Adapting Almond Production to Climate Change through Deficit Irrigation and Foliar Kaolin Application in a Mediterranean Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Plant Material
2.2. Experimental Design
- -
- FI: full irrigation treatment, which received 100% of the crop evapotranspiration (ETc) during the entire irrigation period.
- -
- FI-Kaolin: the same amount of irrigation as FI with kaolin applied.
- -
- RDI: the irrigation was 100% of the ETc until the kernel-filling period, then 35% ETc irrigation until the harvest (100/35).
- -
- RDI-Kaolin: the same amount of irrigation as RDI with kaolin applied.
2.3. Water Status and Leaf Area
2.4. Physiological Measurements
2.5. Yield and Yield Components
2.6. Statistical Analysis
3. Results
3.1. Climate Conditions, Irrigation, and Plant Water Status
3.2. Leaf Morphological Characteristics
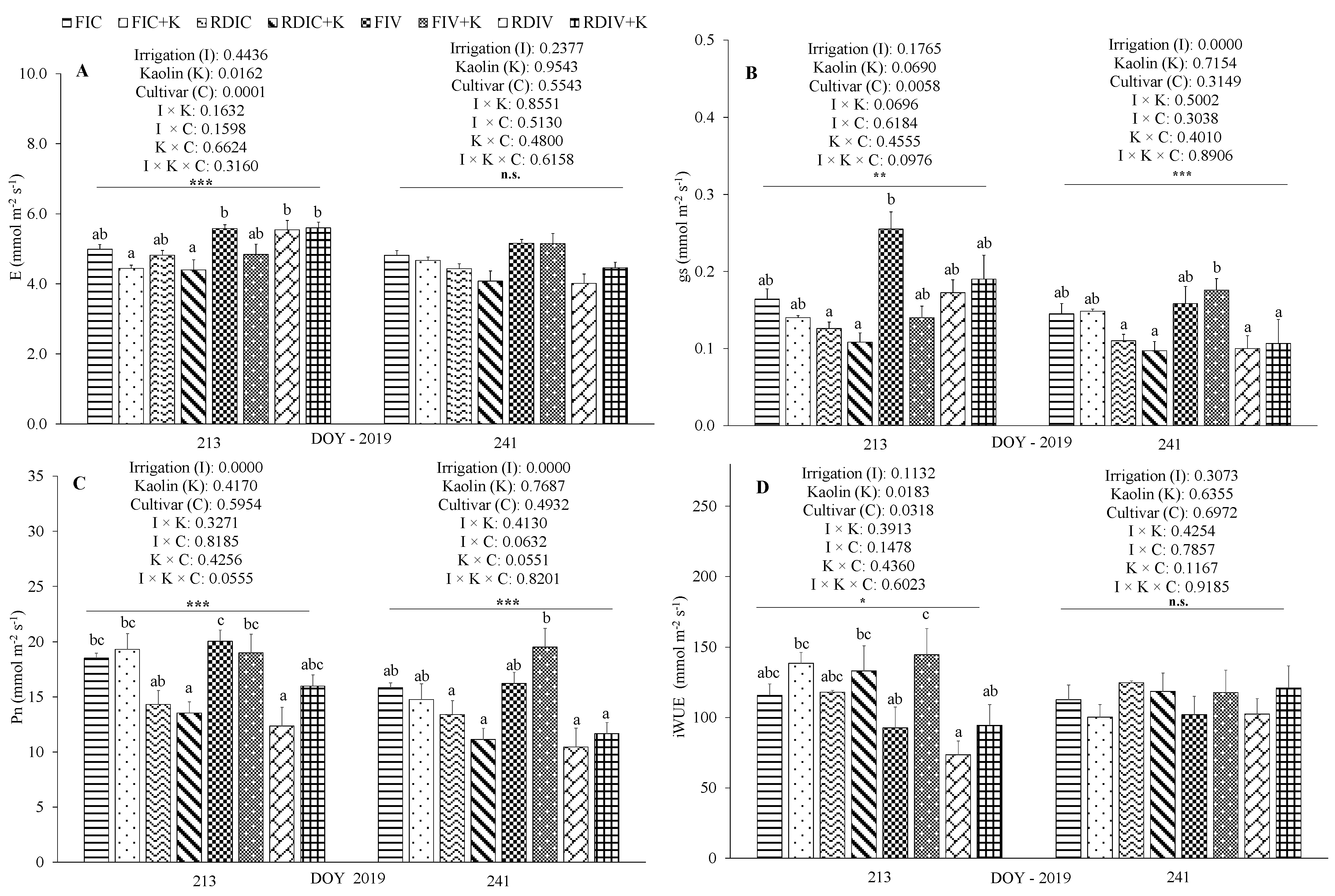
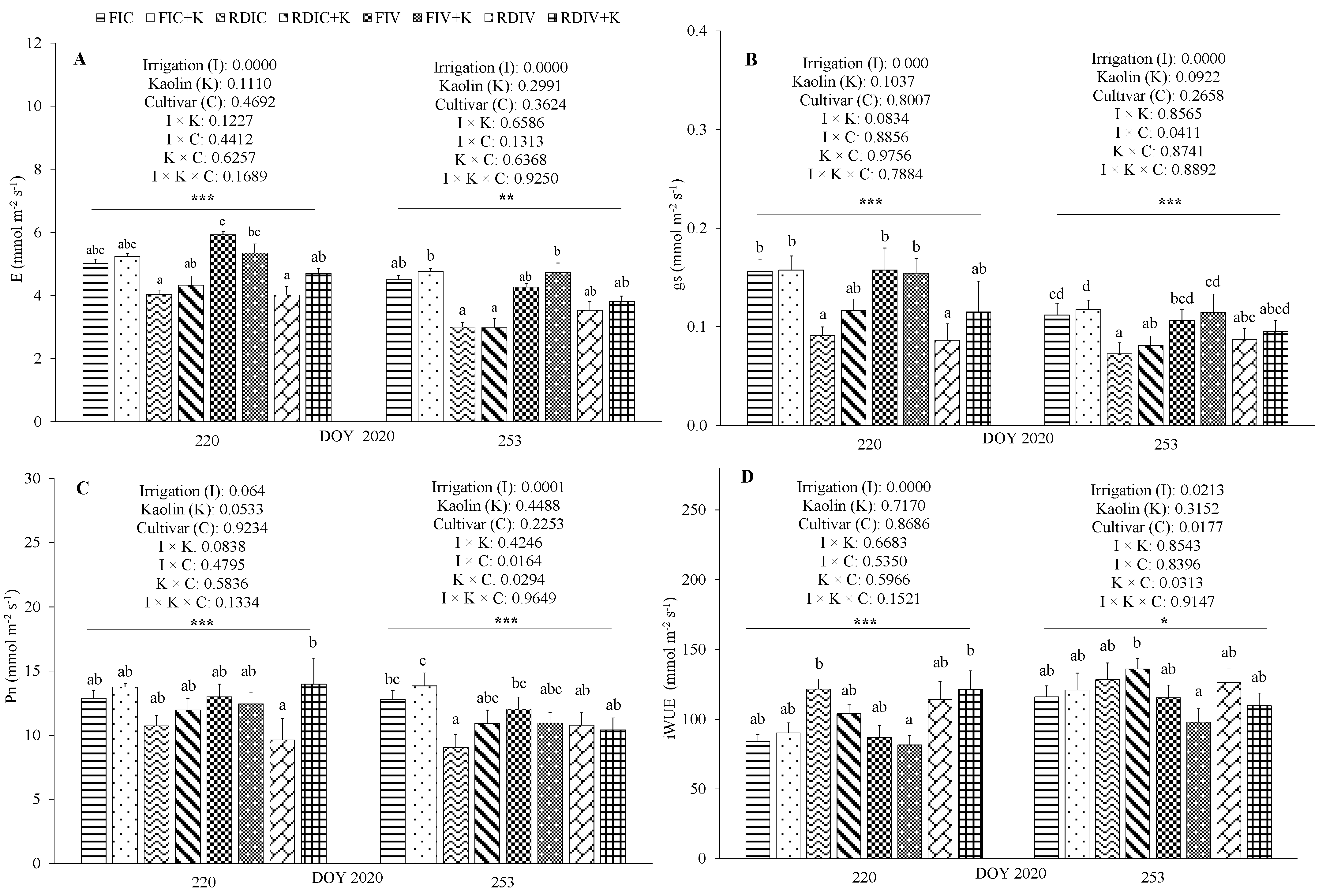
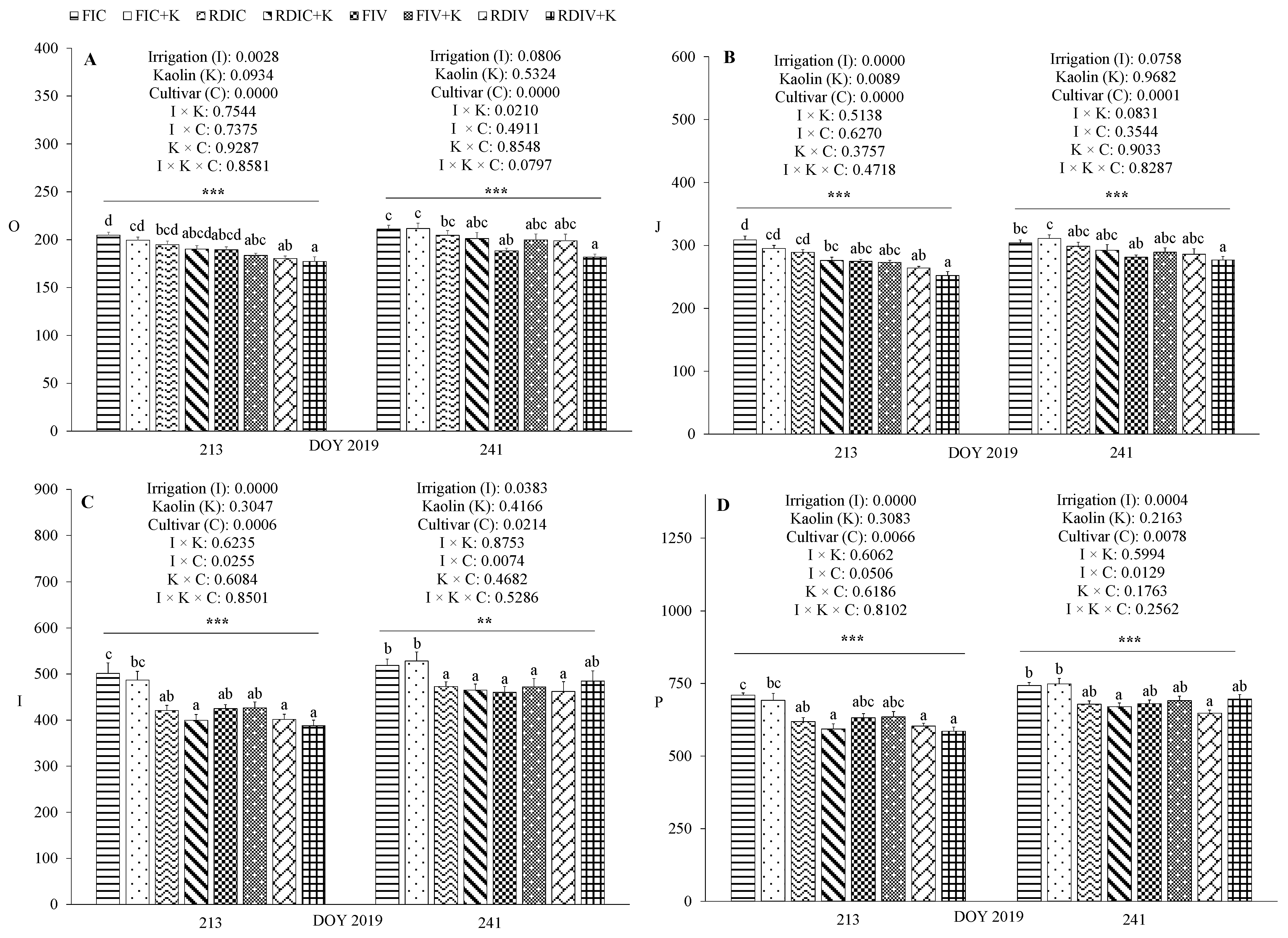
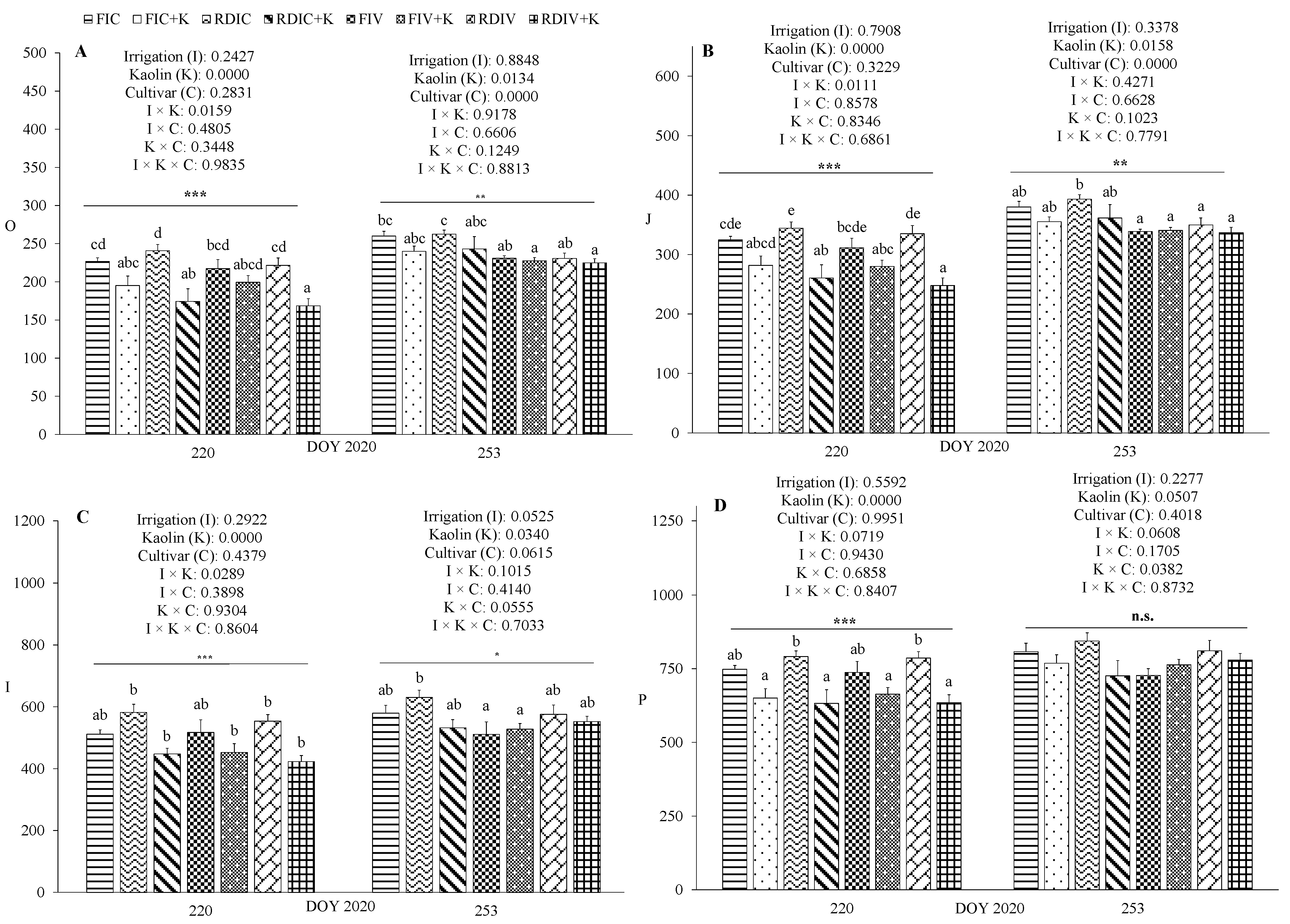
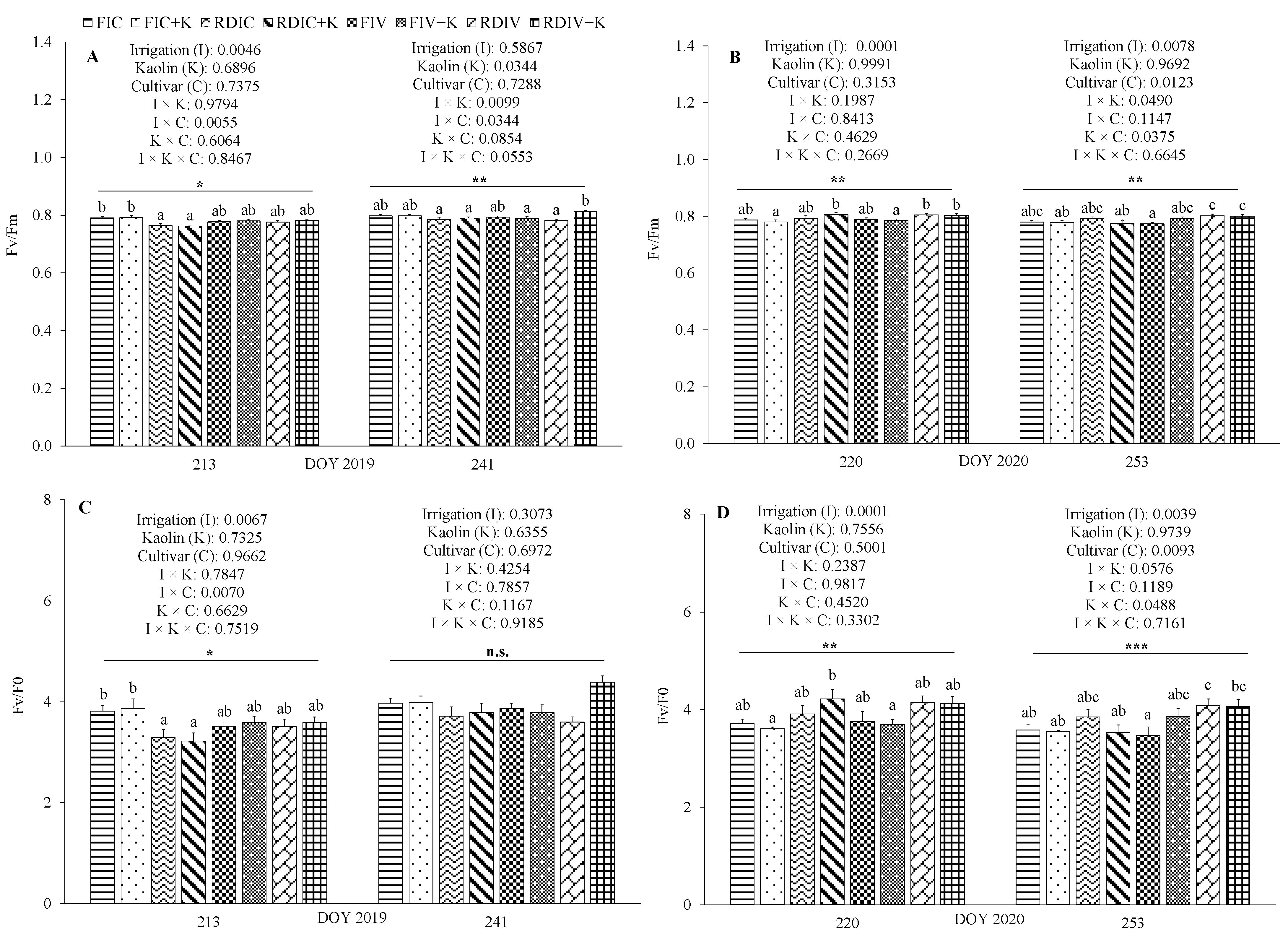
3.3. Physiological Measurements
3.4. Yield and Water Use Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Dugo, V.; Zarco-Tejada, P.; Berni, J.A.; Suarez, L.; Goldhamer, D.; Fereres, E. Almond tree canopy temperature reveals intra-crown variability that is water stress-dependent. Agric. For. Meteorol. 2012, 154, 156–165. [Google Scholar] [CrossRef]
- Arquero, O. Manual del Almendro. Editor. Junta Andal. 2013, 78, 2378. (In Spanish) [Google Scholar]
- Fernandes de Oliveira, A.; Mameli, M.G.; De Pau, L.; Satta, D. Almond Tree Adaptation to Water Stress: Differences in Physiological Performance and Yield Responses among Four Cultivar Grown in Mediterranean Environment. Plants 2023, 12, 1131. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate change impacts and adaptation strategies of agriculture in Mediterranean-climate regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Abernethy, K.E.; Smart, R.; Wilson, R.J.; Maclean, I.M.D. Climate change inputs and adaptative strategies: Lessons from the grapevine. Glob. Chang. Biol. 2016, 22, 3814–3828. [Google Scholar] [CrossRef] [PubMed]
- Lorite, I.J.; Cabezas-Luque, J.M.; Arquero, O.; Gabaldón-Leal, C.; Santos, C.; Rodríguez, A.; Ruiz-Ramos, M.; Lovera, M. The role of phenology in the climate change impacts and adaptation strategies for tree crops: A case study on almond orchards in Southern Europe. Agric. For. Meteorol. 2020, 294, 108142. [Google Scholar] [CrossRef]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fonseca, A.; Fraga, H. Evaluation of historical and future thermal conditions for almond trees in north-eastern Portugal. Clim. Chang. 2023, 176, 89. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. A Report of the Intergovernmental Panel on Climate Change. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Freitas, T.R.; Santos, J.A.; Silva, A.P.; Fraga, H. Reviewing the Adverse Climate Change Impacts and Adaptation Measures on Almond Trees (Prunus dulcis). Agriculture 2023, 13, 1423. [Google Scholar] [CrossRef]
- Fraga, H.; de Cortázar Atauri, I.G.; Santos, J.A. Viticultural irrigation demands under climate change scenarios in Portugal. Agric. Water Manag. 2018, 196, 66–74. [Google Scholar] [CrossRef]
- Galindo, A.; Collado-González, J.; Griñán, I.; Corell, M.; Centeno, A.; Martín-Palomo, M.J.; Girón, I.F.; Rodriguez, P.L.; Cruz, Z.N.; Memmi, H.; et al. Deficit irrigation and emerging fruit crops as a strategy to save water in Mediterranean semiarid agrosystems. Agric. Water Manag. 2018, 202, 311–324. [Google Scholar] [CrossRef]
- Ma, N.; Szilagyi, J.; Zhang, Y. Calibration-free complementary relationship estimates terrestrial evapotranspiration globally. Water Resour. Res. 2021, 57, e2021WR029691. [Google Scholar] [CrossRef]
- Schumacher, D.L.; Keune, J.; Dirmeyer, P.; Miralles, D.G. Drought self-propagation in drylands due to land–atmosphere feedbacks. Nat. Geosci. 2022, 15, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, I.; Foles, P.; Oliveira, C. El cultivo del Almendro en España y Portugal: Situación, innovación tecnológica, costes, rentabilidad y perspectivas. Rev. Frutic. 2021, 81, 6–49. [Google Scholar]
- Girona, J. Estrategias de riego deficitario en el cultivo del almendro. Frutic. Prof. 1992, 47, 38–45. [Google Scholar]
- Goldhamer, D.A.; Fereres, E. Establishing an almond water production function for California using long-term yield response to variable irrigation. Irrig. Sci. 2017, 35, 169–179. [Google Scholar] [CrossRef]
- Mirás-Avalos, J.M.; Gonzalez-Dugo, V.; García-Tejero, I.F.; López-Urrea, R.; Intrigliolo, D.S.; Egea, G. Quantitative analysis of almond yield response to irrigation regimes in Mediterranean Spain. Agric. Water Manag. 2023, 279, 108208. [Google Scholar] [CrossRef]
- Miarnau, X.; Pomar, L.T.; Cambra, M.R.; Caravaca, I.B. Consideraciones para la mejora productiva del almendro en España. Tierras Castilla León Agric. 2018, 267, 112–120. [Google Scholar]
- FAO. The Future of Food and Agriculture–Trends and Challenges; Annual Report; FAO: Rome, Italy, 2017; Volume 296, pp. 1–180. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 August 2022).
- Tous-Martí, J. Frutales mediterráneos cultivados en California. Agric. Rev. Agropecu. Y Ganad. 1995, 769, 680–684. [Google Scholar]
- Miarnau, X.; Torguet, L.; Batlle, I.; Romero, A.; Rovira, M.; Alegre, S. Comportamiento agronómico y productivo de las nuevas variedades de almendro. In Fruticultura; Editorial Tècnica Quatrebcn: Barcelona, Spain, 2016; Volume 49, pp. 42–59. [Google Scholar]
- Morais, M.C.; Aires, A.; Barreales, D.; Rodrigues, M.Â.; Ribeiro, A.C.; Gonçalves, B.; Silva, A.P. Combined soil and foliar nitrogen fertilization effects on rainfed almond tree performance. J. Soil Sci. Plant Nutr. 2020, 20, 2552–2565. [Google Scholar] [CrossRef]
- Torrecillas, A.; Alarcón, J.J.; Domingo, R.; Planes, J.; Sánchez, B.M.J. Strategies for drought resistance in leaves of two almond cultivars. Plant Sci. 1996, 118, 135–143. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Simón, L.; González-Piqueras, J.; Montoya, F.; López-Urrea, R. Monitoring Crop Evapotranspiration and Transpiration/Evaporation Partitioning in a Drip-Irrigated Young Almond Orchard Applying a Two-Source Surface Energy Balance Model. Water 2021, 13, 2073. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, M.D.C.; Domingo, R.; Castel, J.R. Deficit irrigation in fruit trees and vines in Spain. A review. Span. J. Agric. Res. 2010, 8, S5–S20. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M.; Salinas, M. Regulated deficit irrigation in almonds: Effects of variations in applied water and stress timing on yield and yield components. Irrig. Sci. 2006, 242, 101–114. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Gan, Y.; Zhao, C.; Xu, H.; Waskom, R.M.; Niu, Y.; Siddique, K.H.M. Regulated deficit irrigation for crop production under drought stress. A review. Agron. Sustain. Dev. 2016, 36, 3. [Google Scholar] [CrossRef]
- Egea, G.; Nortes, P.A.; Domingo, R.; Baille, A.; Pérez-Pastor, A.; Gónzalez-Real, M.M. Almond agronomic response to long term deficit irrigation applied since orchard establishment. Irrig. Sci. 2013, 31, 445–454. [Google Scholar] [CrossRef]
- Romero, P.; García, J.; Botía, P. Cost–benefit analysis of a regulated deficit-irrigated almond orchard under subsurface drip irrigation conditions in Southeastern Spain. Irrig. Sci. 2006, 24, 175–184. [Google Scholar] [CrossRef]
- Prgomet, I.; Pascual-Seva, N.; Morais, M.C.; Aires, A.; Barreales, D.; Ribeiro, A.C.; Silva, A.P.; Barros, A.I.R.N.A.; Gonçalves, B. Physiological and biochemical performance of almond trees under deficit irrigation. Sci. Hortic. 2020, 261, 108990. [Google Scholar] [CrossRef]
- Gomes-Laranjo, J.; Coutinho, J.P.; Galhano, V.; Cordeiro, V. Responses of five almond cultivars to irrigation: Photosynthesis and leaf water potential. Agric. Water Manag. 2006, 83, 261–265. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán-Zuazo, V.H.; Vélez, L.M.; Hernández, A.; Salguero, A.; Muriel-Fernández, J.L. Improving almond productivity under deficit irrigation in semiarid zones. Open Agric. J. 2011, 5, 56–62. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Gutiérrez Gordillo, S.; Souza, L.; Cuadros-Tavira, S.; Duran Zuazo, V.H. Fostering sustainable water use in almond (Prunus dulcis Mill.) orchards in a semiarid Mediterranean environment. Arch. Agron. Soil Sci. 2019, 65, 164–181. [Google Scholar] [CrossRef]
- Mañas, F.; López-Fuster, P.; López-Urrea, R. Effects of different regulated and sustained deficit irrigation strategies in almond production. Acta Hortic. 2014, 1028, 391–394. [Google Scholar] [CrossRef]
- Gutiérrez-Gordillo, S.; Lipan, L.; Duran Zuazo, V.H.; Sendra, E.; Hernández, F.; Hernández-Zazueta, M.S.; García-Tejero, I.F. Deficit irrigation as a suitable strategy to enhance the nutritional composition of hydrosos almonds. Water 2020, 12, 3336. [Google Scholar] [CrossRef]
- Glenn, D.M.; Puterka, G.J. Particle films: A new technology for agriculture. Hortic. Rev. 2005, 31, 1–44. [Google Scholar]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing kaolin and pinolene to improve sustainable grapevine production during drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef] [PubMed]
- Dinis, L.T.; Bernardo, S.; Matos, C.; Malheiro, A.; Flores, R.; Alves, S.; Costa, C.; Rocha, S.; Correia, C.; Moutinho-Pereira, J. Overview of Kaolin Outcomes from vine to wine: Cerceal white variety case study. Agronomy 2020, 10, 1422. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin particle film modulates morphological, physiological and biochemical olive tree responses to drought and rewatering. Plant Physiol. Biochem. 2018, 133, 29–39. [Google Scholar] [CrossRef]
- Brito, C.; Gonçalves, A.; Silva, E.; Martins, S.; Pinto, L.; Rocha, L.; Arrobas, M.; Rodrigues, M.Â.; Moutinho-Pereira, J.; Correia, C.M. Kaolin foliar spray improves olive tree performance and yield under sustained deficit irrigation. Sci. Hortic. 2021, 277, 109795. [Google Scholar] [CrossRef]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Effects of kaolin application on light absorption and distribution, radiation use efficiency and photosynthesis of almond and walnut canopies. Ann. Bot. 2007, 99, 255–263. [Google Scholar] [CrossRef]
- Luciani, E.; Palliotti, A.; Frioni, T.; Tombesi, S.; Villa, F.; Zadra, C.; Farinelli, D. Kaolin treatments on Tonda Giffoni hazelnut (Corylus avellana L.) for the control of heat stress damages. Sci. Hortic. 2020, 263, 109097. [Google Scholar] [CrossRef]
- Karaat, F.E.; Denizhan, H. The effects of different particle film applications on almond trees. Ciência Rural. 2023, 53, e20210757. [Google Scholar] [CrossRef]
- Barreales, D.; Pereira, J.A.; Casal, S.; Ribeiro, A.C. Influence of sustained deficit irrigation and foliar kaolin application on almond kernel composition. Sci. Hortic. 2023, 321, 112262. [Google Scholar] [CrossRef]
- Mota, N.; Bernardo, S.; Ribeiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T. Silicon application effect on berry quality of Touriga Franca variety in the Douro Demarcated Region. In Proceedings of the VII International Congress of Mountain and Steep Slopes Viticulture, Vila Real, Portugal, 12–14 May 2022; p. 360. [Google Scholar]
- Glenn, D.M.; Cooley, N.; Walker, R.; Clingeleffer, P.; Shellie, K. Impact of kaolin particle film and water deficit on wine grape water use efficiency and plant water relations. HortScience. 2010, 45, 1178–1187. [Google Scholar] [CrossRef]
- Oliveira, D.F.; Benhadi-Marín, J.; Neto, J.; Sanz, L.; Garzo, E.; Aguiar, A.; Fereres, A.; Pereira, J.A. Kaolin particle films disrupt landing, settling behavior and feeding of Trioza erytrae on lemon plants. Pest Manag. Sci. 2022, 78, 4753–4763. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- Glenn, D.M. The mechanisms of plant stress mitigation by kaolin-based particle films and applications in horticultural and agricultural crops. HortScience 2012, 47, 710–711. [Google Scholar] [CrossRef]
- Rosati, A.; Metcalf, S.G.; Buchner, R.P.; Fulton, A.E.; Lampinen, B.D. Physiological effects of kaolin applications in well-irrigated and water-stressed walnut and almond trees. Ann. Bot. 2006, 98, 267–275. [Google Scholar] [CrossRef]
- Barreales, D.; Fernandes, Â.; Barros, L.; Capitão, S.; Castro Ribeiro, A. Effects of regulated deficit irrigation and foliar kaolin application on quality parameters of almond [Prunus dulcis (Mill.) DA Webb]. J. Sci. Food Agric. 2023, 103, 7227–7240. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification update. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration: Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper 56; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998; ISBN 9251042195. [Google Scholar]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Abdi, S.; Abbaspur, N.; Avestan, S.; Barker, A.V. Sana physiological responses of two grapevine (Vitis vinifera L.) cultivars to Cycocel™ treatment during drought. J. Hortic. Sci. Biotechnol. 2016, 91, 211–219. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Afonso, S.; Gonçalves, B. Compared leaf anatomy and water relations of commercial and traditional Prunus dulcis (Mill.) cultivars under rain-fed conditions. Sci. Hortic. 2018, 229, 226–232. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.; Afonso, S.; Ferreira, I.Q.; Arrobas, M. Response of stevia to nitrogen fertilization and harvesting regime in Northeastern Portugal. Arch. Agron. Soil Sci. 2017, 63, 626–637. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Lipan, L.; Gutiérrez-Gordillo, S.; Zuazo, V.H.D.; Jančo, I.; Hernández, F.; Cárceles-Rodríguez, B.; Carbonell-Barrachina, Á.A. Deficit Irrigation and Its Implications for HydroSOStainable Almond Production. Agronomy 2020, 10, 1632. [Google Scholar] [CrossRef]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Purza, L.; Samuel, A.D.; Bungău, S.; Badea, G.E.; Aleya, L. Orchard management under the effects of climate change: Implications for apple, plum, and almond growing. Environ. Sci. Pollut. Res. 2019, 26, 9908–9915. [Google Scholar] [CrossRef]
- Gutiérrez-Gordillo, S.; Lipan, L.; Durán-Zuazo, V.H.; Carbonell-Barrachina, Á.A.; Cárceles Rodríguez, B.; Rubio-Casal, A.E.; Carbonell-Bojollo, R.; Ordoñez-Fernández, R.; García-Tejero, I.F. Linking sustainability and competitiveness of almond plantations under water scarcity and changing climate. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 695–728. [Google Scholar]
- Jacob, D.; Petersen, J.; Eggert, B.; Alias, A.; Christensen, O.B.; Bouwer, L.M.; Braun, A.; Colette, A.; Déqué, M.; Georgievski, G.; et al. EURO-CORDEX: New high-resolution climate change projections for European impact research. Reg. Environ. Chang. 2014, 14, 563–578. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Santos, J.A. Climate change projections for chilling and heat forcing conditions in European vineyards and olive orchards: A multi-model assessment. Clim. Chang. 2019, 152, 179–193. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Fereres, E. Irrigation scheduling of almond trees with trunk diameter sensors. Irrig. Sci. 2004, 23, 11–19. [Google Scholar] [CrossRef]
- Romero, P.; Botia, P.; Garcia, F. Effects of regulated deficit irrigation under subsurface drip irrigation conditions on vegetative development and yield of mature almond trees. Plant Soil. 2004, 260, 169–181. [Google Scholar] [CrossRef]
- Egea, G.; González-Real, M.M.; Baille, A.; Nortes, P.A.; Sánchez-Bel, P.; Domingo, R. The effects of contrasted deficit irrigation strategies on the fruit growth and kernel quality of mature almond trees. Agric. Water Manag. 2009, 96, 1605–1614. [Google Scholar] [CrossRef]
- Egea, G.; Nortes, P.A.; González-Real, M.M.; Baille, A.; Domingo, R. Agronomic response and water productivity of almond trees under contrasted deficit irrigation regimes. Agric. Water Manag. 2010, 97, 171–181. [Google Scholar] [CrossRef]
- Puerto, P.; Domingo, R.; Torres, R.; Pérez-Pastor, A.; García, R.M. Remote management of deficit irrigation in almond trees based n maximum daily trunk shrinkage. Water relations and yield. Agric. Water Manag. 2013, 126, 33–45. [Google Scholar] [CrossRef]
- Hutmacher, R.B.; Nightingale, H.I.; Rolston, D.E.; Biggar, J.W.; Dale, F.; Vail, S.S.; Peters, D. Growth and yield responses of almond (Prunus amygdalus) to trickle irrigation. Irrig. Sci. 1994, 14, 117–126. [Google Scholar] [CrossRef]
- Goldhamer, D.A.; Viveros, M. Effects of preharvest irrigation cutoff durations and postharvest water deprivation on almond tree performance. Irrig. Sci. 2000, 19, 125–131. [Google Scholar] [CrossRef]
- Girona, J.; Mata, M.; Marsal, J. Regulated deficit irrigation during the kernel-filling period and optimal irrigation rates in almond. Agric. Water Manag. 2005, 75, 152–167. [Google Scholar] [CrossRef]
- Gispert, J.R.; de Cartagena, F.R.; Villar, J.M.; Girona, J. Wet soil volume and strategy effects on drip-irrigated olive trees (cv. ‘Arbequina’). Irrig. Sci. 2013, 31, 479–489. [Google Scholar] [CrossRef]
- Gutiérrez-Gordillo, S.; Durán-Zuazo, V.H.; García-Tejero, I. Response of three almond cultivars subjected to different irrigation regimes in Guadalquivir river basin. Agric. Water Manag. 2019, 222, 72–81. [Google Scholar] [CrossRef]
- Nortes, P.A.; Gonzalez-Real, M.M.; Egea, G.; Baille, A. Seasonal effects of deficit irrigation on leaf photosynthetic traits of fruiting and non-fruiting shoots in almond trees. Tree Physiol. 2009, 29, 375–388. [Google Scholar] [CrossRef]
- Alcon, F.; Egea, G.; Nortes, P.A. Financial feasibility of implementing regulated and sustained deficit irrigation in almond orchards. Irrig. Sci. 2013, 31, 931–941. [Google Scholar] [CrossRef]
- Phogat, V.; Skewes, M.A.; Mahadevan, M.; Cox, J.W. Evaluation of soil plant system response to pulsed drip irrigation of an almond tree under sustained stress conditions. Agric. Water Manag. 2013, 118, 1–11. [Google Scholar] [CrossRef]
- Álvarez, S.; Martín, H.; Barajas, E.; Rubio, J.A.; Vivaldi, G.A. Rootstock effects on water relations of young almond trees (cv. Soleta) when subjected to water stress and rehydration. Water 2020, 12, 3319. [Google Scholar] [CrossRef]
- Bellvert, J.; Nieto, H.; Pelechá, A.; Jofre-Čekalović, C.; Zazurca, L.; Miarnau, X. Remote sensing energy balance model for the assessment of crop evapotranspiration and water status in an almond rootstock collection. Front. Plant Sci. 2021, 12, 608967. [Google Scholar] [CrossRef] [PubMed]
- Isaakidis, A.; Sotiropoulos, T.; Almaliotis, D.; Therios, I.; Stylianidis, D. Response to severe water stress of the almond (Prunus amygdalus) ‘Ferragnès’ grafted on eight rootstocks. N. Z. J. Crop Hortic. Sci. 2004, 32, 355–362. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Gonçalves, A.; Silva, E.; Brito, C.; Martins, S.; Pinto, L.; Dinis, L.T.; Luzio, A.; Martins-Gomes, C.; Fernandes-Silva, A.; Ribeiro, C.; et al. Olive tree physiology and chemical composition of fruits are modulated by different deficit irrigation strategies. J. Sci. Food Agric. 2020, 100, 682–694. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Santos, D.L.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Ferreira, H.F.; Correia, C.M. Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: Changes on structure and chemical composition of foliage and oxidative damage. Plant Sci. 2006, 170, 596–605. [Google Scholar] [CrossRef]
- Gispert, J.R.; Vargas, F.J.; Miarnau, F.J.; Alegre, S. Assessment of drought tolerance in almond varieties. In Proceedings of the V International Symposium on Pistachios and Almonds, Sanliurfa, Turkey, 6–10 October 2009; Volume 912, pp. 121–127. [Google Scholar]
- Balestrini, R.; Brunetti, C.; Cammareri, M.; Caretto, S.; Cavallaro, V.; Cominelli, E. Strategies to modulate specialized metabolism in mediterranean crops: From molecular aspects to field. Int. J. Mol. Sci. 2021, 22, 2887. [Google Scholar] [CrossRef]
- Álvarez-Maldini, C.; Acevedo, M.; Estay, D.; Aros, F.; Dumroese, R.K.; Sandoval, S.; Pinto, M. Examining physiological, water relations, and hydraulic vulnerability traits to determine anisohydric and isohydric behavior in almond (Prunus dulcis) cultivars: Implications for selecting agronomic cultivars under changing climate. Front. Plant Sci. 2022, 13, 974050. [Google Scholar] [CrossRef]
- Moutinho-Pereira, J.; Magalhães, N.; Gonçalves, B.; Bacelar, E.; Brito, M.; Correia, C. Gas exchange and water relations of three Vitis vinifera L. cultivars growing under Mediterranean climate. Photosynthetica 2007, 45, 202–207. [Google Scholar] [CrossRef]
- Houghton, E.; Bevandick, K.; Neilsen, D.; Hannam, K.; Nelson, L.M. Effects of postharvest deficit irrigation on sweet cherry (Prunus avium) in five Okanagan Valley, Canada, orchards: I. Tree water status, photosynthesis, and growth. Can. J. Plant Sci. 2022, 103, 73–92. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Maatallah, S.; Guizani, M.; Boughattas, N.E.H.; Guesmi, A.; Ennajeh, M.; Lopez-Lauri, F. Effect of regulated deficit irrigation on agronomic parameters of three plum cultivars (Prunus salicina L.) under semi-arid climate conditions. Plants 2022, 11, 1545. [Google Scholar] [CrossRef] [PubMed]
- Puterka, G.J.; Glenn, D.M.M.; Sekutowski, D.G.; Unruh, T.R.; Jones, S.K. Progress toward liquid formulations of particle films for insect and disease control in pear. Environ. Entomol. 2000, 29, 329–339. [Google Scholar] [CrossRef]
- Marcotegui, A.; Sánchez-Ramos, I.; Pascual, S.; Fernández, C.E.; Cobos, G.; Armendáriz, I.; González-Núñez, M. Kaolin and potassium soap with thyme essential oil to control Monosteira unicostata and other phytophagous arthropods of almond trees in organic orchards. J. Pest Sci. 2015, 88, 753–765. [Google Scholar] [CrossRef]
- Gharaghani, A.; Javarzari, A.M.; Rezaei, A.; Nejati, R. Kaolin Spray Improves Growth, Physiological Functions, Yield, and Nut Quality of ‘Tardy Nonpareil’ Almond under Deficit Irrigation Regimens. Erwerbs-Obstbau 2023, 65, 989–1001. [Google Scholar] [CrossRef]
- Segura-Monroy, S.; Uribe-Vallejo, A.; Ramirez-Godoy, A.; Restrepo-Diaz, H. Effect of kaolin application on growth, water use efficiency, and leaf epidermis characteristics of Physallis peruviana seedlings under two irrigation regimes. J. Agric. Sci. Technol. 2015, 17, 1585–1596. [Google Scholar]
- Dinis, L.-T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Luzio, A.; Silva, E.; Gonçalves, A.; Meijón, M.; Escandón, M.; Arrobas, M.; Rodrigues, M.A.; Moutinho-Pereira, J.; et al. Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical responses. Sci. Hortic. 2019, 246, 201–211. [Google Scholar] [CrossRef]
- Boari, F.; Donadio, A.; Schiattone, M.I.; Cantore, V. Particle film technology: A supplemental tool to save water. Agric. Water Manag. 2015, 147, 154–162. [Google Scholar] [CrossRef]
- Cantore, V.; Pace, B.; Albrizio, R. Kaolin-based particle film technology affects tomato physiology, yield and quality. Environ. Exp. Bot. 2009, 66, 279–288. [Google Scholar] [CrossRef]
- Glenn, D.M.; Prado, E.; Erez, A.; McFerson, J.; Puterka, G.J. A reflective, processed-kaolin particle film affects fruit temperature, radiation reflection, and solar injury in apple. J. Am. Soc. Hortic. Sci. 2002, 127, 188–193. [Google Scholar] [CrossRef]

| Year | Month | T min (°C) | T max (°C) | T avg (°C) | RHavg | Wind Speed (km/h) | Radiation (MJ/m2) | ET0 (mm) | VPD (kPa) | Precipitation (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | January | 0.9 | 9.7 | 4.8 | 80.9 | 4.82 | 216.71 | 23.35 | 0.26 | 38.4 |
| February | 3.3 | 15.1 | 8.5 | 67.4 | 4.76 | 326.95 | 45.72 | 0.58 | 14.4 | |
| March | 5.2 | 17.8 | 10.9 | 58.9 | 5.39 | 560.01 | 87.54 | 0.79 | 37.0 | |
| April | 6.6 | 17.1 | 11.3 | 69.8 | 4.41 | 479.62 | 82.24 | 0.67 | 74.4 | |
| May | 9.9 | 24.5 | 17.0 | 50.7 | 5.37 | 814.20 | 154.18 | 1.35 | 4.2 | |
| June | 12.1 | 25.7 | 18.3 | 57.0 | 4.04 | 679.31 | 136.42 | 1.36 | 27.2 | |
| July | 16.7 | 31.9 | 23.5 | 51.9 | 4.07 | 792.37 | 172.16 | 2.05 | 26.6 | |
| August | 15.7 | 30.5 | 22.8 | 53.1 | 3.88 | 708.41 | 147.36 | 1.81 | 30.0 | |
| September | 13.3 | 27.3 | 19.6 | 56.2 | 4.56 | 559.10 | 114.57 | 1.46 | 14.8 | |
| October | 10.1 | 21.0 | 15.0 | 72.6 | 2.90 | 329.57 | 57.59 | 0.77 | 76.0 | |
| November | 6.7 | 12.9 | 9.5 | 82.0 | 5.82 | 139.87 | 30.00 | 0.29 | 115.0 | |
| December | 3.9 | 10.6 | 7.0 | 86.6 | 5.42 | 133.91 | 21.75 | 0.20 | 218.6 | |
| Total | 676.4 | |||||||||
| 2020 | January | 2.6 | 9.2 | 5.5 | 85.2 | 5.02 | 139.28 | 24.20 | 0.21 | 71.0 |
| February | 6.6 | 13.5 | 10.2 | 82.6 | 5.19 | 272.29 | 35.05 | 0.35 | 2.2 | |
| March | 5.6 | 16.7 | 10.8 | 68.7 | 5.03 | 414.57 | 50.36 | 0.61 | 46.2 | |
| April | 8.4 | 18.2 | 12.6 | 77.1 | 2.75 | 374.41 | 37.18 | 0.55 | 97.6 | |
| May | 12.2 | 26.1 | 18.7 | 60.4 | 3.29 | 653.60 | 140.18 | 1.32 | 20.0 | |
| June | 12.6 | 27.3 | 19.8 | 54.9 | 3.85 | 692.10 | 148.79 | 1.50 | 3.2 | |
| July | 18.4 | 35.5 | 26.7 | 40.0 | 3.48 | 795.16 | 188.92 | 2.77 | 10.6 | |
| August | 15.5 | 30.7 | 22.7 | 51.7 | 3.00 | 654.68 | 149.25 | 1.98 | 34.0 | |
| September | 13.6 | 27.6 | 20.1 | 53.0 | 3.27 | 495.51 | 107.49 | 1.60 | 28.4 | |
| October | 8.3 | 19.1 | 13.3 | 70.5 | 3.86 | 316.08 | 47.76 | 0.71 | 125.8 | |
| November | 7.7 | 14.9 | 10.8 | 82.9 | 3.59 | 159.93 | 26.65 | 0.33 | 132.8 | |
| December | 3.7 | 10.5 | 7.1 | 82.1 | 4.52 | 132.63 | 21.11 | 0.24 | 71.2 | |
| Total | 643.0 | |||||||||
| 2021 | January | 1.6 | 8.8 | 4.8 | 81.5 | 4.69 | 169.14 | 22.77 | 0.23 | 36.8 |
| February | 6.0 | 15.4 | 10.5 | 82.1 | 2.33 | 219.45 | 32.05 | 0.41 | 95.0 | |
| March | 4.1 | 20.1 | 11.9 | 64.4 | 2.68 | 535.79 | 75.20 | 0.84 | 1.0 | |
| April | 8.3 | 22.1 | 14.7 | 64.8 | 2.47 | 562.42 | 93.25 | 0.96 | 68.0 | |
| May | 9.2 | 24.4 | 16.6 | 57.7 | 3.15 | 682.40 | 124.23 | 1.24 | 13.4 | |
| June | 13.0 | 29.5 | 20.9 | 56.5 | 2.74 | 722.58 | 142.72 | 1.74 | 67.0 | |
| July | 14.7 | 30.4 | 22.4 | 50.3 | 3.45 | 769.21 | 157.44 | 1.90 | 3.2 | |
| August | 15.5 | 32.6 | 23.4 | 48.4 | 3.09 | 697.63 | 144.41 | 2.20 | 0.0 | |
| September | 13.6 | 25.5 | 18.8 | 67.3 | 1.99 | 443.42 | 80.33 | 1.13 | 92.0 | |
| October | 8.3 | 19.5 | 15.3 | 69.5 | 1.15 | 351.22 | 46.32 | 0.71 | 24.8 | |
| November | 3.2 | 11.2 | 7.8 | 77.4 | 2.34 | 246.84 | 22.56 | 0.28 | 26.4 | |
| December | 6.1 | 12.6 | 8.4 | 83.4 | 4.06 | 124.33 | 20.79 | 0.23 | 107.0 | |
| Total | 534.6 |
| Treatment | Cultivar | Irrigation Regime | Kaolin |
|---|---|---|---|
| FIC | Constantí | FI | No |
| FIC + K | Constantí | FI | Yes |
| RDIC | Constantí | RDI | No |
| RDIC + K | Constantí | RDI | Yes |
| FIV | Vairo | FI | No |
| FIV + K | Vairo | FI | Yes |
| RDIV | Vairo | RDI | No |
| RDIV + K | Vairo | RDI | Yes |
| Growing Season | Irrigation Dates | Kaolin Application—Reducing Irrigation | Water Applied (m3 ha−1) | Pann (mm) | Peff (mm) | ET0 (mm) | ||
|---|---|---|---|---|---|---|---|---|
| First Irrigation | Last Irrigation | FI | RDI | |||||
| 2019 | 3 June | 26 August | 8 July | 2189.9 | 1287.8 | 676.4 | 265.8 | 952.1 |
| 2020 | 23 June | 3 September | 20 July | 2142.2 | 1254.1 | 643.0 | 318 | 869.9 |
| 2021 | 7 June | 6 September | 14 July | 2458.3 | 1433.6 | 534.6 | 240 | 863.9 |
| Year | DOY | Treatment | Factorial Analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FIC | FIC + K | RDIC | RDIC + K | FIV | FIV + K | RDIV | RDIV + K | I | K | C | I × K | I × C | K × C | I × K × C | |||
| 2020 | 220 | LA (cm2) | 21.49 ± 3.75 | 19.93 ± 4.11 | 19.40 ± 3.26 | 19.70 ± 6.77 | 19.93 ± 3.15 | 20.30 ± 1.93 | 20.82 ± 3.03 | 20.52 ± 5.10 | 0.7186 | 0.7275 | 0.7580 | 0.7238 | 0.3109 | 0.6944 | 0.4545 |
| LMA (g/m2) | 104.40 ± 13.02 | 110.36 ± 6.34 | 113.05 ± 13.94 | 106.16 ± 21.52 | 117.06 ± 11.93 | 118.24 ± 11.88 | 122.88 ± 6.50 | 122.75 ± 36.21 | 0.3088 | 0.9933 | 0.0016 | 0.3297 | 0.6854 | 0.8908 | 0.4263 | ||
| D (g/kg) | 430.87 ± 25.68 ab | 437.30 ± 13.52 ab | 440.93 ± 49.15 ab | 453.55 ± 20.76 b | 412.62 ± 11.29 a | 414.71 ± 33.01 a | 427.47 ± 18.51 ab | 415.64 ± 12.44 a | 0.0503 | 0.6618 | 0.0000 | 0.7165 | 0.6213 | 0.1782 | 0.3458 | ||
| RWC (%) | 86.24 ± 1.66 ab | 88.77 ± 2.38 cd | 85.36 ± 1.27 a | 86.36 ± 1.85 abc | 88.35 ± 1.96 bcd | 90.05 ± 2.76 d | 87.35 ± 1.43 abc | 88.12 ± 1.45 bcd | 0.0001 | 0.0002 | 0.0000 | 0.1192 | 0.8185 | 0.4971 | 0.7017 | ||
| S (mg H2O cm−2) | 13.72 ± 0.69 a | 14.19 ± 0.65 b | 14.35 ± 1.60 b | 12.78 ± 2.43 a | 16.66 ± 1.58 c | 16.70 ± 1.53 c | 16.48 ± 1.14 c | 17.18 ± 4.47 c | 0.7756 | 0.8377 | 0.0000 | 0.4183 | 0.5329 | 0.2880 | 0.1191 | ||
| WSD (%) | 13.76 ± 1.66 bcd | 11.23 ± 2.38 ab | 14.64 ± 1.27 d | 13.64 ± 1.85 bcd | 11.65 ± 1.96 abc | 9.95 ± 2.76 a | 12.65 ± 1.43 bcd | 11.88 ± 1.45 abc | 0.0001 | 0.0002 | 0.0000 | 0.1192 | 0.8185 | 0.4971 | 0.7017 | ||
| WCS (g H2O g−1 DM) | 0.21 ± 0.02 b | 0.16 ± 0.03 a | 0.22 ± 0.04 b | 0.19 ± 0.03 ab | 0.21 ± 0.02 b | 0.16 ± 0.04 a | 0.19 ± 0.02 ab | 0.19 ± 0.02 ab | 0.0045 | 0.0000 | 0.0412 | 0.0637 | 0.9485 | 0.0958 | 0.7469 | ||
| 253 | LA (cm2) | 21.06 ± 3.42 b | 19.97 ± 2.55 ab | 17.13 ± 2.51 a | 19.47 ± 3.49 ab | 24.74 ± 3.56 c | 23.15 ± 2.83 bc | 22.91 ± 2.28 bc | 22.42 ± 2.95 bc | 0.0022 | 0.5330 | 0.0000 | 0.0345 | 0.2931 | 0.2839 | 0.2168 | |
| LMA (g/m2) | 120.86 ± 9.96 a | 122.87 ± 11.30 ab | 136.13 ± 10.51 b | 125.07 ± 7.10 ab | 131.02 ± 7.52 ab | 130.79 ± 14.22 ab | 129.23 ± 11.69 ab | 129.00 ± 11.91 ab | 0.1171 | 0.2822 | 0.0887 | 0.1400 | 0.0186 | 0.3305 | 0.1400 | ||
| D (g/kg) | 477.39 ± 18.99 bc | 502.98 ± 17.44 d | 496.22 ± 10.28 cd | 498.84 ± 7.01 d | 454.46 ± 14.72 a | 458.40 ± 15.57 ab | 457.17 ± 18.26 ab | 460.28 ± 21.53 ab | 0.1465 | 0.0087 | 0.0000 | 0.0736 | 0.4446 | 0.1114 | 0.0958 | ||
| RWC (%) | 78.43 ± 4.74 ab | 77.40 ± 5.27 a | 78.71 ± 2.08 ab | 77.11 ± 2.04 a | 81.91 ± 2.10 bc | 83.43 ± 4.10 c | 82.42 ± 3.07 bc | 78.26 ± 2.48 ab | 0.1006 | 0.0661 | 0.0000 | 0.0296 | 0.1026 | 0.9946 | 0.0734 | ||
| S (mg H2O cm−2) | 13.26 ± 1.40 ab | 12.13 ± 0.93 a | 13.82 ± 1.05 bc | 12.56 ± 0.57 ab | 15.73 ± 0.83 d | 15.44 ± 1.50 d | 15.30 ± 0.63 d | 15.11 ± 0.49 d | 0.7812 | 0.0014 | 0.0000 | 0.9710 | 0.0467 | 0.0304 | 0.7945 | ||
| WSD (%) | 21.57 ± 4.74 bc | 22.60 ± 5.27 c | 21.29 ± 2.08 bc | 22.89 ± 2.04 c | 18.09 ± 2.10 ab | 16.57 ± 4.10 a | 17.58 ± 3.07 ab | 21.74 ± 2.48 bc | 0.1006 | 0.0661 | 0.0000 | 0.0296 | 0.1026 | 0.9946 | 0.0734 | ||
| WCS (g H2O g−1 DM) | 0.30 ± 0.07 bc | 0.29 ± 0.08 abc | 0.27 ± 0.03 abc | 0.30 ± 0.03 bc | 0.27 ± 0.03 abc | 0.24 ± 0.07 a | 0.25 ± 0.04 ab | 0.33 ± 0.03 c | 0.1535 | 0.1424 | 0.0360 | 0.0016 | 0.0220 | 0.4551 | 0.1220 | ||
| 2021 | 224 | LA (cm2) | 20.10 ± 7.15 | 18.87 ± 5.99 | 20.41 ± 5.75 | 19.70 ± 3.88 | 23.15 ± 3.12 | 23.53 ± 3.35 | 21.03 ± 3.07 | 19.05 ± 4.13 | 0.2052 | 0.4107 | 0.0764 | 0.6667 | 0.0740 | 0.9390 | 0.5007 |
| LMA (g/m2) | 108.12 ± 6.97 b | 104.56 ± 9.02 b | 79.95 ± 9.88 a | 78.76 ± 6.50 a | 120.07 ± 11.63 b | 119.18 ± 5.52 b | 121.57 ± 12.25 b | 118.06 ± 14.18 b | 0.0001 | 0.4697 | 0.0000 | 0.9838 | 0.0000 | 0.9779 | 0.6924 | ||
| D (g/kg) | 435.79 ± 12.51 abc | 435.66 ± 20.47 abc | 449.24 ± 10.48 bc | 458.90 ± 18.20 c | 413.98 ± 16.99 a | 425.20 ± 6.15 ab | 421.99 ± 21.70 ab | 409.60 ± 37.14 a | 0.6413 | 0.1077 | 0.0000 | 0.4415 | 0.5509 | 0.0156 | 0.0658 | ||
| RWC (%) | 79.45 ± 2.13 cd | 80.42 ± 3.03 cd | 76.23 ± 2.26 ab | 74.32 ± 2.87 a | 82.39 ± 3.04 d | 81.82 ± 1.33 cd | 79.15 ± 1.39 bc | 80.65 ± 1.37 cd | 0.0000 | 0.9969 | 0.0000 | 0.6942 | 0.0191 | 0.3624 | 0.0182 | ||
| S (mg H2O cm−2) | 14.00 ± 0.84 bc | 13.54 ± 1.04 b | 9.82 ± 3.66 a | 9.28 ± 0.66 a | 16.97 ± 1.15 d | 16.11 ± 0.67 cd | 16.61 ± 1.03 d | 17.01 ± 1.59 d | 0.0000 | 0.3185 | 0.0000 | 0.4207 | 0.0000 | 0.7140 | 0.3591 | ||
| WSD (%) | 20.55 ± 2.13 ab | 19.58 ± 3.03 ab | 23.77 ± 2.26 cd | 25.68 ± 2.87 d | 17.61 ± 3.04 a | 18.18 ± 1.33 ab | 20.85 ± 1.39 bc | 19.35 ± 1.37 ab | 0.0000 | 0.9969 | 0.0000 | 0.6942 | 0.0191 | 0.3624 | 0.0182 | ||
| WCS (g H2O g−1 DM) | 0.33 ± 0.03 ab | 0.32 ± 0.05 a | 0.38 ± 0.04 bc | 0.41 ± 0.05 c | 0.30 ± 0.04 a | 0.30 ± 0.03 a | 0.36 ± 0.02 abc | 0.35 ± 0.07 abc | 0.0000 | 0.9020 | 0.0029 | 0.3637 | 0.4463 | 0.6757 | 0.1784 | ||
| 242 | LA (cm2) | 19.48 ± 6.21 bcd | 15.74 ± 1.79 ab | 14.07 ± 2.07 a | 14.98 ± 3.16 ab | 22.15 ± 3.56 d | 21.12 ± 3.12 d | 19.76 ± 2.13 cd | 19.59 ± 1.91 cd | 0.0010 | 0.1755 | 0.0000 | 0.0655 | 0.4493 | 0.5830 | 0.2035 | |
| LMA (g/m2) | 121.86 ± 8.42 ab | 127.28 ± 16.71 abc | 116.98 ± 10.33 a | 110.86 ± 13.68 a | 127.21 ± 12.57 abc | 126.15 ± 12.44 abc | 137.77 ± 8.41 bc | 138.93 ± 11.09 c | 0.8501 | 0.9553 | 0.0000 | 0.3872 | 0.0001 | 0.9397 | 0.2039 | ||
| D (g/kg) | 482.51 ± 15.90 b | 500.27 ± 21.63 b | 487.36 ± 8.08 b | 489.34 ± 21.62 b | 440.94 ± 16.61 a | 443.07 ± 7.26 a | 457.23 ± 12.15 a | 453.20 ± 17.32 a | 0.1577 | 0.2147 | 0.0000 | 0.1279 | 0.0255 | 0.1331 | 0.5022 | ||
| RWC (%) | 82.17 ± 1.54 abc | 80.05 ± 1.20 a | 80.99 ± 2.67 ab | 81.52 ± 7.41 ab | 86.34 ± 2.30 cd | 87.32 ± 3.35 d | 84.84 ± 1.58 bcd | 85.56 ± 2.50 bcd | 0.3293 | 0.9702 | 0.0000 | 0.4325 | 0.2452 | 0.2790 | 0.3398 | ||
| S (mg H2O cm−2) | 13.06 ± 0.61 b | 12.65 ± 0.98 ab | 12.29 ± 0.89 ab | 11.53 ± 1.05 a | 16.08 ± 0.84 c | 15.84 ± 1.35 c | 16.34 ± 0.65 c | 16.74 ± 0.80 c | 0.3773 | 0.2244 | 0.0000 | 0.7417 | 0.0004 | 0.1121 | 0.2318 | ||
| WSD (%) | 17.83 ± 1.54 bcd | 19.95 ± 1.20 d | 19.01 ± 2.67 d | 18.48 ± 7.41 d | 13.66 ± 2.30 b | 12.68 ± 3.35 a | 15.16 ± 1.58 bc | 14.44 ± 2.50 abc | 0.3293 | 0.9702 | 0.0000 | 0.4325 | 0.2452 | 0.2790 | 0.3398 | ||
| WCS (g H2O g−1 DM) | 0.23 ± 0.03 b | 0.25 ± 0.02 b | 0.25 ± 0.04 b | 0.24 ± 0.10 b | 0.20 ± 0.03 a | 0.19 ± 0.05 a | 0.21 ± 0.02 a | 0.20 ± 0.03 a | 0.4262 | 0.7326 | 0.0002 | 0.7326 | 0.6006 | 0.3755 | 0.5694 | ||
| Year | Treatment | Kernel Yield (kg ha−1) | Percent Kernel (%) | WUE (kg m−3) |
|---|---|---|---|---|
| ANOVA Test | ||||
| Irrigation (I) | 0.0000 | 0.2719 | 0.0002 | |
| Kaolin (K) | 0.2748 | 0.8063 | 0.4339 | |
| Cultivar (C) | 0.2687 | 0.0000 | 0.2553 | |
| I × K | 0.4894 | 0.2040 | 0.7220 | |
| I × C | 0.0000 | 0.4191 | 0.0001 | |
| K × C | 0.5346 | 0.0163 | 0.6437 | |
| I × K × C | 0.5628 | 0.5204 | 0.5936 | |
| Tukey Multiple Range Test | ||||
| 2019 | FIC | 469.03 ± 59.47 ab | 25.51 ± 1.33 a | 0.10 ± 0.01 a |
| FIC + K | 410.83 ± 63.75 a | 25.46 ± 1.77 a | 0.08 ± 0.01 a | |
| RDIC | 458.70 ± 61.79 ab | 26.32 ± 1.60 b | 0.12 ± 0.02 b | |
| RDIC + K | 484.81 ± 56.75 ab | 26.40 ± 1.35 b | 0.12 ± 0.01 b | |
| FIV | 611.04 ± 95.38 d | 28.44 ± 2.80 c | 0.13 ± 0.02 bc | |
| FIV + K | 602.04 ±76.96 d | 28.48 ± 1.97 c | 0.12 ± 0.02 b | |
| RDIV | 556.57 ± 52.06 cd | 28.24 ± 2.04 c | 0.14 ± 0.01 c | |
| RDIV + K | 539.28 ± 80.93 cd | 28.36 ± 1.49 c | 0.14 ± 0.02 c | |
| p-value | 0.0000 | 0.0000 | 0.0000 | |
| 2020 | FIC | 460.62 ± 48.83 b | 23.39 ± 2.70 a | 0.09 ± 0.01 b |
| FIC + K | 462.23 ± 63.87 b | 23.63 ± 2.28 a | 0.09 ± 0.01 b | |
| RDIC | 406.82 ± 41.01 b | 22.75 ± 3.22 a | 0.09 ± 0.01 b | |
| RDIC + K | 475.38 ± 61.86 b | 23.14 ± 2.57 a | 0.11 ± 0.01 c | |
| FIV | 452.65 ± 69.95 b | 26.19 ± 1.90 bc | 0.09 ± 0.01 b | |
| FIV + K | 429.76 ± 77.05 b | 25.76 ± 3.30 b | 0.08 ± 0.01 ab | |
| RDIV | 318.35 ± 61.53 a | 27.17 ± 3.15 c | 0.07 ± 0.01 a | |
| RDIV + K | 301.02 ± 52.24 a | 26.58 ± 2.72 bc | 0.07 ± 0.01 a | |
| p-value | 0.0000 | 0.0000 | 0.0000 | |
| 2021 | FIC | 646.51 ± 92.76 c | 24.05 ± 1.72 a | 0.13 ± 0.02 a |
| FIC + K | 636.47 ± 62.57 c | 23.94 ± 3.34 a | 0.13 ± 0.01 a | |
| RDIC | 667.38 ± 86.11 c | 23.17 ± 2.17 a | 0.17 ± 0.02 b | |
| RDIC + K | 603.26 ± 75.42 c | 24.00 ± 1.98 a | 0.16 ± 0.02 b | |
| FIV | 636.10 ± 106.91 c | 27.80 ± 2.22 c | 0.13 ± 0.02 a | |
| FIV + K | 598.11 ± 87.47 bc | 27.39 ± 1.82 c | 0.12 ± 0.02 a | |
| RDIV | 497.19 ± 89.60 ab | 25.99 ± 2.42 b | 0.13 ± 0.02 a | |
| RDIV + K | 470.55 ± 98.22 a | 26.05 ± 2.50 b | 0.12 ± 0.03 a | |
| p-value | 0.0000 | 0.0000 | 0.0000 | |
| Cumulative—Average | FIC | 1576.16 ± 126.31 cd | 24.31 ± 1.22 a | 0.11 ± 0.01 a |
| FIC + K | 1509.53 ± 59.43 bc | 24.34 ± 1.45 a | 0.10 ± 0.00 a | |
| RDIC | 1532.90 ± 109.94 c | 24.08 ± 1.38 a | 0.13 ± 0.01 b | |
| RDIC + K | 1563.45 ± 128.62 cd | 24.51 ± 1.31 a | 0.13 ± 0.01 b | |
| FIV | 1699.79 ± 193.14 d | 27.48 ± 1.47 b | 0.11 ± 0.01 a | |
| FIV + K | 1629.91 ± 141.74 cd | 27.21 ± 1.48 b | 0.11 ± 0.01 a | |
| RDIV | 1372.11 ± 131.89 a | 27.13 ± 1.53 b | 0.11 ± 0.01 a | |
| RDIV + K | 1310.85 ± 118.01 a | 27.00 ± 1.30 b | 0.11 ± 0.01 a | |
| p-value | 0.0000 | 0.0000 | 0.0000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreales, D.; Capitão, S.; Bento, A.A.; Casquero, P.A.; Ribeiro, A.C. Adapting Almond Production to Climate Change through Deficit Irrigation and Foliar Kaolin Application in a Mediterranean Climate. Atmosphere 2023, 14, 1593. https://doi.org/10.3390/atmos14101593
Barreales D, Capitão S, Bento AA, Casquero PA, Ribeiro AC. Adapting Almond Production to Climate Change through Deficit Irrigation and Foliar Kaolin Application in a Mediterranean Climate. Atmosphere. 2023; 14(10):1593. https://doi.org/10.3390/atmos14101593
Chicago/Turabian StyleBarreales, David, Susana Capitão, Albino António Bento, Pedro A. Casquero, and António Castro Ribeiro. 2023. "Adapting Almond Production to Climate Change through Deficit Irrigation and Foliar Kaolin Application in a Mediterranean Climate" Atmosphere 14, no. 10: 1593. https://doi.org/10.3390/atmos14101593
APA StyleBarreales, D., Capitão, S., Bento, A. A., Casquero, P. A., & Ribeiro, A. C. (2023). Adapting Almond Production to Climate Change through Deficit Irrigation and Foliar Kaolin Application in a Mediterranean Climate. Atmosphere, 14(10), 1593. https://doi.org/10.3390/atmos14101593







