Research Advances on Nitrogen-Doped Carbon Materials in COx Hydrogenation
Abstract
:1. Introduction
2. N-doped Carbon Material and Preparation Methods
2.1. N Type of Doping Species
2.2. Preparation Methods of Nitrogen-Doped Carbon Materials
2.2.1. Post-Doping Method
2.2.2. In Situ Doping Method
3. Catalytic Application of Nitrogen-Doped Carbon Materials in CO Hydrogenation
3.1. Cobalt-Based Catalysts
3.2. Iron-Based Catalysts
4. Catalytic Application of Nitrogen-Doped Carbon Materials in CO2 Hydrogenation
4.1. CO2 Hydrogenation to CO over Nitrogen-Doped Carbon Materials
4.2. CO2 Hydrogenation to CH4 over Nitrogen-Doped Carbon Materials
4.3. CO2 Hydrogenation to C2+ over Nitrogen-Doped Carbon Materials
4.4. CO2 Hydrogenation to CH3OH over Nitrogen-Doped Carbon Materials
4.5. CO2 Hydrogenation to CHOOH over Nitrogen-Doped Carbon Materials
5. Concluding Remarks
- Methods to improve the stability of nitrogen atoms on carbon materials and increase the nitrogen content need to be explored in relation to the material itself. On the one hand, nitrogen atoms serve as effective anchoring sites for active components, but the quantity of nitrogen atoms tends to decrease with the increasing pyrolysis temperature of the material. On the other hand, high pyrolysis temperatures often enhance the graphitization of carbon materials, facilitating electron transfer. These two processes seem contradictory and it appears difficult to achieve both simultaneously. If the interaction between nitrogen atoms and carbon materials can be strengthened (adding functional groups to the surface of the material), allowing nitrogen atoms to remain well-preserved on the surface during high-temperature pyrolysis, it would contribute to further enhancing the catalytic activity of the catalyst. Currently, there are limitations in our understanding of the role of nitrogen species. Nitrogen-doped carbon materials prepared using the current doping methods have complex nitrogen species, and it is difficult to precisely control the content of the corresponding components. This greatly restricts our understanding of the specific roles of nitrogen species (such as which of pyridine N and pyrrole N has the stronger electron-giving ability and the stronger anchoring ability), and causes significant confusion in explorations of the synthesis and mechanisms of subsequent catalytic materials. It is challenging to introduce a specific nitrogen species onto the surface of traditional carbon materials. Therefore, it is crucial to modify the surface of carbon materials or synthesize them through the pyrolysis of raw materials containing specific nitrogen species. Carbon materials with specific nitrogen species can help us to understand the active sites of reactions and provide important support for speculating reaction pathways.
- With the rapid development of porous carbon technology, nitrogen-doped carbon materials with a tunable pore size and hierarchical pore structure can be developed. N-doped carbon materials with adjustable microporous and mesoporous structures can be prepared, featuring a layered structure that connects mesopores and macropores. This facilitates the investigation of the pore structure’s effect on the reaction. Micro-porous structures can spatially restrict the distribution of active components and promote the formation of nanoscale particles. At the same time, the layered structure is beneficial for improving the mass and heat transfer of the catalyst, which has a positive effect on enhancing the reaction activity and improving the product distribution.
- A comprehensive insight into active species and their interactions, along with the interactions among the active centers with support, are of paramount significance for catalyst design and tailoring. Therefore, in situ characterization techniques (such as XRD, TEM, and IR spectroscopy) should be employed to distinguish active sites. Additionally, analysis methods such as XPS and DFT calculations are needed to identify the major surface species and explain the elusive reaction mechanisms of COx hydrogenation to value-added products. Moreover, in situ structural and surface studies can be conducted under working conditions to validate the proposed mechanisms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTS | Fischer–Tropsch synthesis |
| RWGS | Reverse water–gas shift |
| ASF | Anderson–Schulz–Flory |
| CNT | Carbon nanotube |
| NCNT | Nitrogen-doped carbon nanotube |
| CNF | Carbon nanofiber |
| NCNF | Nitrogen-doped carbon nanofiber |
| ZIFs | Zeolitic imidazolate framework materials |
| PANI | Polyaniline |
| PPy | Polypyrrole |
| STY | Space–time yield |
| CVD | Chemical vapor deposition |
| DFT | Density functional theory |
References
- Martínez, L.; Merino, P.; Santoro, G.; Martínez, J.I.; Katsanoulis, S.; Ault, J.; Mayoral, Á.; Vázquez, L.; Accolla, M.; Dazzi, A.; et al. Metal-catalyst-free gas-phase synthesis of long-chain hydrocarbons. Nat. Commun. 2021, 12, 5937. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Cui, W.-G.; Zhang, G.-Y.; Hu, T.-L.; Bu, X.-H. Metal-organic framework-based heterogeneous catalysts for the conversion of C1 chemistry: CO, CO2 and CH4. Coord. Chem. Rev. 2019, 387, 79–120. [Google Scholar] [CrossRef]
- Claeys, M. Cobalt gets in shape. Nature 2016, 538, 44–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, J.; Duyar, M.S.; Ordomsky, V.V.; Khodakov, A.Y.; Liu, J. Carbon-based catalysts for Fischer–Tropsch synthesis. Chem. Soc. Rev. 2021, 50, 2337–2366. [Google Scholar] [CrossRef] [PubMed]
- Mainali, K.; Mood, S.H.; Pelaez-Samaniego, M.R.; Sierra-Jimenez, V.; Garcia-Perez, M. Production and applications of N-doped carbons from bioresources: A review. Catal. Today 2023, 423, 114248. [Google Scholar] [CrossRef]
- Tackett, B.M.; Gomez, E.; Chen, J.G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2019, 2, 381–386. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- Orege, J.I.; Wei, J.; Han, Y.; Yang, M.; Sun, X.; Zhang, J.; Amoo, C.C.; Ge, Q.; Sun, J. Highly stable Sr and Na co-decorated Fe catalyst for high-valued olefin synthesis from CO2 hydrogenation. Appl. Catal. B Environ. 2022, 316, 121640. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, M.; Wu, L.; Guo, S.; Liu, X.; Zhao, J.; Xue, W.; Li, J.; Liu, C.; Li, X.; et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 2022, 5, 388–396. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Chen, J.; Ma, Q.; Fan, S.; Zhao, T.S. Effect of preparation methods on the structure and catalytic performance of Fe–Zn/K catalysts for CO2 hydrogenation to light olefins. Chin. J. Chem. Eng. 2018, 26, 761–767. [Google Scholar] [CrossRef]
- Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, H.; An, K.; Lee, J.S. Recycling Carbon Dioxide through Catalytic Hydrogenation: Recent Key Developments and Perspectives. ACS Catal. 2020, 10, 11318–11345. [Google Scholar] [CrossRef]
- van Ravenhorst, I.K.; Vogt, C.; Oosterbeek, H.; Bossers, K.W.; Moya-Cancino, J.G.; van Bavel, A.P.; van der Eerden, M.J.; Vine, D.; de Groot, F.M.F.; Meirer, F.; et al. Capturing the Genesis of an Active Fischer–Tropsch Synthesis Catalyst with Operando X-ray Nanospectroscopy. Angew. Chem. Int. Ed. 2018, 57, 11957–11962. [Google Scholar] [CrossRef]
- Meng, G.; Sun, J.; Tao, L.; Ji, K.; Wang, P.; Wang, Y.; Sun, X.; Cui, T.; Du, S.; Chen, J. Ru1Con Single-Atom Alloy for Enhancing Fischer–Tropsch Synthesis. ACS Catal. 2021, 11, 1886–1896. [Google Scholar] [CrossRef]
- Hernández Mejía, C.; van der Hoeven, J.E.S.; de Jongh, P.E.; de Jong, K.P. Cobalt–Nickel Nanoparticles Supported on Reducible Oxides as Fischer–Tropsch Catalysts. ACS Catal. 2020, 10, 7343–7354. [Google Scholar] [CrossRef]
- Okonye, L.U.; Yao, Y.; Hildebrandt, D.; Meijboom, R. Contributing to energy sustainability: A review of mesoporous material supported catalysts for Fischer–Tropsch synthesis. Sustain. Energy Fuels 2021, 5, 79–107. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; de Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M.-T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, M.; Huang, C.; Zhong, L.; Fang, K. Carbon-encapsulated highly dispersed FeMn nanoparticles for Fischer–Tropsch synthesis to light olefins. New J. Chem. 2018, 42, 2413–2421. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Supported Iron Nanoparticles as Catalysts for Sustainable Production of Lower Olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, Characterization, and Application of Metal Nanoparti cles Supported on Nitrogen-Doped Carbon: Catalysis beyond Electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef] [PubMed]
- Rangraz, Y.; Heravi, M.M.; Elhampour, A. Recent Advances on Heteroatom-Doped Porous Carbon/Metal Materials: Fascinating Heterogeneous Catalysts for Organic Transformations. Chem. Rec. 2021, 21, 1985–2073. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Wood, K.N.; O’Hayre, R.; Pylypenko, S. Recent progress on nitrogen/carbon structures designed for use in energy and sustainability applications. Energy Environ. Sci. 2014, 7, 1212–1249. [Google Scholar] [CrossRef]
- Williamson, D.L.; Herdes, C.; Torrente-Murciano, L.; Jones, M.D.; Mattia, D. N-Doped Fe@CNT for Combined RWGS/FT CO2 Hydrogenation. ACS Sustain. Chem. Eng. 2019, 7, 7395–7402. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, P.; Cui, Y.; Liu, G.; Wu, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. One-Pot Hydrothermal Synthesis of Nitrogen Functionalized Carbonaceous Material Catalysts with Embedded Iron Nanoparticles for CO2 Hydrogenation. ACS Sustain. Chem. Eng. 2019, 7, 8331–8339. [Google Scholar] [CrossRef]
- Guo, L.; Guo, Z.; Liang, J.; Yong, X.; Sun, S.; Zhang, W.; Sun, J.; Zhao, T.; Li, J.; Cui, Y.; et al. Quick microwave assembling nitrogen-regulated graphene supported iron nanoparticles for Fischer-Tropsch synthesis. Chem. Eng. J. 2022, 429, 132063. [Google Scholar] [CrossRef]
- de Almeida Ribeiro, R.S.; Monteiro Ferreira, L.E.; Rossa, V.; Lima, C.G.; Paixao, M.W.; Varma, R.S.; de Melo Lima, T. Graphitic Carbon Nitride-Based Materials as Catalysts for the Upgrading of Lignocellulosic Biomass-Derived Molecules. ChemSusChem 2020, 13, 3992–4004. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, J.; Yang, W.; Song, S.; Zhang, H. Recent Advances in Graphitic Carbon Nitride Supported Single-Atom Catalysts for Energy Conversion. ChemCatChem 2021, 13, 1250–1270. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vargas, D.X.; Sandoval-Rangel, L.; Campuzano-Calderon, O.; Romero-Flores, M.; Lozano, F.J.; Nigam, K.D.P.; Mendoza, A.; Montesinos-Castellanos, A. Recent Advances in Bifunctional Catalysts for the Fischer–Tropsch Process: One-Stage Production of Liquid Hydrocarbons from Syngas. Ind. Eng. Chem. Res. 2019, 58, 15872–15901. [Google Scholar] [CrossRef]
- Khodakov, A.Y.; Chu, W.; Fongarland, P. Advances in the Development of Novel Cobalt Fischer–Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels. Chem. Rev. 2007, 107, 1692–1744. [Google Scholar] [CrossRef]
- Mota, F.M.; Kim, D.H. From CO2methanation to ambitious long-chain hydrocarbons: Alternative fuels paving the path to sustainability. Chem. Soc. Rev. 2019, 48, 205–259. [Google Scholar] [CrossRef]
- Nie, X.; Li, W.; Jiang, X.; Guo, X.; Song, C. Chapter Two—Recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 65, pp. 121–233. [Google Scholar]
- Zang, G.; Sun, P.; Elgowainy, A.A.; Bafana, A.; Wang, M. Performance and cost analysis of liquid fuel production from H2 and CO2 based on the Fischer-Tropsch process. J. CO2 Util. 2021, 46, 101459. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L.; Jin, Z. Nitrogen-doped graphene: Synthesis, characterizations and energy applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef]
- Ham, K.; Shin, D.; Lee, J. The Role of Lone-Pair Electrons in Pt–N Interactions for the Oxygen Reduction Reaction in Polymer Exchange Membrane Fuel Cells. ChemSusChem 2020, 13, 1751–1758. [Google Scholar] [CrossRef]
- Wu, G.; Mack, N.H.; Gao, W.; Ma, S.; Zhong, R.; Han, J.; Baldwin, J.K.; Zelenay, P. Nitrogen-Doped Graphene-Rich Catalysts Derived from Heteroatom Polymers for Oxygen Reduction in Nonaqueous Lithium–O2 Battery Cathodes. ACS Nano 2012, 6, 9764–9776. [Google Scholar] [CrossRef]
- Li, R.; Wang, D. Understanding the structure-performance relationship of active sites at atomic scale. Nano Res. 2022, 15, 6888–6923. [Google Scholar] [CrossRef]
- Li, O.L.; Prabakar, K.; Kaneko, A.; Park, H.; Ishizaki, T. Exploration of Lewis basicity and oxygen reduction reaction activity in plasma-tailored nitrogen-doped carbon electrocatalysts. Catal. Today 2019, 337, 102–109. [Google Scholar] [CrossRef]
- Feng, H.; Ma, J.; Hu, Z. Nitrogen-doped carbon nanotubes functionalized by transition metal atoms: A density functional study. J. Mater. Chem. 2010, 20, 1702–1708. [Google Scholar] [CrossRef]
- Wang, X.; Pan, H.; Lin, Q.; Wu, H.; Jia, S.; Shi, Y. One-Step Synthesis of Nitrogen-Doped Hydrophilic Mesoporous Carbons from Chitosan-Based Triconstituent System for Drug Release. Nanoscale Res. Lett. 2019, 14, 259. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.-C.; Liang, J.; Zhou, G.-M.; Li, F.; Saito, R.; Cheng, H.-M. Understanding the interactions between lithium polysulfides and N-doped graphene using density functional theory calculations. Nano Energy 2016, 25, 203–210. [Google Scholar] [CrossRef]
- Su, P.; Huang, W.; Zhang, J.; Guharoy, U.; Du, Q.; Sun, Q.; Jiang, Q.; Cheng, Y.; Yang, J.; Zhang, X.; et al. Fe atoms anchored on defective nitrogen doped hollow carbon spheres as efficient electrocatalysts for oxygen reduction reaction. Nano Res. 2021, 14, 1069–1077. [Google Scholar] [CrossRef]
- Chen, S.; Qi, P.; Chen, J.; Yuan, Y. Platinum nanoparticles supported on N-doped carbon nanotubes for the selective oxidation of glycerol to glyceric acid in a base-free aqueous solution. RSC Adv. 2015, 5, 31566–31574. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Jeon, I.; Noh, H.; Baek, J. Nitrogen-Doped Carbon Nanomaterials: Synthesis, Characteristics and Applications. Chem. Asian J. 2020, 15, 2282–2293. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Liang, X.-Q.; Song, W.-G.; Wu, Z.-Y. Identification of the nitrogen species on N-doped graphene layers and Pt/NG composite catalyst for direct methanol fuel cell. Phys. Chem. Chem. Phys. 2010, 12, 12055–12059. [Google Scholar] [CrossRef]
- Luo, W.; Wang, B.; Heron, C.G.; Allen, M.J.; Morre, J.; Maier, C.S.; Stickle, W.F.; Ji, X. Pyrolysis of Cellulose under Ammonia Leads to Nitrogen-Doped Nanoporous Carbon Generated through Methane Formation. Nano Lett. 2014, 14, 2225–2229. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Xu, G.; Guo, Z.; Zhang, Y. In situ synthesis of Fe-N-C catalysts from cellulose for hydrogenation of nitrobenzene to aniline. Chin. J. Catal. 2019, 40, 1557–1565. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Chen, Y.; Wan, H.; Dong, L.; Guan, G. Construction of ultramicropore-enriched N-doped carbons for CO2 capture: Self-decomposition of polyethyleneimine-based precursor to promote pore formation and surface polarity. J. Environ. Chem. Eng. 2021, 9, 105046. [Google Scholar] [CrossRef]
- Ali, M.; Riaz, R.; Anjum, A.S.; Sun, K.C.; Li, H.; Ahn, S.; Jeong, S.H.; Ko, M.J. Microwave-assisted ultrafast in-situ growth of N-doped carbon quantum dots on multiwalled carbon nanotubes as an efficient electrocatalyst for photovoltaics. J. Colloid Interface Sci. 2021, 586, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huang, S.; Han, X.; Chen, J.; Wang, J.; Rykov, A.; Wang, Y.; Wang, M.; Lv, J.; Ma, X. Highly active and controllable MOF-derived carbon nanosheets supported iron catalysts for Fischer-Tropsch synthesis. Carbon 2021, 173, 364–375. [Google Scholar] [CrossRef]
- Gholampour, N.; Zhao, Y.; Devred, F.; Sassoye, C.; Casale, S.; Debecker, D.P. CO2 Methanation over Cobalt Nanoparticles Embedded in ZIF-L–Derived Porous Carbon. ChemCatChem 2023, 15, e202201338. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, J.; Tian, Y.; Zhang, B.; Wu, Y. Preparation and Performances of ZIF-67-Derived FeCo Bimetallic Catalysts for CO2 Hydrogenation to Light Olefins. Catalysts 2020, 10, 455. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.-W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Recent Development of Zeolitic Imidazolate Frameworks (ZIFs) Derived Porous Carbon Based Materials as Electrocatalysts. Adv. Energy Mater. 2018, 8, 1801257. [Google Scholar] [CrossRef]
- Lee, S.-H.; Hwang, Y.-M.; Byun, T.-S.; Ko, J.-H.; Roh, J.-S. Effect of heating rate, temperature, and residence time during graphitization on the mechanical and electrical properties of isotropic graphite blocks. Carbon 2023, 208, 443–451. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, J.; Chen, X.; Wang, Q.; Zhang, S.; Tian, Y.; Liu, C.; Wang, L.; Wei, Z.; Cao, L.; et al. In Situ N,O-Dually Doped Nanoporous Biochar Derived from Waste Eutrophic Spirulina for High-Performance Supercapacitors. Nanomaterials 2023, 13, 2431. [Google Scholar] [CrossRef]
- Su, Y.; Shi, Y.; Jiang, M.; Chen, S. One-Step Synthesis of Nitrogen-Doped Porous Biochar Based on N-Doping Co-Activation Method and Its Application in Water Pollutants Control. Int. J. Mol. Sci. 2022, 23, 14618. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Z.; Kang, R.; Wang, J.; Lu, Q.; Wang, T.; Tian, D.; Xu, Y.; Wang, Z.; Ding, H. N-Rich Algal Sludge Biochar for Peroxymonosulfate Activation toward Sulfadiazine Removal. Coatings 2023, 13, 431. [Google Scholar] [CrossRef]
- Preparation of highly porous nitrogen-doped biochar derived from birch tree wastes with superior dye removal performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 669, 131493. [CrossRef]
- Wang, X.; Liu, Y.; Zhu, L.; Li, Y.; Wang, K.; Qiu, K.; Tippayawong, N.; Aggarangsi, P.; Reubroycharoen, P.; Wang, S. Biomass derived N-doped biochar as efficient catalyst supports for CO2 methanation. J. CO2 Util. 2019, 34, 733–741. [Google Scholar] [CrossRef]
- Zhong, L.; Qiu, X.; Yang, S.; Sun, S.; Chen, L.; Zhang, W. Supermolecule-regulated synthesis strategy of general biomass-derived highly nitrogen-doped carbons toward potassium-ion hybrid capacitors with enhanced performances. Energy Storage Mater. 2023, 61, 102887. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Raju, R.; Youk, J.H. Polypyrrole-derived N-doped CNT nanocomposites decorated with CoNi alloy nanoparticles for high-performance supercapacitor electrodes. Appl. Surf. Sci. 2023, 619, 156796. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Chen, B.; Wang, L.; Yang, J.; Wang, B. Nitrogen-doped hierarchically constructed interconnected porous carbon nanofibers derived from polyaniline (PANI) for highly selective CO2 capture and effective methanol adsorption. J. Environ. Chem. Eng. 2022, 10, 108847. [Google Scholar] [CrossRef]
- Shi, P.-C.; Yi, J.-D.; Liu, T.-T.; Li, L.; Zhang, L.-J.; Sun, C.-F.; Wang, Y.-B.; Huang, Y.-B.; Cao, R. Hierarchically porous nitrogen-doped carbon nanotubes derived from core–shell ZnO@zeolitic imidazolate framework nanorods for highly efficient oxygen reduction reactions. J. Mater. Chem. A 2017, 5, 12322–12329. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, J.; Jin, Y.; Tong, Y.; Lu, Q.; Gao, F. Quantum Effects Allow the Construction of Two-Dimensional Co3O4 -Embedded Nitrogen-Doped Porous Carbon Nanosheet Arrays from Bimetallic MOFs as Bifunctional Oxygen Electrocatalysts. Chem. Eur. J. 2018, 24, 14522–14530. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, S.; Lu, X.; Fan, W.; Chi, B.; Ye, Y.; Shi, X.; Zeng, J.; Li, X.; Liao, S. Two-Dimensional Bimetallic Zn/Fe-Metal-Organic Framework (MOF)-Derived Porous Carbon Nanosheets with a High Density of Single/Paired Fe Atoms as High-Performance Oxygen Reduction Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 13878–13887. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Wang, M.; Wang, Y.; Cheng, K.; Zhang, Q.; Kang, J.; Wang, Y. Functionalized Carbon Materials in Syngas Conversion. Small 2021, 17, 2007527. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Huang, B.; Mortensen, M.K.; Keyvanloo, K.; Fletcher, T.H.; Woodfield, B.F.; Hecker, W.C.; Argyle, M.D. Effect of different alumina supports on performance of cobalt Fischer-Tropsch catalysts. J. Catal. 2018, 359, 92–100. [Google Scholar] [CrossRef]
- Campisi, S.; Chan-Thaw, C.E.; Villa, A. Understanding Heteroatom-Mediated Metal–Support Interactions in Functionalized Carbons: A Perspective Review. Appl. Sci. 2018, 8, 1159. [Google Scholar] [CrossRef]
- Kafrani, M.R.; Beni, A.A.; Nodeh, Z.P. Preparation of cobalt-ruthenium nanocatalysts supported on nitrogen-doped graphene aerogel and carbon nanotubes in Fischer-Tropsch synthesis. Arab. J. Chem. 2023, 16, 104914. [Google Scholar] [CrossRef]
- Wang, B.; Han, Y.; Chen, S.; Zhang, Y.; Li, J.; Hong, J. Construction of three-dimensional nitrogen-doped graphene aerogel (NGA) supported cobalt catalysts for Fischer-Tropsch synthesis. Catal. Today 2020, 355, 10–16. [Google Scholar] [CrossRef]
- Zhan, W.; Wang, Y.; Chen, J.; Chen, Z.; Li, Y. Boosting the Fischer-Tropsch synthesis performances of cobalt-based catalysts via geometric and electronic engineering: Construction of hollow structures. Appl. Catal. B Environ. 2022, 313, 121469. [Google Scholar] [CrossRef]
- Taghavi, S.; Tavasoli, A.; Asghari, A.; Signoretto, M. Loading and promoter effects on the performance of nitrogen functionalized graphene nanosheets supported cobalt Fischer-Tropsch synthesis catalysts. Int. J. Hydrog. Energy 2019, 44, 10604–10615. [Google Scholar] [CrossRef]
- Chernyak, S.; Stolbov, D.; Ivanov, A.; Klokov, S.; Egorova, T.; Maslakov, K.; Eliseev, O.; Maximov, V.; Savilov, S.; Lunin, V. Effect of type and localization of nitrogen in graphene nanoflake support on structure and catalytic performance of Co-based Fischer-Tropsch catalysts. Catal. Today 2020, 357, 193–202. [Google Scholar] [CrossRef]
- Dlamini, M.W.; Phaahlamohlaka, T.N.; Kumi, D.O.; Forbes, R.; Jewell, L.L.; Coville, N.J. Post doped nitrogen-decorated hollow carbon spheres as a support for Co Fischer-Tropsch catalysts. Catal. Today 2020, 342, 99–110. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhao, N.; Lyu, S.; Tian, Y.; Gao, F.; Dong, L.; Jiang, Z.; Zhang, J.; Tsubaki, N.; Li, X. Tuning interaction between cobalt catalysts and nitrogen dopants in carbon nanospheres to promote Fischer-Tropsch synthesis. Appl. Catal. B Environ. 2019, 248, 73–83. [Google Scholar]
- Guo, S.; Niu, C.; Ma, Z.; Wang, J.; Hou, B.; Jia, L.; Li, D. A novel and facile strategy to decorate Al2O3 as an effective support for Co-based catalyst in Fischer-Tropsch synthesis. Fuel 2021, 289, 119780. [Google Scholar] [CrossRef]
- Li, S.; Yao, N.; Zhao, F.; Li, X. Nitrogen-doped carbon species: A promising nonmetallic promoter for the Co/SiO2 Fischer–Tropsch synthesis catalyst. Catal. Sci. Technol. 2016, 6, 2188–2194. [Google Scholar]
- Yang, Y.; Jia, L.; Hou, B.; Li, D.; Wang, J.; Sun, Y. The Correlation of Interfacial Interaction and Catalytic Performance of N-Doped Mesoporous Carbon Supported Cobalt Nanoparticles for Fischer–Tropsch Synthesis. J. Phys. Chem. C 2014, 118, 268–277. [Google Scholar]
- Fu, T.; Liu, R.; Lv, J.; Li, Z. Influence of acid treatment on N-doped multi-walled carbon nanotube supports for Fischer–Tropsch performance on cobalt catalyst. Fuel Process. Technol. 2014, 122, 49–57. [Google Scholar] [CrossRef]
- Fu, T.; Li, Z. Highly dispersed cobalt on N-doped carbon nanotubes with improved Fischer–Tropsch synthesis activity. Catal. Commun. 2014, 47, 54–57. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, L.; Hou, B.; Li, D.; Wang, J.; Sun, Y. The Effect of Nitrogen on the Autoreduction of Cobalt Nanoparticles Supported on Nitrogen-Doped Ordered Mesoporous Carbon for the Fischer-Tropsch Synthesis. ChemCatChem 2014, 6, 319–327. [Google Scholar] [CrossRef]
- Chai, J.; Jiang, J.; Gong, Y.; Wu, P.; Wang, A.; Zhang, X.; Wang, T.; Meng, X.; Lin, Q.; Lv, Y.; et al. Recent Mechanistic Understanding of Fischer-Tropsch Synthesis on Fe-Carbide. Catalysts 2023, 13, 1052. [Google Scholar]
- Liu, G.; Chen, Q.; Oyunkhand, E.; Ding, S.; Yamane, N.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Nitrogen-rich mesoporous carbon supported iron catalyst with superior activity for Fischer-Tropsch synthesis. Carbon 2018, 130, 304–314. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, C.; Si, Z.; Wen, C.; Xu, Y.; Lv, W.; Chen, L.; Zhang, X.; Ma, L. Enhancement of Light Olefins Selectivity Over N-Doped Fischer-Tropsch Synthesis Catalyst Supported on Activated Carbon Pretreated with KMnO4. Catalysts 2019, 9, 505. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.-K.; Chun, D.H.; Choi, H.; Rhim, G.B.; Youn, M.H.; Jeong, H.; Kang, S.W.; Yang, J.-I.; Jung, H.; et al. Phase-controlled synthesis of thermally stable nitrogen-doped carbon supported iron catalysts for highly efficient Fischer-Tropsch synthesis. Nano Res. 2019, 12, 2568–2575. [Google Scholar] [CrossRef]
- Tang, L.; Dong, X.-L.; Xu, W.; He, L.; Lu, A.-H. Iron-based catalysts encapsulated by nitrogen-doped graphitic carbon for selective synthesis of liquid fuels through the Fischer-Tropsch process. Chin. J. Catal. 2018, 39, 1971–1979. [Google Scholar] [CrossRef]
- Hwang, J.; Ejsmont, A.; Freund, R.; Goscianska, J.; Schmidt, B.V.K.J.; Wuttke, S. Controlling the morphology of metal–organic frameworks and porous carbon materials: Metal oxides as primary architecture-directing agents. Chem. Soc. Rev. 2020, 49, 3348–3422. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Hu, M.; Wang, H.; Huang, H.-S.; Fujita, T.; Wu, K.C.-W.; Chen, L.-C.; Yamauchi, Y.; Ariga, K. Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes. Chem. Commun. 2012, 48, 7259–7261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Tan, X.; Guo, L.; Zhang, J.; Liu, S.; Guo, Y.; Zhang, J.; Wang, H.; Chu, W. Monoclinic ZIF-8 Nanosheet-Derived 2D Carbon Nanosheets as Sulfur Immobilizer for High-Performance Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 25239–25249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Han, X.; Liang, H.; Wang, Y.; Lv, J.; Wang, M.-Y.; Huang, S.; Ma, X. Activating nitrogen-doped carbon nanosheets by KOH treatment to promote the Fischer-Tropsch synthesis performance. Chem. Eng. J. 2023, 455, 140810. [Google Scholar] [CrossRef]
- Park, B.J.; Jang, S.; Lee, J.H.; Chun, D.H.; Park, J.C.; Park, H.S. Hyperactive iron carbide@N-doped reduced graphene oxide/carbon nanotube hybrid architecture for rapid CO hydrogenation. J. Mater. Chem. A 2018, 6, 11134–11139. [Google Scholar] [CrossRef]
- Liu, R.; Liu, R.; Ma, X.; Davis, B.H.; Li, Z. Efficient diesel production over the iron-based Fischer–Tropsch catalyst supported on CNTs treated by urea/NaOH. Fuel 2018, 211, 827–836. [Google Scholar] [CrossRef]
- Lama, S.M.; Weber, J.L.; Heil, T.; Hofmann, J.P.; Yan, R.; de Jong, K.P.; Oschatz, M. Tandem promotion of iron catalysts by sodium-sulfur and nitrogen-doped carbon layers on carbon nanotube supports for the Fischer-Tropsch to olefins synthesis. Appl. Catal. A Gen. 2018, 568, 213–220. [Google Scholar] [CrossRef]
- Wu, J.; Qin, L.; Wang, C.; Lv, B.; Wang, L.; Chen, J.; Xu, Y. Ultrathin N-rich boron nitride nanosheets supported iron catalyst for Fischer–Tropsch synthesis. RSC Adv. 2016, 6, 38356–38364. [Google Scholar] [CrossRef]
- An, B.; Cheng, K.; Wang, C.; Wang, Y.; Lin, W. Pyrolysis of Metal–Organic Frameworks to Fe3O4@Fe5C2 Core–Shell Nanoparticles for Fischer–Tropsch Synthesis. ACS Catal. 2016, 6, 3610–3618. [Google Scholar] [CrossRef]
- Li, Z.; Liu, R.; Xu, Y.; Ma, X. Enhanced Fischer–Tropsch synthesis performance of iron-based catalysts supported on nitric acid treated N-doped CNTs. Appl. Surf. Sci. 2015, 347, 643–650. [Google Scholar] [CrossRef]
- Chen, X.; Deng, D.; Pan, X.; Hu, Y.; Bao, X. N-doped graphene as an electron donor of iron catalysts for CO hydrogenation to light olefins. Chem. Commun. 2015, 51, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Moyo, M.; Motchelaho, M.A.; Tetana, Z.N.; Dube, S.M.; Jewell, L.L.; Coville, N.J. Fischer–Tropsch synthesis: Iron catalysts supported on N-doped carbon spheres prepared by chemical vapor deposition and hydrothermal approaches. J. Catal. 2014, 311, 80–87. [Google Scholar] [CrossRef]
- Xiong, H.; Motchelaho, M.A.; Moyo, M.; Jewell, L.L.; Coville, N.J. Fischer–Tropsch synthesis: Iron-based catalysts supported on nitrogen-doped carbon nanotubes synthesized by post-doping. Appl. Catal. A Gen. 2014, 482, 377–386. [Google Scholar] [CrossRef]
- Xiong, H.; Moyo, M.; Rayner, M.K.; Jewell, L.L.; Billing, D.G.; Coville, N.J. Autoreduction and Catalytic Performance of a Cobalt Fischer-Tropsch Synthesis Catalyst Supported on Nitrogen-Doped Carbon Spheres. ChemCatChem 2010, 2, 514–518. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, L.; Zhang, L.; Zhang, C.; Jun, K.-W.; Kim, S.K.; Park, H.-G.; Zhao, T.; Gao, Y.; Zhu, Y.; et al. Upcycling of CO2 into sustainable hydrocarbon fuels via the integration of Fe-based Fischer-Tropsch synthesis and olefin oligomerization: A comparative case study. Fuel 2022, 325, 124855. [Google Scholar] [CrossRef]
- Arslan, M.T.; Tian, G.; Ali, B.; Zhang, C.; Xiong, H.; Li, Z.; Luo, L.; Chen, X.; Wei, F. Highly Selective Conversion of CO2 or CO into Precursors for Kerosene-Based Aviation Fuel via an Aldol–Aromatic Mechanism. ACS Catal. 2022, 12, 2023–2033. [Google Scholar] [CrossRef]
- Li, Y.; Cai, X.; Chen, S.; Zhang, H.; Zhang, K.H.L.; Hong, J.; Chen, B.; Kuo, D.-H.; Wang, W. Highly Dispersed Metal Carbide on ZIF-Derived Pyridinic-N-Doped Carbon for CO2 Enrichment and Selective Hydrogenation. ChemSusChem 2018, 11, 1040–1047. [Google Scholar] [CrossRef]
- Arpini, B.H.; Braga, A.H.; Borges, L.R.; Vidinha, P.; Gonçalves, R.V.; Szanyi, J.; Rossi, L.M. Tuning CO2 Hydrogenation Selectivity by N-Doped Carbon Coating over Nickel Nanoparticles Supported on SiO2. ACS Sustain. Chem. Eng. 2022, 10, 2331–2342. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Lu, W.; Zhu, H.; Sun, F.; Mei, B.; Jiang, Z.; Lyu, Y.; Chen, X.; Guo, L.; et al. Single-atom Co-N-C catalysts for high-efficiency reverse water-gas shift reaction. Appl. Catal. B Environ. 2023, 324, 122298. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, B.; Luo, K.H. Unveiling the mechanism of controllable CO2 hydrogenation by group VIB metal single atom anchored on N-doped graphite: A density functional theory study. Int. J. Hydrog. Energy 2022, 47, 40972–40985. [Google Scholar] [CrossRef]
- Jiang, Y.; Sung, Y.; Choi, C.; Joo Bang, G.; Hong, S.; Tan, X.; Wu, T.-S.; Soo, Y.-L.; Xiong, P.; Li, M.M.-J.; et al. Single-Atom Molybdenum-N3 Sites for Selective Hydrogenation of CO2 to CO. Angew. Chem. 2022, 134, e202203836. [Google Scholar] [CrossRef]
- Hasan, M.F.; Rossi, L.M.; Debecker, D.P.; Leonard, K.C.; Li, Z.; Makhubela, B.C. Can CO2 and Renewable Carbon Be Primary Resources for Sustainable Fuels and Chemicals? ACS Sustain. Chem. Eng. 2021, 9, 12427–12430. [Google Scholar] [CrossRef]
- Hidalgo, D.; Martín-Marroquín, J. Power-to-methane, coupling CO2 capture with fuel production: An overview. Renew. Sustain. Energy Rev. 2020, 132, 110057. [Google Scholar] [CrossRef]
- Di Stasi, C.; Renda, S.; Greco, G.; González, B.; Palma, V.; Manyà, J.J. Wheat-Straw-Derived Activated Biochar as a Renewable Support of Ni-CeO2 Catalysts for CO2 Methanation. Sustainability 2021, 13, 8939. [Google Scholar] [CrossRef]
- Roldán, L.; Marco, Y.; García-Bordejé, E. Origin of the Excellent Performance of Ru on Nitrogen-Doped Carbon Nanofibers for CO2 Hydrogenation to CH4. ChemSusChem 2017, 10, 1139–1144. [Google Scholar] [CrossRef]
- Wu, J.; Wen, C.; Zou, X.; Jimenez, J.; Sun, J.; Xia, Y.; Rodrigues, M.-T.F.; Vinod, S.; Zhong, J.; Chopra, N.; et al. Carbon Dioxide Hydrogenation over a Metal-Free Carbon-Based Catalyst. ACS Catal. 2017, 7, 4497–4503. [Google Scholar] [CrossRef]
- Wang, W.; Duong-Viet, C.; Ba, H.; Baaziz, W.; Tuci, G.; Caporali, S.; Nguyen-Dinh, L.; Ersen, O.; Giambastiani, G.; Pham-Huu, C. Nickel Nanoparticles Decorated Nitrogen-Doped Carbon Nanotubes (Ni/N-CNT); a Robust Catalyst for the Efficient and Selective CO2 Methanation. ACS Appl. Energy Mater. 2019, 2, 1111–1120. [Google Scholar] [CrossRef]
- Gonçalves, L.P.; Meledina, M.; Meledin, A.; Petrovykh, D.Y.; Sousa, J.P.; Soares, O.S.G.; Kolen’Ko, Y.V.; Pereira, M.F.R. Understanding the importance of N-doping for CNT-supported Ni catalysts for CO2 methanation. Carbon 2022, 195, 35–43. [Google Scholar] [CrossRef]
- Gödde, J.; Merko, M.; Xia, W.; Muhler, M. Nickel nanoparticles supported on nitrogen–doped carbon nanotubes are a highly active, selective and stable CO2 methanation catalyst. J. Energy Chem. 2021, 54, 323–331. [Google Scholar] [CrossRef]
- Weber, D.; Rui, N.; Zhang, F.; Zhang, H.; Vovchok, D.; Wildy, M.; Arizapana, K.; Saporita, A.; Zhang, J.Z.; Senanayake, S.D. Carbon Nanosphere-Encapsulated Fe Core–Shell Structures for Catalytic CO2 Hydrogenation. ACS Appl. Nano Mater. 2022, 5, 11605–11616. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Jiang, X.; Zhang, A.; Song, C.; Guo, X. Pyrolyzing ZIF-8 to N-doped porous carbon facilitated by iron and potassium for CO2 hydrogenation to value-added hydrocarbons. J. CO2 Util. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Zhang, P.; Han, F.; Yan, J.; Qiao, X.; Guan, Q.; Li, W. N-doped ordered mesoporous carbon (N-OMC) confined Fe3O4-FeCx heterojunction for efficient conversion of CO2 to light olefins. Appl. Catal. B Environ. 2021, 299, 120639. [Google Scholar] [CrossRef]
- Peng, L.; Jurca, B.; Primo, A.; Gordillo, A.; Parvulescu, V.I.; García, H. High C2-C4 selectivity in CO2 hydrogenation by particle size control of Co-Fe alloy nanoparticles wrapped on N-doped graphitic carbon. iScience 2022, 25, 104252. [Google Scholar] [CrossRef]
- LLimleamthong, P.; Chuchuan, A.; Thepphankulngarm, N.; Kongkachuichay, P. Structured Porous Carbon-Based Catalysts: Cu–ZnO/CMK-3 and Cu–CeO2/CMK-3 for Direct CO2 Conversion to Methanol. Top. Catal. 2023. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Bao, Y.; Wang, G.; Zhang, Y.; Fu, M.; Wu, J.; Ye, D. Roles of nitrogen species on nitrogen-doped CNTs supported Cu-ZrO2 system for carbon dioxide hydrogenation to methanol. Catal. Today 2018, 307, 212–223. [Google Scholar] [CrossRef]
- Deerattrakul, V.; Yigit, N.; Rupprechter, G.; Kongkachuichay, P. The roles of nitrogen species on graphene aerogel supported Cu-Zn as efficient catalysts for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 580, 46–52. [Google Scholar] [CrossRef]
- Ma, Q.; Geng, M.; Zhang, J.; Zhang, X.; Zhao, T.-S. Enhanced Catalytic Performance for CO2 Hydrogenation to Methanol over N-doped Graphene Incorporated Cu-ZnO-Al2O3 Catalysts. ChemistrySelect 2019, 4, 78–83. [Google Scholar] [CrossRef]
- Donphai, W.; Thepphankulngarm, N.; Chaisuwan, T.; Tanangteerapong, D.; Rood, S.C.; Kongkachuichay, P. Catalytic performance of copper and ruthenium loaded on N-doped modified PBZ-derived carbons for CO2 hydrogenation. Chem. Eng. Sci. 2023, 274, 118693. [Google Scholar] [CrossRef]
- Moret, S.; Dyson, P.J.; Laurenczy, G. Direct synthesis of formic acid from carbon dioxide by hydrogenation in acidic media. Nat. Commun. 2014, 5, 4017. [Google Scholar] [CrossRef]
- Yang, G.; Kuwahara, Y.; Mori, K.; Louis, C.; Yamashita, H. PdAg alloy nanoparticles encapsulated in N-doped microporous hollow carbon spheres for hydrogenation of CO2 to formate. Appl. Catal. B Environ. 2021, 283, 119628. [Google Scholar] [CrossRef]
- Ahn, S.; Park, K.; Lee, K.R.; Haider, A.; Van Nguyen, C.; Jin, H.; Yoo, S.J.; Yoon, S.; Jung, K.-D. Atomically dispersed Ru(III) on N-doped mesoporous carbon hollow spheres as catalysts for CO2 hydrogenation to formate. Chem. Eng. J. 2022, 442, 136185. [Google Scholar] [CrossRef]
- Jaleel, A.; Haider, A.; Van Nguyen, C.; Lee, K.R.; Choung, S.; Han, J.W.; Baek, S.-H.; Shin, C.-H.; Jung, K.-D. Structural effect of Nitrogen/Carbon on the stability of anchored Ru catalysts for CO2 hydrogenation to formate. Chem. Eng. J. 2022, 433, 133571. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, Y.; Cao, Z. Rational Design of Main Group Metal-Embedded Nitrogen-Doped Carbon Materials as Frustrated Lewis Pair Catalysts for CO2 Hydrogenation to Formic Acid. ACS Appl. Mater. Interfaces 2022, 14, 1002–1014. [Google Scholar] [CrossRef]



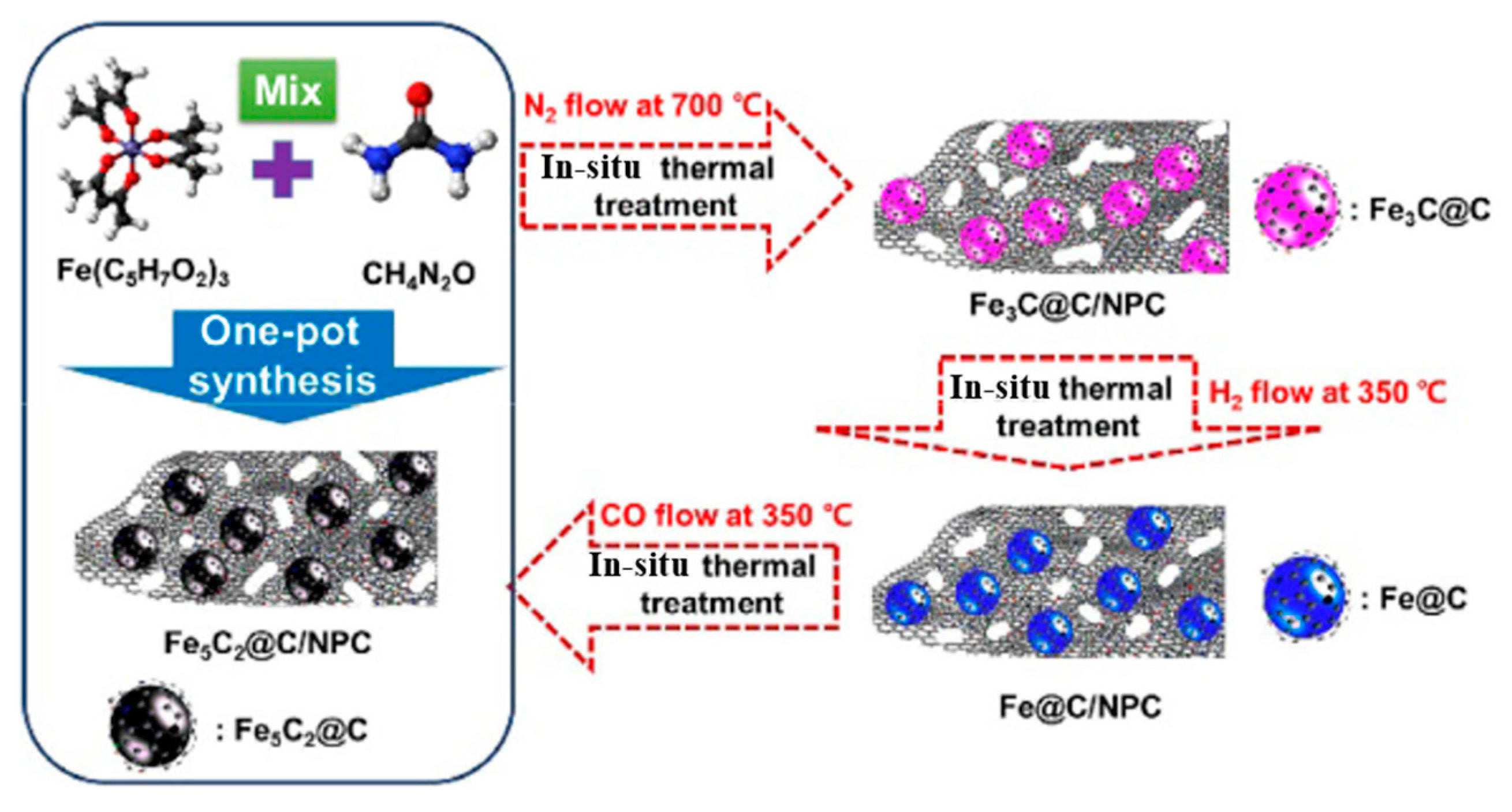

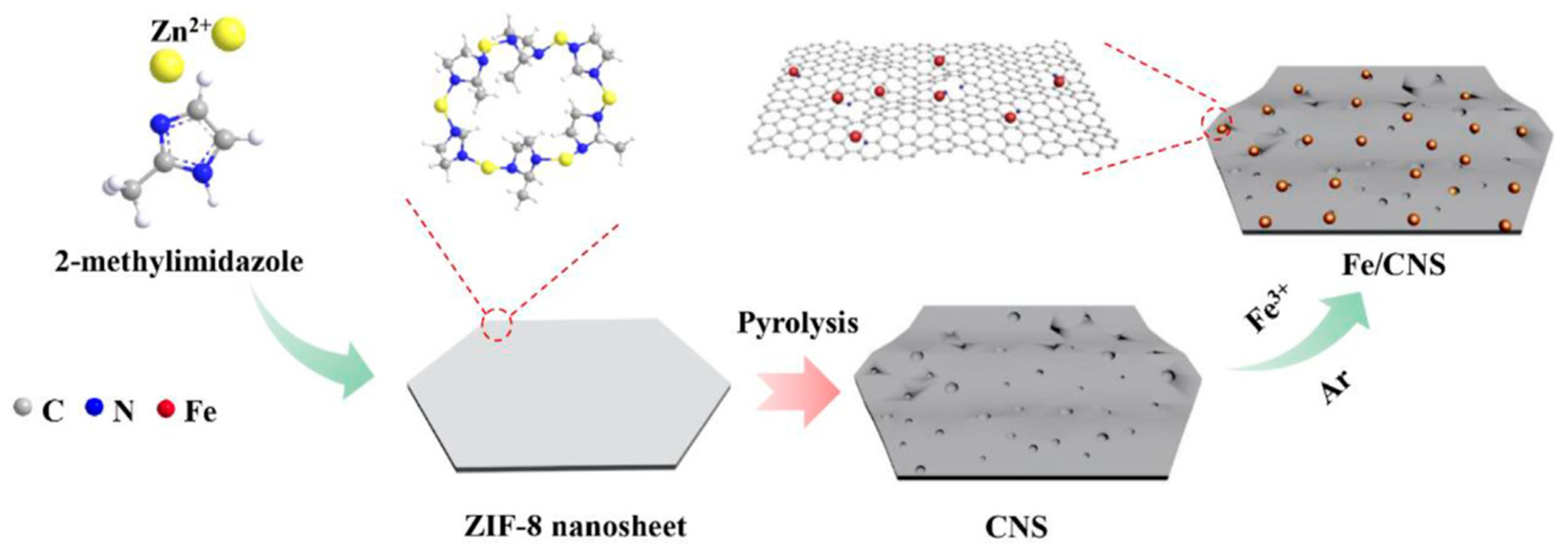
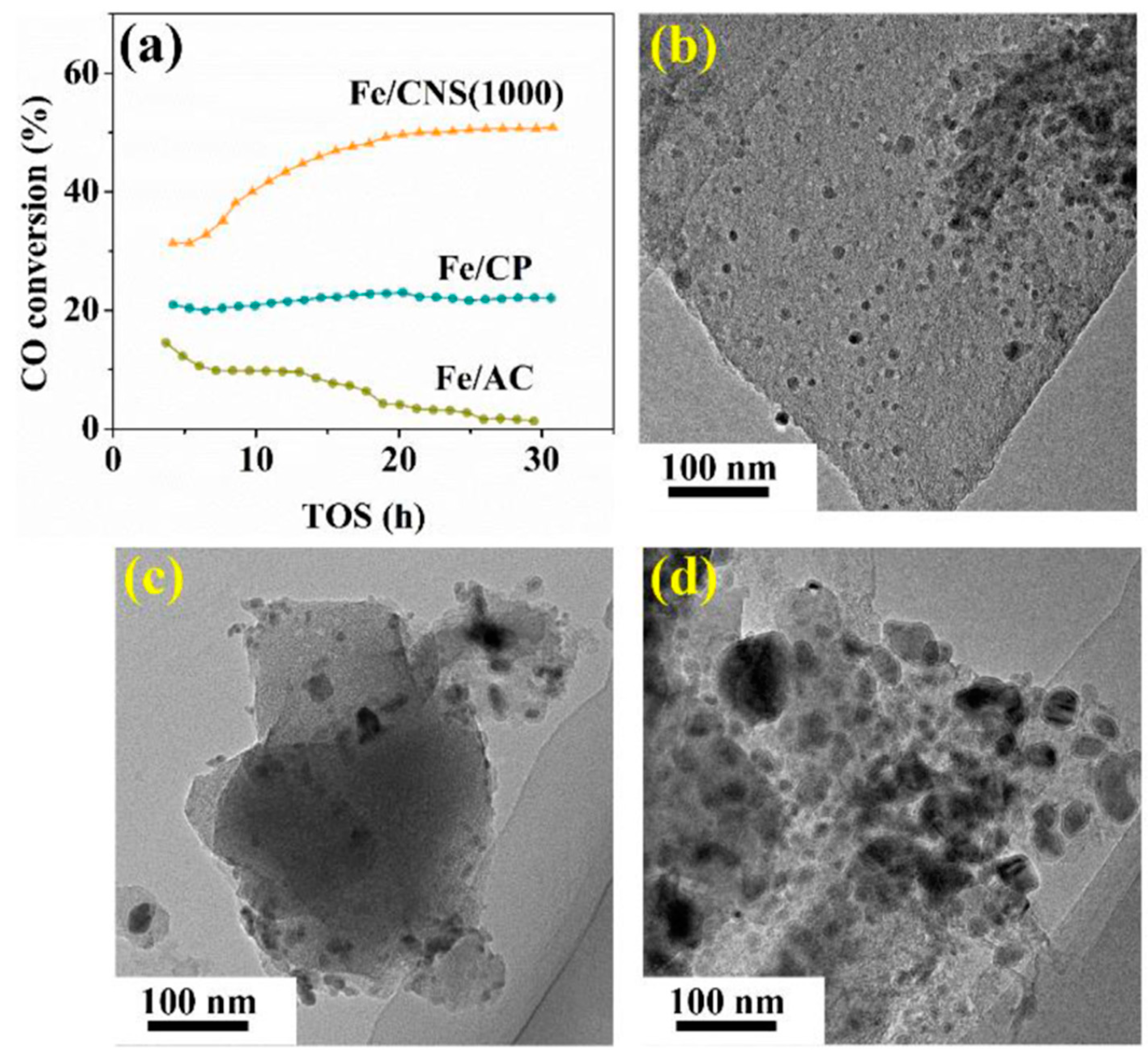

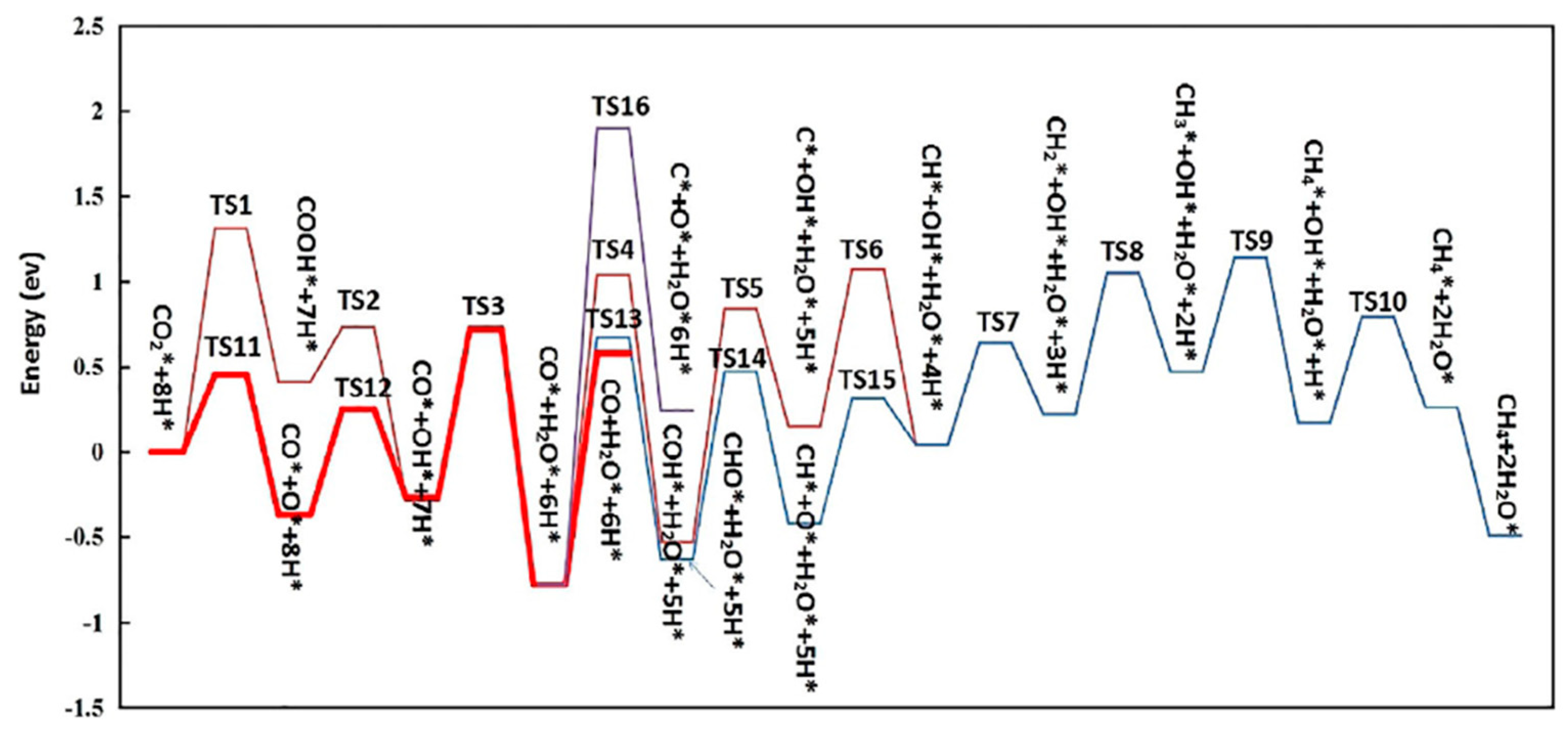

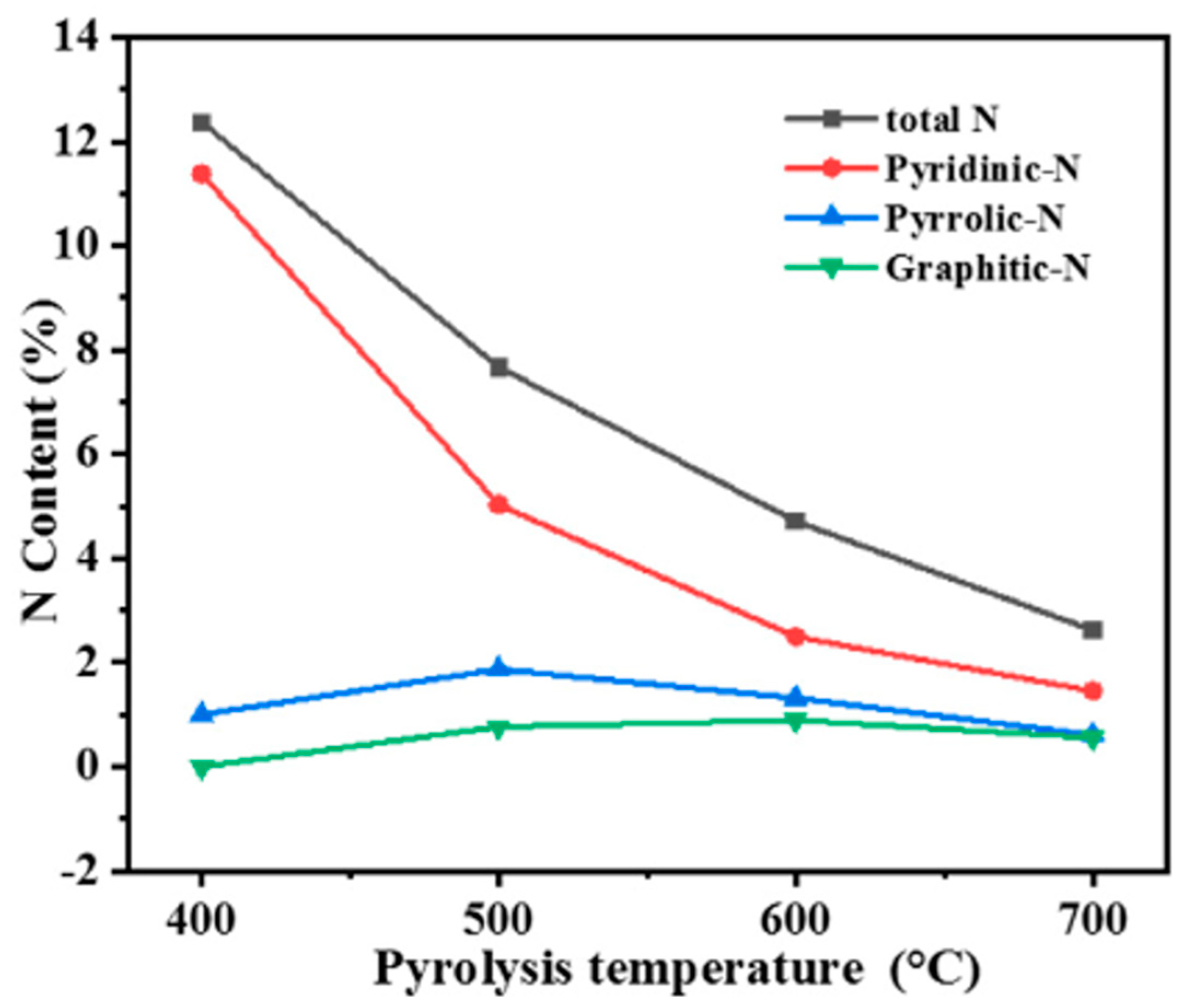
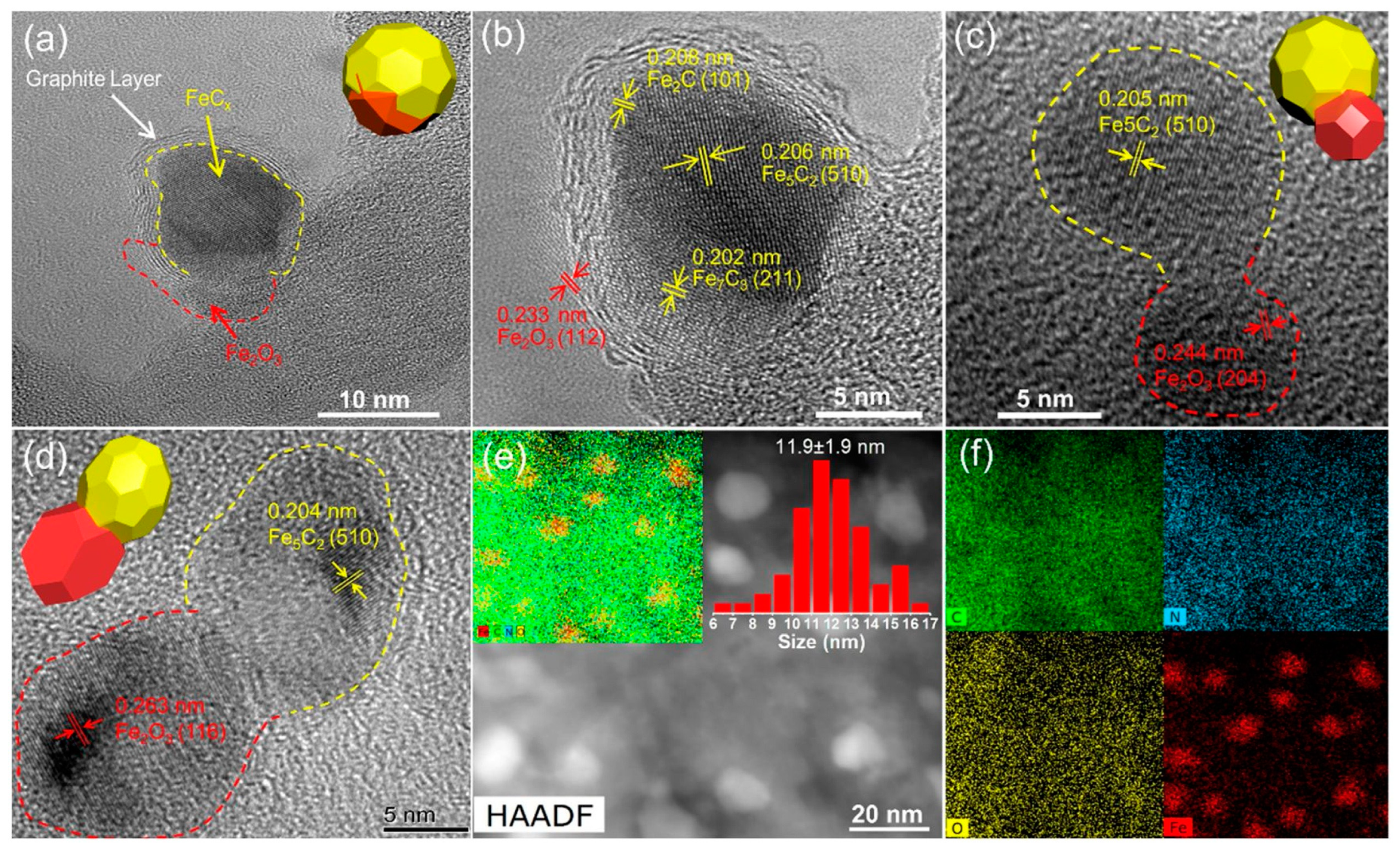

| Catalyst | Pressure (bar) | Temperature (°C) | GHSV | H2:CO Ratio | CO Conversion (%) | C5+ Selectivity | Reference |
|---|---|---|---|---|---|---|---|
| Co-Ru/NGA | 25 | 280 | 10,000 b | 1 | 75.25 | 17.2 | [75] |
| 2.7%Co/NGA | 10 | 250 | 2000 b | 2 | 9.0 | 88.2 | [76] |
| H-Co@NCNHP | 20 | 250 | 28,400 b | 2 | 16.4 | 90.4 | [77] |
| Co/GHFox | 20 | 240 | 6000 * | 2 | 31.9 | 30.0 | [79] |
| 10Co/N-HCSs900 | 10 | 220 | N.A. | 2 | 34.0 | 75.8 | [80] |
| Co/NCS-500 | 20 | 220 | N.A. | 2 | N.A. | 74.4 | [81] |
| Co/Al2O3-15CN | 20 | 250 | N.A. | 2 | 71.3 | 77.8 | [82] |
| Co/SiO2-CN | 20 | 250 | 27,000 b | 2 | 34.4 | 76.3 | [83] |
| 10Co/N-MC | 20 | 230 | 1000 a | 2 | N.A. | 73.0 | [84] |
| Co/NCNTs-2 | 20 | 230 | 6750 b | 2 | 51.2 | 83.4 | [85] |
| Co/A-NCNTs | 20 | 230 | 6750 b | 2 | 74.3 | 80.0 | [86] |
| 15Co/NMC-2 | 20 | 240 | 1000 a | 2 | N.A. | 61.2 | [87] |
| Catalyst | Pressure (bar) | Temperature (°C) | GHSV | H2:Co Ratio | CO Conversion (%) | C5+ Selectivity | Reference |
|---|---|---|---|---|---|---|---|
| Fe/CNS(1000) | 10 | 340 | 9000 b | 1 | 45.9 | 39.2 | [55] |
| 15%Fe/AG(12h)-W(10) | 20 | 320 | 5 # | 2 | 97.2 | 40.0 | [29] |
| 40Fe/N1 | 10 | 260 | 5 # | 1 | 74.7 | 67.0 | [89] |
| FeN-AC | 20 | 320 | 15,000 a | 1 | 59.7 | 18.1 | [90] |
| Fe5C2@C/NPC | 15 | 340 | 42 * | 1 | 96.4 | 33.5 | [91] |
| FeC-800 | 20 | 300 | N.A. | 2 | 47.0 | 52.0 | [92] |
| Fe/CNS-KOH(700, 0.6) | 10 | 340 | 9000 b | 1 | 87.0 | 55.6 | [96] |
| Fe5C2@Ns-rGO/CNT | 15 | 340 | 210 * | 1 | 80.5 | 30.0 | [97] |
| Fe/CNTs-NaU | 20 | 270 | 4500 b | 1 | 60.0 | 90.8 | [98] |
| FeBN2 | 20 | 270 | 1500 a | 2 | 47.0 | 48.0 | [100] |
| Fe-MIL-88B-NH2/C | 20 | 300 | 36,000 a | 1 | 81.8 | 50.7 | [101] |
| Fe/NCNTs-10 | 20 | 270 | 4500 b | 1 | 45.0 | 76.0 | [102] |
| Fe/NG−16.4 | 5 | 340 | 600 a | 1 | 21.1 | 19.8 | [103] |
| Fe/NCSver | 8 | 275 | 2700 a | N.A. | 50.0 | 51.6 | [104] |
| Fe/N-CNT-h | 8 | 275 | 2400 a | 2 | 70.1 | 60.9 | [105] |
| 2.3 wt% Co/N-CSs | 8 | 230 | 1200 a | 2 | N.A. | 47.1 | [106] |
| Catalyst | Pressure (bar) | Temperature (°C) | GHSV | H2:CO Ratio | CO2 Conversion (%) | CO Selectivity | Reference |
|---|---|---|---|---|---|---|---|
| Ni/ZIF-8-C | 1 | 420 | 15,000 b | 4 | 43.8 | 42.7 | [82] |
| Fe/ZIF-8-C | 1 | 420 | 15,000 b | 4 | 43.8 | 42.6 | [82] |
| 5%Co-N-C | 1 | 500 | 6000 b | 4 | 52.4 | 98.3 | [84] |
| Mo/NC | 1 | 500 | 8000 b | 3 | 46.3 | 100 | [86] |
| Catalyst | Pressure (bar) | Temperature (°C) | GHSV | H2:Co Ratio | CO2 Conversion (%) | CH4 Selectivity | Reference |
|---|---|---|---|---|---|---|---|
| BCCe30Ni20 | 1 | 400 | 13,200 a | 4 | 65.0 | 95.0 | [27] |
| NGQDs/Al2O3 | 1 | 400 | 18,000 b | 4 | 61.8 | 55.2 | [117] |
| Ni/N-CNTs | 1 | 360 | 120,000 b | 4 | 75.0 | 94.0 | [118] |
| 60,000 b | 81.0 | 98.0 | [118] | ||||
| Ru/N-ABC-600 | 10 | 380 | 6000 b | 4 | 93.8 | 99.7 | [65] |
| Co/C(L) | 1 | 300 | 12,000 b | 4 | 44.0 | 84.0 | [56] |
| Co/C(67) | 1 | 300 | 12,000 b | 4 | 33.0 | 56.0 | [56] |
| Ni/CNT-N | 1 | 400 | 60,000 b | 4 | 81.2 | 99.2 | [119] |
| 40Ni/NCNT | 1 | 340 | 50,000 b | 4 | 51.4 | 95.8 | [120] |
| Catalyst | Pressure (bar) | Temperature (°C) | GHSV | H2:CO Ratio | CO2 Conversion (%) | C2+ Selectivity | Reference |
|---|---|---|---|---|---|---|---|
| FeZn-NC | 30 | 320 | 7200 b | 3 | 29.3 | 63.5 | [124] |
| FeCo/NC-600 | 20 | 320 | 6240 b | 3 | 37.0 | 49.4 | [58] |
| 0.8Fe@N-OMC | 30 | 320 | 4800 b | 3 | 54.5 | 82.9 | [127] |
| Co-Fe@(N) | 40 | 300 | 6000 a | 4 | 58.0 | 44.0 | [128] |
| Catalyst | Pressure (bar) | Temperature (°C) | GHSV | H2:CO Ratio | CO2 Conversion (%) | STY of Methanol (mg g−1cat h−1) | Reference |
|---|---|---|---|---|---|---|---|
| CZ/NCMK-3U | 15 | 250 | 2444 a | 3 | 43.0 | 512.0 | [127] |
| CC/NCMK-3U | 15 | 250 | 2444 a | 3 | 30.0 | 367.0 | [127] |
| CZ/CNTs-N | 30 | 260 | 3600 b | 3 | 11.5 | 102.2 | [128] |
| 15%CuZn/NrGOae-H | 15 | 250 | 2444 a | 3 | 20.5 | 329.4 | [129] |
| 15%CuZn/NrGOae-A | 15 | 250 | 2444 a | 3 | 16.4 | 264.4 | [129] |
| 15%CuZn/NrGOae-U | 15 | 250 | 2444 a | 3 | 24.2 | 405.5 | [129] |
| 10NG-CZA | 30 | 200 | 10 # | 3 | 8.2 | N.A. | [130] |
| Cu-Ru-Ce/Am-P123 | 15 | 210 | 2400 a | 3 | 27.0 | 530 | [131] |
| Cu-Ru-Zr/Am-P123 | 15 | 210 | 2400 a | 3 | 37.0 | 642 | [131] |
| Cu-Ru-Ce/U-P123 | 15 | 210 | 2400 a | 3 | 26.0 | 504 | [131] |
| Cu-Ru-Zr/U-P123 | 15 | 210 | 2400 a | 3 | 36.0 | 639 | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Xu, L.; Hu, K.; Chen, X.; Gao, R.; Zhang, L.; Wang, L.; Zhang, C. Research Advances on Nitrogen-Doped Carbon Materials in COx Hydrogenation. Atmosphere 2023, 14, 1510. https://doi.org/10.3390/atmos14101510
Deng C, Xu L, Hu K, Chen X, Gao R, Zhang L, Wang L, Zhang C. Research Advances on Nitrogen-Doped Carbon Materials in COx Hydrogenation. Atmosphere. 2023; 14(10):1510. https://doi.org/10.3390/atmos14101510
Chicago/Turabian StyleDeng, Chao, Lujing Xu, Kehao Hu, Xixi Chen, Ruxing Gao, Leiyu Zhang, Lei Wang, and Chundong Zhang. 2023. "Research Advances on Nitrogen-Doped Carbon Materials in COx Hydrogenation" Atmosphere 14, no. 10: 1510. https://doi.org/10.3390/atmos14101510
APA StyleDeng, C., Xu, L., Hu, K., Chen, X., Gao, R., Zhang, L., Wang, L., & Zhang, C. (2023). Research Advances on Nitrogen-Doped Carbon Materials in COx Hydrogenation. Atmosphere, 14(10), 1510. https://doi.org/10.3390/atmos14101510




