Abstract
Carbazole is one of the typical heterocyclic aromatic compounds (NSO-HETs) observed in polluted urban atmosphere, which has become a serious environmental concern. The most important atmospheric loss process of carbazole is the reaction with OH radical. The present work investigated the mechanism of OH-initiated atmospheric oxidation degradation of carbazole by using density functional theory (DFT) calculations at the M06-2X/6-311++G(3df,2p)//M06-2X/6-311+G(d,p) level. The rate constants were determined by the Rice–Ramsperger–Kassel–Marcus (RRKM) theory. The lifetime of carbazole determined by OH was compared with other typical NSO-HETs. The theoretical results show that the degradation of carbazole initiated by OH radical includes four types of reactions: OH additions to “bend” C atoms, OH additions to “benzene ring” C atoms, H abstractions from C-H bonds and the H abstraction from N-H bond. The OH addition to C1 atom and the H abstraction from N-H bond are energetically favorable. The main oxidation products are hydroxycarbazole, dialdehyde, carbazolequinone, carbazole-ol, hydroxy-carbazole-one and hydroperoxyl-carbazole-one. The calculated overall rate constant of carbazole oxidation by OH radical is 6.52 × 10−12 cm3 molecule−1 s−1 and the atmospheric lifetime is 37.70 h under the condition of 298 K and 1 atm. The rate constant of carbazole determined by OH radical is similar with that of dibenzothiophene oxidation but lower than those of pyrrole, indole, dibenzofuran and fluorene. This work provides a theoretical investigation of the oxygenated mechanism of NSO-HETs in the atmosphere and should help to clarify their potential health risk for determining the reaction pathways and environmental influence of carbazole.
1. Introduction
Heterocyclic aromatic compounds (NSO-HETs) are important contaminants characterized by the presence of fused aromatic rings, which pose a great threat to the environment and human health due to their genotoxicity, mutagenesis potential, and carcinogenicity [1,2,3,4]. Carbazole, one of the typical nitrogen-substituted NSO-HETs, has been extensively applied in pesticides, dyes, lubricants, detergents, and pharmaceutical manufacturing [5,6]. The main sources of carbazole include waste incineration [7], biomass burning [8], tobacco smoke [9], synthetic dye production [10], aluminum manufacturing and rubber [11], and oil and coal burning [12]. Carbazole can be considered as a one-nitrogen-substituted dibenzofuran, which exhibits mutagenicity, toxicity, and adverse effect on humans. Therefore, it can be viewed as a dioxin-like compound and listed as a second-class carcinogen by the World Health Organization [9,13,14]. Given these harmful consequences, obtaining detailed insight into the degradation of carbazole in the atmosphere will deepen our understanding of the degradation mechanisms of dioxin-like compounds and could lead to emission-reduction and dioxin-control strategies.
At present, carbazole has been constantly detected in atmospheric samples, river sediments, groundwater, and contaminated sites [5]. In the atmosphere, carbazole can cause the formation of nitrogen oxides by burning, which can accelerate the formation of acid rain and the destruction of the ozone layer [15,16]. The measurement by Esen et al. in Bursa showed that the average gas and particle-phase concentrations of carbazole were 7.6 ± 9.9, 1.1 ± 1.2, 3.3 ± 5.0, and 1.2 ± 0.7 ng m−3 in residential, traffic, industrial and campus areas, respectively [5]. Air samples collected from coal burned indoors showed that the maximum concentration of carbazole in the air was up to 7500–20,168 ng m−3 [3,9]. Heim et al. measured the concentration of carbazole in an undisturbed sediment core collected from the Lippe River in Germany, ranging from 11 to 172 ng g−1 [17]. Carbazole can undergo diverse chemical reactions and long-distance transport in the atmosphere, which may induce more complex contamination in remote sites.
In the troposphere, carbazole can be removed by dry and wet subsidence as well as photochemical reactions, and the main sinks are the oxidation reactions with major oxidants such as OH radical, NO3 radical, and O3. Among these oxidants, OH radical [18] accounts for a quite high proportion in determining the oxidation power of the atmosphere, especially in the daytime [19]. Reaction with OH radical is considered to be the main chemical process for carbazole in the atmosphere [20]. Therefore, it is necessary to investigate the reaction mechanism of carbazole with OH radical to clarify its atmospheric transformations. To date, only one study had been conducted to remove carbazole initiated by OH radical [21]. The reaction mechanism has not been fully elucidated. Clearly, further studies are needed to obtain more comprehensive knowledge of carbazole oxidation by OH radical.
Due to the lack of efficient detection schemes for intermediate radical species, a full analysis of the atmospheric processes of the reaction mechanism of OH-initiated atmospheric oxidation of carbazole is limited in the laboratory studies, which hinders to further evaluate the atmospheric effect of carbazole. Therefore, in this paper, with the aid of the density functional theory (DFT) method, a comprehensive degradation mechanism of carbazole initiated by OH radical in the presence of O2 and NO was carried out. The main products and reaction pathways were determined. Additionally, the rate constants for the vital reactions of carbazole degradation by OH radical were calculated by RRKM theory, and the lifetimes were evaluated [22,23]. The obtained rate constants and lifetime were compared with the literature values of other NSO-HETs (pyrrole/indole/dibenzothiophene/dibenzofuran/fluorine) degradation by OH radical [24,25,26,27,28,29]. The influences of steric structure and element substitution and of NSO-HETs on the lifetimes were discussed. This work provides a theoretical investigation of the oxygenated mechanism of NSO-HETs in the atmosphere and should help to clarify their potential health risk for determining the reaction pathways and atmospheric influence of carbazole.
2. Computational Detail
2.1. Thermodynamic Calculation
All the calculations on the geometries, energies and frequencies for reactants, complexes, transition states, and products were determined using the Gaussian 09 program package [30]. Geometrical optimizations and vibrational frequency calculations were carried out using the M06-2X functional method with a 6-311+G(d,p) basis set [31]. The vibrational frequency calculations were used to determine the nature of each species and provide the zero-point energy (ZPE) values. Transition states have exactly one imaginary frequency, whereas reactants, intermediates, and products have real frequencies. The minimum energy path (MEP) was obtained by the Intrinsic Reaction Coordinate (IRC) calculation to confirm the connections between transition states and the corresponding energy minima along the reaction path [32]. In order to obtain more reliable energy results, based on the geometries obtained at 6-311+G(d,p) level, the single-point energies were calculated by using the more accurate basis set 6-311++G(3df,2p). The potential barriers are the energy differences between transition states and reactants, and the reaction enthalpies are the energy differences between products and reactants. All the relative energies that were quoted and discussed in this paper include zero-point energy (ZPE) corrections.
2.2. Kinetic Calculation
Rice–Ramsperger–Kassel–Marcus (RRKM) theory [22,23] was carried out to calculate rate constants of the crucial elementary reactions discussed in the present work by means of the MESMER program [33]. The RRKM rate constant is given by:
where W(E) is the rovibrational sum of states at the transition state, ρ(E) is the density of states of reactants, and h is Planck’s constant. Then, the canonical rate constant k(T) is determined from the usual equation:
where Q(T) is the reactant partition function, k(E) is the rate constant in a microcanonical ensemble with energy E and kB is Boltzmann’s constant, T is temperature. The collisional parameters are estimated according to Gilbert and Smith [34]. The bath gas is set as nitrogen. The default pressure is 1 atm and the temperature is 298 K. The exponential-down model is assumed for the collisional energy transfer with <△E down> of 250 cm−1.
3. Results and Discussion
The reliability and accuracy of the M06-2X/6-31+G(d,p) level for the geometry parameters of carbazole have been compared with experimental and other calculated values are listed in Table S1 of Supplementary data [35,36]. The optimized geometries of carbazole at the M06-2X/6-31+G(d,p) level are in reasonable accordance with the corresponding experimental values, and the largest discrepancy remains within 4.5% and 2.3% for the bond lengths and bond angles, respectively, except two abnormal values [36]. The optimized geometries of carbazole at the M06-2X/6-31+G(d,p) level match better with the calculated values on the MP2/6-31G* level and the largest discrepancy remains within 0.5% for both bond lengths and bond angles. Typical transition states involved in the degradation of carbazole initiated by OH radical are shown in Figure 1. Imaginary frequencies, zero-point energies, total energies, and cartesian coordinates for the transition states involved in the oxidation reaction of carbazole initiated by OH radical are depicted in Tables S2 and S3 of Supplementary data.
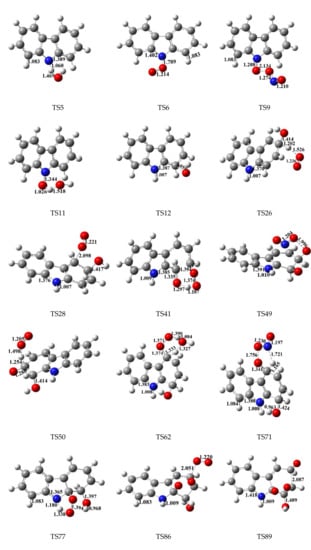
Figure 1.
M06-2X/6-311+G(d,p) optimized geometries for typical transition states involved in the degradation of carbazole initiated by OH radical. Distances are in angstroms. Gray sphere, C; Blue sphere, N; Red sphere, O; White sphere, H.
3.1. Reaction of Carbazole and OH Radical
The molecular structures of carbazole are depicted in Figure 2, from which the C atoms in carbazole are numbered. There are C-H, N-H, and C=H bonds in carbazole. Thus, the reaction pathways of carbazole with OH include H abstractions from C-H or N-H bonds and OH additions on C=C bonds. The OH addition and H abstraction scheme of carbazole embedded with the potential barriers ΔE (in kcal mol−1) and reaction heats ΔH (in kcal mol−1, 0 K) are presented in Figure 2.

Figure 2.
The OH addition and H abstraction reaction scheme of carbazole embedded with the potential barriers ΔE (in kcal mol−1) and reaction heats ΔH (in kcal mol−1, 0 K).
Nine H abstraction pathways of carbazole initiated by OH radical were identified: one H abstraction from the N-H and eight H abstractions from the C-H in benzene rings (C1-H, C2-H, C3-H, C4-H, C5-H, C6-H, C7-H, and C8-H bonds). Owning to carbazole molecule belongs to the C2V symmetrical structure, C1 and C8, C2 and C7, C3 and C6, and C4 and C5 are equivalent. Therefore, only one benzene ring of carbazole is discussed. As shown in Figure 2, at the M06-2X/6-311++G(3df,2p)//M06-2X/6-311+G(d,p) level, all the four C-H abstractions have the potential barriers in the range from 3.06 to 3.66 kcal mol−1, and are exothermic by −5.12 ~ −6.30 kcal mol−1. The H abstraction from the N-H bond is barrierless and is exothermic by 24.13 kcal mol−1, which is more favorable than those from C-H bonds. Thus, in the abstraction reactions, the N-H abstraction is the most favorable reaction pathway, resulting in the formation of intermediate IM5. Only the subsequent reactions of IM5 intermediate are further discussed.
OH radical can also add to the C atoms in the benzene ring of the carbazole molecule. C10, C11, C12, and C13 are inside the “bend” of the carbazole molecule, which can be called the “bend” C atoms. C1, C2, C3, C4, C5, C6, C7, and C8 are in the benzene ring and out of the “bend” of the carbazole molecule, which can be called the “benzene ring” C atoms. Considering the C2V symmetry, six different OH-carbazole adduct isomers are labeled as IM12-IM17 in Figure 2, which can be readily formed through the OH additions to the C1, C2, C3, C4, C10, and C11 atoms. In Figure 2, the OH additions to “benzene ring” C atoms are highly exothermic (−22.30 ~ −14.67 kcal mol−1) with low potential barriers (−1.19 ~ 1.18 kcal mol−1). However, the potential barriers of OH additions to “bend” C atoms are 2.51 and 3.55 kcal mol−1, which are higher than those to C1, C2, C3, and C4 atoms. In addition, OH additions to “bend” C atoms are less exothermic (−11.90 ~ −8.40 kcal mol−1) than those to “bend” C atoms. Thus, OH additions to “benzene ring” C atoms are energetically more favorable than those to “bend” C atoms. The OH additions to these C9 and C10 atoms are sterically hindered and energetically unfavorable. In the “benzene ring” C atoms, the OH addition reaction to the C1 atom has the lowest potential barrier and is the most exothermic reaction among those to other C atoms, resulting in the formation of IM12. In Figure 2, the H abstraction reaction from the N-H bond and the OH addition to C1 atoms have similar potential barriers and reaction heats, which indicates the two reactions are competitive.
Comparison of the OH additions to “benzene ring” C atoms with the H abstractions from C-H bonds shows that the OH additions to “benzene ring” C atoms have lower potential barriers and higher heat releases than the H abstractions from C-H bonds. Thus, the OH additions to “benzene ring” C atoms are energetically more favorable than the H abstractions from C-H bonds. This agrees well with the experimental pattern that the reaction of aromatic compounds with OH is mainly through the OH addition to “benzene ring” C atoms (approximately 90 percent) rather than the H abstraction from C-H bonds, such as in the case of naphthalene [37], anthracene [38], and phenanthrene [39,40]. Hence, the H abstractions in benzene rings are not considered further in the subsequent reactions for the degradation of carbazole under general atmospheric conditions.
3.2. Subsequent Reaction
The intermediates produced by the abstraction and addition reactions of carbazole with OH radical contain unpaired electrons and can subsequently react with O2/NO in the atmosphere.
3.2.1. Reactions with O2
Figure 3 shows the reaction pathways of carbazole-OH adducts (IM12-IM15) with O2. Firstly, carbazole-OH adducts can react via a simple metathesis mechanism to yield hydroxycarbazoles and HO2 radical through H abstractions from the C-H bonds. The potential barriers of these hydroxycarbazole formation processes are in the range of −33.66 ~ −29.03 kcal mol−1. The processes are strongly exothermic by −68.78 ~ −62.43 kcal mol−1. The overall reactions, carbazole + OH + O2 → hydroxycarbazole + HO2 have the total potential barriers of −33.33 ~ −27.84 kcal mol−1, and are strongly exothermic by −86.02 ~ −83.45 kcal mol−1. These indicate that the degradation pathways leading to the hydroxycarbazoles formation are easy to occur under the real atmospheric conditions. A comparison of the reaction pathways presented in Figure 3 shows that the formation of 3-hydroxycarbazole (P4) from carbazole + OH + O2 → hydroxycarbazole + HO2 has the lowest total potential barriers of −33.33 kcal mol−1 and is energetically most favorable relative to the other H abstraction pathways, which means that 3-hydroxycarbazole is the main hydroxycarbazole product.
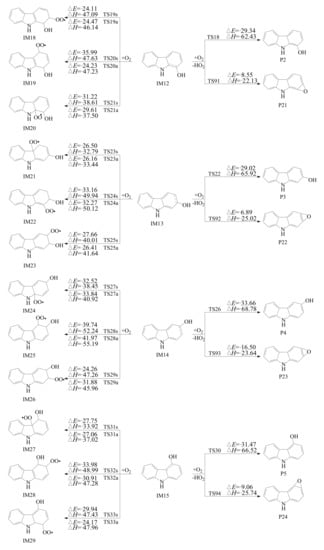
Figure 3.
The O2 addition and abstraction scheme of carbazole-OH adducts embedded with the potential barriers ΔE (in kcal mol−1) and reaction heats ΔH (in kcal mol−1, 0 K).
Secondly, carbazole-OH adducts may react with O2 via H abstractions from the O-H bonds to produce carbazole-epoxides and HO2 radical. As shown in Figure 3, the carbazole-epoxide formations from the H abstractions of the O-H bonds have the potential barriers of −16.5 ~ −6.89 kcal mol−1, which are higher than the hydroxycarbazole formations from the H abstractions of the C-H bonds. In addition, the carbazole-epoxides formation from the H abstractions of the O-H bonds is exothermic by −25.74 ~ −22.13 kcal mol−1, which is less than the hydroxycarbazoles formation from the H abstractions of the C-H bonds. This means that hydroxycarbazole formation is energetically more preferred than carbazole-epoxide formation, e.g., H abstraction from carbazole-OH with O2 on the C-H bond is easier to occur than that on the O-H bond. This may be caused by the differences in bond length and bond energy between the C-H bond and O-H bond in carbazole-OH intermediates. It can be seen from Table S4 that the bond energies of O-H bonds are 40.30 ~ 45.13 kcal mol−1 higher than those of C-H bonds and the bond lengths are about 0.14 angstrom shorter than the C-H bonds, which indicates that the break of C-H bonds is more favorable than that of O-H bonds.
Thirdly, O2 can associate with the C atoms in the OH-carbazole adducts with an unpaired electron from two different directions: the anti- or syn-position with respect to the -OH group. Therefore, as depicted in Figure 3, the O2 addition reactions in the carbazole-OH adducts can form a series of carbazole-OH-O2 adducts (namely IM18s/a-IM29s/a, where ‘s’ denotes syn-position and ‘a’ denotes anti-position O2 addition). The formation of carbazole-OH-O2 adducts IM19, IM22, IM25, and IM28 from precursors IM12, IM13, IM14, and IM15 have lower potential barriers and are more exothermic compared to those of other O2-OH-carbazole isomers. The potential barriers characterizing the formation of syn-position radicals are lower by 0.34 ~ 11.76 kcal mol−1 than those for the formation of anti-position O2-OH-carbazole, except for IM18s/a, IM24s/a, IM25s/a, and IM26s/a. In most cases, O2 additions at syn-positions are energetically favored over those at anti-positions. Therefore, the formations of anti-isomers are of minor importance and will not be further discussed. In addition, considering IM12 is the most favored carbazole-OH adducts, the IM12-O2 adducts (IM18s/a-IM20s/a) will be further discussed.
The scheme of reaction from the H abstraction product of carbazolide anion (IM5) with O2 is depicted in Figure 4. As shown in Figure 4, O2 may be added to the N atom of IM5 containing unpaired electrons to form OH-carbazole-O2 adduct IM6. This process is barrierless and strongly exothermic.

Figure 4.
The reaction scheme of IM5 is embedded with the potential barriers ΔE (in kcal mol−1) and the reaction heats ΔH (in kcal mol−1, 0 K) in the presence of NO and O2.
3.2.2. Subsequent Reaction of Carbazole-OH-O2 Intermediate
- (1)
- Intramolecular H-transfer reaction
As shown in Figure 5, the intramolecular H transfer includes four modes: (1) H transfer from the -CH group to the -OO peroxyl group through a four-membered ring transition state (H-transferCH4); (2) H transfer from the -CH group to the -OO peroxyl group through a five-membered ring transition state (H-transferCH5); (3) H transfer from the O-H group to the -OO peroxyl group through a six-membered ring transition state (H-transferOH); (4) H transfer from the -NH group to the -OO peroxyl group through a five-membered ring transition state (H-transferNH).
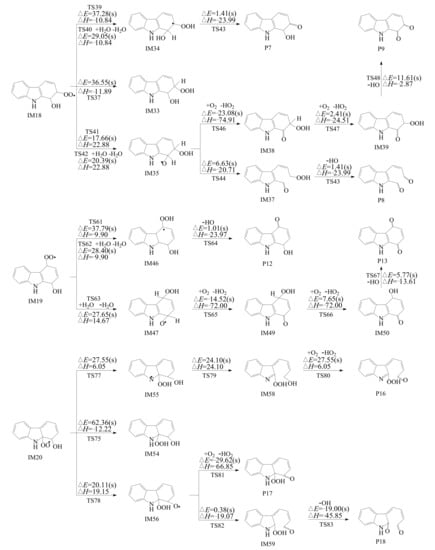
Figure 5.
Intramolecular H-transfer reaction of carbazole-OH-O2 adducts embedded with the potential barrier ΔE (in kcal mol−1) and reaction heat ΔH (in kcal mol−1, 0 K) with and without H2O molecules.
Water molecules can participate in the H-transfer reactions. In TS40 and TS62, water molecules can reduce the potential barriers of the H-transferCH4, which means water molecules promote the H-transferCH4 reactions. In TS42, the water molecule can increase the potential barrier of the H-transferCH4, which indicates water molecule inhibits the H-transferOH. A comparison of all the four H-transfer modes shows that ortho H-transferOH (TS41 and TS78) have lower potential barriers (17.66 kcal mol−1 for TS41 and 20.11 kcal mol−1 for TS78) and is thermodynamically more favorable than other H-transfer modes, resulting in the formation of IM35 and IM56. The secondary reaction from the IM35 and IM56 involves two kinds of pathways. Firstly, IM35 and IM56 can undergo C-C bond cleavage and elimination of OH to form dialdehyde (P8 and P18). Secondly, IM35 can be attacked by O2 to form IM39 and HO2. IM39 can be further attacked by O2 and eliminate OH to form carbazolequinone (P9). IM56 can be attacked by O2 to form hydroperoxyl-carbazole-one (P17).
- (2)
- NO addition reaction
Carbazole-OH-O2 intermediates have unpaired electrons, which can react with NO to form carbazole-OH-OONO adducts. As shown in Figure 6, the reactions of NO addition are barrierless and exothermic. Then, the carbazole-OH-OONO adducts may undergo NO2 elimination reactions and produce intermediates IM40, IM51, and IM60. However, these processes are difficult to occur in atmospheric conditions due to the more than 40 kcal mol−1 potential barriers. Carbazole-OH-OONO adducts can also remove HNO2 to form hydroxy-carbazole-one (P7 and P12). These elementary reactions are more competitive in atmospheric reaction than NO2 removal.
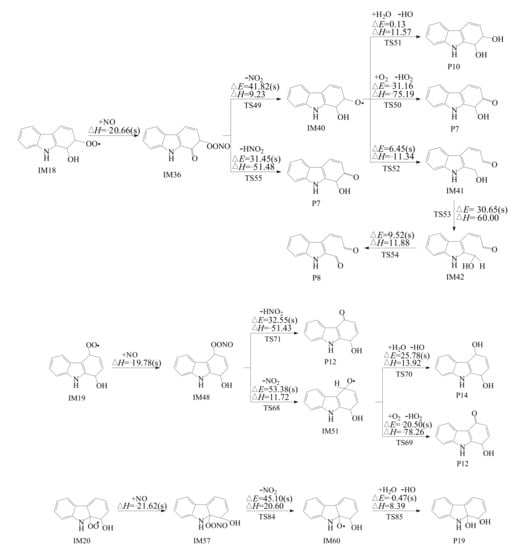
Figure 6.
Intramolecular H-transfer reaction of carbazole-OH-O2 adducts embedded with the potential barrier ΔE (in kcal mol−1) and reaction heat ΔH (in kcal mol−1, 0 K) with and without H2O molecular.
Figure 4 shows the subsequent abstraction reaction of IM5 with O2. IM6 can undergo an addition reaction with NO and O-ONO bond cleavage to form NO2 and IM10 adduct, followed by a ring-opening or reaction with the water molecule. Those processes can provide H for unpaired electrons of O to form the product carbazole-ol (P1). The former has high potential barriers (35.21 kcal mol−1), which is not easy to occur in the atmosphere, so it is not discussed further. The latter is a barrierless reaction, which is more favorable in the atmospheric environment.
- (3)
- Reactions of bicyclic peroxy radicals
The intramolecular cyclization reaction scheme of carbazole-OH-O2 adducts IM18, IM19, and IM20 are illustrated in Figure 7. Four- or five-membered cycle intermediates are formed in the reactions. The formation of five-membered cycle IM32 from the isomerization of IM18 and IM20 is predominating over the others by comparison of the potential barriers and reaction heats. Similarly, the formation of the five-membered cycle IM31 from the isomerization of IM19 is dominated over the formations of IM43-IM45. However, the formation of IM31 from isomerization of IM19 is endothermic by 9.71 kcal mol−1 with high potential barriers of 34.54 kcal mol−1, which indicates that the formation of IM31 is not feasible under common atmospheric conditions. The formations of IM32 from IM18 and IM20 have much lower potential barriers (21.38 and 12.93 kcal mol−1) than the formation of IM31 from IM19. Thus, IM32 is a possible bicyclic peroxy radical in the oxidation process of gaseous carbazole and will be further discussed.

Figure 7.
Intramolecular cyclization and H addition reaction scheme of IM18, IM19 and IM20 embedded with the potential barriers ΔE (in kcal mol−1) and reaction heats ΔH (in kcal mol−1, 0 K) in the presence of H2O.
As shown in Figure 8, IM32 can undergo isomerization through O-O bond cleavage or recombine O2, forming the bicyclic peroxy radical (IM61 and IM62). However, the potential barrier of O-O bond cleavage is 44.50 kcal mol−1 higher than addition reactions with O2. Therefore, the cleavage of O-O bonds can possibly compete with the O2 addition reactions only under the condition of extremely low O2 concentration. The subsequent reaction of IM61 involves five elementary reactions: addition reaction with NO, NO2 removal and the cleavage of C-C bond to produce ring-opening radical, the barrierless O-O bond and C-H bond cleavages and the elimination of CHOCHOH radical to form P20.

Figure 8.
The reaction scheme of IM32 embedded with the potential barriers ΔE (in kcal mol−1) and reaction heats ΔH (in kcal mol−1, 0 K) in the presence of NO and O2.
As depicted in Figure 4, IM6 may also combine the C-O bond to form a four- or five- membered ring intermediates IM8 and IM9. However, the formation of IM8 and IM9 are endothermic and need to cross potential barriers of more than 50 kcal mol−1. Consequently, IM8 and IM9 are generally unimportant in the atmosphere and will not be discussed further. The intermediate IM5 is not easy to undergo cyclization reaction. Figure 3 shows the reaction pathways of carbazole-OH adducts (IM12-IM15) with O2. Firstly, carbazole-OH adducts can react via a simple metathesis mechanism to yield hydroxycarbazoles and HO2 radical through H abstractions from the C-H bonds. The potential barriers of these hydroxycarbazole formation processes are in the range of −33.66 ~ −29.03 kcal mol−1. The processes are strongly exothermic by −68.78 ~ −62.43 kcal mol−1. The overall reactions, carbazole + OH + O2 → hydroxycarbazole + HO2 have the total potential barriers of −33.33 ~ −27.84 kcal mol−1, and are strongly exothermic by −86.02 ~ −83.45 kcal mol−1. These indicate that the degradation pathways leading to the hydroxycarbazoles formation are easy to occur under the real atmospheric conditions. A comparison of the reaction pathways presented in Figure 3 shows that the formation of 3-hydroxycarbazole (P4) from carbazole + OH + O2 → hydroxycarbazole + HO2 has the lowest total potential barriers of −33.33 kcal mol−1 and is energetically most favorable relative to the other H abstraction pathways, which means that 3-hydroxycarbazole is the main hydroxycarbazole product.
Thirdly, O2 can associate with the C atoms in the OH-carbazole adducts with an unpaired electron from two different directions: the anti- or syn-position with respect to the -OH group. Therefore, as depicted in Figure 3, the O2 addition reactions in the carbazole-OH adducts can form a series of carbazole-OH-O2 adducts (namely IM18s/a-IM29s/a, where ‘s’ denotes syn-position and ‘a’ denotes anti-position O2 addition). The formation of carbazole-OH-O2 adducts IM19, IM22, IM25, and IM28 from precursors IM12, IM13, IM14, and IM15 have lower potential barriers and are more exothermic compared to those of other O2-OH-carbazole isomers. The potential barriers characterizing the formation of syn-position radicals are lower by 0.34 ~ 11.76 kcal mol−1 than those for the formation of anti-position O2-OH-carbazole, except for IM18s/a, IM24s/a, IM25s/a, and IM26s/a. In most cases, O2 additions at syn-positions are energetically favored over those at anti-positions. Therefore, the formations of anti-isomers are of minor importance and will not be further discussed. In addition, considering IM12 is the most favored carbazole-OH adducts, the IM12-O2 adducts (IM18s/a-IM20s/a) will be further discussed.
3.3. Rate Constant Calculations
In order to clarify the transport and atmospheric fate of carbazole, it is of great significance to calculate the accurate rate constants of the elementary reactions involved in the atmospheric oxidation degradation of carbazole. On the basis of energy data obtained at M06-2X/6-311+G(3df,2p)//M06-2X/6-311+G(d,p) level, the rate constants of elementary reactions were calculated by using RRKM theory [22,23] and MESMER program at 298 K and 1 atm [33]. The previous studies for the PAHs and dioxin-like compound degradation initiated by OH radical have successfully proved the accuracy of RRKM theory to calculate the rate constants for elementary reactions of PAHs and dioxin-like compound degradations [26,41,42,43]. Thus, it might be inferred that RRKM theory individual rate constants are reasonable in this paper. The calculated rate constants for the elementary reactions in the oxidative degradation of carbazole initiated by OH at 298 K and 1 atm are listed in Table 1.

Table 1.
RRKM calculated rate constants (in cm3 molecule−1 s−1) for the elementary reactions involved in the OH-initiated oxidation degradation of carbazole at 298 K and 1 atm.
In Table 1, the individual rate constants for the H abstraction by OH radical of the C1-H, C2-H, C3-H, C4-H, and N-H bonds of carbazole are noted as k1, k2, k3, k4, and k5, respectively. In the H abstraction reactions, the H abstraction from the N-H bond has the maximum rate constant of 3.70 × 10−12 cm3 molecule−1 s−1, which is four orders of magnitude higher than those from the C-H bond. The overall rate constant of the OH abstraction reaction is denoted as kabs, kabs = (k1 + k2 + k3 + k4) × 2 + k5. The overall rate constant kabs is 3.70 × 10−12 cm3 molecule−1 s−1 at 298 K and 1 atm. The individual rate constants for the OH addition to the C1, C2, C3, C4, C10, and C11 bonds of carbazole are noted as k6, k7, k8, k9, k10 and k11, respectively. In the OH addition reactions, the OH addition on C1 has the maximum rate constant of 1.23 × 10−12 cm3 molecule−1 s−1. The overall rate constant of the OH addition reaction is denoted as kadd, kadd = (k6 + k7 + k8 + k9 + k10 + k11) × 2. The overall rate constant kadd is 2.82 × 10−12 cm3 molecule−1 s−1 at 298 K and 1 atm. The overall rate constant of carbazole with OH is denoted as k, k = kabs + kadd. The overall rate constant k is 6.52 × 10−12 cm3 molecule−1 s−1 at 298 K and 1 atm.
Comparison of the k5 and k1, k5, and k1 show that the H abstraction from the N-H bond and OH addition to the C1 atom are competitive. This agrees well with the thermal analysis above. The OH addition on C1 and H abstraction on N atoms account for 94% of the total reaction rate.
According to the overall rate constants of the reaction of carbazole with OH radical and the average OH concentration in the atmosphere COH = 11.3 × 105 molecule cm−3 [3,18], from the formula:
the atmospheric lifetime of carbazole determined by OH radical is calculated as 37.70 h.
Due to the absence of the available experimental values, it is difficult to make a direct comparison of the calculated rate constants with the experimental data for the reaction of carbazole with OH radical. Therefore, a comparison of the lifetime for the reaction of carbazole and OH radical with the literature values of the other five kinds of NSO-HET compounds and OH radical, and the results are displayed in Table S5. Wallington and Atkinson et al. measured the rate constant of pyrrole and indole with OH were (1.03 ± 0.06) × 10−10 cm3 molecule−1 s−1 and (1.54 ± 0.35) × 10−10 cm3 molecule−1 s−1 [28,29], respectively, which were lower than that of carbazole with OH. This may have originated from the different reaction activities and positions of OH attack from the benzene ring and hetero cyclopentadiene ring. It can be seen from Table S5 that the rate constant of carbazole with OH is similar to that of dibenzothiophene with OH [27], and lower than those of dibenzofuran/fluorene with OH radical [24,26]. The N/S substitution of NSO-HETs can decrease the reactivity of the reaction with OH radical and increase the rate constant than that from the O/C substitution. Jenkin et al. calculated the group rate constants for OH addition to an unsubstituted carbon (karom) and to a methyl-substituted carbon (kipso) are 2.00 × 10−13 and 2.80 × 10−13 cm3 molecule−1 s−1, which are lower than OH addition of carbazole (kadd = 2.82 × 10−12 cm3 molecule−1 s−1) [44].
4. Conclusions
In this study, we investigated the atmospheric oxidation of carbazole initiated by OH radical with quantum chemical calculation methods. The mechanism of the formation of main products was determined. In addition, the rate constants and lifetimes for the vital reactions of carbazole degradation by OH radical were evaluated. The rate constants and lifetime of carbazole were compared with those of the other five kinds of NSO-HETs. The impact of structure and element substitution of NSO-HETs on the lifetimes was also discussed. This work provides a better understanding of the reactivity of carbazole in the atmospheric environment and the chemical degradation and removal of carbazole in the atmosphere. The main conclusions are as follows:
- There are four types of reactions for the degradation of carbazole initiated by OH radical: OH additions to “bend” C atoms, OH additions to “benzene ring” C atoms, H abstractions from C-H bonds, and the H abstraction from N-H bond. Among them, OH additions to “bend” C atoms and H abstractions from C-H bonds are energetically unfavorable. The best pathway of OH addition reactions is OH addition to C1 atom, which is competitive with the H abstraction from the N-H bond.
- The primary products of carbazole oxidation by OH radicals in the atmosphere include hydroxycarbazole, dialdehyde, carbazolequinone, carbazole-ol, hydroxy-carbazole-one, and hydroperoxyl-carbazole-one.
- The degradation of carbazole in the atmosphere is significant, for which the rate constant determined by OH radical is 6.52 × 10−12 cm3 molecule−1 s−1 and the lifetime is 37.70 h. The OH addition on C1 and H abstraction on N atom account for 94% of the total reaction rate. The ranking of the rate constant for the reaction of NSO-HETs with OH is as follows: Carbazole ≈ Dibenzothiophene < Dibenzofuran ≈ Fluorene < Pyrrole ≈ Indole.
Supplementary Materials
The following Supplementary Materials can be downloaded at: https://www.mdpi.com/article/10.3390/atmos13071129/s1, Table S1. Comparison of the geometrical parameters of carbazole degradation by OH radical in M06-2X method with those of other 5 kinds of methods. Table S2. Imaginary frequencies (in cm−1), zero-point energies (ZPE, in a.u.) and total energies (in a.u.) for the transition states involved in the oxidation reaction of carbazole initiated by OH radical. Table S3. Cartesian coordinates for the transition states in the oxidation reaction of carbazole initiated by OH radical. Table S4. Calculated bond lengths (angstrom) and bond energies (kcal mol−1) of C-H bonds and O-H bonds in the intermediates IM12-IM15 at M06-2X/6-311++G(3df,2p)//M06-2X/6-311+G(d,p) level. Table S5. Comparison of the rate constants and lifetime of carbazole degradation by OH radical with those of other 5 kinds of NSO-HETs. Table S6. RRKM calculated rate constants (in cm3 molecule−1 s−1) for TS1, TS5 and TS14 involved in the OH-initiated oxidation degradation of carbazole at 298 K and 10−670 torr.
Author Contributions
Conceptualization, Z.T. and F.X.; Data curation, Z.T. and Y.H.; Formal analysis, Z.T.; Funding acquisition, X.W.; Investigation, Q.Z.; Methodology, Y.S.; Project administration, X.W., M.H.H. and X.Z.; Resources, X.W.; Software, M.H.H. and Q.Z.; Supervision, F.X.; Visualization, X.Z. and H.W.; Writing—original draft, Y.H. and H.W.; Writing—review & editing, Q.Z. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Natural Science Foundation of China (project No. 21876102, 21976107, 22076103), Taishan Scholars (No. ts201712003), the Fundamental Research Funds of Shandong University (project No. 2016WLJH51, 2017JC033).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johansen, S.S.; Hansen, A.B.; Mosbaek, H.; Arvin, E. Identification of Heteroaromatic and Other Organic Compounds in Ground Water at Creosote-Contaminated sites in Denmark. Ground Water Monit. Remediat. 1997, 17, 106–115. [Google Scholar] [CrossRef]
- World Health Organization. Iarc Monographs on the Evaluation of Carcinogenic Risks to Humans Preamble. Monogr. Eval. Carcinog. Risks Hum. 2008, 97, 9–38. [Google Scholar]
- Tovar, C.M.; Barnes, I.; Bejan, I.G.; Wiesen, P. Kinetic study of the atmospheric oxidation of a series of epoxy compounds by OH radicals. Atmos. Chem. Phys. 2022, 22, 6989–7004. [Google Scholar] [CrossRef]
- Eisentraeger, A.; Brinkmann, C.; Hollert, H.; Sagner, A.; Tiehm, A.; Neuwoehner, J. Heterocyclic Compounds: Toxic Effects Using Algae, Daphnids, and the Salmonella/Microsome Test Taking Methodical Quantitative Aspects into Account. Environ. Toxicol. Chem. 2008, 27, 1590–1596. [Google Scholar] [CrossRef]
- Esen, F.; Tasdemir, Y.; Cindoruk, S.S. Dry Deposition, Concentration and Gas/Particle Partitioning of Atmospheric Carbazole. Atmos. Res. 2010, 95, 379–385. [Google Scholar] [CrossRef]
- Roy, J.; Jana, A.K.; Mal, D. Recent Trends in the Synthesis of Carbazoles: An Update. Tetrahedron 2012, 68, 6099–6121. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sakazaki, Y.; Eguchi, Y.; Suetomi, R.; Nakamura, E. Identification of Chemical Substances in Industrial Wastes and Their Pyrolytic Decomposition Products. Chemosphere 2005, 59, 1343–1353. [Google Scholar] [CrossRef]
- Glarborg, P.; Jensen, A.D.; Johnsson, J.E. Fuel Nitrogen Conversion in Solid Fuel Fired Systems. Prog. Energ. Combust. 2003, 29, 89–113. [Google Scholar] [CrossRef]
- Fromme, H.; Mi, W.; Lahrz, T.; Kraft, M.; Aschenbrenner, B.; Bruessow, B.; Ebinghaus, R.; Xie, Z.; Fembacher, L. Occurrence of Carbazoles in Dust and Air Samples from Different Locations in Germany. Sci. Total Environ. 2018, 610–611, 412–418. [Google Scholar] [CrossRef]
- Parette, R.; McCrindle, R.; McMahon, K.S.; Pena-Abaurrea, M.; Reiner, E.; Chittim, B.; Riddell, N.; Voss, G.; Dorman, F.L.; Pearson, W.N. Halogenated Indigo Dyes: A Likely Source of 1,3,6,8-Tetrabromocarbazole and Some Other Halogenated Carbazoles in the Environment. Chemosphere 2015, 127, 18–26. [Google Scholar] [CrossRef]
- Campbell, N.; Barclay, B.M. Recent Advances in the Chemistry of Carbazole. Chem. Rev. 1947, 40, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Y.M.; Kara, M.; Dumanoglu, Y.; Odabasi, M.; Elbir, T. Source Apportionment of Polycyclic Aromatic Hydrocarbons (PAHs) and Polychlorinated Biphenyls (PCBs) in Ambient Air of an Industrial Region in Turkey. Atmos. Environ. 2014, 97, 271–285. [Google Scholar] [CrossRef]
- Shi, S.N.; Qu, Y.Y.; Ma, F.; Zhou, J.T. Bioremediation of Coking Wastewater Containing Carbazole, Dibenzofuran and Dibenzothiophene by Immobilized Naphthalene-Cultivated Arthrobacter sp. W1 in Magnetic Gellan Gum. Bioresour. Technol. 2014, 166, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yu, B.; Li, F.L.; Cai, X.F.; Ma, C.Q. Microbial Degradation of Sulfur, Nitrogen and Oxygen Heterocycles. Trends Microbiol. 2006, 14, 398–405. [Google Scholar] [CrossRef]
- Larentis, A.L.; Sampaio, H.C.C.; Carneiro, C.C.; Martins, O.B.; Alves, T.L.M. Evaluation of Growth, Carbazole Biodegradation and Anthranilic Acid Production by Pseudomonas Stutzeri. Braz. J. Chem. Eng. 2011, 28, 37–44. [Google Scholar] [CrossRef]
- Nojiri, H.; Omori, T. Carbazole Metabolism by Pseudomonads. In Pseudomonas: A Model System in Biology; Filloux, A., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 107–145. [Google Scholar]
- Heim, S.; Schwarzbauer, J.; Kronimus, A.; Littke, R.; Woda, C.; Mangini, A. Geochronology of Anthropogenic Pollutants in Riparian Wetland Sediments of the Lippe River (Germany). Org. Geochem. 2004, 35, 1409–1425. [Google Scholar] [CrossRef]
- Lelieveld, J.; Gromov, S.; Pozzer, A.; Taraborrelli, D. Global tropospheric hydroxyl distribution, budget and reactivity. Atmos. Chem. Phys. 2016, 16, 12477–12493. [Google Scholar] [CrossRef] [Green Version]
- Keyte, I.J.; Harrison, R.M.; Lammel, G. Chemical Reactivity and Long-Range Transport Potential of Polycyclic Aromatic Hydrocarbons-A Review. Chem. Soc. Rev. 2013, 42, 9333–9391. [Google Scholar] [CrossRef]
- Kurylo, M.J.; Orkin, V.L. Determination of Atmospheric Lifetimes via the Measurement of OH Radical Kinetics. Chem. Rev. 2003, 103, 5049–5076. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D. Study an the Generated Mechanism of Carbazole Nitroxyl Radical via Photocatalysis Oxidation of Carbazole. Acta Phys. Chim. Sin. 1995, 11, 1014–1019. [Google Scholar]
- Truhlar, D.G.; Garrett, B.C.; Klippenstein, S.J. Current Status of Transition-State Theory. J. Phys. Chem. 1996, 100, 12771–12800. [Google Scholar] [CrossRef]
- Rice, O.K.; Ramsperger, H.C. Theories of Unimolecular Gas Reactions at Low Pressures. J. Am. Chem. Soc. 1927, 49, 1617–1629. [Google Scholar] [CrossRef]
- Altarawneh, M.; Kennedy, E.M.; Dlugogorski, B.Z.; Mackie, J.C. Computational Study of the Oxidation and Decomposition of Dibenzofuran Under Atmospheric Conditions. J. Phys. Chem. A 2008, 112, 6960–6967. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.V.; Nguyen, H.T.; Huynh, L.K. Ab initio Kinetic Mechanism of OH-initiated Atmospheric Oxidation of Pyrrole. Chemosphere 2021, 263, 127850. [Google Scholar] [CrossRef]
- Ding, Z.Z.; Yi, Y.Y.; Zhang, Q.Z.; Zhuang, T. Theoretical Investigation on Atmospheric Oxidation of Fluorene Initiated by OH Radical. Sci. Total Environ. 2019, 669, 920–929. [Google Scholar] [CrossRef]
- Kwok, E.S.C.; Atkinson, R.; Arey, J. Kinetics of the Gas-phase Reactions of Dibenzothiophene with OH radicals, NO3 Radicals, and O3. Polycycl. Aromat. Comp. 1999, 13, 175–189. [Google Scholar] [CrossRef]
- Wallington, T.J. Kinetics of the gas-phase reaction of OH radicals with Pyrrole and Thiophene. Int. J. Chem. Kinet. 1986, 18, 487–496. [Google Scholar] [CrossRef]
- Atkinson, R.; Tuazon, E.C.; Arey, J.; Aschmann, S.M. Atmospheric and Indoor Chemistry of Gas-phase Indole, Quinoline, and Isoquinoline. Atmos. Environ. 1995, 29, 3423–3432. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09W, Revision A. 02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Fukui, K. The Path of Chemical Reactions-The IRC Approach. Accounts Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Glowacki, D.R.; Liang, C.H.; Morley, C.; Pilling, M.J.; Robertson, S.H. Mesmer: An Open-source Master Equation Solver for Multi-energy Well Reactions. J. Phys. Chem. A 2012, 116, 9545–9560. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.G.; Smith, S.C. Theory of Unimolecular and Recombination Reactions; Blackwell Science Inc.: Hoboken, NY, USA, 1990; pp. 341–350. [Google Scholar]
- Lee, S.Y.; Boo, B.H. Molecular Structures and Vibrational Spectra of Pyrrole and Carbazole by density Functional Theory and Conventional Ab Initio Calculations. J. Phys. Chem. 1996, 100, 15073–15078. [Google Scholar] [CrossRef]
- Batra, I.P.; Bagus, P.S.; Clementi, E.; Seki, H. Ab-initio Calculations for Electronic-structure of Carbazole and Trinitrofluorenone. Theoret. Chim. Acta 1974, 32, 279–293. [Google Scholar] [CrossRef]
- Lorenz, K.; Zellner, R. Kinetics of the reactions of OH-radicals with benzene, benzene-d6 and naphthalene. Ber. Bunsenges. Phys. Chem. 1983, 87, 629–636. [Google Scholar] [CrossRef]
- Ananthula, R.; Yamada, T.; Taylor, P.H. Kinetics of OH Radical Reaction with Anthracene and Anthracene-d10. J. Phys. Chem. A 2006, 110, 3559–3566. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Stevens, P.S.; Hites, R.A. Rate Constants for the Gas-phase Reactions of Methylphenanthrenes with OH as a Function of Temperature. J. Phys. Chem. A 2003, 107, 6603–6608. [Google Scholar] [CrossRef]
- Ananthula, R.; Yamada, T.; Taylor, P.H. Kinetics of OH Radical Reaction with Phenanthrene: New Absolute Rate Measurements and Comparison with Other PAHs. Int. J. Chem. Kinet. 2007, 39, 629–637. [Google Scholar] [CrossRef]
- Dang, J.; Zhang, Q.Z. Gas-phase Reaction of Benzo a Anthracene with Hydroxyl Radical in the Atmosphere: Products, Oxidation Mechanism, and Kinetics. J. Mol. Model. 2018, 24, 320. [Google Scholar] [CrossRef]
- Dang, J.; Shi, X.L.; Zhang, Q.Z.; Wang, W.X. Theoretical Perspectives on the Mechanism and Kinetics of the OH Radical-initiated Gas-phase oxidation of PCB126 in the Atmosphere. Sci. Total Environ. 2015, 517, 1–9. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, Q.; Wang, W. Atmospheric Oxidation of Phenanthrene Initiated by OH Radicals in the Presence of O2 and NOx-A Theoretical Study. Sci. Total Environ. 2016, 563, 1008–1015. [Google Scholar] [CrossRef]
- Jenkin, M.E.; Valorso, R.; Aumont, B.; Rickard, A.R.; Wallington, T.J. Estimation of Rate Coefficients and Branching Ratios for Gas-phase Reactions of OH with Aromatic Organic Compounds for Use in Automated Mechanism Construction. Atmos. Chem. Phys. 2018, 18, 9329–9349. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).