Abstract
Volatile organic compounds (VOCs) emitted from the paint manufacturing industry include substances that are highly volatile, such as toluene, and highly carcinogenic, such as benzene. In the Republic of Korea, the emission of volatile organic compounds is regulated under the Clean Air Conservation Act, but it is found that individual substances are systematically insufficient. Although the Pollutant Release and Transfer Register (PRTR) is maintained to report the expected emissions from each plant every year, actual measurements are not performed. This study measured and analyzed VOCs at the site fenceline boundary. The ratio of PRTR and VOCs speciation results for xylene and toluene was similar to that of xylene 29% and toluene 28%, but ethylbenzene accounted for 2% in PRTR. Still, the actual measurement result showed a big difference of 11%. Because it is a solvent that is treated in large quantities in the resin manufacturing process and the reactivity of ethylbenzene, it is vaporized at high temperature and high pressure, resulting in many measurements. This study classified a large amount of VOCs emitted through the fence line monitoring system in the paint manufacturing industry and confirmed which VOCs were emitted the most. We compared whether this produced similar results to the actual emission survey method conducted by the EPA. Some substances have produced similar results, but certain substances have significant differences. This indicates that priority VOCs should be selected for each location through continuous measurement.
1. Introduction
The chemical industry is an essential component of the economic growth of many countries worldwide. Most of the products used are manufactured by the chemical industry, affecting the surrounding environment (air, water, soil, etc.). Chemical accidents result in severe adverse effects. In 1976, dioxins leaked into the atmosphere in Seveso, Italy, impacting nearby villages. In 1984, methyl isocyanate (MIC) leaked from a factory in Bhopal, India, and resulted in a serious impact on the surrounding environment and residents. In Korea, hydrogen fluoride leaked in Gumi in 2012, causing damage to residents. As a result, respiratory examinations among residents and health examinations for vulnerable people such as the elderly were conducted [1].
In cases of such grave accidents, local residents realized they have the right to be aware of the dangers of chemicals in chemical industries. In line with this public opinion, the world’s first Emergency Planning and Community Right-to-Know Act (EPCRA) was introduced in the United States in 1986. This law requires that the Toxicity Release Inventory be publicly communicated annually [2].
This system served as an opportunity to continuously reduce the release of chemical substances from industrial sites by disclosing the types of chemicals used, the amounts handled, and the associated risks. This has led to minimal usage of dangerous chemicals, such as carcinogens [3].
This system started in the United States and has spread worldwide. Agenda21 adopted at the “United Nations Conference on Environment and Development” (UNCED) held in Brazil in 1992 included the Environmental Safety Management of Hazardous Chemicals (Chapter 19). The Organization for Economic Cooperation and Development (OECD) made recommendations on the Pollutant Release and Transfer Register (PRTR) in 1996 and also recommended it to its member countries [4]. As a result, OECD member countries worldwide have investigated the chemical substances emitted from their industrial sites and have publicly disclosed information on their risks and handling volumes. When the Republic of Korea joined the OECD in 1996, it legislated the contents of PRTR [5].
PRTR is important because it allows residents the right to information and is also related to their social economic status (SES) [6].
Previous studies on chemical exposure and SES in the United States and Australia have reported that exposure to carcinogenic chemicals in mothers living near chemical industrial complexes is associated with birth defects and miscarriages. Apart from revealing information on the emission of chemical substances to the local community, such studies also indicate the necessity of a policy for companies to reduce the use of toxic chemical substances and highlight policy acceptance by local governments for maintaining good public health [7].
However, the PRTR surveyed at industrial sites has limitations in its current methodology. At present, it is difficult to estimate the amount of chemicals released into the atmosphere accurately. There are four typical methods (emission model method, emission factor method, material balance method, and source testing method) for calculating emissions, considering several other methods cannot be applied to the actual industrial sites owing to differences in application methods [8].
Volatile organic compounds (VOCs) can cause various human health hazards, ranging from carcinogenicity to reproductive toxicity or reacting with atmospheric nitrogen to generate nitrogen oxides resulting in smog. In addition, there is always a risk of scattering leakage from storage facilities, transportation means, or processes other than a fixed emission source resulting in the release of various types of VOCs. Therefore, it is necessary to understand the characteristics of VOCs emitted from specific industries and processes [9].
Fenceline monitoring is a public concern for the environment and the health and safety of the communities surrounding chemical companies. The environmental focus area is air quality. At the same time, CEOs continuously look for innovative ways to improve their air monitoring systems to reduce emissions. Finally, fenceline monitoring is included in the Ministry of the Environment and Climate Change (MOECC) Benzene Technical Standard, which came into effect in July 2016. The standard outlines specific maintenance and operating practices to minimize benzene emissions, including a system for fenceline monitoring of benzene, leak detection and repair (LDAR), wastewater monitoring, tank monitoring and inspection, and product loading limits.
Currently, the United States Environmental Protection Agency (US EPA) has introduced a fenceline monitoring system to measure and disclose the workplace boundary concentration of VOCs, especially benzene (benzene is indispensable, other VOCs are selected by state and business). This makes it easier to track leaks in pipes, flanges, and chemical equipment using atmospheric concentration measurement technology and the chemicals released. Essentially, not all VOC pollutants originate from chimneys; therefore, undetected leaks are a significant source of emissions and can occur in industries, refineries, energy production plants, and natural gas pipelines. The purpose of this system is to measure and analyze the annual emissions, as suggested by the PRTR, detect leaks and emissions more accurately, protect the local community against health hazards, and reduce the company’s operating costs [10].
Since the 1970s, Korea has undergone rapid industrial development, and presently, the distinction between residential areas and industrial complexes is unclear due to unorganized development on narrow lands. Moreover, 50% of the population lives in metropolitan areas and other associated regions, where overcrowding has become a serious issue [11].
The present study measured and analyzed VOCs emitted from business sites. This study aimed to emphasize the necessity of continuous measurement and monitoring by comparing and analyzing the actual measurement results with those of the PRTR, which is currently regulated by law in Korea.
2. Materials and Methods
2.1. Research Subject
2.1.1. Selection of Industries and Process
Two small- and medium-sized paint manufacturers were selected as sites for this study. This is because the paint industry uses different solvents and emits different VOCs. In particular, in Korea, paint companies have more medium-sized businesses than large ones. Various chemicals are used as raw materials, such as toluene, ethylbenzene, xylene, ethyl acetate, and additives. The different stages of paint manufacturing are raw material storage, pipe transfer or raw material input, resin manufacturing (reaction), paint manufacturing (mixing), and air prevention facilities (for example, scrubbers or adsorption towers). VOCs may be emitted at any of these stages throughout each process. For example, in the case of the resin manufacturing process, VOCs generated in the manufacturing plant can be collected using a local ventilation system and emitted as a point source through atmospheric prevention facilities, such as adsorption towers or scrubbers. On the other hand, in the case of pipe transfer and manual raw material input processes, there is a possibility of scattering in the form of an area source owing to the deterioration of flanges or valves attached to the transport pipe. Therefore, it is necessary to analyze and compare the theoretical calculation method, and actual measurement results of VOCs emitted from the entire workplace (Table 1) [12].

Table 1.
Characteristics of VOC pollutants that can be emitted by the process of the research targeting the paint manufacturing industry.
2.1.2. VOCs and Photochemical Ozone Creation Potential (POCP) in Paint Manufacturing
VOCs are a major cause of photochemical smog comprising atmospheric nitrogen oxides because of their high vapor pressure and easy evaporation into the atmosphere. Ethylene, propylene, xylene, and toluene, being representative VOCs, are major precursors for ozone (O3) generation. The degree of VOC contribution to ozone generation is generally affected by the concentration of VOCs and POCP. POCP is determined by the degree of reactivity of VOCs with OH− in the atmosphere, and ethylene is considered a reference substance (POCP = 100). Therefore, the contribution of VOCs to ozone generation is calculated using VOC concentration and POCP as variables [12].
This study selected the top 10 VOCs contributing to ozone generation as comparison targets from 56 VOCs from photochemical atmospheric substances that contribute to ozone generation, as listed in Table 2. This study compared the VOCs emitted during the paint manufacturing process from selected paint manufacturing plants. This shows the VOCs likely to occur in the paint manufacturing industry and the VOCs that need to be reduced first.

Table 2.
Top 10 VOCs with high POCP.
2.2. Methods for Calculating Emissions in the PRTR
VOC emissions are calculated based on the clean air policy support system (CAPSS). Although the calculation differs depending on the characteristics of the emission source, the general formula used is as follows Equation (1):
- Equation (1). Emission calculation method.
The calculation of chemical emissions is centered on point emission sources, especially atmospheric emission facilities, rather than area sources. There are four techniques for calculating VOC emissions. These are the mission factor, material balance, source testing, and mission model methods (Table 3). There is also a method of replacing exhausted gases, such as CO and SOx, but the representative method should be applied according to the following four techniques (Table 4) [13].

Table 3.
PRTR calculation technique applied to each process in a paint plant.

Table 4.
Emission calculation method classification.
2.2.1. Emission Model Method for the Storage Process
Emission calculation methods applicable to storage tanks include engineering calculation and direct measurement methods. In this study, an engineering calculation method was applied. This method estimates the volume of emissions to VOCs when chemicals are stored. This refers to a case in which a chemical is discharged into the atmosphere through a vent by the pressure of the chemical evaporated in the space inside the tank when the chemical is introduced into the storage tank.
- -
- Investigation target of chemical molecular weight X: M (kg/kmol = g/mol)
- -
- Imported volume: V m3 (after inflow) volume (V2) before inflow volume (V1))
- -
- Investigation of target chemical vapor pressure: Po mmHg (at temperature T vapor pressure)
- -
- Annual number of inflows: N times/year
- -
- In tank absolute temperature: TK (= in the tank) Temperature (°C) + 273, Applicable to 293 for atmospheric storage tanks)
- -
- Gas constant: R = 0.082 atm L/(kmol)
- -
- Annual discharge from the storage tank into the atmosphere (kg/year): (M × V × Po × N)/(760 × RT)
The storage tank calculation program included domestic TRIwin and the US EPA TANK. In this study, the domestic program TRIwin version. 2 (Toxic Release Inventory) was used for the calculations. The emission calculation program (hereinafter referred to as the calculation program) is a program developed based on the guidelines to make it easy for companies to quantify chemical emissions in relation to the ‘Regulations on the Investigation of Chemical Emissions and Calculation Coefficient’ announced by the Ministry of Environment [14].
2.2.2. Emission Factor Method of Pipe Transfer and Manual Raw Material Input
The emission factor method includes all processes related to the movement of the chemical substances under investigation that occur within the workplace. It refers to all processes related to flow-through pipes (valves, flanges, process drains, etc.) or transportation of raw materials, materials, products, etc., using small containers, tank lorries, tank trucks, trucks, etc. Direct measurements and emission factor methods are also available for this purpose.
Emissions to the atmosphere through fugitive emission sources of the chemical substances to be investigated during pipe transport through valves, pumps, flanges, pressure safety devices, and sampling ports were calculated by identifying the flanges and number of pumps in the business site using the emission coefficient method. However, the emission factors for petroleum refining and other industries are different (Table 5) [15].

Table 5.
In-piping leaking average emission factor.
2.2.3. Material Balance Method of Resin Manufacturing Process
The material balance method or direct measurement method engineering calculation method can be used for the process of chemical reaction to produce a product [16] Equations (2) and (3).
- − Handling such as yearly purchase amount (kg/year)—A
- − Handling such as yearly residual amount (kg/year)—B
- − Handling gadflies containing the subject of investigation
- − Handling in-reaction by consumed amount (kg/year)—C
- − Handling gadfly contained subject of investigation chemical content—D
- − emissions (kg/year) = (A − B − C) × D
We can calculate emissions from the Korean Ministry of Environment to distribute the Emissions Calculation Worksheet used [15].
- Equation (2). Material Balance Method for Total Emission
= emissions of VOC material; U = material usage (kg); W = VOC content (%); R = VOC retained on the substrate (%); K = control efficiency (%); J = capture efficiency (%); V = VOC content; G = material usage (L). C = VOC content (kg/L)
- Equation (3). Emissions from each VOC containing material.
2.2.4. Emission Model Method for Paint Manufacturing Process
The emission model method is the process of physically mixing raw materials to produce a product. When two or more substances are mixed, the processes for separating each substance correspond can use the emission model method. In the case of paint manufacturing, an emission model method was applied to correspond to a batch process. (Equation (4))
Ex = (annual operating hours × 3600 × A × Kx × Mx × Px × x)/(R × T)
Ex = emission amount of the chemical substance x to be investigated as an air pollutant, kg/year; x = mole fraction of the chemical substance x to be investigated in the liquid phase (0–1); Mx = molecular weight of chemical substance x under investigation, kg/kg -mole; Kx = gaseous mass transfer coefficient of chemical substance x under investigation, m/s; A = surface area of tank m2; Px = vapor pressure of x for pure substances at temperature T; partial pressure of x for mixtures; kPa (partial pressure of x in mixture (Px) is calculated separately); H = time, hour of batch of process/batch; R = gas constant, 8.314 (kPa)(m3)/(kg mol)(K); T = temperature of liquid, K; B = number of mixes per year, batches/year; Kx = 0.00211U0.78 D2/3; D = diffusion coefficient for atmospheric chemicals, cm2/s; U = wind speed, km/h.
- Equation (4). Emission model method calculation formula in batch process.
The diffusion coefficient can be found in chemical engineering handbooks, usually in units of cm2/s.
If the diffusion coefficient D is unknown, it can be determined using the following Equation (5):
- Equation (5). Diffusion coefficient D calculation formula.
2.2.5. Source Testing Method and Material Balance Method of Air Prevention Facility
Most air pollutants generated in each process are treated at air pollution prevention facilities and then discharged into the atmosphere. These facilities treat various dust and chemical substances generated in the factory; for example, centrifugal dust removal, filtration dust removal, electric dust removal, combustion (regenerative thermal oxidizer, etc.), absorption (scrubbers), adsorption (adsorption towers), and cleaning dust removal facilities. Direct measurements and mass balance methods are also applicable.
Most air pollutants are directly measured at air pollution prevention facilities at normal plants using the direct measurement method (Source Testing Method calculation Equations (6) and (7)).
Atmospheric emission (kg/year) = treatment after the amount of exhaust gas (m3/year) × treatment after exhaust gas middle subject of investigation chemical concentration (kg/year/m3)
- Equation (6). Process after the actual value of exhaust gas exists
Atmospheric emission (kg/year) = treatment Jeon amount of exhaust gas (m3/year) × treatment Jeon exhaust gas middle subject of investigation chemistry material concentration (kg/m3) × (1–removal rate)
- Equation (7). Process before of exhaust gas concentration measured value
When the material balance method is used in the air prevention facility, the removal rate of RTO (regenerative thermal oxidizer) is 99.5%. On the other hand, the removal rate of the adsorption tower and the scrubber is 80%.
2.3. Fenceline Monitoring Point Setting Technique
The EPA fenceline monitoring defines a sampler’s location and number to be determined according to the size and shape of the site (sampling at 12 points for 1–750 acres, including at least 4 points; sampling at 18 sites for 750–1500 acres; and sampling at 24 sites over 1500 acres) (Figure 1) [17]. In addition, the test guideline EPA method 325A/B for the measurement and analysis of benzene, a target substance, was also presented [18,19].
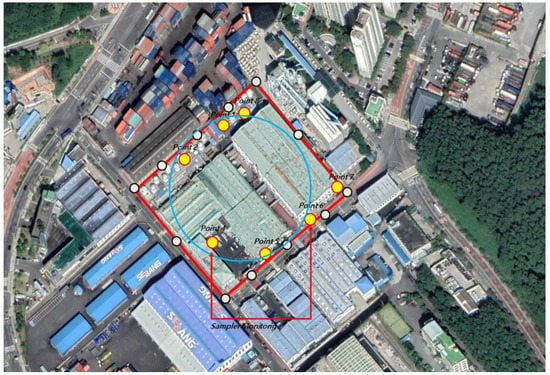
Figure 1.
Linear approach: the regular shape of facilities (Paint Manufacturing business “A”).
If the sub-area is greater than or equal to 750 acres and less than or equal to 1500 acres, it is measured at an angle of 20° from the center and evenly spaced, one for every 20°, with 18 points in total. Unlike the regular-shaped paint manufacturing business “A,” the paint manufacturing business “B” presented a divided structure (Figure 2).
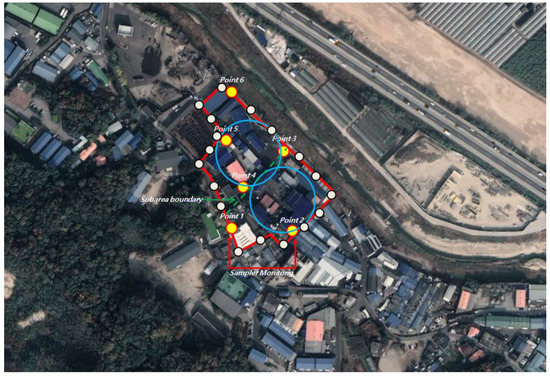
Figure 2.
Angular approach: a facility with two divisions (Paint Manufacturing Plant “B”).
2.4. How to Measure and Analyze Sample in Fenceline Monitoring
The EPA proposed EPA Methods 325A (Volatile organic compounds from fugitive and area sources—Sampler deployment and VOC sample collection) and 325B for measuring and analyzing benzene at the workplace fenceline. The EPA proposed six fenceline monitoring techniques to help refineries with related regulations. These techniques include passive diffusive tube monitoring, active monitoring, ultraviolet differential optical absorption spectroscopy monitoring, open-path Fourier transform infrared spectroscopy, differential absorption light detection and ranging monitoring, and solar occultation flux monitoring. In this study, the active monitoring method was used [19].
2.5. Measurement Point Selection Result and Analysis Method
To measure the concentration of VOCs in two fencelines (A and B) of the paint manufacturing industry considered in this study, the EPA fenceline monitoring technique was partially modified and applied to suit the domestic situation. The process in each workplace was subdivided and measured in compliance with the measurement method.
In the case of the paint manufacturing business A, a 30° angle was measured at the center of the site with an area of 29,812 m2 (2.23 acres). Samples should have been collected from a total of 12 points, one at every 30° interval, but samples were collected at seven points considering the handling process (resin and obstacles). By dividing the process, 35 points were measured and analyzed five times for each of the seven locations. In the case of paint manufacturing business B, samples were collected only at six points for the same reason as in paint manufacturing business A, with an area of 16,097 m2 (1.2 acres). Similarly, 30 points were measured and analyzed five times for each of the six points (Figure 3, Figure 4, Figure 5 and Figure 6).
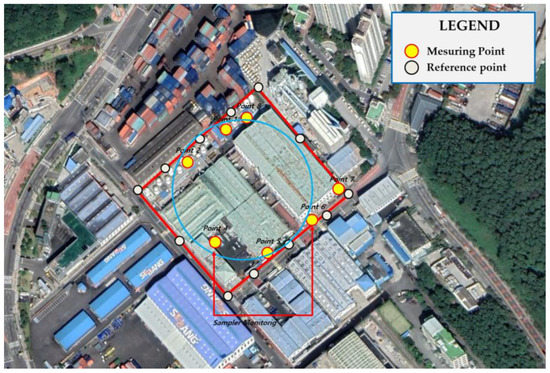
Figure 3.
Measurement location of study site A.
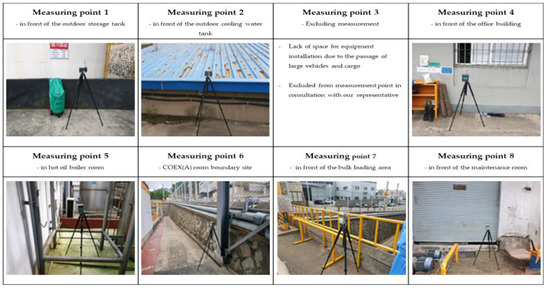
Figure 4.
Installation of measurement equipment for research target study site A.
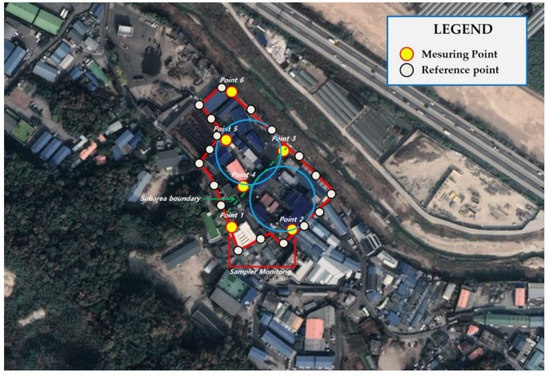
Figure 5.
Measurement location of the site of study B.

Figure 6.
Installation of measurement equipment for research target study site B.
2.6. Analysis Method
Gas phase standards used for VOCs analysis were Retek Ozone Precursor Mixture/PAMS 1 ppm in nitrogen containing 57 ozone precursors and 65 Component Mix 100 ppb in nitrogen for TO-15. Standard dilution and adsorption tube desorption were performed using 99.999% purity nitrogen gas for gaseous standards, and chromatographic analysis was performed using 99.999% pure helium gas. Gas phase standards used for VOCs analysis were Retek Ozone Precursor Mixture/PAMS 1 ppm in nitrogen containing 57 ozone precursors and 65 Component Mix 100 ppb in nitrogen for TO-15. Standard dilution and adsorption tube desorption were performed using 99.999% purity nitrogen gas for gaseous standards, and chromatographic analysis was performed using 99.999% pure helium gas.
For VOC sampling, a sample was collected by actively sampling air using a stainless steel adsorption tube (6.35 mm × 3.5 mm, outer diameter). The adsorption tube used was Carbopack C, Carbopack B, and Carbotrap 300 (Supelco, Bellefonte, PA, USA), containing multiple adsorption layers of Carbosieve S-III. Before using the adsorption tube, impurities were removed for 90 min at 300 °C under the condition that high-purity nitrogen gas was flowing at 100 mL/min using Tube Cleaner CT2000 (Chemtekins, Seoul, Korea) before use. Conditioned tubes were covered with ¼-inch brass long-term storage caps with ¼-inch bonded PTFE ferrule, stored in airtight vials to prevent ambient contamination of the adsorbent, and used for new assays within one week. The sampled adsorption tube was stored at 4 °C until analysis.
For field sampling of VOC, the measurement points were selected according to the US EPA Fenceline monitoring technique. Samples were collected two times in the morning and three times in the afternoon using a sample suction pump. The suction pump used for sampling was MP-Σ30 (SIBATA, Saitama, Japan). The suction flow rate was set to about 200 mL/min, and 6 L of air samples were collected per sample.
To quantify the concentration of VOC, the standard sample impregnation device STD Gas/Liquid Absorber (Chemtekins, Seoul, Korea) was used to impregnate the adsorption tube with the standard mixed gas to prepare a standard adsorption tube for calibration. At this time, the amount of the adsorbed VOC sample was controlled by manipulating the impregnation time while maintaining the flow rate to make it an appropriate concentration level. At this time, the flow rate of the carrier gas (high purity, N2 Gas, purity 99.999%) was maintained in the range of 50 to 100 mL/min. A GC/MS (QP-2020 Plus, Shimadzu, Kyoto, Japan) system connected with an automatic thermal desorption device (TD-20, Shimadzu, Kyoto, Japan) was used to analyze VOC contained in the standard samples and field samples. The operating conditions of each system are shown in Table 6.

Table 6.
Operating conditions of thermal desorption and GC/MS for VOC analysis.
2.7. Quality Control
In order to evaluate the overall performance of the adsorption sampling method and thermal desorption/GC-MS analysis method used for VOC measurement, the method detection limits (MDL) of the sample, the reformation, and the linearity and correlation of the calibration curve were evaluated. The MDL estimation method uses the standard deviation (SD) of each substance’s measured concentration after performing repeated analyses at least seven times for a standard substance with a low concentration of 3–5 times the detection limit of the device. As a result of estimating MDL, it was found to be in the range of 0.06 to 0.16 ug/m3 for most VOCs, assuming 6 L, which is the average value of air collection. In addition, the lowest calibration level for each compound was used as the instrumental limit of quantitation (LOQ) for that compound. The reproducibility of the analysis method using GC/MS was evaluated by the relative standard deviation (RSD) of the standard mixed sample, and the relative standard deviation (%RSD) was between 0.4% and 5.0% (n = 5). The recovery rate of all items was more than 95%. In order to evaluate the linearity of the VOC material to be measured, the standard gas was impregnated with different impregnation times, and the R2 values were all 0.99 or higher, indicating very good linearity.
3. Results and Discussion
3.1. Measurement Results and Analysis with Chromatogram
The analyzed VOCs were analyzed for each hour measurement result. As a result of the analysis, no target chemicals were detected in the blank, as shown in the chromatogram below (Figure 7). Figure 8 shows this study’s chromatogram of the standard material (toluene, ethylbenzene, styrene, xylene). Representative chromatograms of the time detected at Business Sites A and B are shown in Figure 9 and Figure 10. In this study, the average concentration of each measurement point was applied to the boundary of the entire business site, and the types and composition ratios of VOCs emitted in large quantities in the paint manufacturing industry were compared. Therefore, Table 7 shows the average value and standard error range of the measured concentrations of all measurement points for each time.
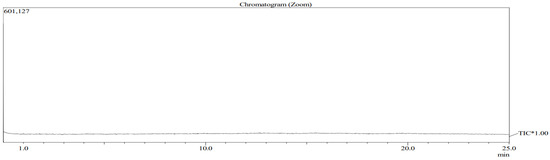
Figure 7.
Chromatogram of blank analysis.
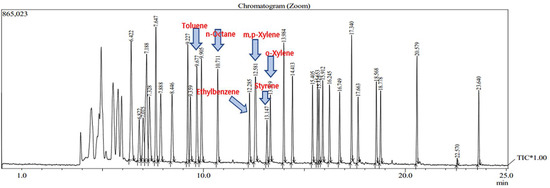
Figure 8.
Chromatogram of standard material analysis.
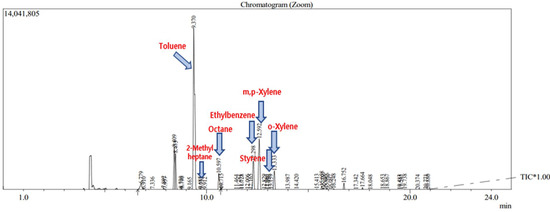
Figure 9.
The chromatogram analyzing the 3–4 pm measurement point, the measuring point 5 of Business site A.
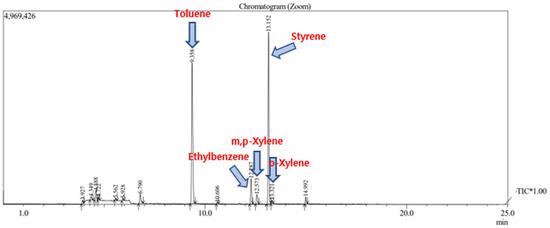
Figure 10.
The chromatogram analyzing the 10–11 am measurement point, the measuring point 4 of Business site B.

Table 7.
VOC speciation concentration by businesses A and B.
The composition ratio was analyzed rather than the measured concentration to determine the ratio between the values calculated by PRTR and the measured VOCs. The limits of the current VOC management in Korea were derived. This was done to analyze the VOCs that greatly affect ozone generation among these VOCs. The concentration at the site is not the concentration to which workers are continuously exposed inside the workplace but the concentration at the fence line. Thus, benzene was 9 µg/m3, but it was not detected in this study. But, regulatory concentrations for other substances have not been established. Some states, such as California, provide lists of substances to be measured and reference concentrations. This study did not detect benzene in the paint manufacturing industry [20].
However, the emission of substances with high POCP values, such as xylene, toluene, and ethylbenzene, must be managed because they contribute significantly to ozone generation [21].
PRTR calculates annual emissions. Accordingly, the average value of all measured points results was converted into a ratio and compared with the ratio of annual emissions.
As a result of measurement and analysis, the composition ratio of xylene (29.8%), toluene (28.6%), ethylbenzene (10.9%), and methylcyclohexane (10%) was measured at the paint manufacturing site A. As a result of measurements at paint manufacturing site B, toluene (61.9%), styrene (21.1%), ethylbenzene (6.02%), xylene (3.66%), and n-octane (1.3%) were detected (Table 8).

Table 8.
VOCs composition ratio by business site as a result of measurement and analysis.
3.2. Comparative Analysis of PRTR and Actual Measurement Results
In addition to the average composition ratio of all business sites, the PRTR data for each process and the process near the measurement point were calculated and compared. Materials used and discharged at paint manufacturing plant A (foreign company) included xylene, toluene, and methylene diphenyl diisocyanate (MDI). After determining the composition ratio based on the actual measurement results, xylene (29%), toluene (28%), and ethylbenzene (11%) were detected. In the case of MDI, the values were unknown because they were excluded from this direct measurement target; however, similarities with the PRTR results were confirmed for xylene and toluene.
From comparison among all business sites, composition ratios of xylene (55%), toluene (15%), and MDI (13%), which are used as major solvents, were observed. The emission results modeled by PRTR were xylene (40%), toluene (50%), and MDI (4%), which showed differences in the ratio in this order. These differences seemed higher than that in the modeling program because toluene has a smaller molecular weight and a larger vapor pressure than xylene and thus, has high volatility.
The substances used and their emissions from Plant B include styrene, toluene, ethylbenzene, ethyl acetate, and xylene. By checking the composition ratio based on actual measurement results, toluene (62%), styrene (21%), ethylbenzene (6%), and xylene (4%) were detected. The emission results for ethyl acetate (53%), toluene (26%), and xylene (12%) were similar to those for toluene and xylene. Paint manufacturing plant B manufactures by ordering paint from a large company rather than its own brand, and the process operation is not constant; thus, the emission result seems to be related to the product manufactured at the workplace on the day of measurement.
Workplace B specializes in the manufacturing of paints, especially for architectural interiors. Accordingly, the PRTR modeling data and annual usage data were compared. Styrene (50%), toluene (19%), ethylbenzene (17%), ethyl acetate (8%), and xylene (5%) were used as solvents at the workplace. The modeling results showed that the PRTR was 53% ethyl acetate, 26% toluene, and 12% xylene. As a result of the analysis of all business sites, toluene accounted for 62%, followed by styrene (21%) and ethylbenzene (6%) (Figure 11 and Figure 12).
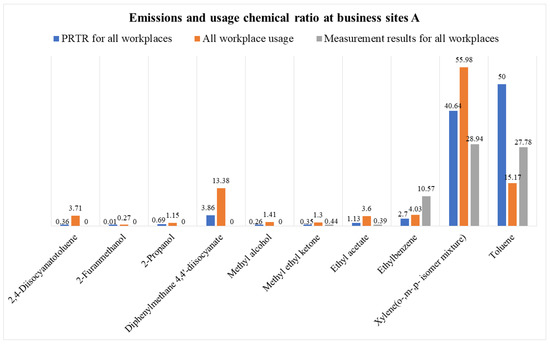
Figure 11.
Emissions and usage chemical ratio at business sites A.
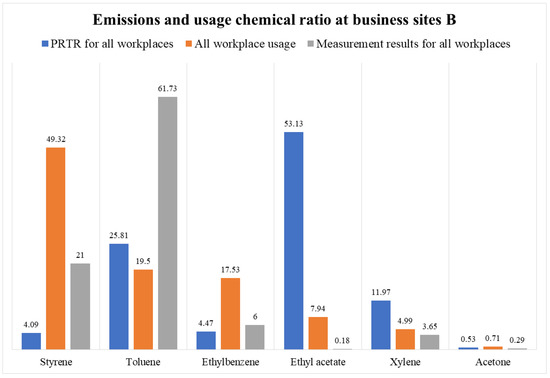
Figure 12.
Emissions and usage chemical ratio at business sites B.
3.3. Characteristics and Management Plan of VOCs in the Paint Manufacturing Industry
According to previous studies before this study, in the case of the petroleum refining industry, the PRTR detailed report has a variety of emissions by process, so there is a limit to the calculation for each measurement point. In the paint manufacturing industry, which is the measurement target of this study, after obtaining detailed drawings and PRTR results for each facility, the theoretical emission and actual measured values were compared by comparing the total amount of emission and the composition of the emission and measurement results for each measurement point.
Business A specialized in manufacturing paints, especially for ships and automobiles. The chemical substances used were cross-analyzed by comparing the PRTR data and annual usage data. As a result, the zone with a particularly low emission concentration was the 6th measurement point between the semi-finished resin and paint manufacturing processes.
The 6th measurement point is the process of producing a secondary polymer resin by reacting the semi-finished resin in the reactor. In this process, the amount of VOCs concentration generated was low because the main component was high molecular compounds rather than solvents.
The actual measurement results detected xylene (30%), toluene (29%), and ethylbenzene (11%). The ratio of xylene to toluene was similar to that in the PRTR modeling method, but there was a significant difference in terms of ethylbenzene. This is because ethylbenzene has a relatively small effect on PRTR modeling owing to its high molecular weight and low volatility. This aspect needs to be confirmed through further measurement and analysis. In particular, as MDI was excluded from the VOC measurement target, its level could not be confirmed.
The present study had limitations with regard to manufacturing site B, which is related to the products manufactured on the day of measurement at the work site. Products manufactured in the workplace differ according to industry characteristics. Rather than owning its own paint brand, site B manufactures by ordering paints from large companies after receiving product orders. Therefore, on the day of the measurement, resin or resin paint for semi-finished products was manufactured using the resin process. As a result, it seemed that large amounts of styrene and toluene used in the resin process were detected.
In particular, because of analysis based on the measurement result chromatogram, toluene was discharged at a high concentration, but in the case of ethyl acetate, the analysis concentration was low. However, since the amount of ethyl acetate used in Business B is high, this point needs to be followed up. However, in the case of ethyl acetate, it was found that the process used in Business B mainly manufactures paints. This indicates that it is important to calculate the emission by considering the process that treats temperature and pressure as high rather than simply estimating the emission through the amount of consumption.
As a result of inquiries at business A, it is estimated that a lot of paint was produced in the first half of the year; hence, ethyl acetate, xylene, methyl ethyl ketone, and styrene (resin, common materials for paints) were used. In the second half of the year, mainly semi-finished resins were manufactured. According to the results of this study, it seemed that toluene and styrene ethylbenzene were generated in large amounts, depending on the process operation characteristics on the particular day of measurement. In addition, the area of the business site was small, and the resin and paint processes were performed close to each other, which could cause significant interference in the characteristics. In particular, as a result of the analysis of the resin and paint manufacturing processes, similar composition ratios were shown in the order of toluene, styrene, ethylbenzene, and xylene. The analysis results for each process were toluene (73%), styrene (16%), ethylbenzene (5%), and xylene (3%) in the resin manufacturing process, whereas that in the paint manufacturing process were toluene (54%), styrene (24%), ethylbenzene (8%), and xylene (4%). This is believed to be the reason for the semi-finished resin manufacturing process at the factory on measurement day. However, the annual consumption and PRTR results showed that during the resin manufacturing process, the values were as follows: styrene (56%), toluene (20%), and ethylbenzene (18%). In the paint manufacturing process, 52% ethyl acetate, 26% toluene, and 12% xylene were used.
The emission characteristics of VOCs vary with the characteristics of the industry, suggesting that it is more important to understand the types of products produced annually at the workplace rather than daily measurements and analysis results. In addition, according to the actual measurement results of the paint manufacturing industry, the material with the highest POCP was ethylbenzene, followed by toluene. The actual measurement result and PRTR result for xylene showed a similar ratio; however, there was a significant difference for ethylbenzene. The composition ratio of ethylbenzene was higher than that of the PRTR. This can be seen as underestimating the generation of secondary air pollution products in terms of PRTR management, according to the current regulatory policy. It can be inferred that substances with high POCP require additional regulation in the process or at the point of their discharge in large quantities. Continuous measurement at each business site makes it possible to replace solvents with high POCP with those with low POCP.
In this study, VOCs that occur in large amounts in the paint manufacturing industry were measured, and the results were compared with PRTR. Substances such as xylene were like PRTR. There was a significant difference between ethylbenzene and ethyl acetate. These substances may be excluded from reduction measures in the workplace. This study compared the results with measurements 5 times a day. This needs to be supplemented through continuous measurement.
4. Conclusions
This study was conducted at the fenceline, targeting two small- and medium-sized paint manufacturing sites in different regions of the Republic of Korea. Differences in emission values were determined by comparing the measured results and theoretically calculated emissions based on the PRTR system. VOCs are diverse compounds containing substances such as benzene, which are highly carcinogenic; some VOCs exhibit high air pollution potential through secondary product generation due to their high POCP. Although the current system regulates the emission of VOCs, the emission concentration of air pollutants is only controlled by the chimney; furthermore, emission of individual VOCs is still unregulated.
In the United States, beyond the total VOC management regulations, VOCs with high carcinogenicity, such as benzene, or high POCP substances, such as ethylene, propylene, xylene, and toluene, are analyzed at the point source of emission in the chimneys and the fencelines; real-time monitoring is being performed to determine the types of pollutants generated. Chemicals mainly used in workplaces are selected and tracked based on: (1) hazardous effect on the human body, (2) high risk of air pollution, and (3) high durability and persistence [22].
Therefore, it is necessary to continuously monitor VOCs as they can directly or indirectly affect the workplace and nearby local communities and characterize and regulate their emissions. Rather than comprehensively regulating individual workplaces, it is necessary to regulate them considering the characteristics of industrial complexes. Therefore, this study emphasizes the necessity and feasibility of applying the fenceline monitoring method to regulate VOC emissions at domestic workplaces.
Author Contributions
M.-G.K., J.Y.L. and K.W.M. conducted and designed the study; H.E.L. performed data processing and coding. J.H.K. and S.H.C. analyzed the results. J.U.Y. and M.-G.K. wrote the manuscript. C.W.K. modeled the PRTR. H.E.L. and J.H.K. were involved in the analysis modeling program and chemicals; K.W.M. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by the Ministry of Environment (MOE) of Korea under the title “Establishment of VOCs specification by emission source (II)” (Project no. NIER-2020-01-02-097).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kleindorfer, P.R.; Belke, J.C.; Elliott, M.R.; Lee, K.; Lowe, R.A.; Feldman, H.I. Accident epidemiology and the US chemical industry: Accident history and worst-case data from RMP* Info. Risk Anal. Int. J. 2003, 23, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Falkenberry, E.M. The Emergency Planning and Community Right-to-Know Act: A Tool for Toxic Release Reduction in the 90’s. Buff. Envtl. LJ 1995, 3, 1. [Google Scholar]
- Konar, S.; Cohen, M.A. Information as regulation: The effect of community right to know laws on toxic emissions. J. Environ. Econ. Manag. 1997, 32, 109–124. [Google Scholar] [CrossRef]
- Hong, J.-S.; Kim, K.-Y.; Kwon, O.-S. A Study on Integrated Approaching Factors of Environmentally-Friendly Companies Certification Scheme and Environmental Impact Assessment of Korea. J. Environ. Impact Assess. 2008, 17, 113–124. [Google Scholar]
- MoE. Chemical Substances Control Act; Article 11 (Pollutant Release and Transfer Registers). Available online: https://elaw.klri.re.kr/kor_service/lawView.do?hseq=55950&lang=ENG (accessed on 15 March 2022).
- Park, J. Environment and health: An overview of current trends at WHO and OECD. J. Environ. Health Sci. 2013, 39, 299–311. [Google Scholar] [CrossRef]
- Currie, J.; Schmieder, J.F. Fetal exposures to toxic releases and infant health. Am. Econ. Rev. 2009, 99, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Huh, E.-H.; Yoon, Y.; Yoon, S.J.; Huh, D.-A.; Moon, K.W. Valuation of estimation toxic chemical release inventory method-focusing on paint manufacturing process. Int. J. Environ. Res. Public Health 2019, 16, 3260. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Henk, J.; Bloemen, T. Chemistry and Analysis of Volatile Organic Compounds in the Environment; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- EIP. Monitoring for Benzene at Refinery Fencelines. Available online: https://environmentalintegrity.org/reports/monitoring-for-benzene-at-refinery-fencelines/ (accessed on 15 March 2022).
- Lee, H.E.; Sohn, J.-R.; Byeon, S.-H.; Yoon, S.J.; Moon, K.W. Alternative risk assessment for dangerous chemicals in South Korea regulation: Comparing three modeling programs. Int. J. Environ. Res. Public Health 2018, 15, 1600. [Google Scholar] [CrossRef] [PubMed]
- Song, B.-J.; Lee, S.-M.; Cho, G.-J.; Cho, J.-G.; You, P.-J.; Kim, G.-G. VOC/HAPs emission characteristics & adsorption evaluation for paint products in Busan area. J. Korean Soc. Environ. Eng. 2012, 34, 316–325. [Google Scholar]
- Yu, B.-G.; Park, S.-H.; Jeong, S.-G.; Ham, Y.-S.; Lee, B.-G. A Study on Concentrations Characteristics of Carbonyl Compounds in Ulsan. In Proceedings of the Korea Air Pollution Research Association Conference, Incheon, Korea, 13–15 May 2010; pp. 438–440. [Google Scholar]
- USEPA. Preferred and Alternative Methods for Estimating Air Emissions from the Printing, Packaging, and Graphic Arts Industry. Available online: https://dep.wv.gov/daq/planning/inventory/Documents/EIIP%20V02%20Ch15%20Printing%20Packaging%20and%20Graphic%20Arts.pdf (accessed on 15 March 2022).
- USEPA. Tanks Emissions Estimation Software, Version 4.09D. Available online: https://www.epa.gov/air-emissions-factors-and-quantification/tanks-emissions-estimation-software-version-409d (accessed on 15 March 2022).
- NICS. Guidelines for the Investigation of Chemical TRI Emissions; NICS: Southaven, MS, USA, 2019; pp. 13–41. [Google Scholar]
- NICS. Guidelines for Estimating TRI Emissions of Volatile Substances; NICS: Southaven, MS, USA, 2013; pp. 4–75. [Google Scholar]
- 40 CFR § 63.658-Fenceline Monitoring Provisions. Available online: https://www.law.cornell.edu/cfr/text/40/63.658 (accessed on 15 March 2022).
- USEPA. Method 325A—Volatile Organic Compounds from Fugitive and Area Sources: Sampler Deployment and VOC Sample Collection. Available online: https://www.epa.gov/sites/default/files/2019-08/documents/method_325a.pdf (accessed on 15 March 2022).
- Rule 364 Refinery Fencline Monitoring Plan Guidelines. Available online: https://documents.pub/document/rule-364-refinery-fenceline-and-community-air-2020-9-25-all-fenceline-air.html (accessed on 29 July 2022).
- Derwent, R.; Jenkin, M. Hydrocarbons and the long-range transport of ozone and PAN across Europe. Atmos. Environ. A Gen. Top. 1991, 25, 1661–1678. [Google Scholar] [CrossRef]
- DeWees, J.M. Refinery fenceline monitoring & method 325A/B. In Proceedings of the National Air Toxics Monitoring and Data Analysis Workshop, Research Triangle Park, NC, USA, 28 October 2015. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).