.; Dice, M.; Sinclair, R.; Ellis, W., Jr.; Powers, L. Taxonomic identification of microorganisms by capture and intrinsic fluorescence detection. Biosens. Bioelectron. 2003, 18, 521–527. [Google Scholar] [CrossRef]
- Savage, N.J.; Krentz, C.E.; Könemann, T.; Han, T.T.; Mainelis, G.; Pöhlker, C.; Huffman, J.A. Systematic characterization and fluorescence threshold strategies for the wideband integrated bioaerosol sensor (WIBS) using size-resolved biological and interfering particles. Atmos. Meas. Tech. 2017, 10, 4279–4302. [Google Scholar] [CrossRef]
- Könemann, T.; Savage, N.; Klimach, T.; Walter, D.; Fröhlich-Nowoisky, F.; Su, H.; Pöschl, U.; Huffman, J.A.; Pöhlker, C. Spectral Intensity Bioaerosol Sensor (SIBS): An instrument for spectrally resolved fluorescence detection of single particles in real time. Atmos. Meas. Tech. 2019, 12, 1337–1363. [Google Scholar] [CrossRef]
- Crawford, I.; Topping, D.; Gallagher, M.; Forde, E.; Lloyd, J.R.; Foot, V.; Stopford, C.; Kaye, P. Detection of Airborne Biological Particles in Indoor Air Using a Real-Time Advanced Morphological Parameter UV-LIF Spectrometer and Gradient Boosting Ensemble Decision Tree Classifiers. Atmosphere 2020, 11, 1039. [Google Scholar] [CrossRef]
- Huffman, J.A.; Perring, A.E.; Savage, N.J.; Clot, B.; Crouzy, B.; Tummon, F.; Shoshanim, O.; Damit, B.; Schneider, J.; Sivaprakasam, V.; et al. Real-time sensing of bioaerosols: Review and current perspectives. Aerosol. Sci. Technol. 2020, 54, 465–495. [Google Scholar] [CrossRef]
- Lieberherr, G.; Auderset, K.; Calpini, B.; Clot, B.; Crouzy, B.; Gysel-Beer, M.; Konzelmann, T.; Manzano, J.; Mihajlovi, A.; Moallemi, A.; et al. Assessment of real-time bioaerosol particle counters using reference chamber experiments. Atmos. Meas. Tech. 2021, 14, 7693–7706. [Google Scholar] [CrossRef]
- Patra, S.S.; Wu, T.; Wnger, D.N.; Jiang, J.; Boor, B.E. Real-time measurements of fluorescent aerosol particles in a living laboratory office under variable human occupancy and ventilation conditions. Build Environ. 2021, 205, 108249. [Google Scholar] [CrossRef]
- Ruske, S.; Topping, D.O.; Foot, V.E.; Kaye, P.H.; Stanley, W.R.; Crawford, I.; Morse, A.P.; Gallagher, M.W. Evaluation of machine learning algorithms for classification of primary biological aerosol using a new UV-LIF spectrometer. Atmos. Meas. Tech. 2017, 10, 695–708. [Google Scholar] [CrossRef]
- Hospodsky, D.; Qian, J.; Nazaroff, W.W.; Yamamoto, N.; Bibby, K.; Rismani-Yazdi, H.; Peccia, J. Human Occupancy as a Source of Indoor Airborne Bacteria. PLoS ONE 2012, 7, e34867. [Google Scholar] [CrossRef]
- Christopher, S.C.; Christopher, M.W. (Eds.) Bioaerosols Handbook; Lewis Publishers: London, UK, 1995. [Google Scholar]
- Odagiri, A.; Yanagi, U.; Kato, S. Comparison of generation of particles and bacteria in endoscopic surgery and thoracotomy. Build Environ. 2021, 193, 107544. [Google Scholar] [CrossRef]
- The Lancet COVID-19 Commission Task Force on Safe Work, Safe School, and Safe Travel. Proposed Non-Infectious Air Delivery Rates (NADR) for Reducing Exposure to Airborne Respiratory Infectious Diseases. 2022. Available online: https://static1.squarespace.com/static/5ef3652ab722df11fcb2ba5d/t/637740d40f35a9699a7fb05f/1668759764821/Lancet+Covid+Commission+TF+Report+Nov+2022.pdf (accessed on 1 August 2023).
- ASHRAE Standard 241-2023: Control of Infectious Aerosols. Approved by the ASHRAE Standards Committee on 24 June 2023. Available online: https://www.ashrae.org/technical-resources/bookstore/ashrae-standard-241-control-of-infectious-aerosols (accessed on 1 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagi, U.; Fukushima, N.; Nagai, H.; Ye, H.; Kano, M. Bioaerosol Sensor for In Situ Measurement: Real-Time Measurement of Bioaerosol Particles in a Real Environment and Demonstration of the Effectiveness of Air Purifiers to Reduce Bioaerosol Particle Concentrations at Hot Spots. Atmosphere 2023, 14, 1656. https://doi.org/10.3390/atmos14111656
Yanagi U, Fukushima N, Nagai H, Ye H, Kano M. Bioaerosol Sensor for In Situ Measurement: Real-Time Measurement of Bioaerosol Particles in a Real Environment and Demonstration of the Effectiveness of Air Purifiers to Reduce Bioaerosol Particle Concentrations at Hot Spots. Atmosphere. 2023; 14(11):1656. https://doi.org/10.3390/atmos14111656
Chicago/Turabian StyleYanagi, U, Nobuhiko Fukushima, Hideyasu Nagai, Haoyi Ye, and Minoru Kano. 2023. "Bioaerosol Sensor for In Situ Measurement: Real-Time Measurement of Bioaerosol Particles in a Real Environment and Demonstration of the Effectiveness of Air Purifiers to Reduce Bioaerosol Particle Concentrations at Hot Spots" Atmosphere 14, no. 11: 1656. https://doi.org/10.3390/atmos14111656
APA StyleYanagi, U., Fukushima, N., Nagai, H., Ye, H., & Kano, M. (2023). Bioaerosol Sensor for In Situ Measurement: Real-Time Measurement of Bioaerosol Particles in a Real Environment and Demonstration of the Effectiveness of Air Purifiers to Reduce Bioaerosol Particle Concentrations at Hot Spots. Atmosphere, 14(11), 1656. https://doi.org/10.3390/atmos14111656





