Climate and the Radial Growth of Conifers in Borderland Natural Areas of Texas and Northern Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Study Area
2.2. Tree-Ring Chronology Development
2.3. Correlation of the Tree-Ring Chronologies with Regional Precipitation and Temperature
2.4. Principal Component Analysis
3. Results
3.1. The Borderlands Tree-Ring Chronologies
3.2. Co-Variability of the Borderlands Tree-Ring Chronologies
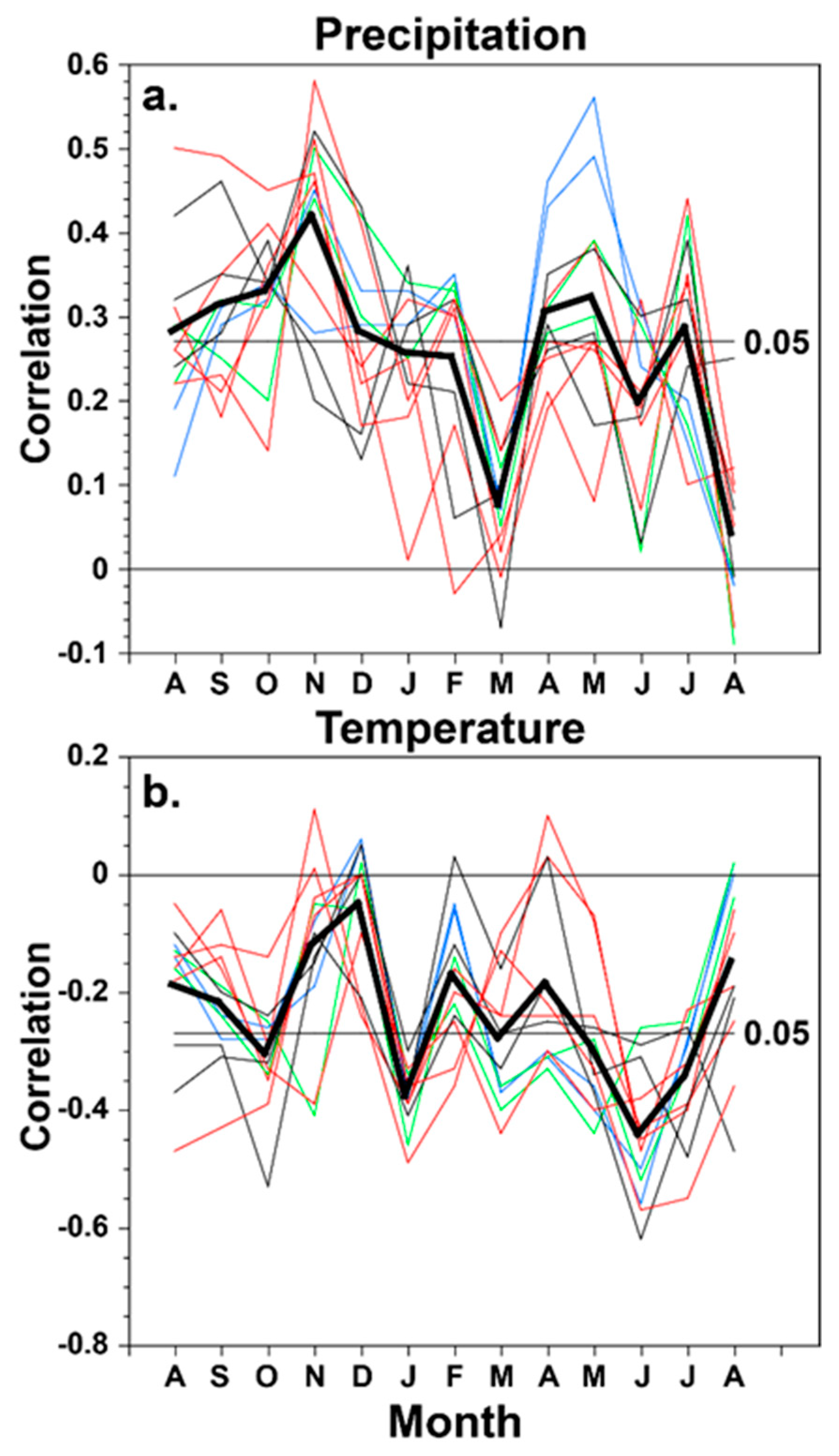
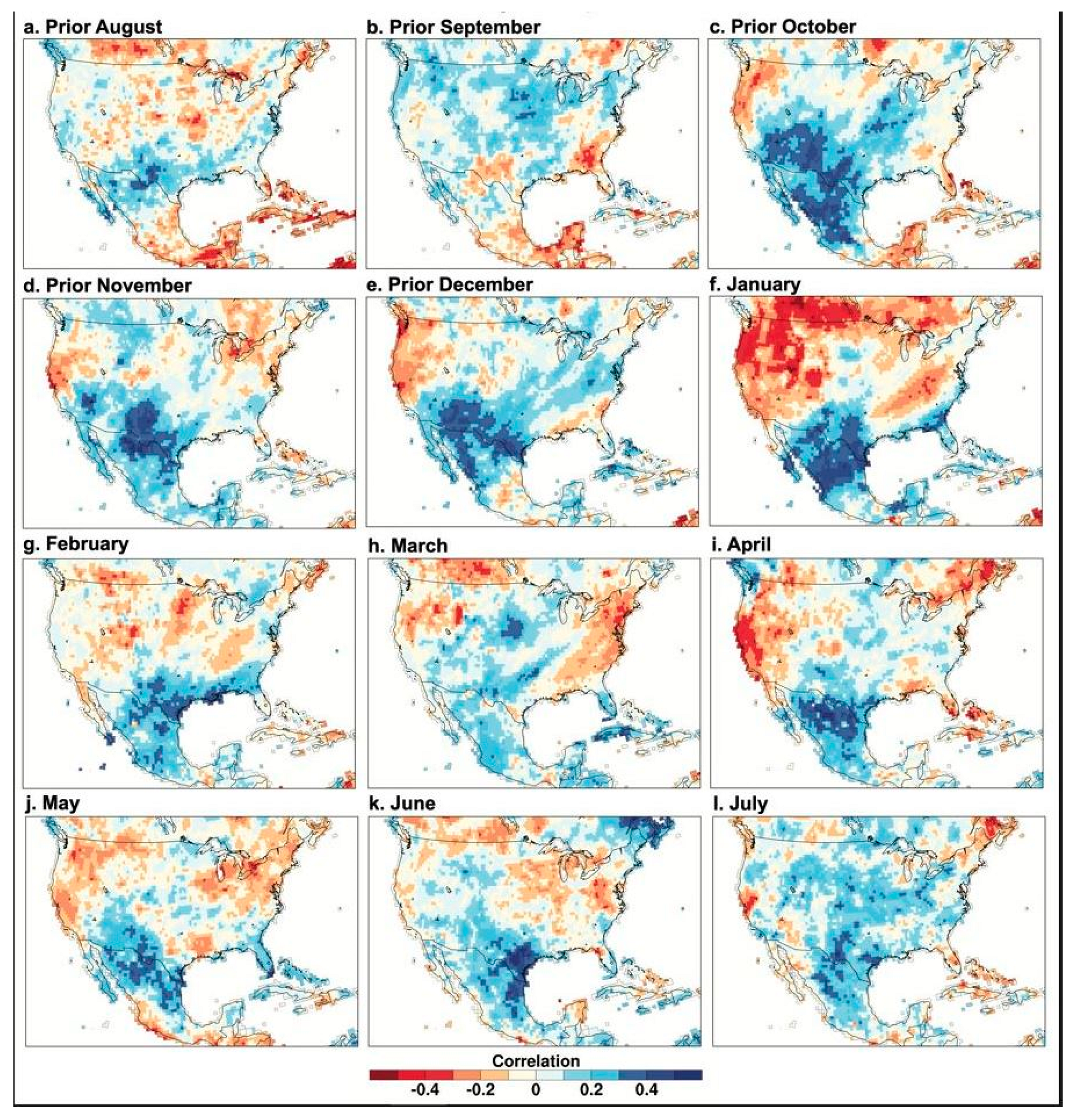
3.3. Climatic Response of the Borderlands Tree-Ring Chronologies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ortega-Rubio, A.; Pinkus-Rendón, M.J.; Espitia-Moreno, I.C.; Las Áreas Naturales Protegidas y la Investigación Científica en México. Centro de Investigaciones Biológicas del Noroeste S.C., La Paz, B.C.S., Universidad Autónoma de Yucatán, Mérida, Yucatán y Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, México. 2015. Available online: https://cobi.org.mx/wp-content/uploads/2016/01/2015_LIBRO-Las-%C3%A1reas-naturales-protegidas-y-la-investigaci%C3%B3n-cient%C3%ADfica-en-M%C3%A9xico.pdf (accessed on 12 July 2020).
- Adams, V.M.; Setterfield, S.A.; Douglas, M.M.; Kennard, M.J.; Ferdinands, K. Measuring benefits of protected area management: Trends across realms and research gaps for freshwater systems. Phil. Trans. R. Soc. B 2015, 370, 20140274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seager, R.; Ting, M.; Davis, M.; Cane, M.; Nike, M.; Nakumara, J.; Lie, C.; Cook, E.; Stahle, D.W. Mexican drought: An observational modeling and tree ring study of variability and climate change. Atmosfera 2009, 22, 1–31. [Google Scholar]
- Magaña, V.; Zermeño, D.; Neri, C. Climate change scenarios and potential impacts on water availability in northern Mexico. Clim. Res. 2012, 51, 171–184. [Google Scholar] [CrossRef]
- Allen, C.A.; Macalady, A.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.; Hogg, E.H.; Gonzalez, O.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Adams, H.A.; Barron-Gafford, G.A.; Minor, R.L.; Gardea, A.A.; Bentley, L.P.; Dorin, L.; Breshears, D.D.; McDowell, N.G.; Huxman, T.C. Temperature response surfaces for mortality risks of tree species with future drought. Environ. Res. Lett. 2017, 12, 115014. [Google Scholar] [CrossRef]
- Truettner, C.; Anderegg, R.L.; Biondi, F.; Koch, G.W.; Ogle, K.; Schwalm, C.; Livtak, M.E.; Shaw, J.D.; Ziaco, E. Conifer radial growth response to recent seasonal warming and droughts from the Southwestern USA. For. Ecol. Manag. 2018, 48, 55–62. [Google Scholar] [CrossRef]
- Marshet, N.G.; Fekadu, H.H. Review on effect of climate change on forest ecoystems. Environ. Sci. Nat. Resour. 2019, 17, 555968. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; Contribution of Working Group, 1 to the Sixth Assessment Report of the Intergovernmental Panel of Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Muldavin, E.H.; Harper, G.; Neville, P.; Wood, S. A vegetation classification of the Sierra del Carmen, U.S.A. and México. In Proceedings of the Sixth Symposium on the Natural Resources of the Chihuahuan Desert Region; Hoyt, C.A., Karges, J., Eds.; Chihuahuan Desert Research Institute: Fort Davis, TX, USA, 2014; pp. 117–150. Available online: http://cdri.org/publications/proceedings-of-the-symposium-on-the-naturalresources-of-the-chihuahuan-desert-region/ (accessed on 14 June 2020).
- Munson, S.M.; Raiser, M.H. Chihuahuan Desert Plant Responses to Climate Change; Chihuahuan Desert Network I&M Program: Big Ben National Park, TX, USA, 2013. Available online: https://www.nps.gov/articles/chihuahuan-desert-plant-responses-to-climate-change.htm (accessed on 13 June 2020).
- Williams, A.P.; Allen, C.D.; Millar, C.I.; Swetnam, T.W.; Michaelsen, J.; Still, C.J.; Leavitt, S.W. Forest responses to increasing aridity and warmth in the southwestern United States. Proc. Natl. Acad. Sci. USA 2010, 107, 21289–21294. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, P.E.; Miranda, B.R.; Telewski, F.W. Shifts in climate-growth relationships of sky island pines. Forests 2019, 10, 1011. [Google Scholar] [CrossRef] [Green Version]
- Ferrusquia-Villafranca, I.; González-Guzman, L.I.; Cartron, J.E. Northern Mexico’s landscape, Part I: The physical setting and constraints on modeling biotic evolution. In Biodiversity, Ecosystems, and Conservation in Northern Mexico; Cartron, J.E., Ceballos, G., Felger, R.S., Eds.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- National Park Service, U.S. Department of the Interior. Foundation Document, Big Bend National Park, Texas. 2016. Available online: http://www.npshistory.com/publications/foundation-documents/bibe-fd-2016.pdf (accessed on 12 July 2020).
- Poulos, H.M.; Gatewood, R.G.; Camp, A.E. Fire-regimes of the pinyon-juniper woodlands of Big Bend national park, and the Davis Mountains West Texas, USA. Can. J. For. Res. 2009, 39, 1236–1246. [Google Scholar] [CrossRef]
- Instituto Nacional de Ecología. Programa de Manejo del Área de Protección de Flora y Fauna Cañón de Santa Elena México. México, D.F., 1997. Available online: https://simec.conanp.gob.mx/pdf_libro_pm/144_libro_pm.pdf (accessed on 20 July 2020).
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Chicago Press: Chicago, IL, USA, 1968. [Google Scholar]
- Holmes, R.L. Computer-assisted quality in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. Available online: https://repository.arizona.edu/handle/10150/261223 (accessed on 3 June 2020).
- Grissino-Mayer, H.D. Evaluating crossdating accuracy: A manual and tutorial for the computer program COFECHA. Tree-Ring Res. 2001, 57, 205–221. [Google Scholar]
- Melvin, T.M.; Briffa, K.R. A “signal-free” approach to dendroclimatic standardization. Dendrochronologia 2008, 26, 71–86. [Google Scholar] [CrossRef]
- Cook, E.R.; Krusic, P.J.; Melvin, T.; Program RCSigFree. Tree-Ring Laboratory, Lamont Doherty Earth Observatory of Columbia University. 2014. Available online: https://www.ldeo.columbia.edu/tree-ring-laboratory/resources/software (accessed on 15 May 2020).
- Meko, D.; Cook, E.R.; Stahle, D.W.; Stockton, C.W.; Hughes, M.K. Spatial patterns of tree-growth anomalies in the United States and Southeastern Canada. J. Clim. 1993, 6, 1773–1786. [Google Scholar] [CrossRef] [Green Version]
- Stahle, D.W.; Cook, E.R.; Burnette, D.J.; Torbenson, M.C.; Howard, I.M.; Griffin, D.; Villanueva-Diaz, J.; Cook, B.I.; Willimas, A.P.; Watson, E.; et al. Dynamics, Variability, and Change in Seasonal Precipitation Reconstructions for North America. J. Clim. 2020, 33, 3173–3194. [Google Scholar] [CrossRef]
- Becker, A.; Finger, P.; Meyer-Christoffer, A.; Rudolf, B.; Schamm, K.; Schneider, U.; Ziese, M. A description of the global land-surface precipitation data products of the Global Precipitation Climatology Centre with sample applications including centennial (trend) analysis from 1901–present. Earth Syst. Sci. Data 2013, 5, 71–99. [Google Scholar] [CrossRef] [Green Version]
- Schneider, U.; Becker, A.; Finger, P.; Meyer-Christoffer, A.; Ziese, M. GPCC Full Data Monthly Product Version 2018 at 0.5°: Monthly Land-Surface Precipitation from Rain-Gauges built on GTS-based and Historical Data. Glob. Precip. Climatol. Cent. 2018. [Google Scholar] [CrossRef]
- Willmott, C.J.; Matsuura, K. Terrestrial Air Temperature and Precipitation: Monthly and Annual Time Series (1950–1999); Version 1.02; Center for Climatic Research, Department of Geography, University of Delaware: Newark, DE, USA, 2001. [Google Scholar]
- Woodhouse, C.; Lukas, J. Drought, tree rings and water resource management in Colorado. Can. Water Resour. J. 2006, 31, 297–310. Available online: https://www.tandfonline.com/doi/pdf/10.4296/cwrj3104297 (accessed on 20 July 2020). [CrossRef]
- Constante, V.; Villanueva, J.; Cerano, J.; Cornejo, E.; Valencia, S. Dendrocronología de Pinus cembroides Zucc. y reconstrucción de precipitación estacional para el sureste de Coahuila. Rev. Cienc. For. Méx. 2009, 34, 17–39. Available online: http://cienciasforestales.inifap.gob.mx/editorial/index.php/forestales/article/view/685 (accessed on 15 June 2020).
- Dettinger, M.D.; Cayan, D.R.; Diaz, H.F.; Meko, D.M. North-South precipitation patterns in Western North America on interannual-to-decadal timescales. J. Clim. 1998, 11, 3095–3111. [Google Scholar] [CrossRef]
- Pederson, N.; Bell, A.R.; Knight, K.N.; Leland, C.; Malcomb, N.; Anchukaitis, K.J.; Tackett, K.; Scheff, J.; Brice, A.; Carton, B.; et al. A long-term perspective on a modern drought in the American Southeast. Environ. Res. Lett. 2012, 7, 014034. [Google Scholar] [CrossRef]
- Wilder, M.; Garfin, G.; Ganster, P.; Eakin, H.; Romero-Lankao, P.; Lara-Valencia, F.; Cortez-Lara, A.A.; Mumme, S.; Neri, C.; Muñoz-Arriola, F. Climate Change and U.S.-Mexico Border Communities. In Assessment of Climate Change in the Southwest United States: A Report Prepared for the National Climate Assessment; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Maxwell, J.T.; Grant, G.L.; Matheusa, Y.J. Dendroclimatic reconstructions from multiple co-occurring species: A case study from an old growth deciduous forest in Indiana, USA. Int. J. Climatol. 2015, 35, 860–870. [Google Scholar] [CrossRef]
- Poulos, H.M.; Camp, A.E. Vegetation-Environmental Relations of the Chisos Mountains, Big Bend National Park, Texas. USDA Forest Service Proceedings RMRS-P-36. 2005. Available online: https://www.fs.fed.us/rm/pubs/rmrs_p036/rmrs_p036_539_544.pdf (accessed on 10 January 2022).
- Cook, E.R.; Seager, R.; Heim, R.; Vose, R.S.; Herweijer, C.; Woodhouse, C. Megadroughts in North America: Placing IPCC projections of hydroclimatic change in a long-term palaeoclimate context. J. Quat. Sci. 2010, 25, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Mendez, M.; Magaña, V. Regional aspects of meteorological droughts over Mexico and Central America. J. Clim. 2010, 23, 1175–1188. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.K. Dendroclimatology in High-Resolution Paleoclimatology. In Dendroclimatology; Developments in Paleoenvironmental Research; Hughes, M., Swetnam, T., Diaz, H., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 11. [Google Scholar] [CrossRef]
- Torbenson, M.C.; Stahle, D.W.; Howard, I.M.; Burnette, D.J.; Villanueva-Diaz, J.; Cook, E.R.; Griffin, D. Multidecadal modulation of the ENSO teleconnection to precipitation and tree growth over subtropical North America. Paleoceanogr. Paleoclimatol. 2019, 34, 886–900. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.; Meko, D.M.; Swetnam, T.W.; Rausscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a potential driver of regional forest drought stress and tree mortality. Nat. Clim. Change 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Thorne, J.H.; Choe, H.; Stine, P.A.; Chambers, J.C.; Holguin, A.; Kerr, A.C.; Schwartz, M.W. Climate change vulnerability assessment of forests in the Southwest USA. Clim. Change 2018, 148, 387–402. [Google Scholar] [CrossRef] [Green Version]
- Stefanidis, S.; Alexandridis, V. Precipitation and potential evapotranspiration temporal variability and their relationship in two forest ecosystems in Greece. Hydrology 2021, 8, 160. [Google Scholar] [CrossRef]
- Brand, R.; Srur, A.M.; Villalba, R. Contrasting growth trends in Nothofagus pumilio upper-elevation forests induced by climate warming in the Southern Andes. Agric. For. Meteorol. 2022, 323, 109083. [Google Scholar] [CrossRef]
- Proutsos, N.; Tigkas, D. Growth response of endemic black pine trees to meteorological variations and drought episodes in a mediterranean region. Atmosphere 2020, 11, 554. [Google Scholar] [CrossRef]
- Archer, S.R.; Predick, K.I. Climate change and ecosystems of the Southwestern United States. Rangelands 2008, 30, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Pavek, D.S. Pinus cembroides. Fire Effects Information System ; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer), 1994. Available online: https://www.fs.fed.us/database/feis/plants/tree/pincem/all.html (accessed on 28 February 2021).
- CONANP. Programa de Manejo Área de Protección de Flora y Fauna Maderas del Carmen. Secretaría de Medio Ambiente y Recursos Naturales. México, D.F., 2013; p. 151. Available online: https://simec.conanp.gob.mx/pdf_libro_pm/158_libro_pm.pdf (accessed on 15 July 2020).
- Garfin, G.; Jardine, A.; Merideth, R.; Black, M.; LeRoy, S. Assessment of Climate Change in the Southwest United States: A Report Prepared for the National Climate Assessment; A Report by the Southwest Climate Alliance; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Kolb, T.E. A new drought tipping point for conifer mortality. Environ. Res. Lett. 2018, 10, 0311002. [Google Scholar] [CrossRef]
- Waring, K.M.; Reboletti, D.M.; Mork, L.A.; Huang, E.H.; Hofstetter, R.W.; Garcia, A.M.; Fulé, P.Z.; Davis, T.S. Modeling the impacts of two bark beetle species under a warming climate in the Southwestern USA: Ecological and economic consequences. Environ. Manag. 2009, 44, 824–835. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Allen, C.D. Darcy’s law predicts widespread forest mortality under climate warming. Nat. Clim. Change 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Langdon, G.R.; Lawler, J.J. Assessing the impacts of projected climate change on biodiversity in the protected areas of western North America. Ecosphere 2015, 6, 87. [Google Scholar] [CrossRef]
- Stahle, D.W.; Cook, E.R.; Villanueva-Diaz, J.; Fye, F.K.; Burnett, D.J.; Griffin, R.D.; Acuña-Soto, R.; Seager, R.; Heim, R.R., Jr. Early 21st-century drought in Mexico. Eos 2009, 90, 89–90. [Google Scholar] [CrossRef]
- Florescano, E.; Swan, S.; Menegus, M.; Galindo, I. Breve Historia de la Sequía en Mexico; Universidad Veracruzana: Veracruz, Mexico, 1995. [Google Scholar]
- Liverman, D.M. Vulnerability and adaptation to drought in Mexico. Nat. Resour. J. 1999, 39, 99. Available online: https://digitalrepository.unm.edu/nrj/vol39/iss1/7 (accessed on 15 December 2021).
- Endfield, G.H.; Fernández-Tejedo, I. Decades of drought years of hunger: Archival investigations of multiple year droughts in late colonial Chihuahua. Clim. Change 2006, 75, 391–419. [Google Scholar] [CrossRef]
- Nace, R.L.; Pluhowski, E.J. Drought of the 1950’s with Special Reference to the Midcontinent; Geological Survey Wayer Supply Paper 1804; United States Government Printing Office: Washington. DC, USA, 1965. Available online: https://pubs.usgs.gov/wsp/1804/report.pdf (accessed on 15 December 2021).
- Gutzler, D.S. Drought in New Mexico: History, Causes, and Future Prospects. 2003. Available online: https://digitalrepository.unm.edu/eps-fsp/3 (accessed on 12 January 2022).
- Cook, B.I.; Cook, E.R.; Smerdon, J.E.; Seager, R.; Parks, W.A.; Coats, S.; Stahle, D.W.; Villanueva-Diaz, J. North American megadroughts in the Common Era: Reconstructions and simulations. Wiley Interdiscip. Rev. Clim. Change 2016, 7, 411–432. [Google Scholar] [CrossRef] [Green Version]




| Site Name | Site Code | Species | Dated Series | Dating |
|---|---|---|---|---|
| 1 Big Bend | BIGTXPSME | P. menziesii | 101 | 1473–2014 |
| ABCTXABDU | A. durangensis | 17 | 1903–2004 | |
| Maderas del Carmen | MDCMXPSME | P. menziesii | 30 | 1807–2004 |
| MDCMXPIAZ | P. arizonica | 24 | 1810–2001 | |
| MDCMXPICE | P. cembroides | 34 | 1827–2004 | |
| MDCMXABDU | A. durangensis | 17 | 1797–2003 | |
| Santa Elena | ELEMXPICE | P. cembroides | 14 | 1725–2014 |
| Serrania del Burro | SBUMXPIAZ | P. arizonica | 46 | 1798–2015 |
| Sierra Rica | SRIMXPICE | P. cembroides | 53 | 1670–2015 |
| Pajaritos | PAJMXPICE | P. cembroides | 13 | 1850–2014 |
| Santa Elena | ELEMXPICE | P. cembropides | 14 | 1725–2014 |
| Sandillal de San Marcos | SANMXPIAZ | P. arizonica | 18 | 1810–2014 |
| Namiquiapa | NAMMXPICE | P. cembroides | 18 | 1627–2014 |
| Comp | Eigen | PropVar | Cumvar |
|---|---|---|---|
| PC1 | 6.717 | 0.56 | 0.56 |
| PC2 | 1.166 | 0.097 | 0.657 |
| PC3 | 0.963 | 0.08 | 0.737 |
| PC4 | 0.779 | 0.065 | 0.802 |
| PC5 | 0.598 | 0.05 | 0.852 |
| PC6 | 0.433 | 0.036 | 0.888 |
| PC7 | 0.391 | 0.033 | 0.921 |
| PC8 | 0.322 | 0.027 | 0.947 |
| PC9 | 0.193 | 0.016 | 0.963 |
| PC10 | 0.173 | 0.014 | 0.978 |
| PC11 | 0.158 | 0.013 | 0.991 |
| PC12 | 0.109 | 0.09 | 1.000 |
| Chronology | PC1 | PC2 | PC3 |
|---|---|---|---|
| ABCTXABDU | −0.299 | −0.212 | 0.242 |
| BIGTXPSME | −0.271 | 0.372 | 0.171 |
| ELEMXPICE | −0.277 | 0.232 | −0.078 |
| MDCMXABDU | −0.321 | −0.198 | 0.357 |
| MDCMXPIAZ | −0.284 | 0.349 | 0.324 |
| MDCMXPICE | −0.321 | −0.329 | 0.077 |
| MDCMXPSME | −0.329 | −0.296 | 0.164 |
| NAMMXPICE | −0.284 | 0.349 | −0.324 |
| PAJMXPICE | −0.244 | 0.516 | −0.181 |
| SANMXPIAZ | −0.31 | 0.22 | −0.575 |
| SBUMXPIAZ | −0.228 | −0.141 | −0.575 |
| SRIMXPICE | −0.28 | −0.091 | −0.502 |
| 1 Species | ABDU | PSME | PIAZ | PICE |
|---|---|---|---|---|
| ABDU | 1.0 | |||
| PSME | 0.727 | 1.0 | ||
| PIAZ | 0.607 | 0.738 | 1.0 | |
| PICE | 0.567 | 0.731 | 0.729 | 1.0 |
| Tree Ring Series | Precipitation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pAug | pSep | pOct | pNov | pDic | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | |
| ABCTXABDU | 0.19 | 0.31 | 0.34 | 0.28 | 0.29 | 0.29 | 0.35 | 0.07 | 0.46 | 0.56 | 0.24 | 0.20 | 0.02 |
| BIGTXPSME | 0.29 | 0.25 | 0.20 | 0.50 | 0.42 | 0.34 | 0.33 | 0.12 | 0.28 | 0.30 | 0.02 | 0.42 | −0.09 |
| ELEMXPICE | 0.31 | 0.18 | 0.36 | 0.46 | 0.22 | 0.25 | −0.03 | 0.04 | 0.21 | 0.08 | 0.32 | 0.10 | 0.12 |
| MDCMXABDU | 0.11 | 0.29 | 0.32 | 0.45 | 0.33 | 0.33 | 0.30 | 0.08 | 0.43 | 0.39 | 0.31 | 0.15 | −0.02 |
| MDCMXPIAZ | 0.42 | 0.46 | 0.34 | 0.26 | 0.13 | 0.29 | 0.32 | 0.14 | 0.26 | 0.28 | 0.03 | 0.24 | 0.25 |
| MDCMXPICE | 0.26 | 0.35 | 0.41 | 0.33 | 0.24 | 0.32 | 0.30 | 0.14 | 0.32 | 0.39 | 0.17 | 0.28 | 0.09 |
| MDCMXPSME | 0.22 | 0.32 | 0.31 | 0.44 | 0.30 | 0.25 | 0.34 | 0.05 | 0.31 | 0.39 | 0.29 | 0.17 | −0.01 |
| NAMMXPICE | 0.22 | 0.23 | 0.14 | 0.58 | 0.41 | 0.20 | 0.32 | 0.20 | 0.25 | 0.27 | 0.07 | 0.35 | −0.07 |
| PAJMXPICE | 0.26 | 0.21 | 0.32 | 0.51 | 0.25 | 0.01 | 0.17 | −0.01 | 0.19 | 0.27 | 0.21 | 0.34 | 0.05 |
| SANMXPIAZ | 0.32 | 0.35 | 0.34 | 0.52 | 0.43 | 0.22 | 0.21 | −0.07 | 0.35 | 0.38 | 0.30 | 0.32 | 0.07 |
| SRUMXPIAZ | 0.24 | 0.28 | 0.39 | 0.20 | 0.16 | 0.36 | 0.06 | 0.09 | 0.29 | 0.17 | 0.18 | 0.30 | −0.01 |
| SRIMXPICE | 0.50 | 0.49 | 0.45 | 0.47 | 0.17 | 0.18 | 0.31 | 0.02 | 0.27 | 0.16 | 0.19 | 0.44 | 0.10 |
| Temperature | |||||||||||||
| ABCTXABDU | −0.14 | −0.28 | −0.28 | −0.08 | 0.06 | −0.38 | −0.06 | −0.36 | −0.31 | −0.40 | −0.50 | −0.29 | 0.02 |
| BIGTXPSME | −0.13 | −0.19 | −0.25 | −0.41 | 0.02 | −0.34 | −0.22 | −0.40 | −0.33 | −0.44 | −0.26 | −0.25 | −0.02 |
| ELEMXPICE | −0.16 | −0.06 | −0.29 | 0.11 | −0.22 | −0.49 | −0.36 | −0.10 | 0.03 | −0.07 | −0.47 | −0.23 | −0.19 |
| MDCMXABDU | −0.12 | −0.24 | −0.26 | −0.19 | 0.05 | −0.36 | −0.05 | −0.37 | −0.30 | −0.36 | −0.56 | −0.28 | 0.00 |
| MDCMXPIAZ | −0.29 | −0.29 | −0.53 | −0.14 | 0.00 | −0.30 | −0.12 | −0.27 | −0.25 | −0.26 | −0.29 | −0.26 | −0.47 |
| MDCMXPICE | −0.18 | −0.14 | −0.35 | −0.07 | 0.00 | −0.39 | −0.20 | −0.24 | −0.24 | −0.24 | −0.43 | −0.39 | −0.25 |
| MDCMXPSME | −0.16 | −0.24 | −0.34 | −0.05 | −0.06 | −0.46 | −0.14 | −0.36 | −0.31 | −0.28 | −0.52 | −0.33 | −0.04 |
| NAMMXPICE | −0.05 | −0.16 | −0.33 | −0.39 | −0.10 | −0.33 | −0.25 | −0.44 | −0.30 | −0.40 | −0.38 | −0.32 | −0.10 |
| PAJMXPICE | −0.14 | −0.12 | −0.14 | 0.01 | −0.24 | −0.39 | −0.16 | −0.24 | 0.10 | −0.08 | −0.45 | −0.40 | −0.06 |
| SANMXPIAZ | −0.37 | −0.31 | −0.32 | −0.10 | −0.21 | −0.41 | −0.24 | −0.33 | −0.18 | −0.37 | −0.62 | −0.39 | −0.19 |
| SRUMXPIAZ | −0.10 | −0.20 | −0.24 | −0.15 | 0.05 | −0.37 | 0.03 | −0.16 | 0.03 | −0.34 | −0.31 | −0.48 | −0.21 |
| SRIMXPICE | −0.47 | −0.43 | −0.39 | −0.04 | 0.00 | −0.36 | −0.33 | −0.13 | −0.22 | −0.33 | −0.57 | −0.55 | −0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva-Díaz, J.; Stahle, D.W.; Poulos, H.M.; Therrell, M.D.; Howard, I.; Martínez-Sifuentes, A.R.; Hermosillo-Rojas, D.; Cerano-Paredes, J.; Estrada-Ávalos, J. Climate and the Radial Growth of Conifers in Borderland Natural Areas of Texas and Northern Mexico. Atmosphere 2022, 13, 1326. https://doi.org/10.3390/atmos13081326
Villanueva-Díaz J, Stahle DW, Poulos HM, Therrell MD, Howard I, Martínez-Sifuentes AR, Hermosillo-Rojas D, Cerano-Paredes J, Estrada-Ávalos J. Climate and the Radial Growth of Conifers in Borderland Natural Areas of Texas and Northern Mexico. Atmosphere. 2022; 13(8):1326. https://doi.org/10.3390/atmos13081326
Chicago/Turabian StyleVillanueva-Díaz, José, David W. Stahle, Helen Mills Poulos, Matthew D. Therrell, Ian Howard, Aldo Rafael Martínez-Sifuentes, David Hermosillo-Rojas, Julián Cerano-Paredes, and Juan Estrada-Ávalos. 2022. "Climate and the Radial Growth of Conifers in Borderland Natural Areas of Texas and Northern Mexico" Atmosphere 13, no. 8: 1326. https://doi.org/10.3390/atmos13081326
APA StyleVillanueva-Díaz, J., Stahle, D. W., Poulos, H. M., Therrell, M. D., Howard, I., Martínez-Sifuentes, A. R., Hermosillo-Rojas, D., Cerano-Paredes, J., & Estrada-Ávalos, J. (2022). Climate and the Radial Growth of Conifers in Borderland Natural Areas of Texas and Northern Mexico. Atmosphere, 13(8), 1326. https://doi.org/10.3390/atmos13081326








