Advances in Oxidative Coupling of Methane
Abstract
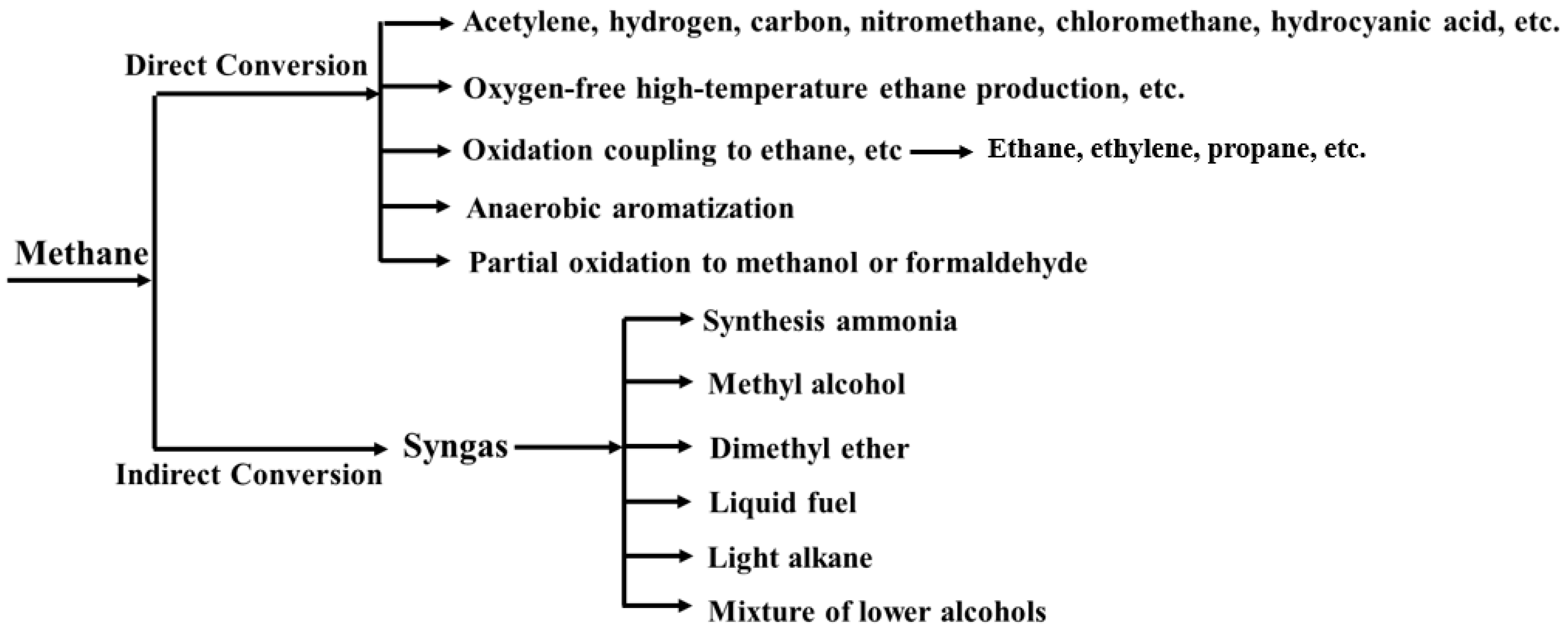
1. Introduction
2. Catalysts for OCM
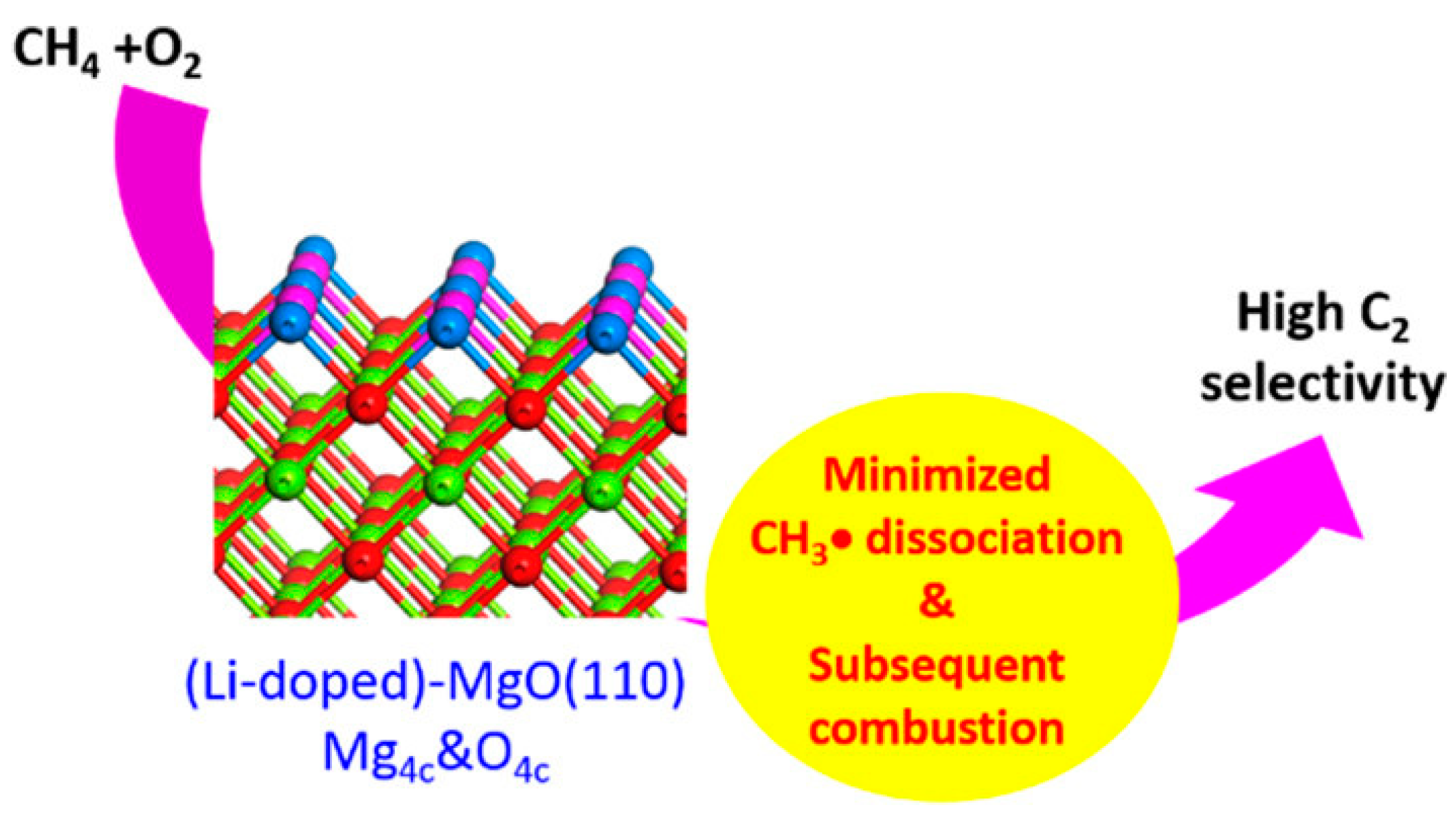
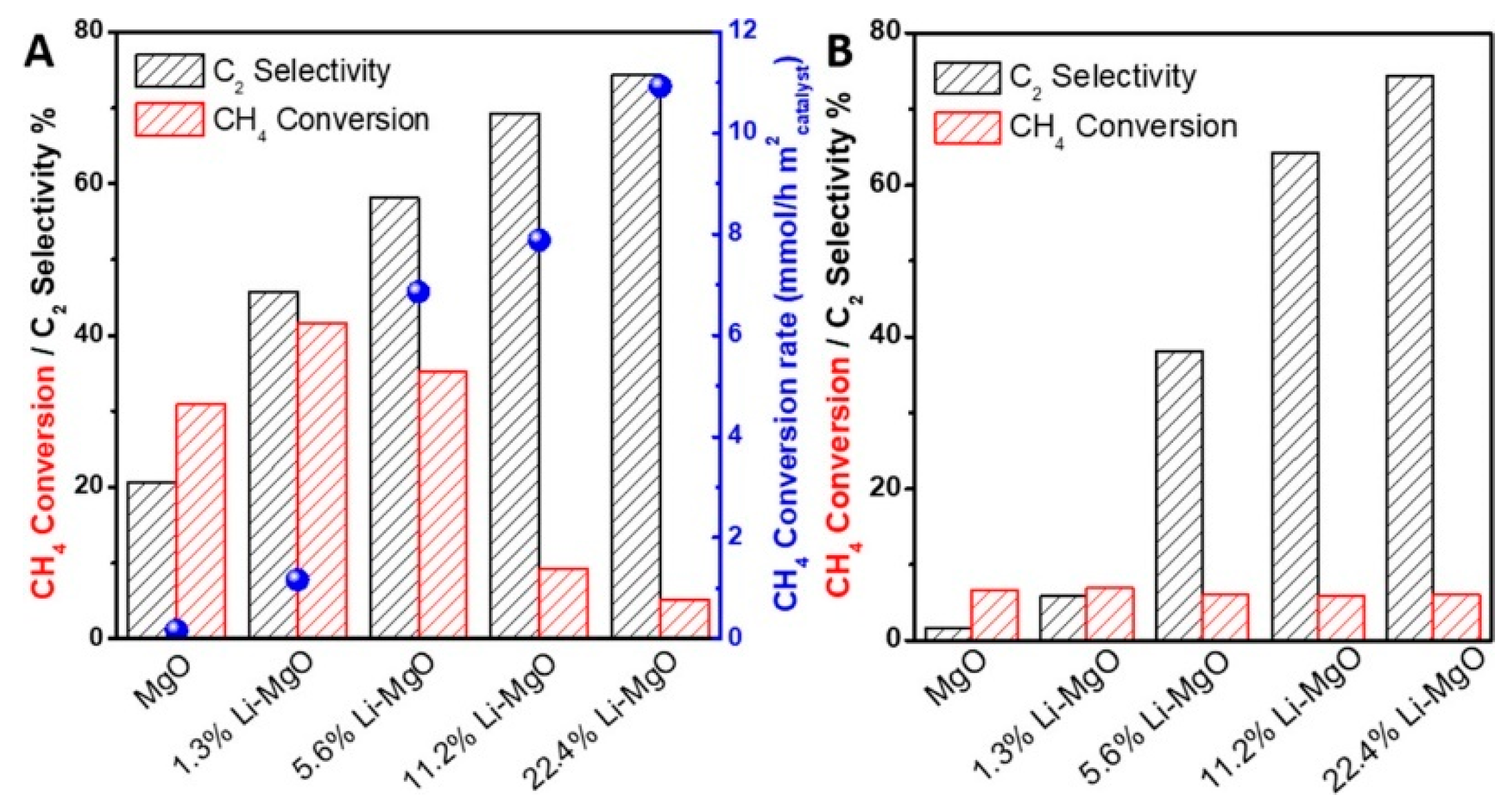
2.1. Alkali-Metal-Modified Alkaline Earth Metal Oxide
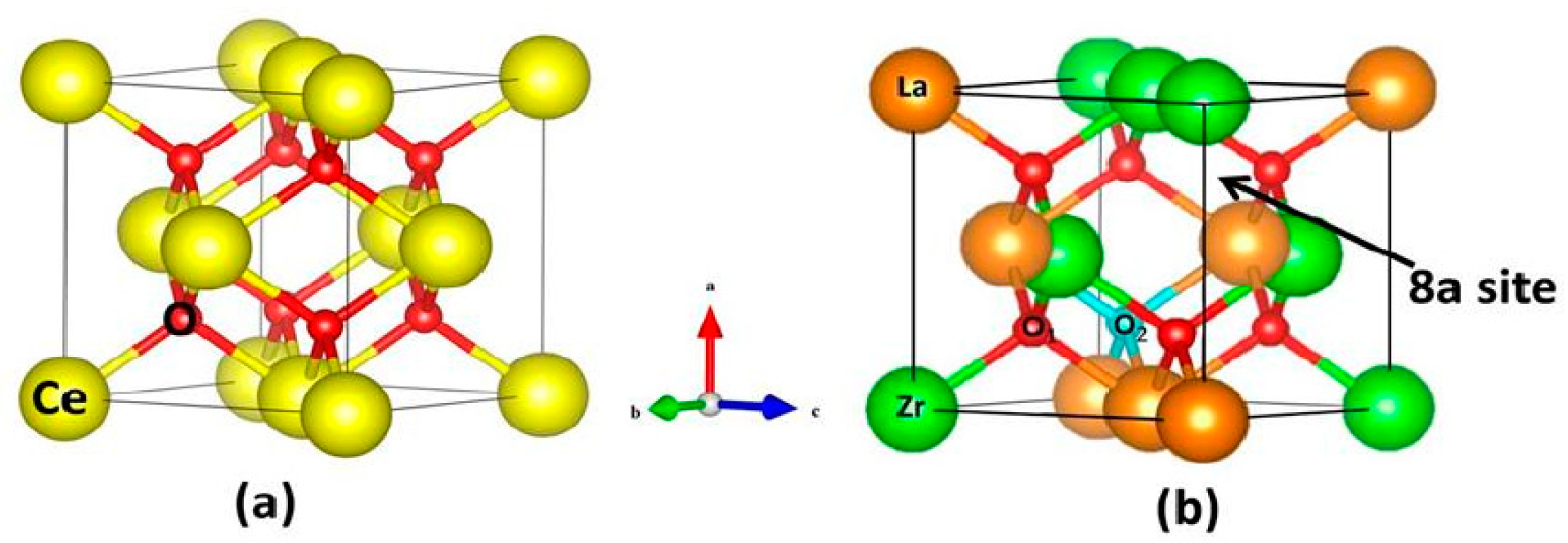
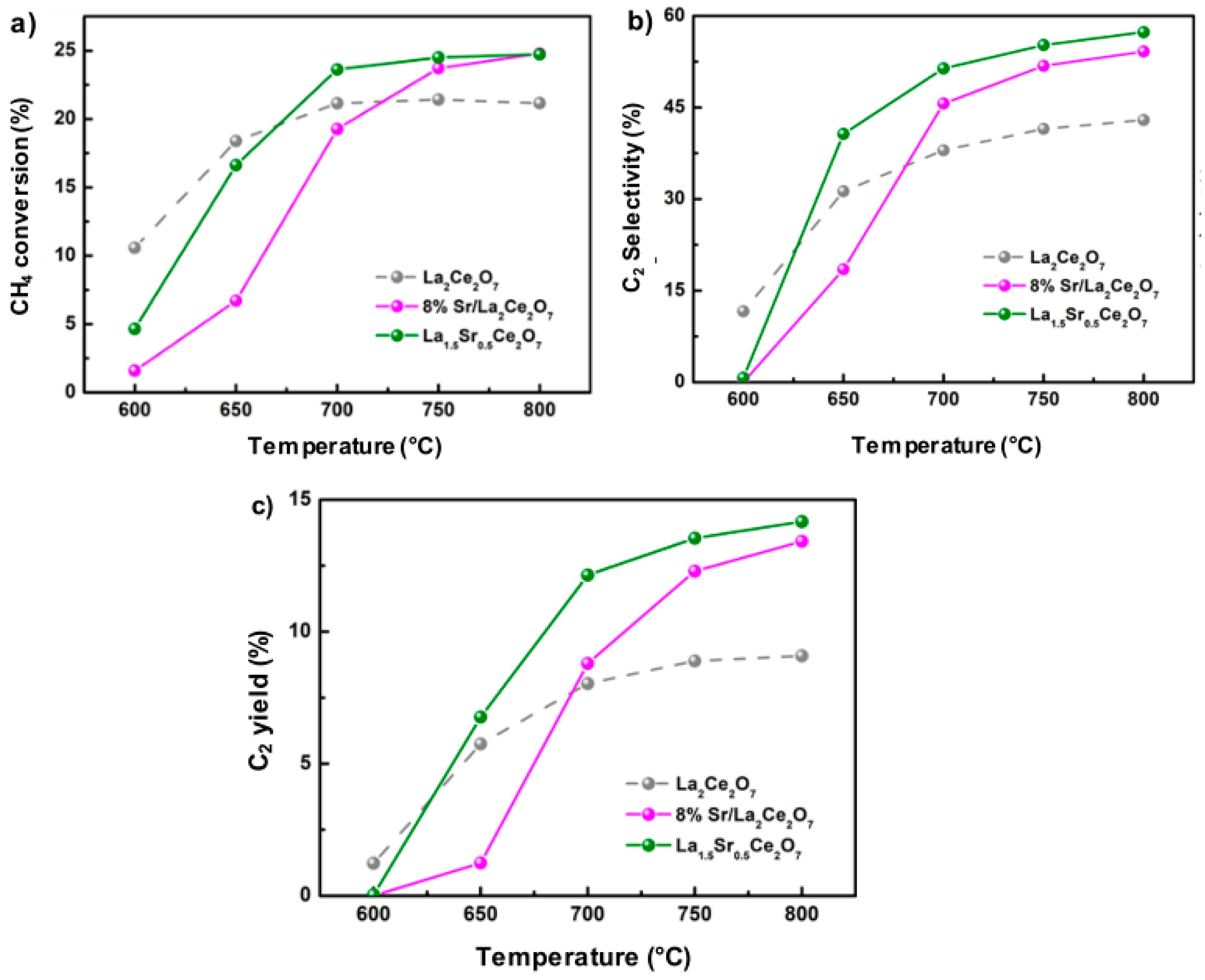
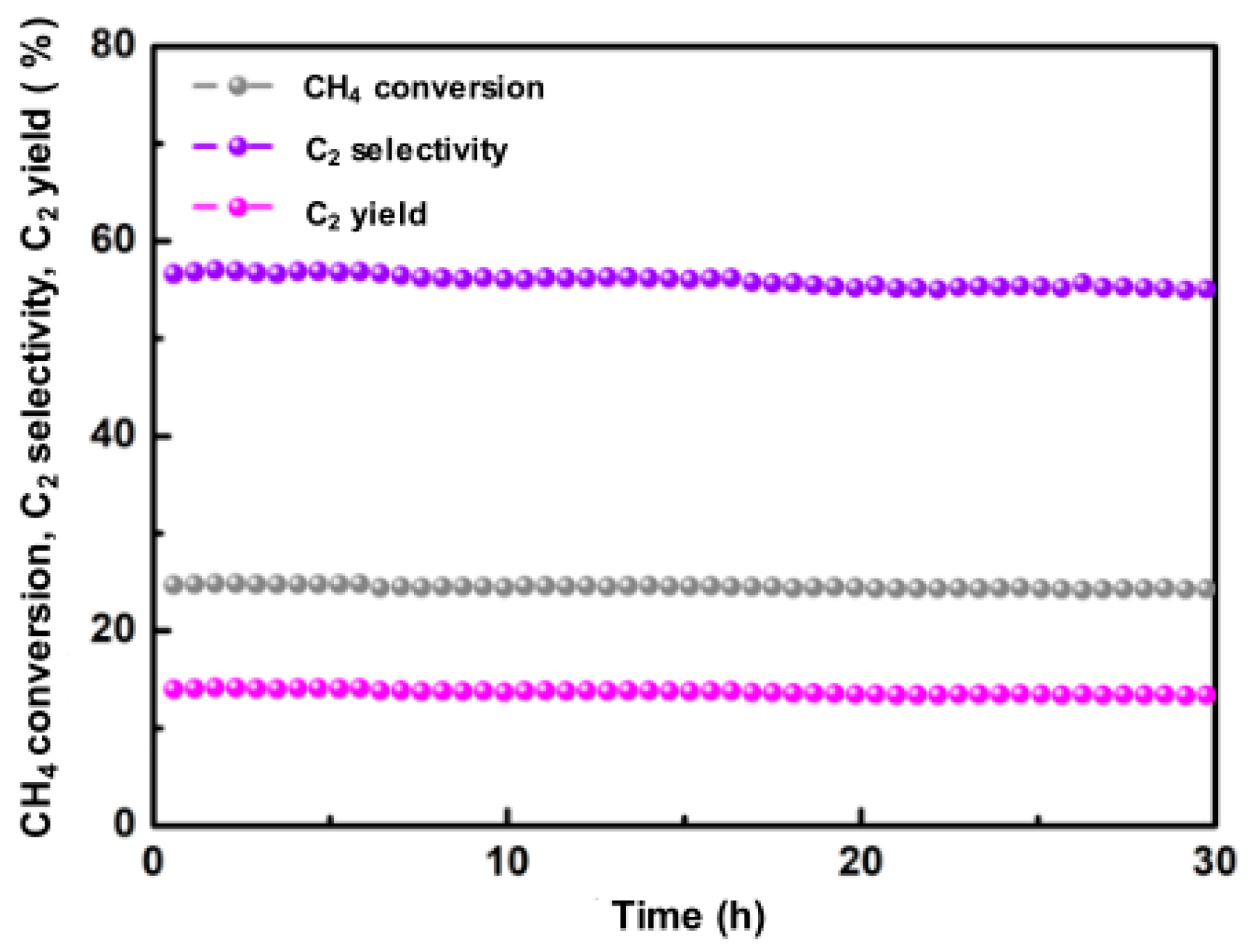
2.2. Rare Earth Metal Oxides
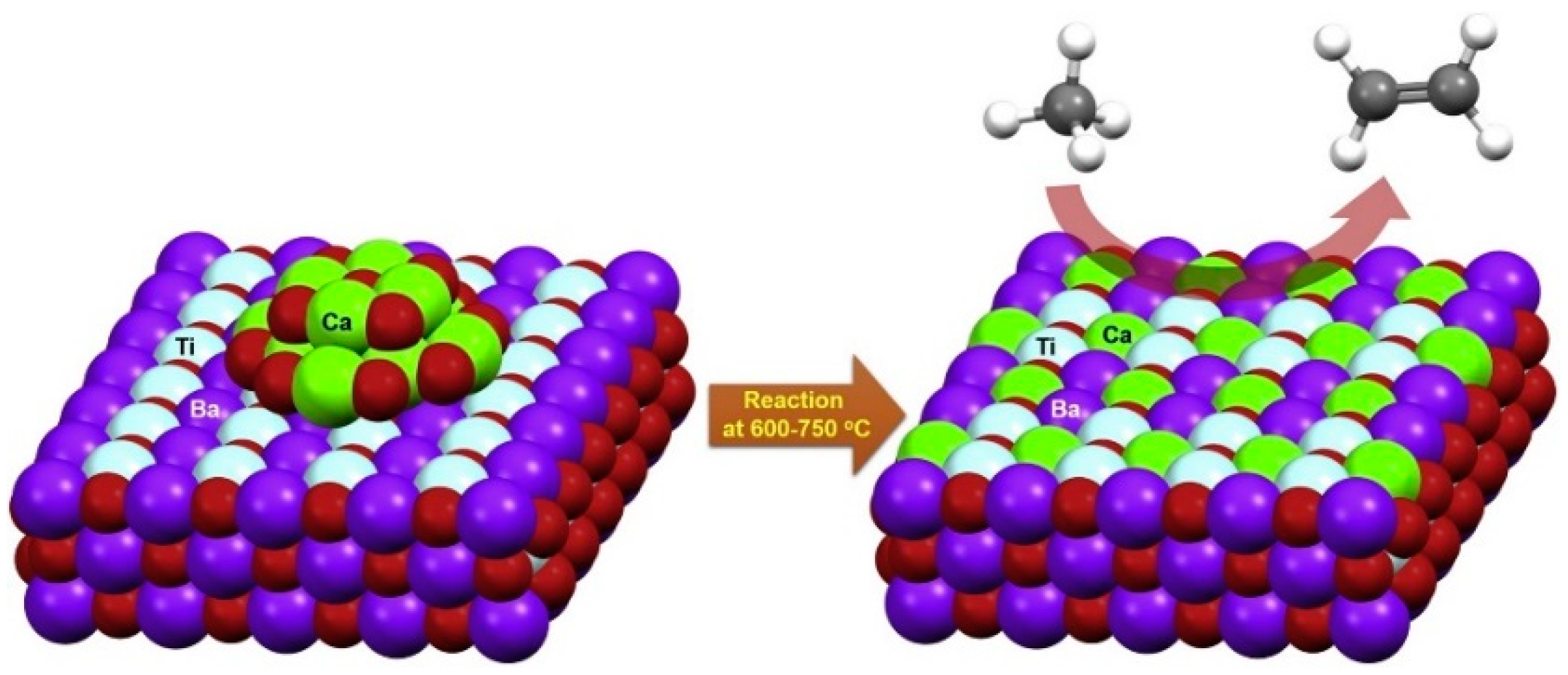
2.3. Perovskite-Type Oxides
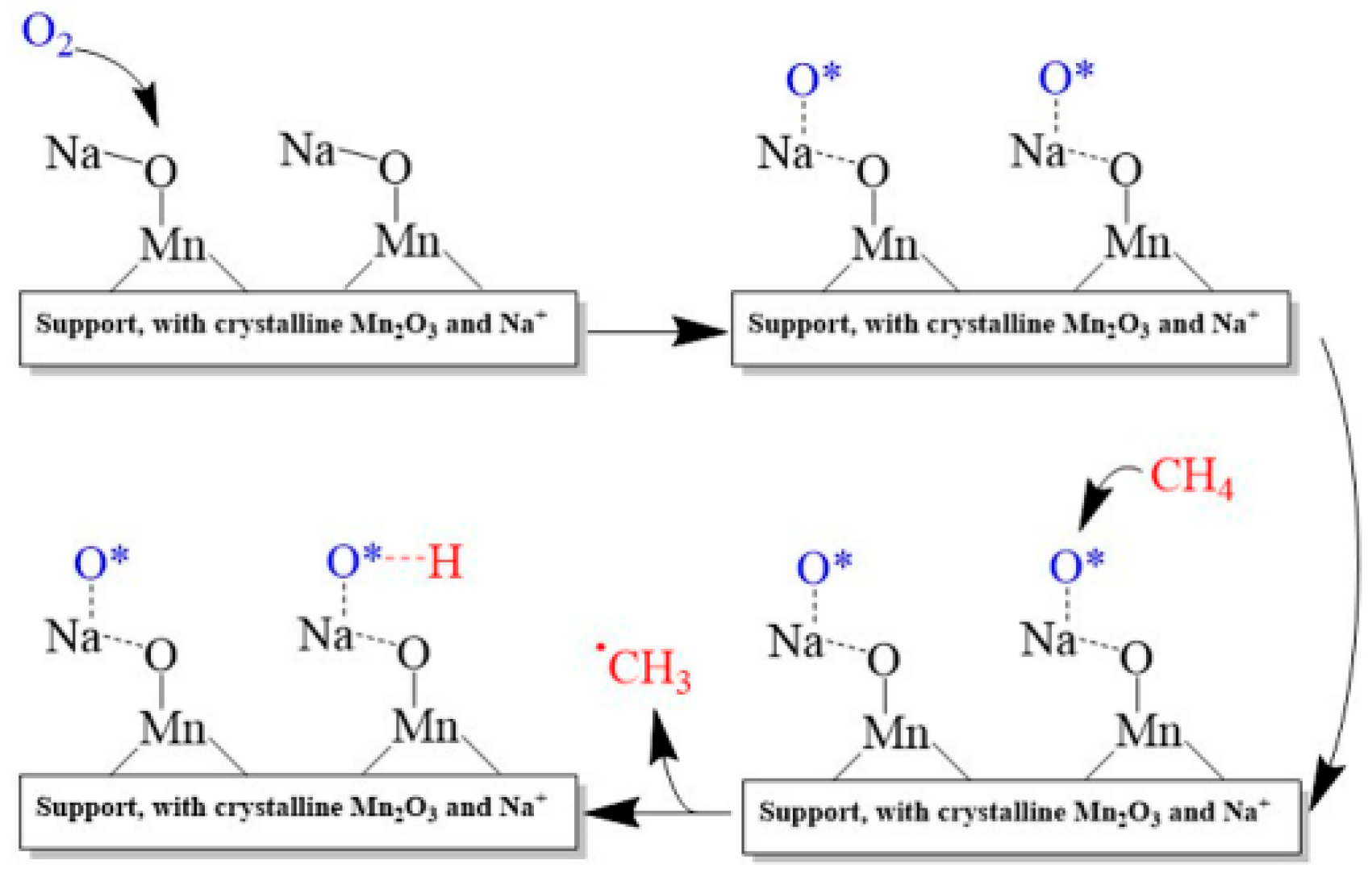
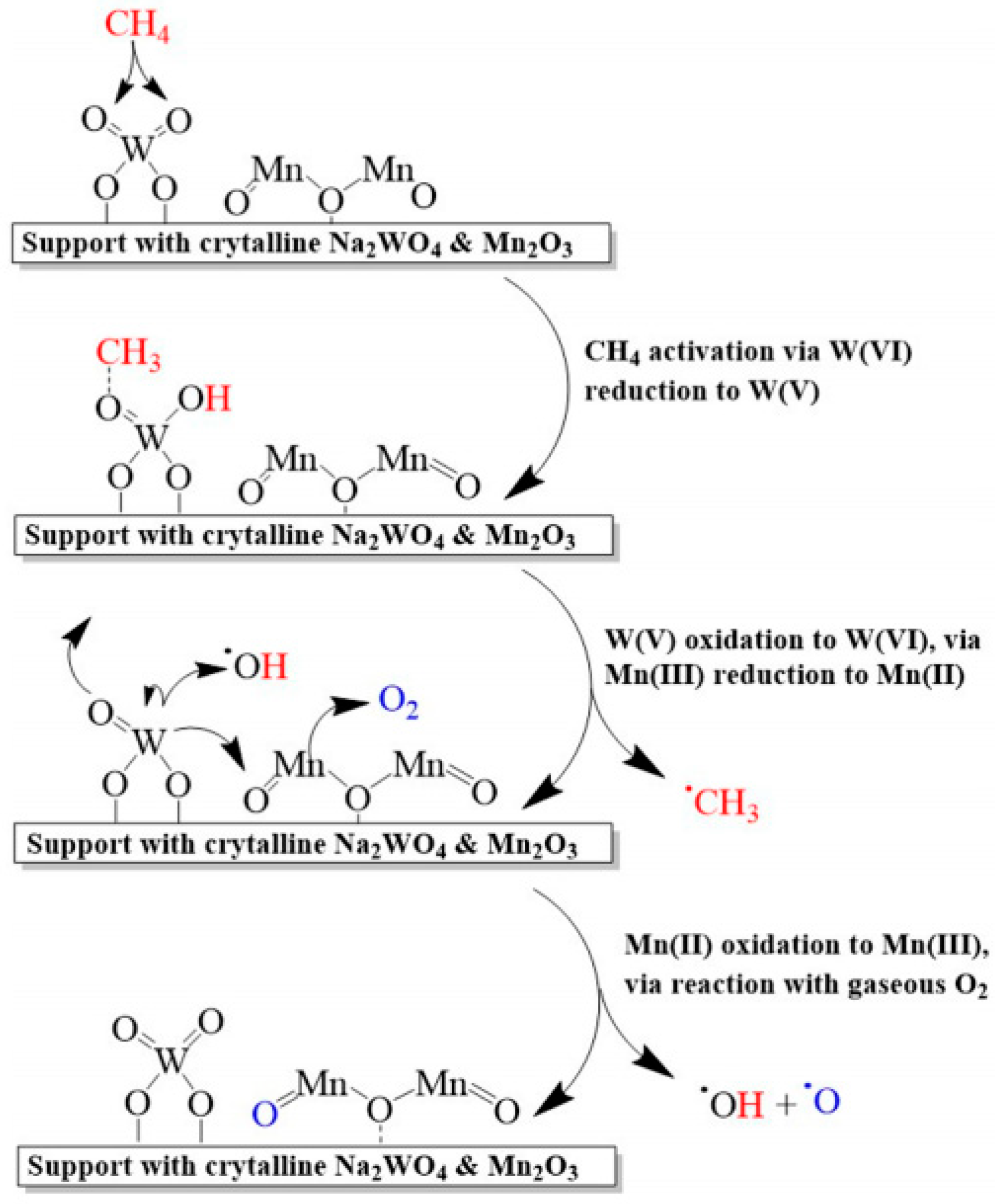
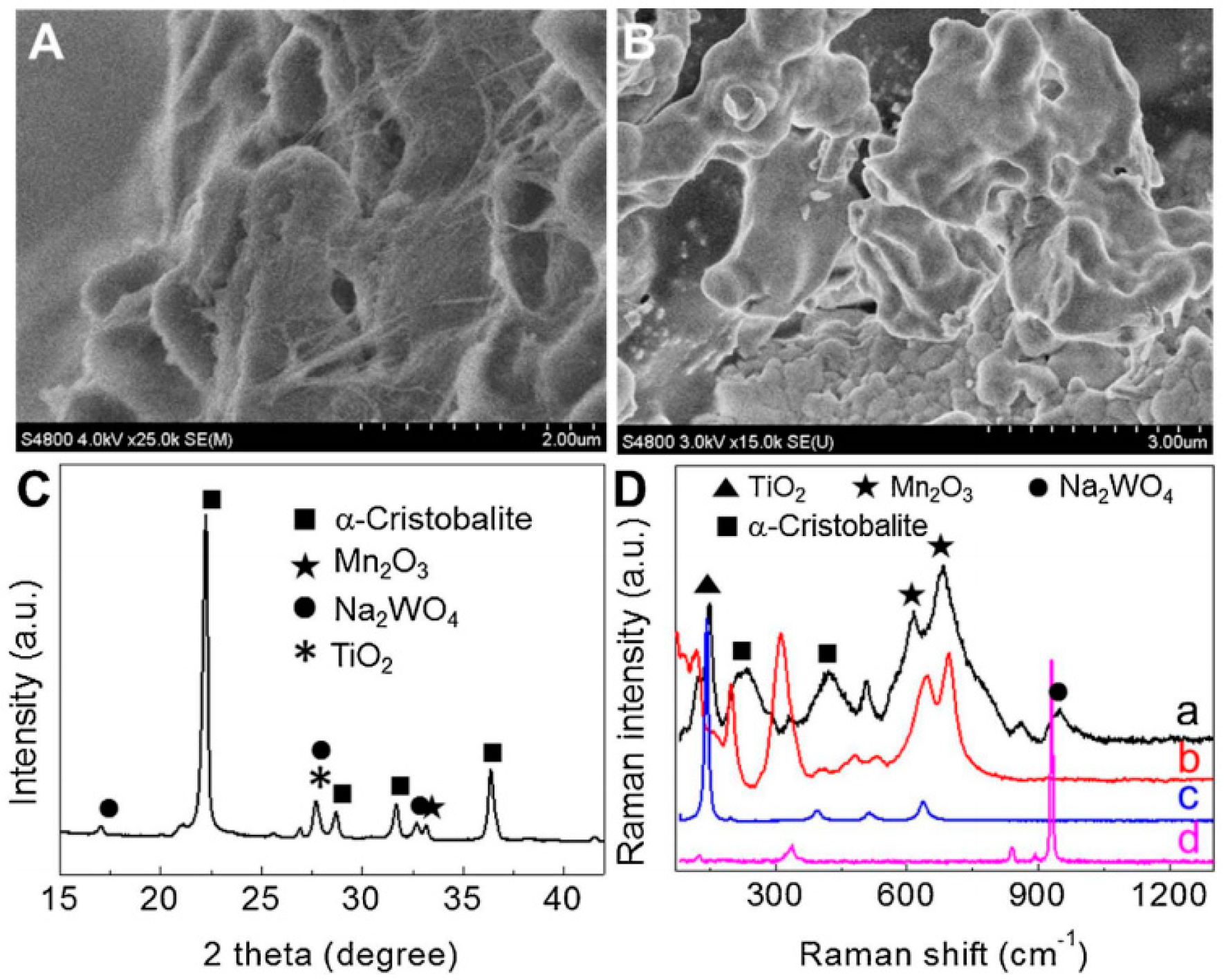
2.4. Mn/Na2WO4/SiO2 Catalysts
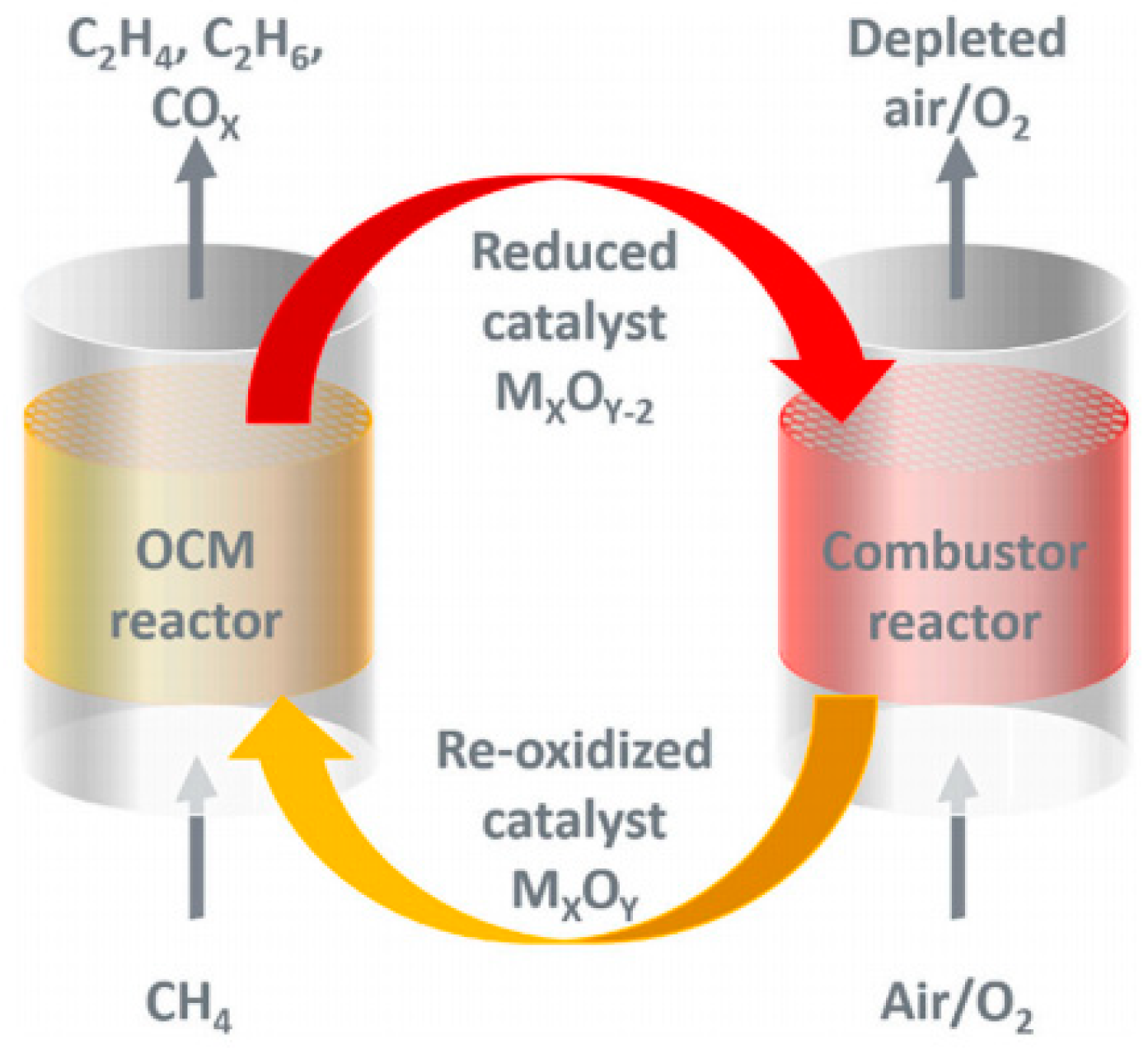
3. Chemical Looping Oxidative Coupling of Methane
Catalysts for CLOCM
4. Mechanism of OCM
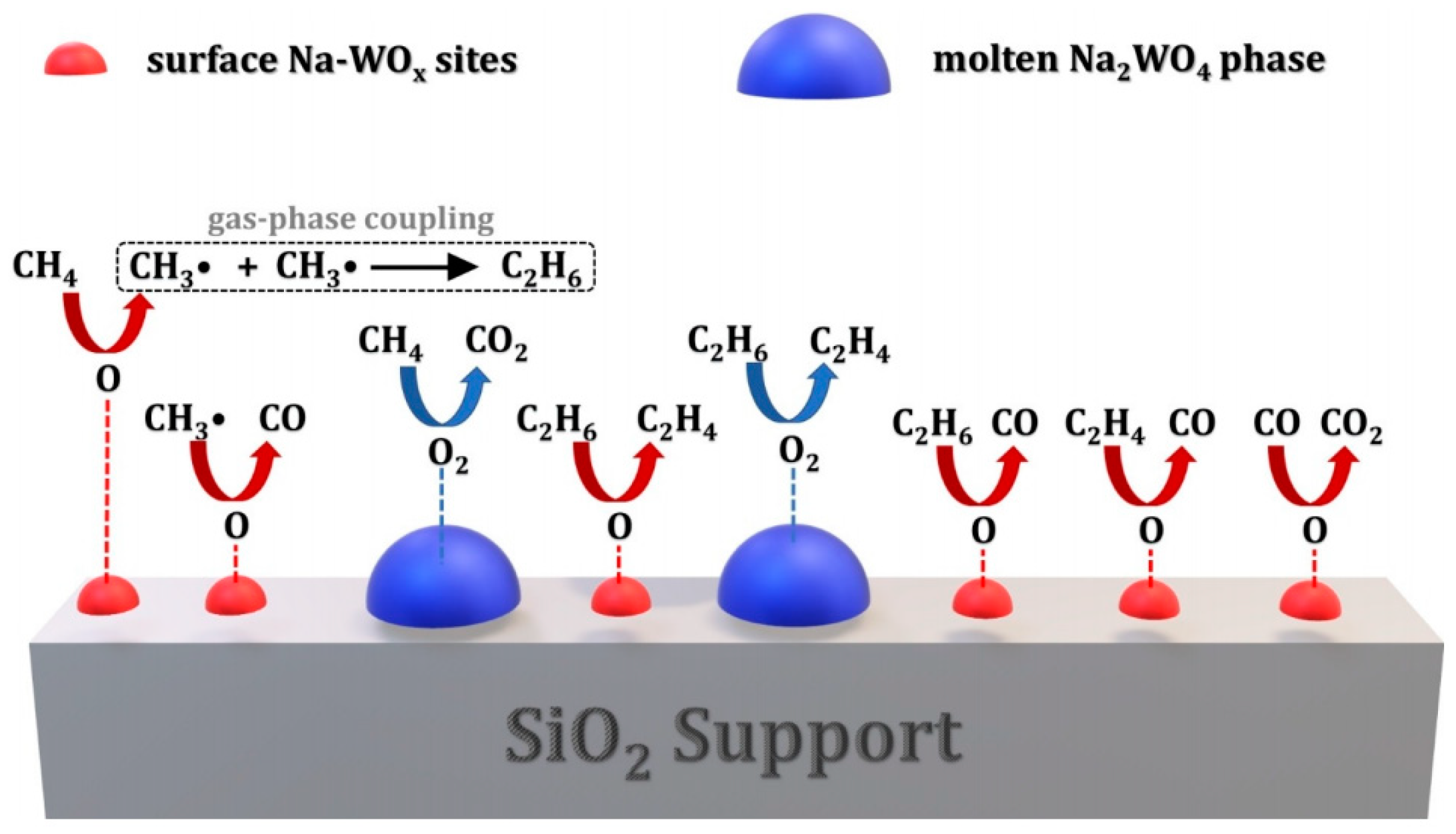
4.1. Activation of Methane
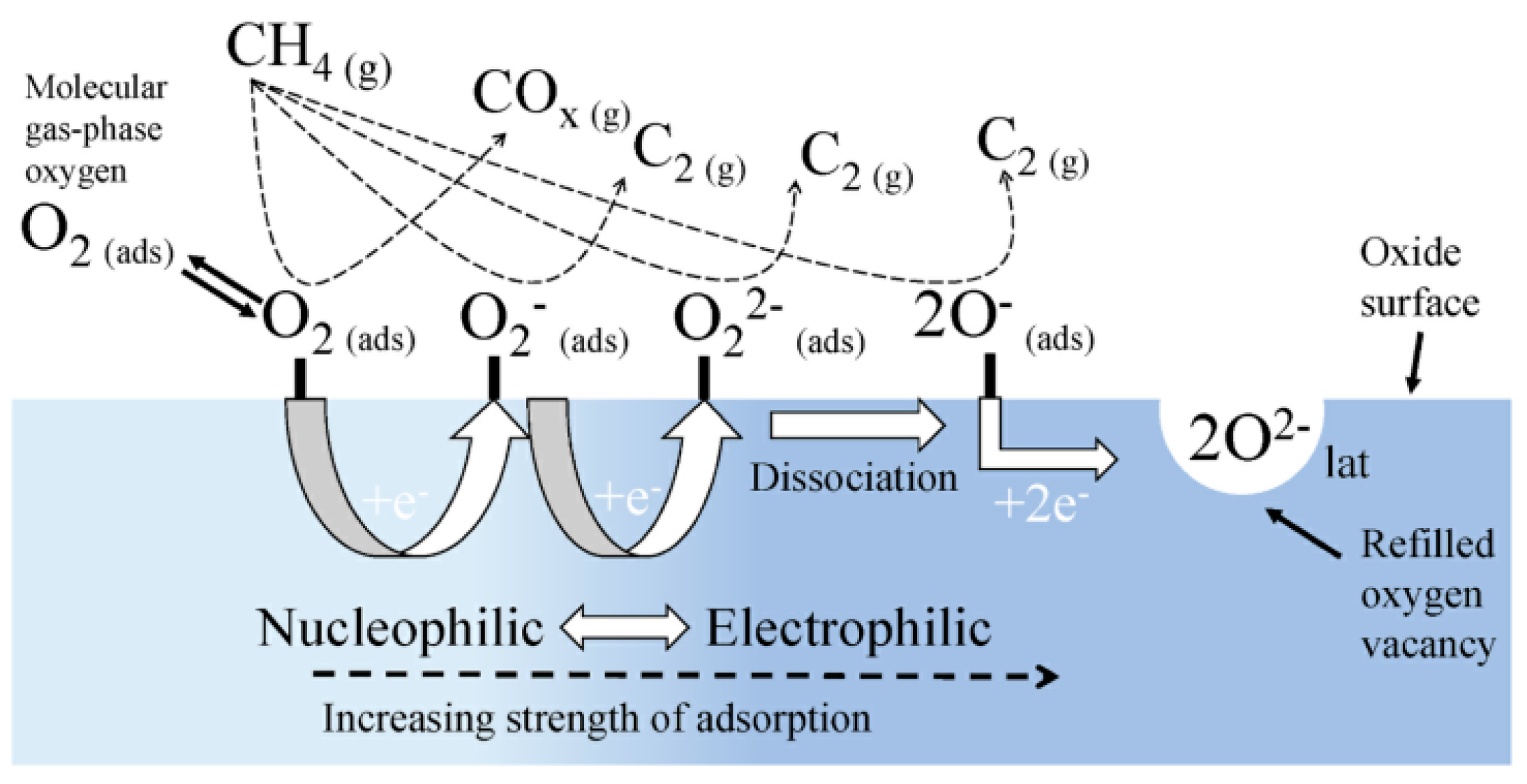
4.2. Gas-Phase Oxygen Activation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar]
- Holmen, A. Direct conversion of methane to fuels and chemicals. Catal. Today 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Arinaga, A.M.; Ziegelski, M.C.; Marks, T.J. Alternative Oxidants for the Catalytic Oxidative Coupling of Methane. Angew. Chem. Int. Ed. 2021, 60, 10502–10515. [Google Scholar] [CrossRef]
- Ming, T.; Richter, R.d.; Dietrich Oeste, F.; Tulip, R.; Caillol, S. A nature-based negative emissions technology able to remove atmospheric methane and other greenhouse gases. Atmos. Pollut. Res. 2021, 12, 101035. [Google Scholar] [CrossRef]
- Jaworski, J.; Dudek, A. Study of the Effects of Changes in Gas Composition as Well as Ambient and Gas Temperature on Errors of Indications of Thermal Gas Meters. Energies 2020, 13, 5428. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Guan, N.; Li, L. Methane Activation and Utilization: Current Status and Future Challenges. Energy Technol. 2019, 8, 1900826. [Google Scholar] [CrossRef]
- Horn, R.; Schlogl, R. Methane Activation by Heterogeneous Catalysis. Catal. Lett. 2015, 145, 23–39. [Google Scholar] [CrossRef]
- Kolesnichenko, N.V.; Ezhova, N.N.; Snatenkova, Y.M. Single-atom catalysts in methane chemistry. Russ. Chem. Rev. 2023, 92, RCR5079. [Google Scholar] [CrossRef]
- Chen, R.; Weng, G.M. Sustainable Energy Resources for Driving Methane Conversion. Adv. Energy Mater. 2023, 13, 2301734. [Google Scholar] [CrossRef]
- Meng, X.; Cui, X.; Rajan, N.P.; Yu, L.; Deng, D.; Bao, X. Direct Methane Conversion under Mild Condition by Thermo-, Electro-, or Photocatalysis. Chem 2019, 5, 2296–2325. [Google Scholar] [CrossRef]
- Schwach, P.; Pan, X.; Bao, X. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 2017, 117, 8497–8520. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Kosinov, N.; Hensen, E.J.M. Non-oxidative coupling of methane over Mo-doped CeO2 catalysts: Understanding surface and gas-phase processes. Chin. J. Catal. 2023, 49, 68–80. [Google Scholar] [CrossRef]
- DeBoy, J.M.; Hicks, R.F. The oxidative coupling of methane over alkali, alkaline earth, and rare earth oxides. Ind. Eng. Chem. Res. 1988, 27, 1577–1582. [Google Scholar]
- Hutchings, G.J.; Scurrell, M.S.; Woodhouse, J.R. Oxidative coupling of methane using oxide catalysts. Chem. Soc. Rev. 1988, 18, 251–283. [Google Scholar] [CrossRef]
- Keller, G.E.; Bhasin, M.M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts. J. Catal. 1982, 73, 9–19. [Google Scholar]
- Gao, Y.; Neal, L.; Ding, D.; Wu, W.; Baroi, C.; Gaffney, A.M.; Li, F. Recent Advances in Intensified Ethylene Production—A Review. ACS Catal. 2019, 9, 8592–8621. [Google Scholar] [CrossRef]
- Zhang, Y.; Cho, Y.; Yamaguchi, A.; Peng, X.; Miyauchi, M.; Abe, H.; Fujita, T. CO(2) oxidative coupling of methane using an earth-abundant CaO-based catalyst. Sci. Rep. 2019, 9, 15454. [Google Scholar] [CrossRef] [PubMed]
- Ampansang, S.; Sringam, S.; Somchuea, P.; Witoon, T.; Wattanakit, C.; Chareonpanich, M.; Sohn, H.; Seubsai, A. Direct Conversion of Methane to Value-Added Hydrocarbons over Alumina-Supported Cobalt Modified by Alkaline Earth Catalysts. Top. Catal. 2023, 1–15. [Google Scholar] [CrossRef]
- Matras, D.; Jacques, S.D.M.; Poulston, S.; Grosjean, N.; Estruch Bosch, C.; Rollins, B.; Wright, J.; Di Michiel, M.; Vamvakeros, A.; Cernik, R.J.; et al. Operando and Postreaction Diffraction Imaging of the La–Sr/CaO Catalyst in the Oxidative Coupling of Methane Reaction. J. Phys. Chem. C 2019, 123, 1751–1760. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, J.; Wang, R. Formation of hydrogen in oxidative coupling of methane over BaCO3 and MgO catalysts. J. Nat. Gas Chem. 2008, 17, 238. [Google Scholar]
- Xu, J.; Ouyang, R.; Zhong, X.; Fang, X.; Xu, X.; Wang, X. Alkali metal and alkaline earth metal perovskites for oxidative coupling of methane and oxidative dehydrogenation of ethane. Mol. Catal. 2023, 547, 113386. [Google Scholar] [CrossRef]
- Lim, S.; Choi, J.-W.; Jin Suh, D.; Lee, U.; Song, K.H.; Ha, J.-M. Low-temperature oxidative coupling of methane using alkaline earth metal oxide-supported perovskites. Catal. Today 2020, 352, 127–133. [Google Scholar] [CrossRef]
- Qian, K.; You, R.; Guan, Y.; Wen, W.; Tian, Y.; Pan, Y.; Huang, W. Single-Site Catalysis of Li-MgO Catalysts for Oxidative Coupling of Methane Reaction. ACS Catal. 2020, 10, 15142–15148. [Google Scholar] [CrossRef]
- Ito, T.; Wang, J.; Lin, C.H.; Lunsford, J.H. Oxidative Dimerization of Methane over a Lithium-Promoted Magnesium Oxide Catalyst. J. Am. Chem. Soc. 1985, 107, 5062. [Google Scholar] [CrossRef]
- Lanza, R.; Canu, P.; Järås, S.G. Methane partial oxidation over Pt–Ru catalyst: An investigation on the mechanism. Appl. Catal. A Gen. 2010, 375, 92–100. [Google Scholar] [CrossRef]
- Pham, T.T.P.; Ro, K.S.; Chen, L.; Mahajan, D.; Siang, T.J.; Ashik, U.P.M.; Hayashi, J.-i.; Pham Minh, D.; Vo, D.-V.N. Microwave-assisted dry reforming of methane for syngas production: A review. Environ. Chem. Lett. 2020, 18, 1987–2019. [Google Scholar] [CrossRef]
- Yang, K.; Gu, Z.; Long, Y.; Lin, S.; Lu, C.; Zhu, X.; Wang, H.; Li, K. Hydrogen production via chemical looping reforming of coke oven gas. Green Energy Environ. 2021, 6, 678–692. [Google Scholar] [CrossRef]
- Elkins, T.W.; Roberts, S.J.; Hagelin-Weaver, H.E. Effects of alkali and alkaline-earth metal dopants on magnesium oxide supported rare-earth oxide catalysts in the oxidative coupling of methane. Appl. Catal. A Gen. 2016, 528, 175–190. [Google Scholar] [CrossRef]
- Luo, L.; You, R.; Liu, Y.; Yang, J.; Zhu, Y.; Wen, W.; Pan, Y.; Qi, F.; Huang, W. Gas-Phase Reaction Network of Li/MgO-Catalyzed Oxidative Coupling of Methane and Oxidative Dehydrogenation of Ethane. ACS Catal. 2019, 9, 2514–2520. [Google Scholar] [CrossRef]
- Luo, L.; Jin, Y.; Pan, H.; Zheng, X.; Wu, L.; You, R.; Huang, W. Distribution and role of Li in Li-doped MgO catalysts for oxidative coupling of methane. J. Catal. 2017, 346, 57–61. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, X.; Zhang, Z.; Shen, X. Methane activation on single-atom Ir-doped metal nanoparticles from first principles. Phys. Chem. Chem. Phys. 2021, 23, 15564–15573. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhao, Y.; Zhang, J.; Zhu, Y.; Sun, Y. Exploiting shape effects of La2O3 nanocatalysts for oxidative coupling of methane reaction. Nanoscale 2013, 5, 10844–10848. [Google Scholar] [CrossRef]
- Hou, Y.-H.; Han, W.-C.; Xia, W.-S.; Wan, H.-L. Structure Sensitivity of La2O2CO3 Catalysts in the Oxidative Coupling of Methane. ACS Catal. 2015, 5, 1663–1674. [Google Scholar] [CrossRef]
- Ferreira, V.J.; Tavares, P.; Figueiredo, J.L.; Faria, J.L. Effect of Mg, Ca, and Sr on CeO2 Based Catalysts for the Oxidative Coupling of Methane: Investigation on the Oxygen Species Responsible for Catalytic Performance. Ind. Eng. Chem. Res. 2012, 51, 10535–10541. [Google Scholar] [CrossRef]
- Noon, D.; Seubsai, A.; Senkan, S. Oxidative Coupling of Methane by Nanofiber Catalysts. ChemCatChem 2013, 5, 146–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Xu, X.; Xi, R.; Liu, Y.; Fang, X.; Wang, X. Tailoring La2Ce2O7 catalysts for low temperature oxidative coupling of methane by optimizing the preparation methods. Catal. Today 2020, 355, 518–528. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Mota, N.; Ojeda, M.; Rojas, S.; Navarro, R.M.; Fierro, J.L.G. Direct methane conversion routes to chemicals and fuels. Catal. Today 2011, 171, 15–23. [Google Scholar] [CrossRef]
- Zohour, B.; Noon, D.; Senkan, S. New Insights into the Oxidative Coupling of Methane from Spatially Resolved Concentration and Temperature Profiles. ChemCatChem 2013, 5, 2729. [Google Scholar] [CrossRef]
- Jiang, T.; Song, J.; Huo, M.; Yang, N.; Liu, J.; Zhang, J.; Sun, Y.; Zhu, Y. La2O3 catalysts with diverse spatial dimensionality for oxidative coupling of methane to produce ethylene and ethane. RSC Adv. 2016, 6, 34872–34876. [Google Scholar] [CrossRef]
- Kiatsaengthong, D.; Jaroenpanon, K.; Somchuea, P.; Chukeaw, T.; Chareonpanich, M.; Faungnawakij, K.; Sohn, H.; Rupprechter, G.; Seubsai, A. Effects of Mg, Ca, Sr, and Ba Dopants on the Performance of La2O3 Catalysts for the Oxidative Coupling of Methane. ACS Omega 2022, 7, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Xu, X.; Fang, X.; Xi, R.; Liu, Y.; Zheng, R.; Wang, X. Constructing La2B2O7 (B = Ti, Zr, Ce) Compounds with Three Typical Crystalline Phases for the Oxidative Coupling of Methane: The Effect of Phase Structures, Superoxide Anions, and Alkalinity on the Reactivity. ACS Catal. 2019, 9, 4030–4045. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Wang, D.; Gong, X.-Q. Strategies To Improve the Activity While Maintaining the Selectivity of Oxidative Coupling of Methane at La2O3: A Density Functional Theory Study. ACS Catal. 2019, 10, 586–594. [Google Scholar] [CrossRef]
- Chrétien, S.; Metiu, H. Acid–Base Interaction and Its Role in Alkane Dissociative Chemisorption on Oxide Surfaces. J. Phys. Chem. C 2014, 118, 27336–27342. [Google Scholar] [CrossRef]
- Chu, C.; Zhao, Y.; Li, S.; Sun, Y. Correlation between the acid–base properties of the La2O3 catalyst and its methane reactivity. Phys. Chem. Chem. Phys. 2016, 18, 16509–16517. [Google Scholar] [CrossRef]
- Korf, S.; Roos, J.; Diphoorn, J.; Veehof, R.; van Ommen, J.; Ross, J. The selective oxidation of methane to ethane and ethylene over doped and un-doped rare earth oxides. Catal. Today 1989, 4, 279–292. [Google Scholar]
- Rane, V.H.; Chaudhari, S.T.; Choudhary, V.R. Comparison of the surface and catalytic properties of rare earth-promoted CaO catalysts in the oxidative coupling of methane. J. Chem. Technol. Biotechnol. 2006, 81, 208–215. [Google Scholar] [CrossRef]
- Yu, C.; Weng, W.; Shu, Q.; Meng, X.; Zhang, B.; Chen, X.; Zhou, X. Additive effects of alkaline-earth metals and nickel on the performance of Co/γ-Al2O3 in methane catalytic partial oxidation. J. Nat. Gas Chem. 2011, 20, 135–139. [Google Scholar] [CrossRef]
- Ferreira, V.J.; Tavares, P.; Figueiredo, J.L.; Faria, J.L. Ce-Doped La2O3 based catalyst for the oxidative coupling of methane. Catal. Commun. 2013, 42, 50–53. [Google Scholar] [CrossRef]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef]
- Nipan, G.D. Phase states of Li/W/Mn/SiO2 composites in catalytic oxidative coupling of methane. Inorg. Mater. 2015, 51, 389–395. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, Q.; Liu, R.; Gui, J. Oxidative Coupling of Methane over SrO/La2O3 Catalyst in an Oxygen-Permeable Separation Membrane Reactor. Catal. Lett. 2020, 151, 1805–1809. [Google Scholar] [CrossRef]
- Boivin, J.C.; Mairesse, G. Recent material developments in fast oxide ion conductors. Chem. Mater. 1998, 10, 2870–2888. [Google Scholar]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B Environ. 2013, 134–135, 251–257. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Yang, W. Oxidative coupling of methane in Ba0.5Sr0.5Co0.8Fe0.2O3−δ tubular membrane reactors. Catal. Today 2005, 104, 160–167. [Google Scholar] [CrossRef]
- Xu, J.; Xi, R.; Xu, X.; Zhang, Y.; Feng, X.; Fang, X.; Wang, X. A2B2O7 pyrochlore compounds: A category of potential materials for clean energy and environment protection catalysis. J. Rare Earths 2020, 38, 840–849. [Google Scholar] [CrossRef]
- Zhang, F.X.; Tracy, C.L.; Lang, M.; Ewing, R.C. Stability of fluorite-type La2Ce2O7 under extreme conditions. J. Alloys Compd. 2016, 674, 168–173. [Google Scholar] [CrossRef]
- Xu, J.; Peng, L.; Fang, X.; Fu, Z.; Liu, W.; Xu, X.; Peng, H.; Zheng, R.; Wang, X. Developing reactive catalysts for low temperature oxidative coupling of methane: On the factors deciding the reaction performance of Ln2Ce2O7 with different rare earth A sites. Appl. Catal. A Gen. 2018, 552, 117–128. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, G.; Jiang, D.; Wang, S. Recent development of A2B2O7 system transparent ceramics. J. Adv. Ceram. 2018, 7, 289–306. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Hou, C.; Yin, F.; Wang, G.; Zhu, X.; Jiang, G.; Li, C. Improved Catalytic Activity and Stability of Ba Substituted SrTiO3 Perovskite for Oxidative Coupling of Methane. ChemCatChem 2021, 13, 4182–4191. [Google Scholar] [CrossRef]
- Sim, Y.; Kwon, D.; An, S.; Ha, J.-M.; Oh, T.-S.; Jung, J.C. Catalytic behavior of ABO3 perovskites in the oxidative coupling of methane. Mol. Catal. 2020, 489, 110925. [Google Scholar] [CrossRef]
- Gan, R.; Nishida, Y.; Haneda, M. Effect of B Site Substitution on the Catalytic Activity of La—Based Perovskite for Oxidative Coupling of Methane. Phys. Status Solidi B 2022, 259, 2100544. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Liu, Y.; Fang, X.; Xu, X.; Liu, W.; Zheng, R.; Wang, X. Optimizing the Reaction Performance of La2Ce2O7—Based Catalysts for Oxidative Coupling of Methane (OCM) at Lower Temperature by Lattice Doping with Ca Cations. Eur. J. Inorg. Chem. 2018, 2019, 183–194. [Google Scholar] [CrossRef]
- Xu, J.; Xi, R.; Xiao, Q.; Xu, X.; Liu, L.; Li, S.; Gong, Y.; Zhang, Z.; Fang, X.; Wang, X. Design of strontium stannate perovskites with different fine structures for the oxidative coupling of methane (OCM): Interpreting the functions of surface oxygen anions, basic sites and the structure–reactivity relationship. J. Catal. 2022, 408, 465–477. [Google Scholar] [CrossRef]
- Yu, C.Y.; Li, W.Z.; Martin, G.A.; Mirodatos, C. Studies of CaTiO3 based catalysts for the oxidative coupling of methane. Appl. Catal. A Gen. 1997, 158, 201–214. [Google Scholar]
- Katouzian, F.; Fakhroueian, Z.; Bidhendi, S.M. The Interesting of Antifungal Effects of Novel In Vitro Fabrics of Stabilized ZnO Nanofluids. Adv. Nanopart. 2016, 5, 206–223. [Google Scholar] [CrossRef][Green Version]
- Kiani, D.; Sourav, S.; Baltrusaitis, J.; Wachs, I.E. Oxidative Coupling of Methane (OCM) by SiO2-Supported Tungsten Oxide Catalysts Promoted with Mn and Na. ACS Catal. 2019, 9, 5912–5928. [Google Scholar] [CrossRef]
- Pak, S.; Lunsford, J.H. Thermal effects during the oxidative coupling of methane over Mn/Na2WO4/SiO2 and Mn/Na2WO4/MgO catalysts. Appl. Catal. A Gen. 1998, 168, 131–137. [Google Scholar] [CrossRef]
- Wu, J.; Li, S. The Role of Distorted W04 in the Oxidative Coupling of Methane on Tungsten Oxide Supported Catalysts. J. Phys. Chem. 1995, 99, 4566–4568. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, G.; Liu, Y.; Lu, Y. TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst for oxidative coupling of methane: Solution combustion synthesis and MnTiO3-dependent low-temperature activity improvement. Appl. Catal. A Gen. 2017, 544, 77–83. [Google Scholar] [CrossRef]
- Fleischer, V.; Steuer, R.; Parishan, S.; Schomäcker, R. Investigation of the surface reaction network of the oxidative coupling of methane over Na2WO4/Mn/SiO2 catalyst by temperature programmed and dynamic experiments. J. Catal. 2016, 341, 91–103. [Google Scholar] [CrossRef]
- Gordienko, Y.; Usmanov, T.; Bychkov, V.; Lomonosov, V.; Fattakhova, Z.; Tulenin, Y.; Shashkin, D.; Sinev, M. Oxygen availability and catalytic performance of NaWMn/SiO2 mixed oxide and its components in oxidative coupling of methane. Catal. Today 2016, 278, 127–134. [Google Scholar] [CrossRef]
- Sourav, S.; Wang, Y.X.; Kiani, D.; Baltrusaitis, J.; Fushimi, R.R.; Wachs, I.E. Resolving the Types and Origin of Active Oxygen Species Present in Supported Mn-Na2WO4/SiO2 Catalysts for Oxidative Coupling of Methane. ACS Catal. 2021, 11, 10288–10293. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef] [PubMed]
- Sourav, S.; Wang, Y.; Kiani, D.; Baltrusaitis, J.; Fushimi, R.R.; Wachs, I.E. New Mechanistic and Reaction Pathway Insights for Oxidative Coupling of Methane (OCM) over Supported Na2WO4/SiO2 Catalysts. Angew. Chem. Int. Ed. 2021, 60, 21502–21511. [Google Scholar] [CrossRef] [PubMed]
- Sourav, S.; Kiani, D.; Wang, Y.; Baltrusaitis, J.; Fushimi, R.R.; Wachs, I.E. Molecular structure and catalytic promotional effect of Mn on supported Na2WO4/SiO2 catalysts for oxidative coupling of methane (OCM) reaction. Catal. Today 2023, 416, 113837. [Google Scholar] [CrossRef]
- Qin, L.; Cheng, Z.; Baser, D.; Goldenbaum, T.; Fan, J.A.; Fan, L.-S. Cyclic redox scheme towards shale gas reforming: A review and perspectives. React. Chem. Eng. 2020, 5, 2204–2220. [Google Scholar] [CrossRef]
- Chung, E.Y.; Wang, W.K.; Nadgouda, S.G.; Baser, D.S.; Sofranko, J.A.; Fan, L.-S. Catalytic Oxygen Carriers and Process Systems for Oxidative Coupling of Methane Using the Chemical Looping Technology. Ind. Eng. Chem. Res. 2016, 55, 12750–12764. [Google Scholar] [CrossRef]
- Damasceno, S.; Trindade, F.J.; Fonseca, F.C.; Florio, D.Z.d.; Ferlauto, A.S. Oxidative coupling of methane in chemical looping design. Fuel Process. Technol. 2022, 231, 107255. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Li, X.; Li, Z.; Zhu, X.; Li, K. Chemical Looping Conversion of Gaseous and Liquid Fuels for Chemical Production: A Review. Energy Fuels 2020, 34, 5381–5413. [Google Scholar] [CrossRef]
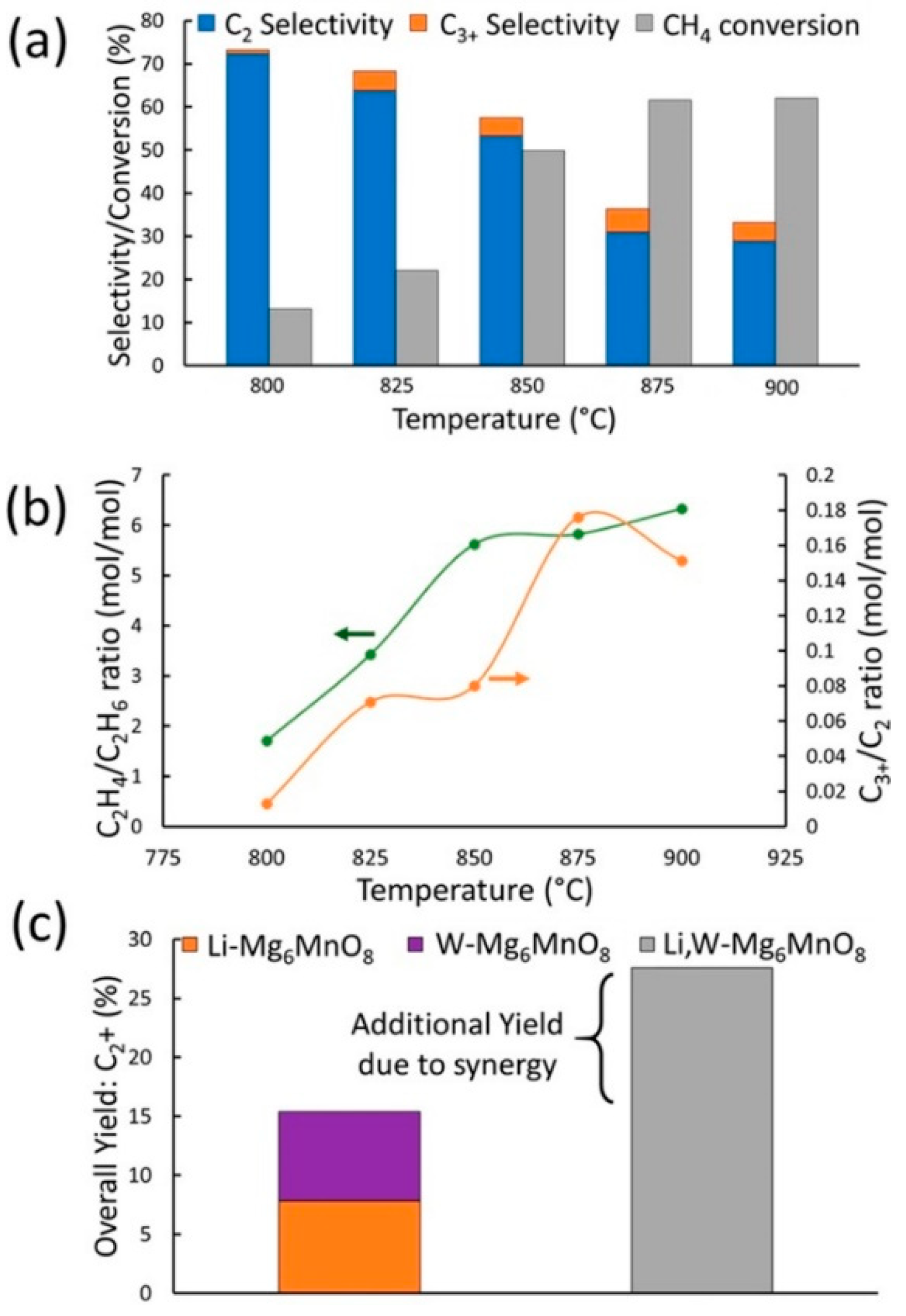
- Huang, J.; Zhao, K.; Jiang, S.; Kang, S.; Lin, Y.; Huang, Z.; Zheng, A.; Zhao, Z. Heteroatom-doping regulated Mg6MnO8 for improving C2+ hydrocarbons during chemical looping oxidative coupling of methane. Fuel Process. Technol. 2022, 235, 107352. [Google Scholar] [CrossRef]
- Baser, D.S.; Cheng, Z.; Fan, J.A.; Fan, L.-S. Codoping Mg-Mn Based Oxygen Carrier with Lithium and Tungsten for Enhanced C2 Yield in a Chemical Looping Oxidative Coupling of Methane System. ACS Sustain. Chem. Eng. 2021, 9, 2651–2660. [Google Scholar] [CrossRef]
- Jiang, S.; Ding, W.; Zhao, K.; Huang, Z.; Wei, G.; Feng, Y.; Lv, Y.; He, F. Enhanced Chemical looping oxidative coupling of methane by Na-doped LaMnO3 redox catalysts. Fuel 2021, 299, 120932. [Google Scholar] [CrossRef]
- Fleischer, V.; Littlewood, P.; Parishan, S.; Schomäcker, R. Chemical looping as reactor concept for the oxidative coupling of methane over a Na2WO4/Mn/SiO2 catalyst. Chem. Eng. J. 2016, 306, 646–654. [Google Scholar] [CrossRef]
- Fleischer, V.; Simon, U.; Parishan, S.; Colmenares, M.G.; Görke, O.; Gurlo, A.; Riedel, W.; Thum, L.; Schmidt, J.; Risse, T.; et al. Investigation of the role of the Na2WO4/Mn/SiO2 catalyst composition in the oxidative coupling of methane by chemical looping experiments. J. Catal. 2018, 360, 102–117. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, G.; Gao, Y.; Si, J.; Liu, Y.; Lu, Y. An oxygen carrier catalyst toward efficient chemical looping-oxidative coupling of methane. Appl. Catal. B Environ. 2022, 304, 120948. [Google Scholar] [CrossRef]
- Sung, J.S.; Choo, K.Y.; Kim, T.H.; Greish, A.; Glukhov, L.; Finashina, E.; Kustov, L. Peculiarities of oxidative coupling of methane in redox cyclic mode over Ag–La2O3/SiO2 catalysts. Appl. Catal. A Gen. 2010, 380, 28–32. [Google Scholar] [CrossRef]
- Greish, A.A.; Glukhov, L.M.; Finashina, E.D.; Kustov, L.M.; Sung, J.-S.; Choo, K.-Y.; Kim, T.-H. Oxidative coupling of methane in the redox cyclic mode over the catalysts on the basis of CeO2 and La2O3. Mendeleev Commun. 2010, 20, 28–30. [Google Scholar] [CrossRef]
- Cheng, Z.; Baser, D.S.; Nadgouda, S.G.; Qin, L.; Fan, J.A.; Fan, L.-S. C2 Selectivity Enhancement in Chemical Looping Oxidative Coupling of Methane over a Mg–Mn Composite Oxygen Carrier by Li-Doping-Induced Oxygen Vacancies. ACS Energy Lett. 2018, 3, 1730–1736. [Google Scholar] [CrossRef]
- Noon, D.; Zohour, B.; Senkan, S. Oxidative coupling of methane with La2O3–CeO2 nanofiber fabrics: A reaction engineering study. J. Nat. Gas Sci. Eng. 2014, 18, 406–411. [Google Scholar] [CrossRef]
- Lomonosov, V.I.; Sinev, M.Y. Oxidative coupling of methane: Mechanism and kinetics. Kinet. Catal. 2016, 57, 647–676. [Google Scholar] [CrossRef]
- Chen, S.; Ma, X. The role of oxygen species in the selective oxidation of methanol to dimethoxymethane over VOx/TS-1 catalyst. J. Ind. Eng. Chem. 2017, 45, 296–300. [Google Scholar] [CrossRef]
- Kim, I.; Lee, G.; Na, H.B.; Ha, J.-M.; Jung, J.C. Selective oxygen species for the oxidative coupling of methane. Mol. Catal. 2017, 435, 13–23. [Google Scholar] [CrossRef]
- Phung, T.K.; Garbarino, G. On the use of infrared spectrometer as detector for Temperature Programmed (TP) techniques in catalysts characterization. J. Ind. Eng. Chem. 2017, 47, 288–296. [Google Scholar] [CrossRef]
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review. J. Ind. Eng. Chem. 2018, 59, 218–229. [Google Scholar] [CrossRef]
- Borchert, H.; Baerns, M. The effect of oxygen-anion conductivity of metal–oxide doped lanthanum oxide catalysts on hydrocarbon selectivity in the oxidative coupling of methane. J. Catal. 1997, 168, 315–320. [Google Scholar]
- Ortiz-Bravo, C.A.; Chagas, C.A.; Toniolo, F.S. Oxidative coupling of methane (OCM): An overview of the challenges and opportunities for developing new technologies. J. Nat. Gas Sci. Eng. 2021, 96, 104254. [Google Scholar] [CrossRef]
- Yoon, S.; Lim, S.; Choi, J.W.; Suh, D.J.; Song, K.H.; Ha, J.M. Study on the unsteady state oxidative coupling of methane: Effects of oxygen species from O(2), surface lattice oxygen, and CO2 on the C2+ selectivity. RSC Adv. 2020, 10, 35889–35897. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Matras, D.; Jacques, S.D.M.; di Michiel, M.; Price, S.W.T.; Senecal, P.; Aran, M.A.; Middelkoop, V.; Stenning, G.B.G.; Mosselmans, J.F.W.; et al. Real-time multi-length scale chemical tomography of fixed bed reactors during the oxidative coupling of methane reaction. J. Catal. 2020, 386, 39–52. [Google Scholar] [CrossRef]
- Dissanayake, D.; Lunsford, J.H.; Rosynek, M.P. Oxidative coupling of methane over oxide-supported barium catalysts. J. Catal. 1993, 143, 286–298. [Google Scholar]
- Kondratenko, E.V.; Peppel, T.; Seeburg, D.; Kondratenko, V.A.; Kalevaru, N.; Martin, A.; Wohlrab, S. Methane conversion into different hydrocarbons or oxygenates: Current status and future perspectives in catalyst development and reactor operation. Catal. Sci. Technol. 2017, 7, 366–381. [Google Scholar] [CrossRef]

| Catalyst | Temperature (K) | CH4 Conversion (%) | C2+ Selectivity (%) | C2+ Yield (%) |
|---|---|---|---|---|
| CaO | 1023 | 10.4 | 38.0 | 4.0 |
| 5% Na/CaO | 1023 | 10.2 | 75.7 | 7.7 |
| 50% Ce/CaO | 1023 | 10.6 | 73.8 | 4.8 |
| 10% La/CaO | 1073 | 37.0 | 15.0 | 5.6 |
| 10% La–20% Sr/CaO | 1073 | 43.0 | 20.0 | 8.6 |
| 7% wt Li/MgO | 1073 | 22.6 | 72.5 | 12.8 |
| 3% wt Li/MgO | 973 | 39.4 | 37.6 | 14.8 |
| 5.3% wt Li/MgO | 803 | 23.0 | 54.0 | 12.4 |
| Li/Mg/Zn (3/75/25, wt) | 948 | 19 | 54.0 | 10.3 |
| 50% LiNiO2–50% MgO | 953 | 36.1 | 59.0 | 21.3 |
| 50% NaLnO2–50% MgO | 953 | 34.2 | 51.1 | 17.5 |
| 50 wt% LiCl-50 wt% Na2MoO4 | 843 | 11.0 | 55.0 | 6.0 |
| 9 wt% K2CO3/7 wt% Bi2O3/Al2O3 | 913 | 7.3 | 29.0 | 2.1 |
| Catalyst | Temperature(K) | CH4 Conversion (%) | C2+ Selectivity (%) | C2+ Yield (%) |
|---|---|---|---|---|
| Sm2O3 | 1035 | 21.9 | 47.0 | 10.3 |
| 55 mol% Na/Sm2O3 | 1035 | 21.2 | 61.0 | 12.1 |
| 30 mol% Ca/Sm2O3 | 1035 | 22.6 | 50.0 | 11.3 |
| ZnO/Sm2O3 | 1048 | 21.7 | 41.9 a | 9.1 |
| MgO/Sm2O3 | 1048 | 23.2 | 49.2 a | 11.4 |
| CaO/Sm2O3 | 1048 | 25.0 | 57.2 a | 14.3 |
| SrO/Sm2O3 | 1048 | 25.9 | 59.8 a | 15.5 |
| CaO | 1058 | 13.3 | 49.3 | 6.6 |
| La–CaO | 1002 | 19.8 | 67.2 | 13.3 |
| Ce–CaO | 982 | 17.7 | 65.0 | 11.5 |
| Sm–CaO | 1013 | 18.7 | 62.5 | 11.7 |
| Nd–CaO | 973 | 19.5 | 70.8 | 13.8 |
| Yb–CaO | 999 | 20.1 | 66.4 | 13.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Chen, P.; Xia, S.; Zheng, M.; Song, D.; Lin, Y.; Liu, A.; Wang, X.; Zhao, K.; Zheng, A. Advances in Oxidative Coupling of Methane. Atmosphere 2023, 14, 1538. https://doi.org/10.3390/atmos14101538
Deng J, Chen P, Xia S, Zheng M, Song D, Lin Y, Liu A, Wang X, Zhao K, Zheng A. Advances in Oxidative Coupling of Methane. Atmosphere. 2023; 14(10):1538. https://doi.org/10.3390/atmos14101538
Chicago/Turabian StyleDeng, Jinlin, Peili Chen, Shengpeng Xia, Min Zheng, Da Song, Yan Lin, Anqi Liu, Xiaobo Wang, Kun Zhao, and Anqing Zheng. 2023. "Advances in Oxidative Coupling of Methane" Atmosphere 14, no. 10: 1538. https://doi.org/10.3390/atmos14101538
APA StyleDeng, J., Chen, P., Xia, S., Zheng, M., Song, D., Lin, Y., Liu, A., Wang, X., Zhao, K., & Zheng, A. (2023). Advances in Oxidative Coupling of Methane. Atmosphere, 14(10), 1538. https://doi.org/10.3390/atmos14101538









